Abstract
Dynamic remodeling of the extracellular matrix (ECM) is essential for development, wound healing and normal organ homeostasis. Life-threatening pathological conditions arise when ECM remodeling becomes excessive or uncontrolled. In this Perspective, we focus on how ECM remodeling contributes to fibrotic diseases and cancer, which both present challenging obstacles with respect to clinical treatment, to illustrate the importance and complexity of cell-ECM interactions in the pathogenesis of these conditions. Fibrotic diseases, which include pulmonary fibrosis, systemic sclerosis, liver cirrhosis and cardiovascular disease, account for over 45% of deaths in the developed world. ECM remodeling is also crucial for tumor malignancy and metastatic progression, which ultimately cause over 90% of deaths from cancer. Here, we discuss current methodologies and models for understanding and quantifying the impact of environmental cues provided by the ECM on disease progression, and how improving our understanding of ECM remodeling in these pathological conditions is crucial for uncovering novel therapeutic targets and treatment strategies. This can only be achieved through the use of appropriate in vitro and in vivo models to mimic disease, and with technologies that enable accurate monitoring, imaging and quantification of the ECM.
Introduction
The extracellular matrix (ECM) is one of the most important regulators of cellular and tissue function in the body. Tightly controlled ECM homeostasis is essential for development, wound healing and normal organ homeostasis, and sustained dysregulation can result in life-threatening pathological conditions. The importance of correct biochemical and biophysical ECM properties on the regulation of cell and tissue homeostasis is illustrated by the fact that the ECM is dysregulated in many different types of disease. In this Perspective, we focus on how ECM composition and remodeling is now thought to be crucial for tumorigenesis and metastatic progression in cancer, as well as how disruption of normal ECM homeostasis leads to fibrotic diseases such as pulmonary fibrosis, systemic sclerosis, liver cirrhosis and cardiovascular disease. We also discuss recent progress in developing physiologically relevant qualitative and quantitative models, as well as advancements in technologies that enable accurate monitoring, imaging and quantification of the ECM. Together, these technologies will help us dissect both the spatial and temporal dynamics of ECM homeostasis, and promote our understanding of the underlying mechanisms that influence cell-ECM interactions in the context of multiple disease types. Finally, we close by examining how recent advances in this field might allow targeting of the ECM to provide new therapeutic approaches for treating fibrotic diseases and cancer.
ECM composition and function
Matrix components
The ECM is defined as the diverse collection of proteins and sugars that surrounds cells in all solid tissues. This tissue compartment provides structural support by maintaining an insoluble scaffold, and this in turn defines the characteristic shape and dimensions of organs and complex tissues. The ECM is mainly composed of an intricate interlocking mesh of fibrillar and non-fibrillar collagens, elastic fibers and glycosaminoglycan (GAG)-containing non-collagenous glycoproteins (hyaluronan and proteoglycans). Although the ECM has historically been perceived as fulfilling a primarily structural and hence biomechanical role, the ability of the ECM to provide the contextual information responsible for controlling both individual and collective cellular behavior has been increasingly recognized in recent years.
Following intracellular synthesis, ECM components are secreted into the interstitial matrix that surrounds and supports cells, and is the main provider of structural scaffolding for tissue. This matrix also plays a key role in protecting cells by acting as a compression buffer when tissues are subjected to deforming stresses. The interstitial matrix found in most but not all tissues consists mainly of the fibrous collagen type I, which, together with fibronectin, confers mechanical strength to tissues (Erler and Weaver, 2009). Although collagens are collectively the most abundant component of the ECM, the differential expression of individual interstitial ECM components underpins the specific functions of many organs and tissues. For example, chondroitin sulfate, a sulfated GAG that is usually found attached to proteins as part of a proteoglycan, is highly expressed in the ECMs of connective tissues such as cartilage, tendons, ligaments and major arteries, where it helps to maintain the structural integrity of the tissue. By contrast, secreted protein acidic and rich in cysteine (SPARC), a matricellular glycoprotein that was initially termed osteonectin, was originally identified in bone, where it binds collagen and Ca2+, initiating nucleation during bone mineralization (Termine et al., 1981). However, SPARC has also been shown to be secreted by non-epithelial cells in non-ossifying tissues (Sage et al., 1984) during both development and tissue repair, where it mediates ECM remodeling and turnover, and cell-ECM interactions (Engel et al., 1987; Sage et al., 1989; Funk and Sage, 1991; Lane and Sage, 1994; Murphy-Ullrich et al., 1995; Chlenski and Cohn, 2010). External mechanical loading of tissues can also modulate ECM composition in some tissues. For example, in situations in which mobility is impaired, there is a decrease in the proteoglycan content of articular collagen and in bone mineral density, but these increase with exercise (Bird et al., 2000; Rittweger et al., 2006; Rittweger et al., 2009), suggesting that ECM composition is modulated by both intrinsic and extrinsic stimuli.
In addition to the interstitial matrix, extracellular basement membranes (BMs) are a specialized form of sheet-like ECM to which epithelial cells can anchor and which interact directly with the epithelium and endothelium. These membranes mainly consist of collagen IV, laminins, entactin (also known as nidogen) and heparan sulfate proteoglycans (Erler and Weaver, 2009). BMs play a key role in epithelial cell function, providing cues for orientation that help to establish and maintain apicobasal polarity and cell differentiation.
The ECM serves many functions in addition to providing structural support. Macroscopically, the ECM physically segregates cells and organs and acts as a protective cushion – for example, by regulating hydrostatic pressure within tissues and organs. At the microscopic level, this highly dynamic molecular network is also capable of regulating cellular behavior through modulation of, among other things, proliferation, cytoskeletal organization, cellular differentiation and receptor signaling (Paszek and Weaver, 2004; Kass et al., 2007). Such ‘outside-in’ biochemical signaling mechanisms rely on the precise spatial organization of ECM ligands to integrate complex signals in a regulated manner (Hynes, 2009). The biophysical properties of the matrix also regulate cellular mechanosensory pathways – through global substrate rigidity (a phenomenon defined as mechanotaxis or durotaxis) (Lo et al., 2000; Wong et al., 2003; Hadjipanayi et al., 2009) or extracellular tension (known as tensotaxis) (Beloussov et al., 2000) – that prompt cells to detect and respond to changes in tissue biomechanics (Yu et al., 2010). In addition, the ECM also sequesters and hence acts as a ‘local depot’ for a wide range of growth factors and cytokines. For example, tissue injury can trigger protease activities, leading to a rapid release of signaling molecules [such as transforming growth factor-β (TGFβ) (Wipff et al., 2007; Wells and Discher, 2008)], which in turn allows a swift and local growth-factor-mediated activation of cellular functions without de novo synthesis.
Structure and mechanical function
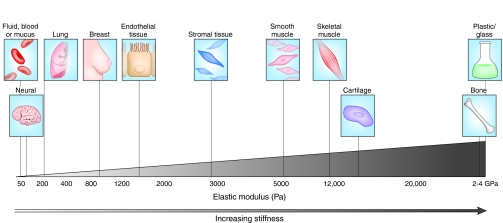
Functionally discrete tissues and organs have markedly distinct biomechanical properties (Fig. 1), which are subject to change during the course of development or during pathogenesis (Butcher et al., 2009). The biomechanical properties of the ECM are tightly controlled by the specific composition and concentration of matrix components, and also by post-translational modifications, such as glycosylation, transglutamination and cross-linking (Erler and Weaver, 2009).
Fig. 1.
Variations in tissue stiffness. The biomechanical properties of a tissue in terms of stiffness (elastic modulus), measured in pascals (Pa), vary markedly between organs and tissues, and are inherently related to tissue function. Mechanically static tissues such as brain or compliant tissues such as lung exhibit low stiffness, whereas tissues exposed to high mechanical loading, such as bone or skeletal muscle, exhibit elastic moduli with a stiffness that is several orders of magnitude greater. Tumorigenesis is typically associated with an increase in matrix and tissue stiffness, as in breast cancer. Adapted, with permission, from Butcher et al. (Butcher et al., 2009).
Collagen cross-linking occurs in both a regulated and non-regulated manner, typically via enzyme-mediated or non-enzyme-mediated processes, respectively. Regulated collagen cross-linking is almost exclusively mediated by lysyl oxidase (LOX) and the LOX family of secreted amine oxidases (Csiszar, 2001; Kagan and Li, 2003), and primarily occurs during developmental processes and wound healing. LOX family members catalyze the cross-linking of collagens (and elastin) through oxidative deamination of lysine residues. The importance of correct LOX expression and the resulting collagen cross-linking is exemplified by the fact that LOX-knockout mice die at birth due to collapse of their lethally fragile diaphragm and cardiovascular system (Maki et al., 2002; Hornstra et al., 2003). Clinical manifestations of reduced LOX activity (which can result from nutritional copper assimilation deficiencies) are also seen in two X-linked recessively inherited disorders: Menkes disease and occipital horn syndrome (OHS) (Royce et al., 1980; Kaler et al., 1994). Osteolathyrism is another collagen cross-linking deficiency, and is brought on by chronic ingestion of Lathyrus sativus, a plant that is rich in the LOX inhibitor β-aminopropionitrile (BAPN). Conversely, elevated LOX activity levels are clinically associated with increased fibrosis and can be detected in the sera of patients with liver cirrhosis (Murawaki et al., 1991; Kagan, 1994; Kagan, 2000). Chronic increases in the level of circulating LOX have also been shown to increase collagen cross-linking and result in stiffening of heart tissues, compromising cardiac function (Sivakumar et al., 2008).
Non-enzymatic collagen cross-linking usually occurs through glycation (Schnider and Kohn, 1980) and transglutamination (Mosher and Schad, 1979; Mosher et al., 1979), or as a result of increased biglycan and proteoglycan levels (Wiberg et al., 2003). Such collagen cross-linking acts to stiffen the ECM, although this process occurs much more slowly than its enzymatic alternative (Avery and Bailey, 2006). However, structural ECM proteins exhibit a remarkable longevity in vivo, often measured in years as opposed to hours for intracellular proteins: types I and II collagen in human skin, articular cartilage and intervertebral disc exhibit 15-, 95- and 117-year half-lives, respectively (Verzijl et al., 2000; Sivan et al., 2008). On such a timescale, glycation-mediated collagen cross-linking becomes important and is thought to play a key role in many age-associated diseases, including degenerative eye disease, pulmonary fibrosis, arterial stiffening and cardiovascular disease, and neurodegeneration; such manifestations are increasingly accelerated in diabetes patients who exhibit chronically elevated blood-glucose levels (Bunn et al., 1978; Frank, 1991; Vitek et al., 1994; Sasaki et al., 1998; Glenn and Stitt, 2009).
At a microscopic level, the precise organization and orientation of ECM components creates a highly organized topology that contributes to the functional properties of the matrix (Paszek and Weaver, 2004; Kass et al., 2007). Although it was previously assumed that the ECM was a static structure that changed only in response to growth or repair, it is now known that the microscopic topology of the ECM is determined by continuous dynamic remodeling, even while macroscopic topology remains mostly unchanged. Such remodeling is regulated by a careful balance between matrix synthesis, secretion, modification and enzymatic degradation. ECM components are degraded by matrix-degrading enzymes, including heparanase, cathepsins, hyaluronidases, matriptases, various serine and threonine proteases (Roycik et al., 2009), and the large superfamily of metzincins, which includes ADAMs (a disintegrin and metalloproteinases), ADAMTSs (ADAMs with thrombospondin motifs), and matrix metalloproteases (MMPs) and their inhibitors [tissue inhibitor of MMPs (TIMPs)] (Mott and Werb, 2004). The tightly controlled ECM homeostasis is sensitive to altered expression of these proteases, which, if altered for prolonged periods of time, can result in excessive ECM remodeling, as is frequently observed in both fibrotic diseases and cancer (see later) (Wynn, 2007; Butcher et al., 2009). Changes in matrix homeostasis affect not only the biochemical properties of the matrix but also the resulting biophysical properties, both of which are crucial for development and normal tissue function.
Cell-ECM interactions
Cellular responses are tissue and context dependent in terms of both biochemical and biomechanical cues (Bissell and Radisky, 2001; Yu et al., 2010). Hence, understanding the complex processes surrounding ECM production, modification and remodeling, and relating these processes to physiological changes in the biochemical and biomechanical properties of the ECM, are key to determining how microenvironmental changes influence cellular responses. These considerations are especially important in the development of anti-fibrotic and anti-cancer therapies, which might be able to target aspects that are dysregulated in both types of disease.
Biochemical cues
Correct ECM composition and organization is important for normal cellular behavior. An excellent example of this is the biochemical orientational cues provided to cells by the BM. BMs are responsible for maintaining apicobasal polarity of epithelial cells and have organ-specific compositions. Changes in biochemical BM composition can lead to changes in the physical properties of the BM and hence to changes in cellular shape and behavior, which, in turn, can drive cell proliferation and tumorigenesis by inducing alterations in the binding activities or spatial distribution of cell-surface receptors (Roskelley et al., 1995; Giancotti and Ruoslahti, 1999; Schwartz and Baron, 1999) (for a review, see Radisky et al., 2001). Although for many years it was thought that loss of apicobasal polarity caused by BM disruption was a secondary consequence of oncogenic transformation, later investigations of Drosophila mutants have shown that the loss of polarity determinants might in fact be a driver of tumorigenesis (Bilder et al., 2000). Because more than 80% of human cancers are derived from epithelia, the contextual biochemical environmental components of tumor initiation and progression are a crucial part of finding commonalities between seemingly distinct tumor types. Notably, some hematological tumors arise from defects in bone marrow stroma that lead to an abnormal microenvironment, which is thought to contribute to tumorigenesis and drive tumor progression, providing an example of how ECM changes might promote non-epithelial malignancies (Dror and Freedman, 1999; Michigami et al., 2000).
Biomechanical cues
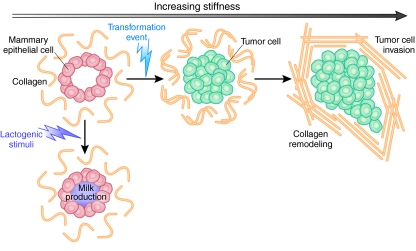
Recent research has focused on how perturbations in ECM stiffness can affect the behavior of tissue cells. This issue has gained substantial importance in light of evidence that the biomechanical properties and, in particular, the nature and tensional force of the three-dimensional (3D) matrix in vitro can have important implications for cellular behavior and might act synergistically with biochemical cues to modulate cell and tissue behavior. For example, increasing matrix stiffness through increasing collagen concentration enhances Rho-generated cytoskeletal tension to promote focal adhesion (FA) assembly and increase growth-factor-dependent ERK activation (Paszek et al., 2005). Mammary epithelial cells (MECs) grown within reconstituted BM (Matrigel) form polarized mammary acini and differentiate in response to lactogenic hormones in a manner that recapitulates the in vivo situation (Fig. 2) (Barcellos-Hoff et al., 1989). Transformation events trigger cells to invade the acini lumen (Weaver et al., 1996), and thus the system can be used to model tumorigenesis. Studies have shown that transformation can be induced by increasing BM matrix stiffness [from 170 pascals (Pa) to 1200 Pa], without altering biochemical composition, and is regulated through integrin- and Rho-kinase-mediated signaling events. These mechanical alterations lead to increases in cell growth, loss of polarity, increased FA contacts, and activation of focal adhesion kinase (FAK), vinculin and p130CAS, as well as a compromised cell-cell junction integrity that impedes lumen formation (Paszek et al., 2005).
Fig. 2.
Modeling mammary epithelial cells in vitro. Schematic to show modeling of mammary epithelial cells (MECs) in 3D assays in vitro. MECs form organized and polarized acini structures when grown in reconstituted basement membrane in vitro. Milk production can be induced through stimulation with lactogenic hormones. A transformation event results in cell invasion into the lumen. Increased invasive ability correlates with the development of disorganized and branching structures. Increasing matrix stiffness can also induce these events. Adapted, with permission, from Kass et al. (Kass et al., 2007) and Butcher et al. (Butcher et al., 2009).
Gene expression studies of mesenchymal stem cells plated onto matrices of different compliances have shown that matrix stiffness can drive cellular differentiation down alternative lineages (Engler et al., 2006; Discher et al., 2009). In addition, altered expression of more than 1500 genes with diverse functions has been observed in human mammary epithelial cells (HMECs) in response to perturbations in matrix stiffness (Alcaraz et al., 2008). Furthermore, microarray studies showed that gene expression changes that are typically associated with cardiovascular disease occur in cells of the ascending aorta in response to changes in arterial stiffening (Durier et al., 2003).
In summary, biochemical and biophysical cues from the ECM directly influence cells in multiple ways, from transcription and cell-cycle control to differentiation, migration and cell-cell interactions. These cues and the cellular responses to them are directly modulated by ECM composition, structure and organization. As discussed in the following sections, investigating precisely how different ECM cues are generated and, in turn, how they induce cellular responses in both normal and pathological environments will greatly help our understanding of disease progression.
Studying the ECM
The microenvironmental conditions that cells find themselves in are key regulators of disease progression. Cells continuously adapt to their environment by modifying their behavior but also by remodeling their microenvironment. Therefore, to investigate cell-ECM interactions and gain an understanding of their role in diseases such as cancer and fibrosis, it is necessary to develop and apply biomimetic model systems that accurately recapitulate the physiological aspects of the ECM and the cell-ECM interface.
In vitro versus in vivo approaches
Almost all cells in the human body grow within organized 3D matrices that are surrounded by other cells of both similar and distinct origin. However, most cell culture experiments are performed with monocultures under 2D adherent conditions. It is well established that cells grown in a 3D tissue context are under different mechanical and microenvironmental conditions, and it is the complex summation of these multiple signals that determines whether cells undergo processes such as differentiation, apoptosis, proliferation and invasion. In 2D monocultures, many of these complex interactions are completely lost and the ‘organ context’ is ignored, resulting in the conflicting reports on cellular response that we so frequently see regarding phenotype, cellular signaling, migration and drug response. Over the last decade it has become increasingly apparent that cells must be cultured in a physiologically relevant topographical context if they are to respond in a physiologically relevant manner to signaling molecules (Weaver et al., 1996; Weaver et al., 1997; Muthuswamy et al., 2001), so that both the environmental biochemical and biomechanical cues mimic the in vivo situation.
There are currently numerous 3D and quasi-3D in vitro systems in which cells can be grown on or within 3D matrices. The matrix components, matrix remodeling and matrix stiffness can be manipulated to perturb cellular behaviors, including survival, differentiation, migration and invasion. However, most conventional 3D cultures are often performed in large multi-well plates in which volumes and dimensions necessitate large numbers of cells. Thus, there is still the issue of whether these macro-culture systems provide an appropriate cellular context in which to study environmental cues.
Despite advances in cell culture methodologies, it must also be considered that effects of the matrix on connective tissue flow (the movement of molecules through tissues) at both micro- and macroscopic scales can also perturb the spatial distribution of oxygen, metabolites and other signaling molecules. Consequently, even sophisticated 3D in vitro models might not represent the physiological conditions found in vivo (Schmeichel and Bissell, 2003; Pampaloni et al., 2007; Yamada and Cukierman, 2007). Gradients of biological molecules are commonly found in tissues, and are modulated by vasculature and angiogenesis, but also both directly and indirectly by the ECM. Gradients of oxygen and the diffusion of oxygen to cells are major determinants of cell survival in 3D cultures (Derda et al., 2009). As a more specific example, not all cells in a solid tumor are exposed to the same microenvironmental cues: cells at the leading edge of a tumor receive more nutrients and oxygen, are more metabolically active, and are more likely to undergo active proliferation and invasion than those closer to the tumor core. This diversity in the tumor microenvironment probably contributes to the high heterogeneity of tumor cell populations in vivo (Vaupel et al., 1989), and is difficult to reproduce in vitro.
It is certain that the best way to accurately study the involvement of cell-ECM interactions in disease is in vivo. Mouse models of disease have provided great biochemical insight into the mechanisms behind numerous diseases, including fibrosis and cancer progression (discussed later). However, many in vivo studies focus primarily on manipulations of cellular aspects, rather than of the ECM directly. Methodological limitations generally preclude the experimental perturbation of ECM structure in vivo and, as such, most ECM manipulations are induced indirectly and provide a limited ability to precisely control the biochemical and biomechanical changes. Examples include the manipulation of matrix cross-linking by modulating LOX expression and/or activity (Erler et al., 2006; Erler et al., 2009; Levental et al., 2009), or influencing ECM degradation by knocking out MMPs or by manipulating TIMP expression or activity (Sternlicht and Werb, 2001; Egeblad and Werb, 2002). Many in vivo approaches also require sophisticated and sensitive imaging and quantification techniques to maximize the information that can be obtained regarding the dynamics of cell-ECM interactions (discussed below).
Through carefully combining 3D in vitro and in vivo models, it has been possible to dissect many aspects of cell-ECM interactions. This approach has enabled investigations of the proteins that are involved in cell-ECM interactions, of the role of mechanosensing and signal transduction pathways, and of the effects on cell cycle, behavior, phenotype, migration and invasion (for a review, see Kumar and Weaver, 2009).
Manipulating and measuring changes in the ECM
To study the effects of the ECM on cellular behavior in vitro in a meaningful way, it is necessary to manipulate and quantify the biochemical and biomechanical properties of the ECM in a controlled manner. Matrix stiffness, for example, can easily be modulated in vitro by overlaying a thin layer of matrix on either tunable polyacrylamide (Wong et al., 2003; Paszek et al., 2005; Levental et al., 2009) or polydimethylsiloxane (PDMS) (Cortese et al., 2009) substrates of a specific pre-defined stiffness. Matrix stiffness can also be modulated in vitro through modulating the in situ cross-linking of native ECM components (Butcher et al., 2009) either enzymatically, using LOX (Erler et al., 2009) and LOX family proteins (Barry-Hamilton et al., 2010), or through non-enzymatic reactions such as glycation using ribose or glucose (Paszek et al., 2005; Erler et al., 2006; Kass et al., 2007; Erler et al., 2009; Levental et al., 2009). ECM properties can also be similarly modulated in vivo, to some extent, either through overexpression of cross-linking enzymes, such as LOX, or by inhibition of matrix-degrading enzymes (Erler et al., 2006; Ahn and Brown, 2008; Levental et al., 2009). Processes such as fibrosis can be induced using irradiation or other agents, such as bleomycin to induce pulmonary fibrosis and carbon tetrachloride or dimethylnitrosamine to induce liver fibrosis. These treatments induce rapid inflammatory and fibrotic responses in target organs, leading to increased ECM deposition and remodeling that mimic pathological progression of the disease. Such approaches have greatly facilitated the identification of key players that mediate fibrotic disease progression (Kagan, 1994; Ebihara et al., 2000; Friedman, 2004; Pardo and Selman, 2006; Iredale, 2007).
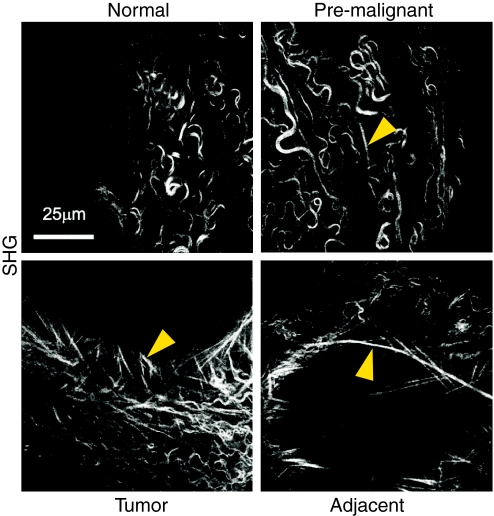
The quantitative assessment of ECM properties is a requirement if the functional effects of complex remodeling processes are to be understood. Changes in matrix composition and stiffness can be measured in vitro, in vivo and ex vivo. Common methods include staining tissues for biochemical markers by immunohistochemistry or immunofluorescence to characterize changes in ECM composition. In addition, histological methods such as the use of Masson’s trichrome and picrosirius red to stain collagens can be used to examine collagen structure and quantify collagen linearization and orientation (Levental et al., 2009) (see Fig. 3). Although such standard procedures can provide highly informative data on matrix changes during development and disease progression, they give only a static snapshot of the ECM and cannot capture its complex dynamics. To address this limitation, specialist techniques [such as echocardiography and sonoelastography (using sound) and second harmonics imaging (SHG; using light) with two-photon microscopy of whole tissues ex vivo and in vivo] can be used to analyze the ECM, particularly the collagen structure, and quantify collagen linearization in a non-invasive manner (Levental et al., 2009). Similarly, these techniques have been used to monitor the interactions of epithelial and stromal cells with tumors, as well as the initiation of collagen remodeling (Brown et al., 2003; Condeelis and Segall, 2003; Perentes et al., 2009; Wolf et al., 2009). Most important, however, is the ability to monitor events on a temporal scale rather than relying on endpoint assays to help understand both ECM dynamics and the resulting cellular behavior. Recent work by Giampieri et al. involving non-invasive intravital SHG imaging identified the paradigmatic role of TGFβ during the intermediate steps of tumor progression. This work highlights how temporal switches in TGFβ expression induced by local microenvironmental cues can dramatically affect cell migration, intravasation into blood and lymphatics systems, and colonization of secondary sites (Giampieri et al., 2009).
Fig. 3.
Second harmonic generation (SHG) imaging of collagen fibril linearization during mammary gland tumorigenesis. Images are representative of whole, unfixed mammary glands of MMTV-neu mice [which carry an activated neu oncogene driven by a mouse mammary tumor virus (MMTV) promoter] and show that collagen fibril linearity increases with malignant progression, correlating with increased tissue stiffness. Arrowheads indicate linearized collagen fibrils. Image adapted, with permission, from Levental et al. (Levental et al., 2009).
Techniques such as atomic force microscopy (AFM) can be used to generate extremely high-resolution images of ECM structure (Graham et al., 2010) and, with the upcoming force-mapping modality experiments that many atomic force microscopes are capable of, local force measurements can be taken to evaluate matrix elasticity and stiffness at the micron scale. Non-destructive tissue AFM can be carried out on standard cryosections in a manner that preserves biomolecular structure and allows visualization of both intra- and extracellular structure at a micro- to nanometer resolution. Owing to the nondestructive nature of the technique, experimental intervention can be applied both pre- and post-imaging and, more importantly, it allows the combining of ultrastructural imaging with current clinicopathological microscopy techniques (Graham et al., 2010).
Also of interest are the viscoelastic (i.e. time-dependent) properties of tissues and matrix at the cellular level. Shear rheology is a standard technique used for measuring ECM and tissue stiffness at the macroscopic level. At its simplest, this approach measures torsional stress and strain, from which it is possible to calculate the elastic modulus (in Pa) as a function of strain rate. At the same time, measurements of mechanical compression and nano-indentation (a commonly applied means of testing the mechanical properties of materials by indenting the test material with a diamond tip while measuring the force-displacement response) can also provide high-resolution measurements of matrix and tissue elasticity, because nano-indentation can achieve lateral resolutions of ∼15 μm (Akhtar et al., 2009b). However, a common problem with all of these techniques is that the method of interrogation can greatly affect outcome, so careful experimental design must be implemented; stiffness values are typically calculated as a function of experimental measurements and depend precisely on how and which variables are measured.
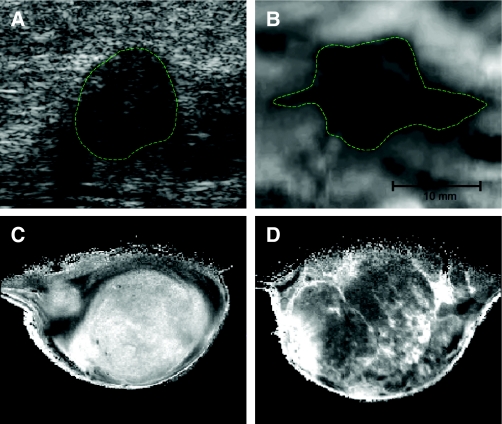
Although the techniques described above provide accurate and useful quantitative data on the biomechanical properties of matrix and tissue, most are generally considered invasive and/or destructive methodologies (Gueta et al., 2006). Hence, there is a need to develop methods to measure elastic properties and stiffness of tissues and matrix in a non-invasive manner for clinical application. The elastic properties of tissues is one of the elements that provides the image contrast in technologies such as magnetic resonance and ultrasound elastography, which are routinely used in the clinic (Barbone and Bamber, 2002). For example, clinical in vivo imaging of malignant breast tumors tend, on elastography, to appear stiffer than benign breast tumors; in particular, a halo of stiffer tissue is frequently observed at the tumor margin (invasive) edge of the tumor (Jeff Bamber, personal communication). These observations can prove invaluable in helping to guide treatment regimens at the bedside. At the bench, however, science seeks to quantify such observations to increase our understanding of how tissue biomechanics are related to disease progression.
New technologies based on fluorescence resonance energy transfer (FRET) (Jiang et al., 2004), magnetic resonance imaging (MRI), positron emission tomography (PET) and single photon emission computed tomography (SPECT) (for a review, see Scherer et al., 2008a) are being developed to image the dynamic status of ECM remodeling, including visualization of MMP activity (Scherer et al., 2008b; Littlepage et al., 2010). Similarly, advances in μ-ultrasound, optical coherence tomography (OCT), optical acoustic microscopy and scanning acoustic microscopy (SAM) (Akhtar et al., 2009a) are currently under development to facilitate quantitative measurement and imaging of stiffness at the microscopic scale (Jeff Bamber, personal communication) (Low et al., 2006). In addition, increasing the resolution and versatility of many of the above techniques will be possible with improved contrast agents, such as so-called ‘smart probes’, which are MRI contrast agents that can be used to study ECM components (Spuentrup et al., 2005; Stracke et al., 2007; Miserus et al., 2009), soluble proteins such as growth factors and MMPs (Tsien, 2005; Scherer et al., 2008a; Scherer et al., 2008b), specific immune- or tumor-cell populations (Reynolds et al., 2006; Korosoglou et al., 2008; McAteer et al., 2008; Radermacher et al., 2009), and even physiological conditions such as hypoxia (Fig. 4) (McPhail and Robinson, 2010). These contrast agents are typically highly selective, specific and undergo enhanced activation on interacting with their target. In summary, new techniques that image the dynamics of cell-ECM interactions to non-invasively quantify remodeling of the ECM at the sub-millimeter level – and, more importantly, on a temporal scale – will ultimately provide additional resources for basic research and in the clinic.
Fig. 4.
Imaging the biomechanical properties of the matrix. Breast tumors are typically identified by changes in tissue mechanics, which can be detected physically, through palpitation or via imaging modalities that exploit tumor-associated changes. (A,B) Images of human breast tumor identified by ultrasound echogram (A) and mammary elastography imaging (B). The dashed lines roughly outline the imaged lesion boundary. The elastogram seems to shows a larger apparent lesion width but a similar height, relative to the echogram. This seems to be due to lateral protrusions, which are consistent with a desmoplastic response associated with local invasion that takes advantage of existing ductal and vascular anatomy. (C,D) MRI images of a 1-methyl-1-nitrosourea (MNU)-induced mammary carcinoma in rat. Here, not only do the changes in tumor ECM provide enhanced tissue contrast, but the intrinsic susceptibility of MRI exploits the paramagnetic properties of deoxyhemoglobin in erythrocytes. Deoxyhemoglobin therefore acts as an intrinsic, blood-oxygenation-level-dependent contrast agent, further highlighting the highly vascular nature of tumors. Ultrasound and elastography images were supplied courtesy of Jeff Bamber (The Institute of Cancer Research, UK). MRI images were supplied courtesy of Simon P. Robinson (The Institute of Cancer Research, UK).
The ECM and disease
The highly dynamic nature of the ECM plays a crucial role in disease progression. The ECM is essential for normal wound healing processes, but excessive deposition – as is observed in the case of fibrotic and degenerative diseases – can lead to organ dysfunction. Similarly, the ECM plays a key role in the development of cancer, modulating processes such as tumor cell invasion and metastasis, among others. In the sections below, we discuss how perturbations in normal cell-ECM interactions contribute to fibrotic diseases and cancer. We highlight how in vitro and in vivo models have helped to unravel the relationships between the biochemical and biophysical properties of the ECM and the physiological functions of cells and tissues during disease progression.
Fibrotic diseases
Tissue fibrosis is the result of abnormal responses to organ injury or to irritation, and is typically characterized by the hyperproliferation of fibroblasts, their differentiation into myofibroblasts, and excessive ECM synthesis and secretion. The increased ECM synthesis, assembly and subsequent cross-linking lead to altered biochemical and biomechanical matrix properties, compromising normal tissue function and further driving disease progression. It is becoming increasingly apparent that it is important to distinguish between increased amounts and increased concentrations of an ECM component in disease progression, as first highlighted in a study by Cattell and coworkers (Cattell et al., 1996). In their study, aortic collagen and elastin concentrations increased with age, whereas the absolute amounts decreased. The authors attributed this discrepancy to the age-related differential loss of other tissue components. Consistently, in aged bone, skin and breast, there is reduced collagen deposition and increased MMP activity over time (Butcher et al., 2009). However, this is accompanied by increases in tissue stiffness (i.e. loss of elasticity), and is probably associated with a disproportionate increase in inappropriate post-translational cross-linking and modification of ECM proteins, such as by glycation (discussed earlier). In fibrotic diseases such as pulmonary fibrosis, liver cirrhosis, cardiovascular disease and systemic sclerosis, evidence suggests that the constitutive activation of collagen-secreting myofibroblast-like cells is ultimately responsible for increasing both collagen amount and concentration. In such cases, these cells can synthesize and secrete excessive ECM components, particularly collagens, leading to increased tissue stiffness and progressive organ dysfunction.
Variations in ECM composition substantially affect the biomechanical properties of a tissue, as is clearly seen in fibrotic diseases (Birk and Bruckner, 2005). For example, elastin and collagen fibers form the main structural components of pulmonary connective tissue matrix, but their elastic properties are markedly different; the elastic modulus of collagen fibrils is in the region of 1200 megapascals (MPa), whereas that of elastin is closer to 1 MPa (Sherratt et al., 2003; Akhtar et al., 2009a). Together, they form a continuous network in the lung that provides the forces necessary for passive recoil during expiration. During pulmonary fibrosis, increases in the amount and concentration of collagen lead to changes in tissue mechanics and particularly a loss of elastic properties, resulting in progressive dyspnea (shortness of breath) (for reviews, see Rocco et al., 2001; Suki and Bates, 2008).
In pulmonary fibrosis, an important source of activated myofibroblast-like cells is circulatory fibrocytes, which are derived from bone marrow (Keeley et al., 2009). Under normal situations, these fibrocytes extravasate into the lungs and promote wound healing together with resident mesenchymal cells. In mice, C-X-C chemokine receptor 4 (CXCR4) and C-C chemokine receptors 2 and 7 (CCR2 and CCR7) have been shown to mediate lung healing and fibrosis by recruiting these fibrocytes (Phillips et al., 2004; Moore et al., 2005; Strieter et al., 2007). During hepatic cirrhosis, hepatic stellate cells provide the source of myofibroblast-like cells and have been shown to contribute to the fibrotic response that leads to portal hypertension and liver failure (Iredale, 2007). In such situations, the balance of matrix production and degradation tips in favor of production, which can be facilitated by overexpression of TIMPs or loss of MMP expression, resulting in incomplete matrix remodeling and irreversible fibrosis (Issa et al., 2004).
Inappropriate fibroblast activation can also manifest as other fibrotic diseases, such as systemic sclerosis (SSc). Fibroblasts experimentally explanted from fibrotic lungs or skin lesions of SSc patients have been shown to have a constitutively activated myofibroblast-like phenotype, further implicating this cell type as a principal cause of disease (Varga and Abraham, 2007). In SSc, the constitutive activation of myofibroblast-like cells is thought to result from circulating auto-antibodies, connective tissue growth factor (CTGF) and interleukin-6 (IL-6) produced in response to viral infections (Markiewicz et al., 2004; Abraham and Varga, 2005; Hasegawa et al., 2005). Autocrine TGFβ signaling and TGFβ- and SMAD3-independent mechanisms have also been implicated in fibroblast activation (Kaviratne et al., 2004; Moustakas and Heldin, 2005). TGFβ is also involved in ECM remodeling accompanying arterial hypertension. Sustained hypertension results in structural and functional alterations in the ECM of the heart and vessels, which ultimately lead to progressive dysfunction. Local TGFβ levels are upregulated by activated macrophages and myofibroblasts in and around vessels; TGFβ then acts in an autocrine manner to promote fibroblast-to-myofibroblast differentiation and activation, and further induce TGFβ production. This results in an increase in the production and secretion of interstitial collagens, fibronectin and proteoglycans, leading to excessive ECM deposition, tissue stiffening and ultimately scarring within the heart (Li et al., 1997; Denton and Abraham, 2001; Rosenkranz et al., 2002; Watanabe et al., 2005; Wynn, 2007).
Cancer
Tumor development is a complex, dynamic and progressive process that involves both cellular and environmental cues. The tumor microenvironment is mechanically and biologically active and, more importantly, is dynamic, as is highlighted by the fact that it is continuously and progressively remodeled (Yu et al., 2010). It is well known that interactions between cells and an altered microenvironment can drive malignancy. Conversely, tumor cells can manipulate their microenvironment to enhance their own survival, thereby creating a positive tumorigenic feedback loop. Thus, it has been proposed that, once established, tumors should be considered functionally discrete organs (Bissell and Radisky, 2001). Interestingly, cells with a tumorigenic genotype can become phenotypically normal if the environmental context is appropriately manipulated, and there is increasing evidence that it might be possible to restore aggressive breast cancer cell lines to a near-normal phenotype by manipulating environmental cues and simultaneously inhibiting multiple signaling pathways (Bissell and Radisky, 2001). Thus, it is becoming increasingly clear that tumors should be studied in a physiologically relevant context.
It has long been known that tumor-derived ECM is biochemically distinct in its composition compared with normal ECM. Furthermore, reports have demonstrated that the tumor stroma is typically stiffer than normal stroma (∼400 Pa compared with 150 Pa, respectively), and that breast cancer tissue can be tenfold stiffer than normal breast tissue (150 Pa versus 1.5 kPa, respectively) (Kass et al., 2007; Butcher et al., 2009; Levental et al., 2009). More recently, increased matrix stiffness and ECM remodeling were observed in pre-malignant tissue (∼350 Pa in pre-malignant tissue versus 150 Pa in normal tissue), and this increase was shown to contribute to malignant transformation in the breast (Levental et al., 2009). Pronounced changes in ECM homeostasis, which in some respects mimic those that occur in fibrotic diseases, play a crucial role in tumor progression. They occur owing to disruption of the balance between ECM synthesis and secretion, and owing to alterations in the normal levels of matrix-remodeling enzymes such as LOX (Payne et al., 2007) and MMPs (Jodele et al., 2006; Strongin, 2006; Kessenbrock et al., 2010).
Alterations in matrix-remodeling enzymes
As discussed earlier, LOX cross-links newly synthesized collagen, and its expression and activity are elevated in response to increased collagen deposition. Elevated LOX expression is significantly correlated with metastasis and decreased survival in cancer patients and mouse models of cancer (Erler et al., 2006), and LOX has been validated as a prognostic marker in patients with head and neck cancer (Le et al., 2007; Le et al., 2009). Increased LOX activity results in increased ECM stiffness (Levental et al., 2009), and has been shown to increase the invasiveness of many cancer cell types (Kirschmann et al., 2002; Erler et al., 2006; Erler et al., 2009). The roles of LOX and LOX-mediated matrix modifications are summarized in Fig. 5. Although the exact mechanisms by which LOX activity promotes invasiveness remain to be elucidated, they are thought to involve increased cell-matrix adhesion, increased integrin clustering and FAK activation (Payne et al., 2005; Erler et al., 2006). Integrins are one of the most widely studied families of mechanosensing proteins and are highly implicated in tumor progression (Baker and Zaman, 2010). Through changing their avidity, conformation, clustering and recruitment, integrins transduce biomechanical cues by modulating intracellular signaling cascades. In particular, the increase in ECM stiffness in and around tumors leads to increased integrin clustering (Paszek et al., 2005), resulting in enhanced mechanotransduction that consequently promotes cell migration. A detailed discussion of integrins in cancer is beyond the scope of this article (for a recent review, see Desgrosellier and Cheresh, 2010).
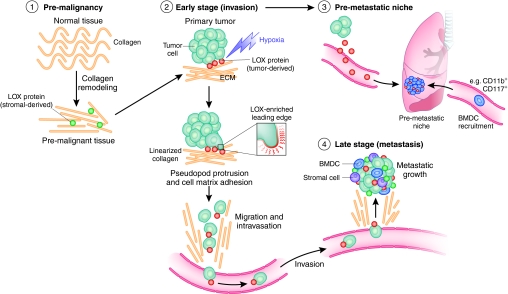
Fig. 5.
The role of LOX in tumor progression. Schematic to summarize the role of LOX in tumor progression. (1) Increased LOX expression from stromal cells results in increased collagen linearization and tissue stiffness in pre-malignant tissue. These changes increase tumor incidence and tumor burden, and drive malignant progression. (2) LOX secreted by hypoxic tumor cells increases invasion, enabling metastatic dissemination. (3) LOX secreted by hypoxic tumor cells accumulates at distant sites of future metastasis and recruits BMDCs to form the pre-metastatic niche. This greatly enables establishment and growth of metastases. (4) LOX-expressing tumor cells have an enhanced ability to colonize distant organs and form metastases. LOX secreted by both tumor and stromal cells supports metastatic tumor growth. Adapted, with permission, from Erler and Giaccia (Erler and Giaccia, 2008).
MMPs degrade the ECM and are involved in promoting the inflammatory response, normal tissue remodeling, wound healing and angiogenesis. However, their expression is often highly upregulated in many solid tumors, and the sustained presence of these proteases (which can be produced by activated stromal cells, infiltrating innate immune cells and cancer cells in the tumor microenvironment), coupled with increased ECM synthesis and secretion, leads to the progressive destruction of normal ECM and its replacement by tumor-derived ECM. This degradation and the resulting new microenvironmental cues stimulate proliferation and apoptotic mechanisms, which are thought to lead to the selection of apoptosis-resistant tumor cells with enhanced invasive potential (Sethi et al., 1999; Coussens and Werb, 2001; Mitsiades et al., 2001). More recently, however, MMPs have been shown to regulate not only ECM turnover, but also cell signaling pathways controlling cell growth, inflammation and angiogenesis. They might also act in a non-proteolytic manner through various non-catalytic domains (Dufour et al., 2008; Glasheen et al., 2009; Sakamoto and Seiki, 2009) (for a review, see Kessenbrock et al., 2010).
This is of particular importance because MMP expression and activity levels are elevated in cells resident within the tumor environment. MMP1 (also known as collagenase-1) degrades collagen IV and its expression is increased in highly metastatic cancer cells (Morikawa et al., 1988; Chakraborti et al., 2003). Similarly, overexpression of MMP2 has been shown in transformed mammary epithelial cells, whereas overexpression of MMP3, MMP11, MMP12 and MMP13 has been demonstrated in tumor stroma (Butcher et al., 2009). Polymorphisms in the human MMP3 promoter that increase its expression have been clinically associated with an increased tumor incidence, highlighting the important nature of MMPs in cancer (Biondi et al., 2000). Consistent with this, transgenic mice overexpressing MMP3 exhibit increased desmoplasia (the growth of fibrous or connective tissue), display abnormal and defective branching of the mammary epithelium, and develop tumors with genetic abnormalities (Sternlicht et al., 1999). MMP degradation of the ECM not only facilitates cell movement but also generates numerous bioactive cleaved peptides and releases growth factors and chemokines that are contained within the ECM (Egeblad and Werb, 2002).
Alterations in the biochemical and biomechanical properties of the ECM
The majority of increased tumor and adjacent tissue stiffness occurs as a result of increased ECM deposition. Type I collagen and fibronectin are the most common and abundant ECM components deposited in cancer (Provenzano et al., 2008) as a result of desmoplasia. This results in a pervasive dense fibrous tissue that typically surrounds the tumor, increasing local tissue stiffness. This is generally only associated with malignant tumors and is rarely observed in benign tumors (Butcher et al., 2009). The mechanisms and key players implicated in tumor-associated fibrosis are much the same as those involved in pathological fibrosis. For example, CCL2, CXCR4, CCR2, CCR7, TGFβ, CTGF and IL-6 have all been reported to play a role in tumor progression and its associated fibrosis (Ben-Baruch, 2006; Stover et al., 2007; Massague, 2008; Aggarwal and Gehlot, 2009; Bennewith et al., 2009). Other ECM proteins, such as fibronectin, tenascin, decorin, fibromodulin, SPARC, lumican and osteopontin, have also been shown to be involved in tumor development, modifying both biochemical and biomechanical properties of the tumor ECM; the precise roles of these proteins in tumor progression are currently under investigation (Oldberg et al., 2007; Hynes, 2009; Klotzsch et al., 2009; Arnold et al., 2010).
Remodeling of the BM is also commonly associated with cancer and, moreover, with malignant progression and metastatic dissemination. Disruption of the BM abrogates apicobasal polarity and also allows tumor cells to escape the primary tumor. Remodeling of fibrillar collagens surrounding the tumor is also observed and associated with metastatic progression (Fig. 3). The linearization and reorientation of collagen fibers surrounding cells at the invasive front of the tumor is a classic example of malignant transformation (Levental et al., 2009) and metastatic dissemination (Provenzano et al., 2006), and transformed mammary epithelial cells are often found on bundles of linear collagen fibers adjacent to blood vessels (Ingman et al., 2006). Consistent with this, intravital imaging has shown that tumor cells can travel along these realigned collagen fibers to facilitate invasion through tissue and intravasation into the bloodstream (Condeelis and Pollard, 2006).
The ECM and metastasis
As discussed, changes in the tumor microenvironment through remodeling of the ECM are important for metastatic dissemination. Specifically, the breakdown of normal ECM and its replacement with tumor ECM in the microenvironment leads to altered physiological cues that act as key drivers for malignant progression. We discussed above the role of the ECM in driving tumor progression at the primary site; more recently, primary-tumor-mediated ECM remodeling has been implicated in the systemic appropriation of future sites of metastasis – so-called pre-metastatic niches.
We recently demonstrated a crucial role for tumor-secreted LOX and matrix remodeling in pre-metastatic niche formation (Erler et al., 2009). We showed that tumor-driven ECM remodeling and matrix stiffening at sites distant from the primary tumor is LOX dependent and acts to recruit bone-marrow-derived cells (BMDCs), and also to facilitate tumor cell colonization and growth. Concomitantly, matrix stiffening either enzymatically (with LOX) or chemically (through glycation) increases invasiveness of myeloid (CD11b+) precursor BMDCs and increases MMP activity in vitro (Erler et al., 2009).
Another ECM component, fibronectin, plays an important role in the formation of the pre-metastatic niche. At 3 days following orthotopic tumor implant, increased pulmonary fibroblast fibronectin expression (Kaplan et al., 2005) and increased tumor-secreted deposition of fibronectin (Erler et al., 2009) are observed in metastatic target organs, indicating that at least some of the fibronectin observed in pre-metastatic niches comes directly from the tumors. The aforementioned aggregates of BMDCs [many of which express the fibronectin receptor VLA-4 (also known as α4β1)] are also found adjacent to regions of elevated fibronectin, suggesting a potential mechanism for their site-specific recruitment and role in pre-metastatic niche formation.
Although metastasis has traditionally been described as the autonomous spread of renegade cells throughout the body, it is now becoming clear that this is no longer the case. It is clear that ECM remodeling is crucial for facilitating primary tumor escape, but it is now also apparent that the successful colonization of secondary sites of metastasis relies in part on the correct appropriation of local environments through ECM remodeling, which results in the creation of niches that are permissive to tumor cell colonization and outgrowth. The possibility that tumor cells can achieve this appropriation systemically before they arrive at the site further highlights the therapeutic potential of targeting ECM remodeling to prevent the metastatic dissemination of solid tumors.
Targeting the ECM: clinical significance
The dysregulation of ECM homeostasis is a common driving factor in both fibrotic diseases and cancer. In line with this, the manifestations of these diseases can overlap: organ fibrosis has been shown to drive malignant transformation, and cancer-associated fibrosis and desmoplasia are implicated in primary tumor growth and metastatic dissemination. It has long been known, for example, that there is a clear clinical correlation between breast tissue density and cancer risk (Wolfe, 1976b; Wolfe, 1976a; Boyd et al., 2002; Boyd et al., 2007). However, although there are numerous treatments available to treat cancer, there are currently no approved treatments that directly target the mechanisms of fibrosis. Given that the ECM plays a pivotal role in the progression of both types of disease, we discuss below the potential of the ECM as a therapeutic target.
The targeting of ECM-remodeling-enzymes to prevent the changes in ECM homeostasis that promote disease progression has received much interest in terms of developing antifibrotic therapies and is now becoming an increasingly attractive therapeutic approach for preventing cancer progression. However, this strategy is not straightforward: for example, early attempts to treat hypertrophic fibrotic scarring and keloidal scars focused on targeting collagen cross-linking with β-aminopropionitrile (BAPN; an inhibitor of LOX). Although this therapy effectively reduced collagen cross-linking and scarring when applied topically, clinical trials were halted owing to toxicity of the drugs, and there has been limited progress since. However, targeting LOX activity has shown more promise in treating cancer: inhibition of LOX was shown to reduce primary tumor growth and mechanotransduction in the mammary epithelium (Levental et al., 2009). Furthermore, LOX inhibition prevents the formation of invasive branching structures in collagen in vitro, the invasion of tumors in vivo, and abrogates BMDC recruitment and the establishment of metastases in vivo. It also destabilizes already-formed metastases by reducing their growth, prevents further metastases and increases host survival (Erler et al., 2006; Erler et al., 2009). Although this suggests that the tumor progression in these models directly depends on LOX and its effects on ECM properties, the exact mechanism by which LOX inhibition is protective remains unknown and is currently being investigated. Nevertheless, these data support the idea that antagonizing matrix modifications, and cross-linking in particular, is a promising cancer prevention strategy. Indeed, LOX seems an excellent therapeutic target and inhibitors are now in development for use in the clinic. The potential of targeting LOX and LOX-mediated matrix modifications in cancer treatment is schematized in Fig. 5.
Other strategies to target the ECM in cancer have been less successful. For example, although there is substantial evidence that MMPs play multiple roles in metastasis, clinical trials of MMP inhibitors have failed to show significant efficacy, because they failed to increase survival rates in patients (Coussens et al., 2002), prompting a re-examination of MMP function and its complex roles. The poor success of MMP inhibitors has largely been due to unexpected toxicity of the drugs on normal tissues, conflicting roles in both promoting and reducing metastatic dissemination, and a lack of robust preclinical models to reliably interrogate the efficacy of MMP inhibitors. In addition, most MMP inhibitors are nonspecific, targeting multiple MMPs, and, more importantly, MMPs are implicated in a wide variety of functions that are both pro- and anti-tumorigenic. Finally, there is thought to be functional redundancy between MMP family members, meaning that a combination of precisely administered inhibitors might be required for clinical efficacy. Although a new generation of highly specific MMP inhibitors, and inhibitors of other matrix-modifying proteases, hold promise (Mack and Marshall, 2010), targeting the specific enzymes involved in ECM remodeling while avoiding unwanted side effects is an ongoing challenge.
Although LOX is an encouraging therapeutic target for fibrotic diseases and cancer, lessons from the clinic have taught us that targeting a single molecule in a disease network can result in ‘network compensation’ and subsequent drug resistance. In the case of cancer, the 3D tumor microenvironment contains many overlapping mechanisms that help to maintain its functional disorder. Therefore, there is a need to better understand the position of key individual and collective players within cellular networks, how these nodes respond to the dynamics of the network and, more importantly, how microenvironmental cues modulate these networks. Such studies can only be done through extensive, multi-disciplinary collaborative modeling of disease, yet will ultimately provide insight regarding the most promising strategies for drug combinations and the timing of treatments (Pawson and Linding, 2008; Erler and Linding, 2010). A greater understanding of how microenvironmental cues drive tumorigenesis will pave the way to developing therapies that target both a tumor and its microenvironment. Key examples of research that suggest that this approach will be beneficial are that involving hepatocarcinoma, in which targeting the underlying liver fibrosis can increase efficacy of chemotherapy (Friedman et al., 2000), breast cancer, in which fibrotic breast disease predisposes individuals to cancer (Jacobs et al., 1999), and situations in which environmentally induced lung fibrosis can increase the incidence of lung cancer (Mossman and Churg, 1998).
Conclusion
Tightly controlled ECM homeostasis is crucial for regulating many essential cellular processes, allowing for correct organism development, wound healing and normal tissue homeostasis. When this homeostasis is perturbed, an aberrant ECM can contribute to pathological conditions, including fibrotic disease, tumor progression and metastasis (as discussed in this Perspective). The pathological conditions caused by aberrant ECM changes are responsible for millions of deaths worldwide, and present a challenging obstacle with respect to clinical treatment. Investigating the processes underlying perturbation of homeostasis, the consequential changes in biochemical and biomechanical ECM properties, and the resulting nature of altered cell-ECM interactions will allow us to identify therapeutic targets for clinical benefit across multiple diseases. Among the challenges is to identify effective ways to spatially and temporally monitor these events in a non-invasive and quantitative manner. The hope is that monitoring and therapeutically targeting the abnormalities in the ECM and cell-ECM interactions that are associated with pathological conditions will soon become standard clinical practice.
Given that evidence implicating the ECM in disease progression is rapidly accumulating, new therapies are continuously entering pre-clinical and clinical trials. Studies across multiple disease types, particularly cancer, are aiming to target various factors that regulate ECM homeostasis; several of these factors have shown promise, including the previously mentioned collagen cross-linker LOX (Erler et al., 2006; Le et al., 2007; Erler et al., 2009; Le et al., 2009; Levental et al., 2009), the MMP inhibitor Marimastat (Goffin et al., 2005; Rosenbaum et al., 2005), anti-tenascin-C therapies (Hicke et al., 2006; Reardon et al., 2008) and anti uPA/uPAR (Berkenblit et al., 2005), among others. Such studies continue to unravel the commonalities between diseases involving the ECM, including fibrosis and cancer; identifying these commonalities will not only help to develop novel therapeutics, but will also increase our understanding of the underlying pathological mechanisms. As we move forward, we must always be aware that tissues and organs are made up of both cells and surrounding ECM. Furthermore, we must recognize that conditions such as cancer and fibrotic diseases are complex tissue diseases in which all of these components should ideally be studied simultaneously.
Acknowledgments
We thank Michael J. Sherratt and Erik Sahai for their helpful comments on this manuscript; and Jeff Bamber, Simon P. Robinson and Valerie Weaver for providing images and their useful discussions. T.R.C. and J.T.E. are funded by the CRUK, ICR, and a kind donation from Justin and Lucy Bull. J.T.E. is also funded by the AICR, MRC and the Breast Cancer Campaign.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing or financial interests.
REFERENCES
- Abraham D. J., Varga J. (2005). Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 26, 587–595 [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Gehlot P. (2009). Inflammation and cancer: how friendly is the relationship for cancer patients? Curr. Opin. Pharmacol. 9, 351–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn G. O., Brown J. M. (2008). Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 13, 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar R., Sherratt M. J., Watson R. E., Kundu T., Derby B. (2009a). Mapping the micromechanical properties of cryo-sectioned aortic tissue with scanning acoustic microscopy. Mater. Res. Soc. Symp. Proc. 1132E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar R., Schwarzer N., Sherratt M. J., Watson R. E., Graham H. K., Trafford A. W., Mummery P. M., Derby B. (2009b). Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J. Mater. Res. 24, 638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J., Xu R., Mori H., Nelson C. M., Mroue R., Spencer V. A., Brownfield D., Radisky D. C., Bustamante C., Bissell M. J. (2008). Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27, 2829–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. A., Rivera L. B., Miller A. F., Carbon J. G., Dineen S. P., Xie Y., Castrillon D. H., Sage E. H., Puolakkainen P., Bradshaw A. D., et al. (2010). Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis. Model. Mech. 3, 57–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery N. C., Bailey A. J. (2006). The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol. Biol. (Paris) 54, 387–395 [DOI] [PubMed] [Google Scholar]
- Baker E. L., Zaman M. H. (2010). The biomechanical integrin. J. Biomech. 43, 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbone P. E., Bamber J. C. (2002). Quantitative elasticity imaging: what can and cannot be inferred from strain images. Phys. Med. Biol. 47, 2147–2164 [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. (1989). Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105, 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H. M., Oyasu M., Mikels A., Vaysberg M., Ghermazien H., Wai C., et al. (2010). Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 [DOI] [PubMed] [Google Scholar]
- Beloussov L. V., Louchinskaia N. N., Stein A. A. (2000). Tension-dependent collective cell movements in the early gastrula ectoderm of Xenopus laevis embryos. Dev. Genes. Evol. 210, 92–104 [DOI] [PubMed] [Google Scholar]
- Ben-Baruch A. (2006). The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 25, 357–371 [DOI] [PubMed] [Google Scholar]
- Bennewith K. L., Huang X., Ham C. M., Graves E. E., Erler J. T., Kambham N., Feazell J., Yang G. P., Koong A., Giaccia A. J. (2009). The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 69, 775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenblit A., Matulonis U. A., Kroener J. F., Dezube B. J., Lam G. N., Cuasay L. C., Brunner N., Jones T. R., Silverman M. H., Gold M. A. (2005). A6, a urokinase plasminogen activator (uPA)-derived peptide in patients with advanced gynecologic cancer: a phase I trial. Gynecol. Oncol. 99, 50–57 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 [DOI] [PubMed] [Google Scholar]
- Biondi M. L., Turri O., Leviti S., Seminati R., Cecchini F., Bernini M., Ghilardi G., Guagnellini E. (2000). MMP1 and MMP3 polymorphisms in promoter regions and cancer. Clin. Chem. 46, 2023–2024 [PubMed] [Google Scholar]
- Bird J. L., Platt D., Wells T., May S. A., Bayliss M. T. (2000). Exercise-induced changes in proteoglycan metabolism of equine articular cartilage. Equine Vet. J. 32, 161–163 [DOI] [PubMed] [Google Scholar]
- Birk D. E., Bruckner P. (2005). Collagen Suprastructures. Top. Curr. Biol. 247, 185–205 [Google Scholar]
- Bissell M. J., Radisky D. (2001). Putting tumours in context. Nat. Rev. Cancer 1, 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. F., Dite G. S., Stone J., Gunasekara A., English D. R., McCredie M. R., Giles G. G., Tritchler D., Chiarelli A., Yaffe M. J., et al. (2002). Heritability of mammographic density, a risk factor for breast cancer. N. Engl. J. Med. 347, 886–894 [DOI] [PubMed] [Google Scholar]
- Boyd N. F., Guo H., Martin L. J., Sun L., Stone J., Fishell E., Jong R. A., Hislop G., Chiarelli A., Minkin S., et al. (2007). Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 356, 227–236 [DOI] [PubMed] [Google Scholar]
- Brown E., McKee T., diTomaso E., Pluen A., Seed B., Boucher Y., Jain R. K. (2003). Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 9, 796–800 [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. (1978). The glycosylation of hemoglobin: relevance to diabetes mellitus. Science 200, 21–27 [DOI] [PubMed] [Google Scholar]
- Butcher D. T., Alliston T., Weaver V. M. (2009). A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell M. A., Anderson J. C., Hasleton P. S. (1996). Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin. Chim. Acta 245, 73–84 [DOI] [PubMed] [Google Scholar]
- Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. (2003). Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 253, 269–285 [DOI] [PubMed] [Google Scholar]
- Chlenski A., Cohn S. L. (2010). Modulation of matrix remodeling by SPARC in neoplastic progression. Semin. Cell Dev. Biol. 21, 55–65 [DOI] [PubMed] [Google Scholar]
- Condeelis J., Pollard J. W. (2006). Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 [DOI] [PubMed] [Google Scholar]
- Condeelis J., Segall J. E. (2003). Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 3, 921–930 [DOI] [PubMed] [Google Scholar]
- Cortese B., Gigli G., Riehle M. (2009). Mechanical gradient cues for guided cell motility and control of cell behavior on uniform substrates. Adv. Funct. Mater. 19, 2961–2968 [Google Scholar]
- Coussens L. M., Werb Z. (2001). Inflammatory cells and cancer: think different! J. Exp. Med. 193, F23–F26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L. M., Fingleton B., Matrisian L. M. (2002). Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295, 2387–2392 [DOI] [PubMed] [Google Scholar]
- Csiszar K. (2001). Lysyl oxidases: a novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 70, 1–32 [DOI] [PubMed] [Google Scholar]
- Denton C. P., Abraham D. J. (2001). Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr. Opin. Rheumatol. 13, 505–511 [DOI] [PubMed] [Google Scholar]
- Derda R., Laromaine A., Mammoto A., Tang S. K., Mammoto T., Ingber D. E., Whitesides G. M. (2009). Paper-supported 3D cell culture for tissue-based bioassays. Proc. Natl. Acad. Sci. USA 106, 18457–18462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier J. S., Cheresh D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D. E., Mooney D. J., Zandstra P. W. (2009). Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror Y., Freedman M. H. (1999). Shwachman-Diamond syndrome: an inherited preleukemic bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. Blood 94, 3048–3054 [PubMed] [Google Scholar]
- Dufour A., Sampson N. S., Zucker S., Cao J. (2008). Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 217, 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durier S., Fassot C., Laurent S., Boutouyrie P., Couetil J. P., Fine E., Lacolley P., Dzau V. J., Pratt R. E. (2003). Physiological genomics of human arteries: quantitative relationship between gene expression and arterial stiffness. Circulation 108, 1845–1851 [DOI] [PubMed] [Google Scholar]
- Ebihara T., Venkatesan N., Tanaka R., Ludwig M. S. (2000). Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Temporal aspects. Am. J. Respir. Crit. Care Med. 162, 1569–1576 [DOI] [PubMed] [Google Scholar]
- Egeblad M., Werb Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174 [DOI] [PubMed] [Google Scholar]
- Engel J., Taylor W., Paulsson M., Sage H., Hogan B. (1987). Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry 26, 6958–6965 [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 [DOI] [PubMed] [Google Scholar]
- Erler J. T., Giaccia A. J. (2008). The cellular microenvironment and metastases. In Abeloff’s Clinical Oncology, Fourth Edition (eds Abeloff M. D., Armitage J. O., Niederhuber J. E., Kastan M. B., McKenna W. G.), pp. 33–47 Philadelphia: Elsevier Publications Ltd; (Churchill Livingstone). [Google Scholar]
- Erler J. T., Linding R. (2010). Network-based drugs and biomarkers. J. Pathol. 220, 290–296 [DOI] [PubMed] [Google Scholar]
- Erler J. T., Weaver V. M. (2009). Three-dimensional context regulation of metastasis. Clin. Exp. Metastasis 26, 35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler J. T., Bennewith K. L., Nicolau M., Dornhofer N., Kong C., Le Q. T., Chi J. T., Jeffrey S. S., Giaccia A. J. (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 [DOI] [PubMed] [Google Scholar]
- Erler J. T., Bennewith K. L., Cox T. R., Lang G., Bird D., Koong A., Le Q. T., Giaccia A. J. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. N. (1991). On the pathogenesis of diabetic retinopathy. A 1990 update. Ophthalmology 98, 586–593 [DOI] [PubMed] [Google Scholar]
- Friedman S. L. (2004). Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastroenterol. Hepatol. 1, 98–105 [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Maher J. J., Bissell D. M. (2000). Mechanisms and therapy of hepatic fibrosis: report of the AASLD single topic basic research conference. Hepatology 32, 1403–1408 [DOI] [PubMed] [Google Scholar]
- Funk S. E., Sage E. H. (1991). The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA 88, 2648–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri S., Manning C., Hooper S., Jones L., Hill C. S., Sahai E. (2009). Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat. Cell Biol. 11, 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. (1999). Integrin signaling. Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- Glasheen B. M., Kabra A. T., Page-McCaw A. (2009). Distinct functions for the catalytic and hemopexin domains of a Drosophila matrix metalloproteinase. Proc. Natl. Acad. Sci. USA 106, 2659–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. V., Stitt A. W. (2009). The role of advanced glycation end products in retinal ageing and disease. Biochim. Biophys. Acta 1790, 1109–1116 [DOI] [PubMed] [Google Scholar]
- Goffin J. R., Anderson I. C., Supko J. G., Eder J. P., Jr, Shapiro G. I., Lynch T. J., Shipp M., Johnson B. E., Skarin A. T. (2005). Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 11, 3417–3424 [DOI] [PubMed] [Google Scholar]
- Graham H. K., Hodson N. W., Hoyland J. A., Millward-Sadler S. J., Garrod D., Scothern A., Griffiths C. E., Watson R. E., Cox T. R., Erler J. T., et al. (2010). Tissue section AFM: In situ ultrastructural imaging of native biomolecules. Matrix Biol. 29, 254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueta R., Barlam D., Shneck R. Z., Rousso I. (2006). Measurement of the mechanical properties of isolated tectorial membrane using atomic force microscopy. Proc. Natl. Acad. Sci. USA 103, 14790–14795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipanayi E., Mudera V., Brown R. A. (2009). Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil. Cytoskeleton 66, 121–128 [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Fujimoto M., Takehara K., Sato S. (2005). Pathogenesis of systemic sclerosis: altered B cell function is the key linking systemic autoimmunity and tissue fibrosis. J. Dermatol. Sci. 39, 1–7 [DOI] [PubMed] [Google Scholar]
- Hicke B. J., Stephens A. W., Gould T., Chang Y. F., Lynott C. K., Heil J., Borkowski S., Hilger C. S., Cook G., Warren S., et al. (2006). Tumor targeting by an aptamer. J. Nucl. Med. 47, 668–678 [PubMed] [Google Scholar]
- Hornstra I. K., Birge S., Starcher B., Bailey A. J., Mecham R. P., Shapiro S. D. (2003). Lysyl oxidase is required for vascular and diaphragmatic development in mice. J. Biol. Chem. 278, 14387–14393 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2009). The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingman W. V., Wyckoff J., Gouon-Evans V., Condeelis J., Pollard J. W. (2006). Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn. 235, 3222–3229 [DOI] [PubMed] [Google Scholar]
- Iredale J. P. (2007). Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 117, 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa R., Zhou X., Constandinou C. M., Fallowfield J., Millward-Sadler H., Gaca M. D., Sands E., Suliman I., Trim N., Knorr A., et al. (2004). Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126, 1795–1808 [DOI] [PubMed] [Google Scholar]
- Jacobs T. W., Byrne C., Colditz G., Connolly J. L., Schnitt S. J. (1999). Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N. Engl. J. Med. 340, 430–436 [DOI] [PubMed] [Google Scholar]
- Jiang T., Olson E. S., Nguyen Q. T., Roy M., Jennings P. A., Tsien R. Y. (2004). Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 101, 17867–17872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodele S., Blavier L., Yoon J. M., DeClerck Y. A. (2006). Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 25, 35–43 [DOI] [PubMed] [Google Scholar]
- Kagan H. M. (1994). Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Pathol. Res. Pract. 190, 910–919 [DOI] [PubMed] [Google Scholar]
- Kagan H. M. (2000). Intra- and extracellular enzymes of collagen biosynthesis as biological and chemical targets in the control of fibrosis. Acta Trop. 77, 147–152 [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Li W. (2003). Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88, 660–672 [DOI] [PubMed] [Google Scholar]
- Kaler S. G., Gallo L. K., Proud V. K., Percy A. K., Mark Y., Segal N. A., Goldstein D. S., Holmes C. S., Gahl W. A. (1994). Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat. Genet. 8, 195–202 [DOI] [PubMed] [Google Scholar]
- Kaplan R. N., Riba R. D., Zacharoulis S., Bramley A. H., Vincent L., Costa C., MacDonald D. D., Jin D. K., Shido K., Kerns S. A., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L., Erler J. T., Dembo M., Weaver V. M. (2007). Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int. J. Biochem. Cell Biol. 39, 1987–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaviratne M., Hesse M., Leusink M., Cheever A. W., Davies S. J., McKerrow J. H., Wakefield L. M., Letterio J. J., Wynn T. A. (2004). IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J. Immunol. 173, 4020–4029 [DOI] [PubMed] [Google Scholar]
- Keeley E. C., Mehrad B., Strieter R. M. (2009). Fibrocytes: Bringing new insights into mechanisms of inflammation and fibrosis. Int. J. Biochem. Cell Biol. 42, 535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. (2010). Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann D. A., Seftor E. A., Fong S. F., Nieva D. R., Sullivan C. M., Edwards E. M., Sommer P., Csiszar K., Hendrix M. J. (2002). A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 62, 4478–4483 [PubMed] [Google Scholar]
- Klotzsch E., Smith M. L., Kubow K. E., Muntwyler S., Little W. C., Beyeler F., Gourdon D., Nelson B. J., Vogel V. (2009). Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc. Natl. Acad. Sci. USA 106, 18267–18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosoglou G., Weiss R. G., Kedziorek D. A., Walczak P., Gilson W. D., Schar M., Sosnovik D. E., Kraitchman D. L., Boston R. C., Bulte J. W., et al. (2008). Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using “positive contrast” magnetic resonance imaging. J. Am. Coll. Cardiol. 52, 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Weaver V. M. (2009). Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. (1994). The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 8, 163–173 [PubMed] [Google Scholar]
- Le Q. T., Kong C., Lavori P. W., O’Byrne K., Erler J. T., Huang X., Chen Y., Cao H., Tibshirani R., Denko N., et al. (2007). Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 69, 167–175 [DOI] [PubMed] [Google Scholar]
- Le Q. T., Harris J., Magliocco A. M., Kong C. S., Diaz R., Shin B., Cao H., Trotti A., Erler J. T., Chung C. H., et al. (2009). Validation of lysyl oxidase as a prognostic marker for metastasis and survival in head and neck squamous cell carcinoma: radiation therapy oncology group trial 90–03. J. Clin. Oncol. 27, 4281–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. K., Li G., Mickle D. A., Weisel R. D., Merante F., Luss H., Rao V., Christakis G. T., Williams W. G. (1997). Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation 96, 874–881 [DOI] [PubMed] [Google Scholar]
- Littlepage L. E., Sternlicht M. D., Rougier N., Phillips J., Gallo E., Yu Y., Williams K., Brenot A., Gordon J. I., Werb Z. (2010). Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 70, 2224–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. M., Wang H. B., Dembo M., Wang Y. L. (2000). Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A. F., Tearney G. J., Bouma B. E., Jang I. K. (2006). Technology Insight: optical coherence tomography-current status and future development. Nat. Clin. Pract. Cardiovasc. Med. 3, 154–162 [DOI] [PubMed] [Google Scholar]
- Mack G. S., Marshall A. (2010). Lost in migration. Nat. Biotechnol. 28, 214–229 [DOI] [PubMed] [Google Scholar]
- Maki J. M., Rasanen J., Tikkanen H., Sormunen R., Makikallio K., Kivirikko K. I., Soininen R. (2002). Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509 [DOI] [PubMed] [Google Scholar]
- Markiewicz M., Smith E. A., Rubinchik S., Dong J. Y., Trojanowska M., LeRoy E. C. (2004). The 72-kilodalton IE-1 protein of human cytomegalovirus (HCMV) is a potent inducer of connective tissue growth factor (CTGF) in human dermal fibroblasts. Clin. Exp. Rheumatol. 22, S31–S34 [PubMed] [Google Scholar]
- Massague J. (2008). TGFbeta in Cancer. Cell 134, 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer M. A., Schneider J. E., Ali Z. A., Warrick N., Bursill C. A., von zur Muhlen C., Greaves D. R., Neubauer S., Channon K. M., Choudhury R. P. (2008). Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler. Thromb. Vasc. Biol. 28, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. D., Robinson S. P. (2010). Intrinsic susceptibility MR imaging of chemically induced rat mammary tumors: relationship to histologic assessment of hypoxia and fibrosis. Radiology 254, 110–118 [DOI] [PubMed] [Google Scholar]
- Michigami T., Shimizu N., Williams P. J., Niewolna M., Dallas S. L., Mundy G. R., Yoneda T. (2000). Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood 96, 1953–1960 [PubMed] [Google Scholar]
- Miserus R. J., Herias M. V., Prinzen L., Lobbes M. B., Van Suylen R. J., Dirksen A., Hackeng T. M., Heemskerk J. W., van Engelshoven J. M., Daemen M. J., et al. (2009). Molecular MRI of early thrombus formation using a bimodal alpha2-antiplasmin-based contrast agent. JACC Cardiovasc. Imaging 2, 987–996 [DOI] [PubMed] [Google Scholar]
- Mitsiades N., Yu W. H., Poulaki V., Tsokos M., Stamenkovic I. (2001). Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 61, 577–581 [PubMed] [Google Scholar]
- Moore B. B., Kolodsick J. E., Thannickal V. J., Cooke K., Moore T. A., Hogaboam C., Wilke C. A., Toews G. B. (2005). CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am. J. Pathol. 166, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K., Walker S. M., Nakajima M., Pathak S., Jessup J. M., Fidler I. J. (1988). Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 48, 6863–6871 [PubMed] [Google Scholar]
- Mosher D. F., Schad P. E. (1979). Cross-linking of fibronectin to collagen by blood coagulation Factor XIIIa. J. Clin. Invest. 64, 781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Schad P. E., Kleinman H. K. (1979). Inhibition of blood coagulation factor XIIIa-mediated cross-linking between fibronectin and collagen by polyamines. J. Supramol. Struct. 11, 227–235 [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Churg A. (1998). Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 157, 1666–1680 [DOI] [PubMed] [Google Scholar]
- Mott J. D., Werb Z. (2004). Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 16, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A., Heldin C. H. (2005). Non-Smad TGF-beta signals. J. Cell Sci. 118, 3573–3584 [DOI] [PubMed] [Google Scholar]
- Murawaki Y., Kusakabe Y., Hirayama C. (1991). Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatology 14, 1167–1173 [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lane T. F., Pallero M. A., Sage E. H. (1995). SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J. Cell. Biochem. 57, 341–350 [DOI] [PubMed] [Google Scholar]
- Muthuswamy S. K., Li D., Lelievre S., Bissell M. J., Brugge J. S. (2001). ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3, 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Kalamajski S., Salnikov A. V., Stuhr L., Morgelin M., Reed R. K., Heldin N. E., Rubin K. (2007). Collagen-binding proteoglycan fibromodulin can determine stroma matrix structure and fluid balance in experimental carcinoma. Proc. Natl. Acad. Sci. USA 104, 13966–13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni F., Reynaud E. G., Stelzer E. H. (2007). The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845 [DOI] [PubMed] [Google Scholar]
- Pardo A., Selman M. (2006). Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc. Am. Thorac. Soc. 3, 383–388 [DOI] [PubMed] [Google Scholar]
- Paszek M. J., Weaver V. M. (2004). The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia 9, 325–342 [DOI] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 [DOI] [PubMed] [Google Scholar]
- Pawson T., Linding R. (2008). Network medicine. FEBS Lett. 582, 1266–1270 [DOI] [PubMed] [Google Scholar]
- Payne S. L., Fogelgren B., Hess A. R., Seftor E. A., Wiley E. L., Fong S. F., Csiszar K., Hendrix M. J., Kirschmann D. A. (2005). Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 65, 11429–11436 [DOI] [PubMed] [Google Scholar]
- Payne S. L., Hendrix M. J., Kirschmann D. A. (2007). Paradoxical roles for lysyl oxidases in cancer-a prospect. J. Cell. Biochem. 101, 1338–1354 [DOI] [PubMed] [Google Scholar]
- Perentes J. Y., McKee T. D., Ley C. D., Mathiew H., Dawson M., Padera T. P., Munn L. L., Jain R. K., Boucher Y. (2009). In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat. Methods 6, 143–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. J., Burdick M. D., Hong K., Lutz M. A., Murray L. A., Xue Y. Y., Belperio J. A., Keane M. P., Strieter R. M. (2004). Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 114, 438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P. P., Eliceiri K. W., Campbell J. M., Inman D. R., White J. G., Keely P. J. (2006). Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Knittel J. G., Yan L., Rueden C. T., White J. G., Keely P. J. (2008). Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher K. A., Beghein N., Boutry S., Laurent S., Vander Elst L., Muller R. N., Jordan B. F., Gallez B. (2009). In vivo detection of inflammation using pegylated iron oxide particles targeted at E-selectin: a multimodal approach using MR imaging and EPR spectroscopy. Invest. Radiol. 44, 398–404 [DOI] [PubMed] [Google Scholar]
- Radisky D., Hagios C., Bissell M. J. (2001). Tumors are unique organs defined by abnormal signaling and context. Semin. Cancer Biol. 11, 87–95 [DOI] [PubMed] [Google Scholar]
- Reardon D. A., Zalutsky M. R., Akabani G., Coleman R. E., Friedman A. H., Herndon J. E., 2nd, McLendon R. E., Pegram C. N., Quinn J. A., Rich J. N., et al. (2008). A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro. Oncol. 10, 182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Larkman D. J., Haskard D. O., Hajnal J. V., Kennea N. L., George A. J., Edwards A. D. (2006). Detection of vascular expression of E-selectin in vivo with MR imaging. Radiology 241, 469–476 [DOI] [PubMed] [Google Scholar]
- Rittweger J., Winwood K., Seynnes O., de Boer M., Wilks D., Lea R., Rennie M., Narici M. (2006). Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension. J. Physiol. 577, 331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittweger J., Simunic B., Bilancio G., De Santo N. G., Cirillo M., Biolo G., Pisot R., Eiken O., Mekjavic I. B., Narici M. (2009). Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone 44, 612–618 [DOI] [PubMed] [Google Scholar]
- Rocco P. R., Negri E. M., Kurtz P. M., Vasconcellos F. P., Silva G. H., Capelozzi V. L., Romero P. V., Zin W. A. (2001). Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am. J. Respir. Crit. Care Med. 164, 1067–1071 [DOI] [PubMed] [Google Scholar]
- Rosenbaum E., Zahurak M., Sinibaldi V., Carducci M. A., Pili R., Laufer M., DeWeese T. L., Eisenberger M. A. (2005). Marimastat in the treatment of patients with biochemically relapsed prostate cancer: a prospective randomized, double-blind, phase I/II trial. Clin. Cancer Res. 11, 4437–4443 [DOI] [PubMed] [Google Scholar]
- Rosenkranz S., Flesch M., Amann K., Haeuseler C., Kilter H., Seeland U., Schluter K. D., Bohm M. (2002). Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am. J. Physiol. Heart Circ. Physiol. 283, H1253–H1262 [DOI] [PubMed] [Google Scholar]
- Roskelley C. D., Srebrow A., Bissell M. J. (1995). A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr. Opin. Cell Biol. 7, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce P. M., Camakaris J., Danks D. M. (1980). Reduced lysyl oxidase activity in skin fibroblasts from patients with Menkes’ syndrome. Biochem. J. 192, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roycik M. D., Fang X., Sang Q. X. (2009). A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr. Pharm. Des. 15, 1295–1308 [DOI] [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. (1984). Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J. Biol. Chem. 259, 3993–4007 [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. (1989). SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 109, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T., Seiki M. (2009). Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells 14, 617–626 [DOI] [PubMed] [Google Scholar]
- Sasaki N., Fukatsu R., Tsuzuki K., Hayashi Y., Yoshida T., Fujii N., Koike T., Wakayama I., Yanagihara R., Garruto R., et al. (1998). Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 153, 1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer R. L., McIntyre J. O., Matrisian L. M. (2008a). Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 27, 679–690 [DOI] [PubMed] [Google Scholar]
- Scherer R. L., VanSaun M. N., McIntyre J. O., Matrisian L. M. (2008b). Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol. Imaging 7, 118–131 [PMC free article] [PubMed] [Google Scholar]
- Schmeichel K. L., Bissell M. J. (2003). Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci. 116, 2377–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. (1980). Glucosylation of human collagen in aging and diabetes mellitus. J. Clin. Invest. 66, 1179–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Baron V. (1999). Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11, 197–202 [DOI] [PubMed] [Google Scholar]
- Sethi T., Rintoul R. C., Moore S. M., MacKinnon A. C., Salter D., Choo C., Chilvers E. R., Dransfield I., Donnelly S. C., Strieter R., et al. (1999). Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 5, 662–668 [DOI] [PubMed] [Google Scholar]
- Sherratt M. J., Baldock C., Haston J. L., Holmes D. F., Jones C. J., Shuttleworth C. A., Wess T. J., Kielty C. M. (2003). Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J. Mol. Biol. 332, 183–193 [DOI] [PubMed] [Google Scholar]
- Sivakumar P., Gupta S., Sarkar S., Sen S. (2008). Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol. Cell. Biochem. 307, 159–167 [DOI] [PubMed] [Google Scholar]
- Sivan S. S., Wachtel E., Tsitron E., Sakkee N., van der Ham F., Degroot J., Roberts S., Maroudas A. (2008). Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J. Biol. Chem. 283, 8796–8801 [DOI] [PubMed] [Google Scholar]
- Spuentrup E., Buecker A., Katoh M., Wiethoff A. J., Parsons E. C., Jr, Botnar R. M., Weisskoff R. M., Graham P. B., Manning W. J., Gunther R. W. (2005). Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation 111, 1377–1382 [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D., Werb Z. (2001). How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M. D., Lochter A., Sympson C. J., Huey B., Rougier J. P., Gray J. W., Pinkel D., Bissell M. J., Werb Z. (1999). The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98, 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover D. G., Bierie B., Moses H. L. (2007). A delicate balance: TGF-beta and the tumor microenvironment. J. Cell. Biochem. 101, 851–861 [DOI] [PubMed] [Google Scholar]
- Stracke C. P., Katoh M., Wiethoff A. J., Parsons E. C., Spangenberg P., Spuntrup E. (2007). Molecular MRI of cerebral venous sinus thrombosis using a new fibrin-specific MR contrast agent. Stroke 38, 1476–1481 [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Gomperts B. N., Keane M. P. (2007). The role of CXC chemokines in pulmonary fibrosis. J. Clin. Invest. 117, 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y. (2006). Mislocalization and unconventional functions of cellular MMPs in cancer. Cancer Metastasis Rev. 25, 87–98 [DOI] [PubMed] [Google Scholar]
- Suki B., Bates J. H. (2008). Extracellular matrix mechanics in lung parenchymal diseases. Respir. Physiol. Neurobiol. 163, 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. (1981). Mineral and collagen-binding proteins of fetal calf bone. J. Biol. Chem. 256, 10403–10408 [PubMed] [Google Scholar]
- Tsien R. Y. (2005). Building and breeding molecules to spy on cells and tumors. FEBS Lett. 579, 927–932 [DOI] [PubMed] [Google Scholar]
- Varga J., Abraham D. (2007). Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. (1989). Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 [PubMed] [Google Scholar]
- Verzijl N., DeGroot J., Thorpe S. R., Bank R. A., Shaw J. N., Lyons T. J., Bijlsma J. W., Lafeber F. P., Baynes J. W., TeKoppele J. M. (2000). Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 275, 39027–39031 [DOI] [PubMed] [Google Scholar]
- Vitek M. P., Bhattacharya K., Glendening J. M., Stopa E., Vlassara H., Bucala R., Manogue K., Cerami A. (1994). Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 91, 4766–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Barker T. A., Berk B. C. (2005). Angiotensin II and the endothelium: diverse signals and effects. Hypertension 45, 163–169 [DOI] [PubMed] [Google Scholar]
- Weaver V. M., Fischer A. H., Peterson O. W., Bissell M. J. (1996). The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem. Cell Biol. 74, 833–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver V. M., Petersen O. W., Wang F., Larabell C. A., Briand P., Damsky C., Bissell M. J. (1997). Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. G., Discher D. E. (2008). Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci. Signal. 1, pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg C., Klatt A. R., Wagener R., Paulsson M., Bateman J. F., Heinegard D., Morgelin M. (2003). Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J. Biol. Chem. 278, 37698–37704 [DOI] [PubMed] [Google Scholar]
- Wipff P. J., Rifkin D. B., Meister J. J., Hinz B. (2007). Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Alexander S., Schacht V., Coussens L. M., von Andrian U. H., van Rheenen J., Deryugina E., Friedl P. (2009). Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 20, 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J. N. (1976a). Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 37, 2486–2492 [DOI] [PubMed] [Google Scholar]
- Wolfe J. N. (1976b). Breast patterns as an index of risk for developing breast cancer. AJR Am. J. Roentgenol. 126, 1130–1137 [DOI] [PubMed] [Google Scholar]
- Wong J. Y., Velasco A., Rajagopalan P., Pham Q. (2003). Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir 19, 1908–1913 [Google Scholar]
- Wynn T. A. (2007). Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Cukierman E. (2007). Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 [DOI] [PubMed] [Google Scholar]
- Yu H., Mouw J. K., Weaver V. M. (2010). Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 21, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]