Abstract
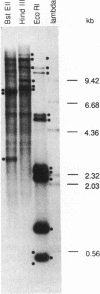
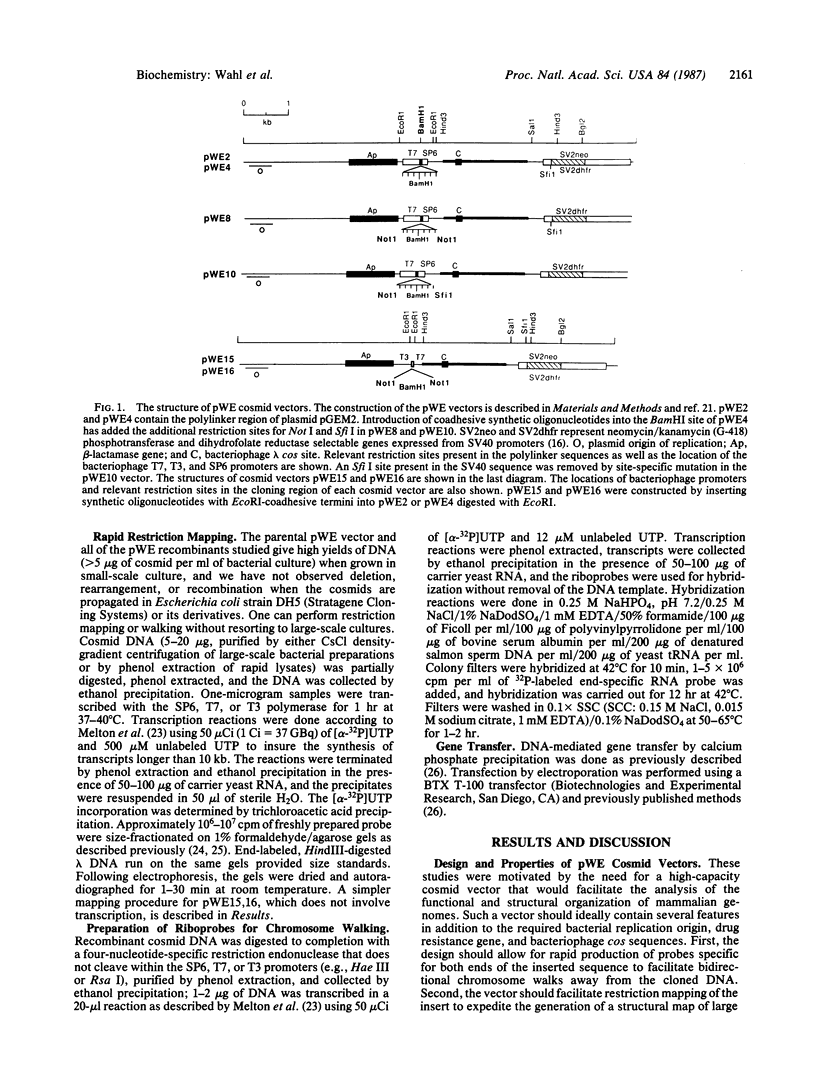
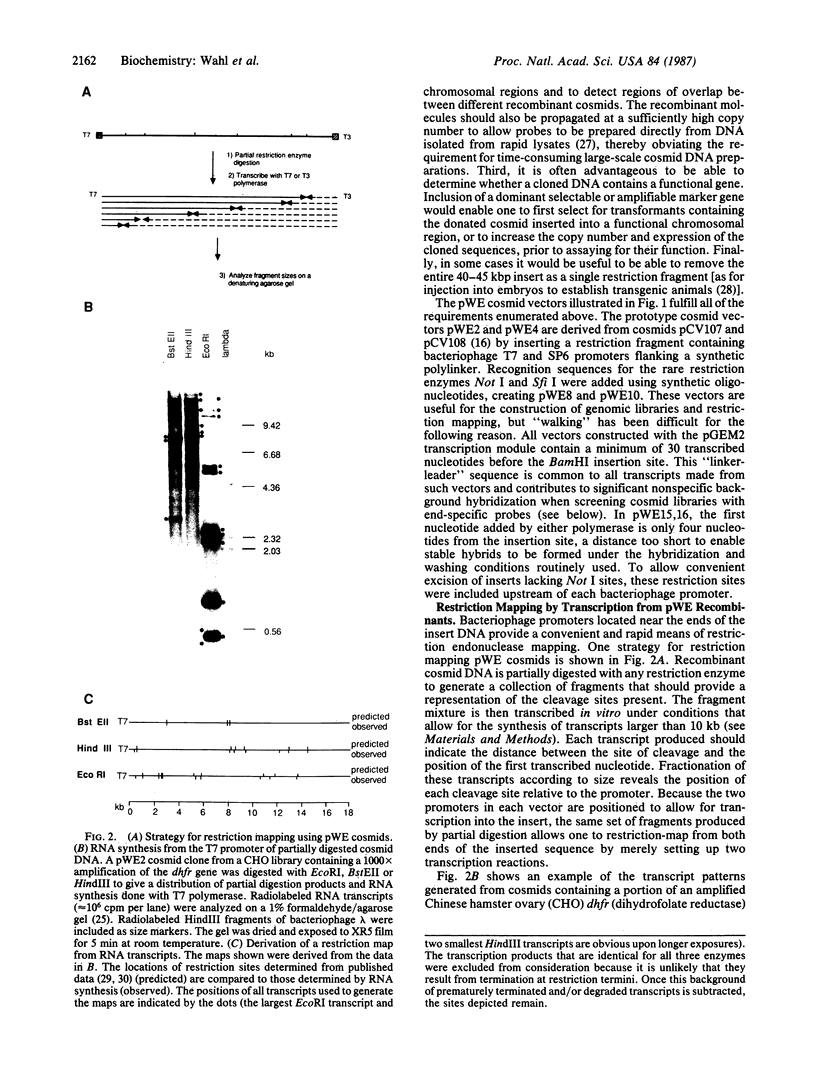
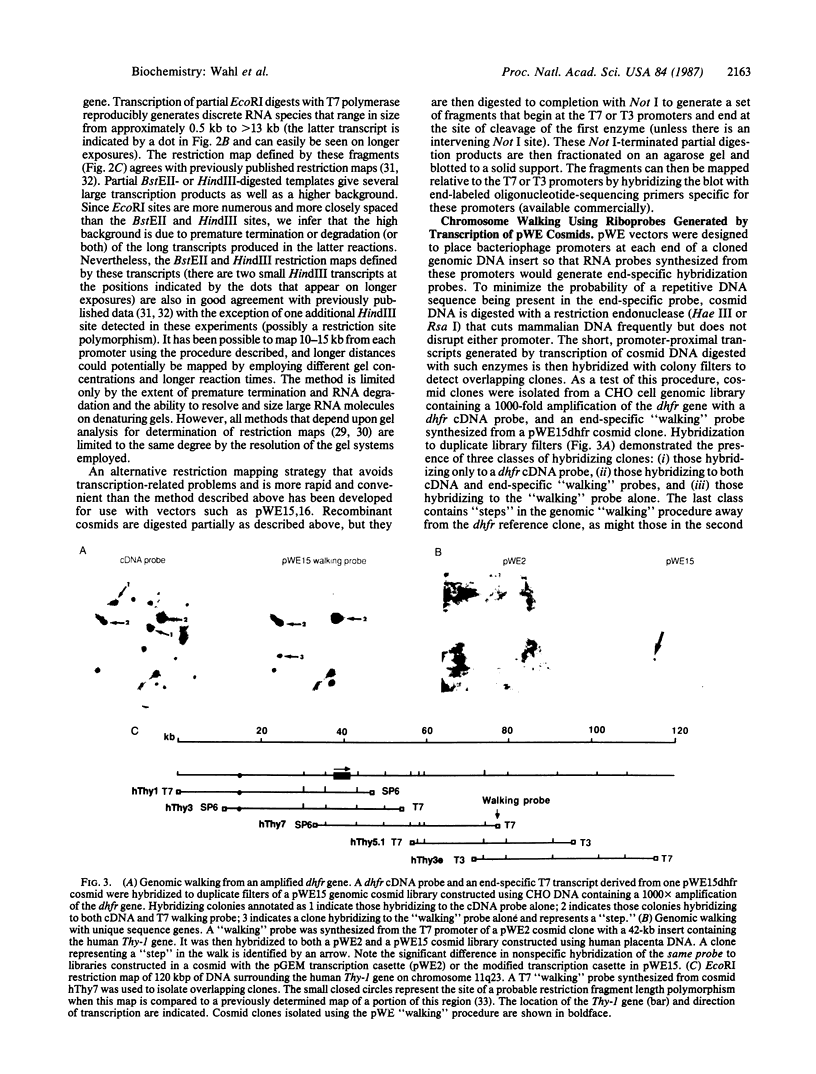
We have designed cosmid vectors for rapid genomic "walking" and restriction mapping. These vectors contain the transcription promoters from either bacteriophage SP6, T7, or T3 flanking a unique BamHI cloning site. Mammalian expression modules encoding the dominant marker neomycin phosphotransferase or the amplifiable dihydrofolate reductase gene expressed from SV40 promoters were inserted for use in gene transfer studies. Restriction sites for the enzymes Not I and Sfi I, which cut mammalian DNA very infrequently, have been engineered near the transcriptional promoters to enable the excision of most inserts as single, full-length fragments. Genomic libraries representative of mouse, human, and hamster genomes were constructed by inserting 33- to 44-kilobase-pair (kbp) DNA fragments, generated by partial cleavage of genomic DNA with Mbo I or Sau3A, into the unique BamHI site. Digestion of recombinant cosmids with restriction enzymes that cleave frequently but do not disrupt the transcriptional promoters generates two small DNA templates for the synthesis of end-specific RNA probes to facilitate directional "walking." Cosmid restriction maps can be determined rapidly by one of several methods. The cosmids and methods we describe should have wide utility in determining the functional and structural organization of complex eukaryotic genomes and for physically linking distant genetic loci.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Ellis N., Chasin L. A. Structure of the dihydrofolate reductase gene in Chinese hamster ovary cells. Nucleic Acids Res. 1983 Apr 11;11(7):1997–2012. doi: 10.1093/nar/11.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- Chia W., Scott M. R., Rigby P. W. The construction of cosmid libraries of eukaryotic DNA using the Homer series of vectors. Nucleic Acids Res. 1982 Apr 24;10(8):2503–2520. doi: 10.1093/nar/10.8.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Ingraham H. A., Lewis K., Cunningham K., Seki T., Moriuchi T., Chang H. C., Silver J., Hyman R. Expression of the Thy-1 glycoprotein gene by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5532–5536. doi: 10.1073/pnas.81.17.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Fox H. S., Martin G. R., Lyon M. F., Herrmann B., Frischauf A. M., Lehrach H., Silver L. M. Molecular probes define different regions of the mouse t complex. Cell. 1985 Jan;40(1):63–69. doi: 10.1016/0092-8674(85)90309-5. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J. F., Wexler N. S., Conneally P. M., Naylor S. L., Anderson M. A., Tanzi R. E., Watkins P. C., Ottina K., Wallace M. R., Sakaguchi A. Y. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983 Nov 17;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Lawless G. M., Evans G. A. The mouse Thy-1.2 glycoprotein gene: complete sequence and identification of an unusual promoter. J Immunol. 1986 Feb 15;136(4):1482–1489. [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Versatile cosmid vectors for the isolation, expression, and rescue of gene sequences: studies with the human alpha-globin gene cluster. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5225–5229. doi: 10.1073/pnas.80.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Little P. F., Cross S. H. A cosmid vector that facilitates restriction enzyme mapping. Proc Natl Acad Sci U S A. 1985 May;82(10):3159–3163. doi: 10.1073/pnas.82.10.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T., Grosveld F. G., Flavell R. A. Isolation of transforming DNA by cosmid rescue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):520–524. doi: 10.1073/pnas.79.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Guild G. M., Prestidge L. S., Hogness D. S. A new high-capacity cosmid vector and its use. Gene. 1980 Nov;11(3-4):271–282. doi: 10.1016/0378-1119(80)90067-0. [DOI] [PubMed] [Google Scholar]
- Montoya-Zavala M., Hamlin J. L. Similar 150-kilobase DNA sequences are amplified in independently derived methotrexate-resistant Chinese hamster cells. Mol Cell Biol. 1985 Apr;5(4):619–627. doi: 10.1128/mcb.5.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Murialdo H., Delius H., Chai J. H., Poustka A., Frischauf A., Lehrach H. Analysis of cosmids using linearization by phage lambda terminase. Gene. 1985;40(2-3):259–266. doi: 10.1016/0378-1119(85)90048-4. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Schmidtke J., Watson E. A., Law H. Y., Farrall M., Cooke H. J., Eiberg H., Williamson R. Localization of cystic fibrosis locus to human chromosome 7cen-q22. 1985 Nov 28-Dec 4Nature. 318(6044):384–385. doi: 10.1038/318384a0. [DOI] [PubMed] [Google Scholar]
- White R., Woodward S., Leppert M., O'Connell P., Hoff M., Herbst J., Lalouel J. M., Dean M., Vande Woude G. A closely linked genetic marker for cystic fibrosis. 1985 Nov 28-Dec 4Nature. 318(6044):382–384. doi: 10.1038/318382a0. [DOI] [PubMed] [Google Scholar]
- van Rijs J., Giguère V., Hurst J., van Agthoven T., Geurts van Kessel A., Goyert S., Grosveld F. Chromosomal localization of the human Thy-1 gene. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5832–5835. doi: 10.1073/pnas.82.17.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]