SUMMARY
The family of matrix metalloproteinases (MMPs) is responsible for extracellular matrix degradation during physiological and pathophysiological tissue remodeling processes such as embryogenesis, tissue repair and cancer progression. Despite these important roles of MMPs, inhibition or ablation of individual members of the MMP family in animal models have been shown to have little effect. It has been speculated that this results from a functional overlap between individual MMPs and (as-yet-unclassified) functional overlaps between MMPs and other protease systems. We here present genetic data showing that concomitant ablation of MMP9 (gelatinase B) and the serine protease plasmin results in lethal inflammatory mass lesions in the colon. These lesions possessed several histological attributes that are characteristic of mucosal prolapse seen in humans, and they were found to be associated with splenomegaly, enlarged mesenteric lymph nodes, decreased thymus size and altered populations of circulating immune cells. A time-course study provided evidence that the massive lymphoid hyperplasia and reactive changes were secondary to discrete fibrinous lesions also observed in mice only deficient for plasminogen (Plg), the zymogen for plasmin. These data demonstrate a non-appreciated vital protective role for MMP9 in the absence of Plg.
INTRODUCTION
Increased expression of several different matrix metalloproteinases (MMPs), the main role of which is to degrade extracellular matrix (ECM) proteins, has been associated with a poor prognosis in various diseases, including cancer, arthritis and cardiovascular pathologies, as well as in cerebral infarction (Fingleton, 2008). In contrast to their well-documented involvement in pathological events, their role during normal physiological processes still remains poorly understood. One reason for this is that genetically engineered mice lacking functional expression of individual MMPs generally have subtle phenotypes, a phenomenon that could be explained by enzymatic redundancy, compensation or adaption (Page-McCaw et al., 2007). Concerning enzymatic redundancy, various members of the MMP family might have a functional overlap: they share a long range of substrates and are active during the same physiological and pathological events (Sternlicht and Werb, 2001; Greenlee et al., 2006; Hattori et al., 2009).
In addition to functional overlaps among individual MMPs, a functional overlap between the MMP system and the central serine protease plasmin, which is essential for fibrin clearance (Bugge et al., 1996), has been proposed (Danø et al., 1999). This notion is supported by the synergistic effects of broad-spectrum pharmacological MMP inhibition and plasminogen (Plg) deficiency on events such as embryonic development and wound healing (Lund et al., 1999; Solberg et al., 2003; Lund et al., 2006). However, the particular MMP(s) whose dysfunction is responsible for these synergistic effects in Plg-deficient mice, as well as the decisive substrate, remains to be determined. A key candidate is MMP9, which has been shown to have several substrates in common with plasmin, including fibrin (Lelongt et al., 2001).
Although the most noticeable effects of Plg deficiency are reverted by a lack of fibrinogen (Bugge et al., 1996), plasmin has been shown to have the capacity to proteolytically activate other extracellular proteases, including MMP9 (Heissig et al., 2007; Gong et al., 2008) and vital cytokines, such as transforming growth factor-β (TGFβ) (Sato and Rifkin, 1989; Dallas et al., 2002). However, these actions of plasmin are conducted by other means in the absence of plasmin. This notion is clearly substantiated in the case of TGFβ activation because, in contrast to TGFβ-receptor-deficient mice, mice deficient for Plg are viable and furthermore they do not carry any phenotypical resemblances with mice lacking TGFβ or TGFβ-receptor downstream signaling proteins (Bugge et al., 1995; Dunker and Krieglstein, 2000). It is not inconceivable that activation of cytokines that have diverse and crucial activities, such as TGFβ, can be regulated by different proteases under various conditions (Annes et al., 2003), and, in addition to plasmin, a limited number of MMPs, including MMP9, have been shown in vitro to possess TGFβ activation capacities (Dallas et al., 2002).
It is well documented that, besides having substrates in common, plasmin and MMP9 are both active following pathophysiological events, such as cancer invasion and wound healing (Green et al., 2008; Hattori et al., 2009), in which they are likely to have both distinct and overlapping functions. Nevertheless, studies based on Plg−/− and MMP9−/− mice have also shown that the mice have distinctive phenotypes. This includes the development of scattered microscopic lesions in the colon and degeneration of the gastric mucosa along with rectal prolapse in Plg−/− mice (Bugge et al., 1995), whereas MMP9−/− mice have not been reported to suffer from any of these pathological changes. However, MMP9−/− mice are known to have a small decrease in bone length compared with wild-type mice (Vu et al., 1998) owing to an MMP9-dependent decrease in vascular endothelial growth factor bioavailability during early bone development (Engsig et al., 2000).
In contrast to the limited impact of MMP9 deficiency during normal physiological development, the importance of active MMP9 during cell migration and cytokine activation are emphasized by the detrimental effects of MMP9 in diverse pathological alterations, including colitis (Santana et al., 2006; Garg et al., 2009), neuroinflammation (Kawasaki et al., 2008) and aneurysm formation (Pyo et al., 2000), as well as by the beneficial effects on epidermal regeneration following wounding (Hattori et al., 2009).
To clarify the importance of the proposed substrate redundancy between plasmin and MMP9 in relation to embryogenesis, development and tissue remodeling following pathological events, we mated MMP9−/+:Plg−/+ mice. Despite any putative dependencies on the combined functions of plasmin and MMP9 during embryogenesis, viable MMP9−/−:Plg−/− pups were born, although at a low frequency. These pups grew to adulthood, expressing a mixed phenotype resembling both Plg- and MMP9-deficient mice. Surprisingly, MMP9−/−:Plg−/− mice started wasting at an age of approximately 20 weeks, leading to spontaneous deaths or necessitated euthanization. In addition to features associated with deficiency of either MMP9 or Plg, necropsy revealed large obstructive mass lesions in the colon of these mice but not in MMP9−/−, Plg−/− or wild-type (WT) controls. Systematic investigation of these lesions showed that small ulcers associated with lymphoid aggregates in the mucosa might represent the initial visible event; these ulcers then expanded over weeks by simultaneous recruitment of inflammatory cells and degradation of the normal healthy mucosa. Histologically, these lesions resemble those observed in mucosal-prolapse-related diseases in humans, and clearly demonstrate that combined protease activity is crucial for the homeostasis of the intestinal mucosa.
RESULTS
Skewed genetic distribution of MMP9 and Plg alleles in the offspring of MMP9−/+:Plg−/+ mice
To test the existence of a putative embryonic lethal phenotype of MMP9 and Plg double deficiency, MMP9−/+:Plg−/+ mice were mated and their offspring were genotyped at weaning. Surprisingly, MMP9−/−:Plg−/− pups were found to be viable and resembled WT littermates at gross inspection. However, at time of weaning, MMP9−/−:Plg−/− pups were obtained at a lower frequency than expected based on a normal mendelian distribution pattern. Moreover, a decreased proportion of MMP9−/− and MMP9−/−:Plg−/+ pups was observed, whereas the other genotypes were obtained in an expected ratio (Fig. 1A).
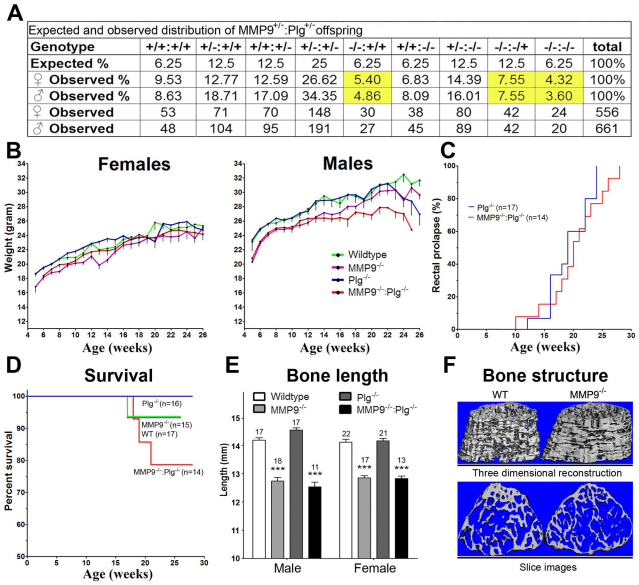
Fig. 1.
Offspring of MMP9−/+:Plg−/+ mice express a mixed phenotype and additional traits. (A) The offspring of MMP9−/+:Plg−/+ mice were genotyped at weaning. A total of 1217 pups were tested for the mutant alleles. Genotypes are presented as follows (MMP9:Plg): + indicates a WT allele and – indicates the dysfunctional mutant allele. Highlighted areas indicate a decreased fraction of pups compared with the expected fraction on the basis of a theoretical mendelian distribution. MMP9−/− pups were observed at a decreased frequency, which was further decreased by the lack of one or two functional Plg alleles down to 69% and 57% of the expected number for females and males, respectively. P<0.001 for both males and females. (B) From time of weaning, mice were weighed weekly until the age of 26 weeks. MMP9−/− and MMP9−/−:Plg−/− mice were generally smaller than WT and Plg−/− mice (P<0.05). Male Plg−/− mice showed a sudden steep decline in weight around week 22, by which time point they had also developed severe rectal prolapses. In contrast to male Plg−/− mice, male MMP9−/−:Plg−/− mice stopped gaining weight around 12 weeks of age (P<0.05). A similar development of weight over time was not evident in female mice. (C) Plg−/− and MMP9−/−:Plg−/− mice were examined weekly for the presence of rectal lesions. The time of onset of rectal prolapse development took place from 10 to 24 weeks of age. The Kaplan-Meier analysis showed that Plg−/− (7 males and 8 females) and MMP9−/−:Plg−/− (8 males and 5 females) mice both followed the same time course of rectal prolapse development, and furthermore, no significant effect of gender was observed. (D) Spontaneous deaths of unknown cause were monitored in male and female MMP9−/−:Plg−/− mice and compared with controls. Among the included MMP9−/−:Plg−/− mice (7 males and 7 females), three male mice died from unknown causes. The tendency for a decreased survival rate of MMP9−/−:Plg−/− mice did not reach statistical significance (P=0.22). (E) Bone-length measurements showed that the short-bone phenotype observed in MMP9−/− mice is unaffected by the lack of functional Plg. The graph shows the length of femoral bones isolated from adult mice. The number of mice in each group is shown above the columns. ***P<0.0001 compared with respective controls. (F) Reconstruction of cancellous bone from distal femurs of WT and MMP9−/− mice, in which significant differences in the microarchitecture can be observed. WT and Plg−/− mice had a more plate-like structure with thicker trabeculae compared with MMP9−/− and MMP9−/−:Plg−/− mice, which had a combination of plate- and rod-like structure with relatively thin trabeculae. Data are presented as means ± s.e.m. and analyzed by ANOVA with a Newman-Keuls post-test.
MMP9 and Plg deficiency have additive effects on development
To investigate the effect of concurrent ablation of MMP9 and Plg on mouse development and during adulthood, we set up a cohort study including MMP9−/−:Plg−/− mice and their WT, MMP9−/− and Plg−/−littermate controls. The cohort included: 47 WT, 45 MMP9−/−, 51 Plg−/− and 39 MMP9−/−:Plg−/− mice (a total of 182 mice). The mice included in the cohort were weighed weekly and monitored for development of rectal prolapses, which are known to appear in mice lacking functional Plg (Bugge et al., 1995). At the age of 8 and 12 weeks, approximately one-third of the mice at each time point were randomly picked out and euthanized, examined and their tissues prepared for analyses. Owing to ethical considerations concerning the impact of rectal prolapse development on the general welfare of the animals, the remaining Plg−/− and MMP9−/−:Plg−/− mice were euthanized, at an average age of 24 weeks, along with the remaining WT and MMP9−/− mice to create age-matched controls.
For both males and females, we found that MMP9−/− and MMP9−/−:Plg−/− mice weighed less than both WT and Plg−/− mice upon entry to the cohort at the age of approximately 6 weeks (P<0.05). For female mice this weight difference was evident throughout the experiment. However, for males, MMP9−/−:Plg−/− mice stopped gaining weight at week 12 (P<0.05) and started wasting around 21 weeks of age (P=0.0796). In contrast to male MMP9−/−:Plg−/− mice, male Plg−/− mice continued to gain weight similar to WT mice until an age of approximately 22 weeks, at which point they also showed a steep decline in weight (P<0.001) (Fig. 1B). As expected, Plg−/− and MMP9−/−:Plg−/− mice started to develop rectal prolapses when they reached adulthood. However, no difference was found in the time of onset of prolapse development in mice that did develop a rectal prolapse (Plg−/− mice: 7/9 females and 7/8 males; MMP9−/−:Plg−/−mice: 5/8 females and 7/7 males) between the two groups and the time of onset was furthermore independent of gender (Fig. 1C; supplementary material Fig. S1). Survival analyses showed that spontaneous deaths not related to either tumor or rectal prolapse development reached 22% in MMP9−/−:Plg−/− mice (deaths: 3/7 males and 0/7 females), whereas only 6% of MMP9−/− and WT, and none of the included Plg−/−, mice died (Fig. 1D).
A known phenotype of MMP9−/− mice is the decreased length of the long bones. To investigate the impact of Plg deficiency on this phenotype, the isolated femoral and tibial bones were measured with a caliper. Plg deficiency did not affect the length of the long bones nor exacerbate the effect of MMP9 deficiency on bone length. Because long bones in mice reach their final length before the age of 8 weeks, no difference was observed between the age groups (Fig. 1E). However, micro-CT (μCT) scannings revealed significant microarchitectural changes in the distal femur cancellous bone. There was a decreased trabecular thickness and an increased cancellous bone surface:volume ratio in MMP9−/− mice compared with WT mice regardless of Plg deficiency. Moreover, the structure model index, a parameter monitoring bone remodeling and describing a dominate trabecular structure of cancellous bone, whether it is rod-like or plate-like, was increased by 50% in MMP9−/− mice (Fig. 1F). Thus, cancellous bone structure was more rod-like in MMP9−/− mice compared with WT. However, no differences were seen in the microarchitecture of distal femur cortical bone, in the midshaft cortical bone, in the cross-sectional areas of midshaft cortical bone or in the marrow cavity. In addition, hematoxylin and eosin (H&E)-stained sections of femurs and tibias did not reveal any difference between the various genotypes (data not shown).
MMP9−/−:Plg−/− mice develop inflammatory mass lesions in the colon
Necropsy of MMP9−/−:Plg−/− mice revealed a completely unanticipated phenotype characterized by the formation of severe inflammatory mass lesions in the colon. Macroscopically these mass lesions appeared as large obstructing tumors, which in all cases developed near the same location at the upper part of the sigmoid colon, and resulted in proximal distention of the colon, cecum and small intestines owing to a complete blockade of the bowel lumen (Fig. 2A,B). The volume of these tumorous mass lesions ranged from approximately 50–250 mm3. In some cases, they were associated with several smaller mass lesions proximal to the central mass lesion. The tissue between these smaller mass lesions seemed normal. Gross inspection of the lesions, colon and feces did not reveal any signs of hemorrhage or perforation of the colon. In some cases, the mass lesions were pedunculated, whereas in others they had a more flattened appearance. Examination of mice euthanized at various time points revealed that the formation of these mass lesions in MMP9−/−:Plg−/− mice commenced around the age of 12 weeks, at which time point 20% of the mice (0/6 males and 3/9 females) carried tumors. At an average age of approximately 24 weeks, 85% of the MMP9−/−:Plg−/− mice (5/5 males and 6/8 females) had developed large and obstructing inflammatory mass lesions in their colons, whereas only a single Plg−/− mouse (0/7 males and 1/9 females) showed signs of having such an inflammatory mass lesion in the colon (Fig. 2C). No WT or MMP9−/− mice developed any signs of colon abnormalities.
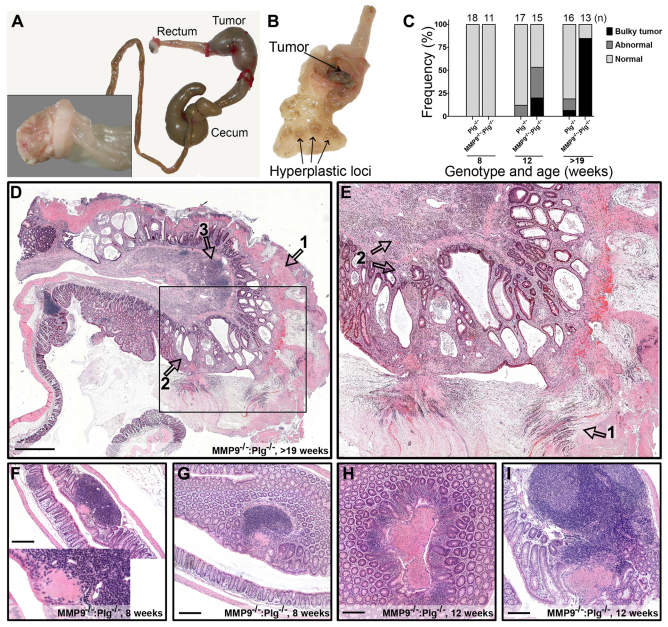
Fig. 2.
MMP9−/−:Plg−/− mice develop large inflammatory mass lesions in the colon, mimicking prolapsed mucosa in humans. Dissection of MMP9−/−:Plg−/− mice revealed that large pedunculated tumorous lesions occupied the colon lumen, resulting in complete obstruction of fecal flow. By contrast, macroscopic inspection of Plg−/− mice only revealed smaller abnormalities in a fraction of the investigated mice. (A) A representative picture of intestines isolated from an MMP9−/−:Plg−/− mouse, in which blockade of the colon resulted in the accumulation of feces in the proximal colon (including the cecum) and even the ileum. Inset shows the solid tumor that was visible after removal of the distal colon while the dilated proximal colon is still attached. (B) Dissection of a colon from an MMP9−/−:Plg−/− mouse revealed a pedunculated tumorous lesion as well as smaller inflammatory lesions located proximal to the obstructing mass lesion. (C) In a time-course study, colons were isolated from WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice at the age of 8 and 12 weeks, and at the time point at which they had to be euthanized owing to ethical considerations (between the age of 19 and 26 weeks). Colons were classified as normal, abnormal or containing a bulky tumorous lesion. ‘Abnormal’ refers to small hyperplastic lesions in the mucosal wall and ‘bulky tumorous lesion’ refers to the presence of at least one large obstructing mass lesion. All WT and MMP9−/− mice appeared normal and were thus not included in the graph. The graph shows the fraction of the mice in each group expressing the three different phenotypes. (D) Microscopic scanning of a large obstructing mass lesion covered by a broad pseudomembrane (arrow 1). The subjacent mucosa displayed a markedly distorted crypt pattern, including several cystically dilated and angulated crypts (arrow 2). Remnants of a lymphoid follicle occupied the superficial portion of the submucosa (arrow 3). (E) Close-up view of the boxed area in D illustrates the streaming pattern of inflammatory cells in the mucinous material of the pseudomembrane (arrow 1). Additionally, inflammatory cells expanded the surrounding lamina propria and extended for a limited distance into the subjacent submucosa (arrow 2). (F,G) Small lesions in 8-week-old MMP9−/−:Plg−/− mice were closely associated with enlarged lymphoid follicles. These lesions are speculated to develop into the inflammatory mass lesions over time. (H,I) Lesions in 12-week-old MMP9−/−:Plg−/− mice have now reached a considerable size and recruitment of additional inflammatory cells was evident along with some distortion of the associated crypt pattern surrounding the lesions. Scale bars: 1 mm (D); 200 μm (F–I).
Histological examination of the inflammatory mass lesions revealed that their polypoid structures were largely composed of mucosa and submucosa. The mucosal crypt pattern was markedly distorted, including angulated and cystically dilated crypts. Mucinous material and cellular debris filled some of the crypts. The lining epithelium was focally attenuated. The surrounding lamina propria was hemorrhagic and richly endowed with inflammatory cells, and the luminal parts of the mass lesions were necrotic. Some of the dilated crypts were expanded by an inflammatory infiltrate. Additionally, a broad pseudomembrane of mucin with admixed inflammatory cells covered the top of the polypoid lesion. The muscularis mucosa did not appear hypertrophied, but was focally splayed with discrete bundles of smooth muscle cells extending for a short distance into the lamina propria. Verhoeff-van Gieson (VVG) and orcein staining came out negative, showing a lack of elastic tissues (supplementary material Fig. S2). Some of the dilated crypts abutted the muscularis mucosa, but inversion into the submucosa was not a feature. The submucosa and the muscularis propria were largely unremarkable; specifically, vascular proliferation was not a feature. No signs of dysplasia were detected (Fig. 2D,E).
The inflammatory mass lesion that developed in a single Plg−/−mouse (age=27 weeks) resembled the lesions found in the MMP9−/−:Plg−/− mice. Dilated crypt structures were surrounded by an inflamed lamina propria and the submucosa was enlarged and heavily infiltrated by inflammatory cells. In distinct areas, the lumenal part of the mucosa was necrotized (supplementary material Fig. S3).
Inflammatory mass lesions develop in association with lymphoid follicles
To reveal the exact site and time of origin of the intestinal mass lesions, we performed detailed histological analyses on colon samples from MMP9−/−:Plg−/− mice of 8 and 12 weeks of age. In the colon of 12-week-old MMP9−/−:Plg−/− mice, mass lesions of various dimensions were detected. They ranged in size, from occupying a single crypt to expanding over hundreds of crypts. These mass lesions seemingly started to develop lumenally in the mucosa in close proximity to solitary lymphoid follicles. With increasing size of the mass lesions, a pronounced recruitment of inflammatory cells was evident. In addition, the crypts surrounding the expanding mass lesion were clearly distorted (Fig. 2F–I). Interestingly, the lymphoid follicles in MMP9−/−:Plg−/− mice seemed to be vastly enlarged and increased in numbers compared with WT mice. In 8-week-old MMP9−/−:Plg−/− mice, in which lesions were hardly detectable, a detailed analysis of the lymphoid follicles revealed that, already at this age, their total combined volume was increased several fold (data not shown).
As previously published (Bugge et al., 1995), Plg−/− mice develop small lesions throughout the colon mucosa. At the age of 12 weeks, two out of 17 Plg−/− mice had developed macroscopically visible lesions. Detailed inspection of these mice revealed that the lesions were composed of large lymphoid aggregates associated with a slightly distorted crypt pattern and an inflamed lamina propria (supplementary material Fig. S3).
Mass lesions express only scarce fibrin depositions and are infiltrated by MMP9-expressing immune cells
To further characterize the cellular and extracellular components of the mass lesions, immunohistological stainings for fibrinogen, the leukocyte marker CD45, the granulocyte marker Gr1, the macrophage markers CD204 and F4/80, and toluidine blue staining for mast cells were conducted. In addition, because MMP9 clearly rescues Plg−/− mice from developing lethal inflammatory mass lesions in the colon, we investigated the expression pattern of MMP9 in inflamed colon lesions of Plg−/− mice.
The microscopic lesions in 8- and 12-week-old MMP9−/−:Plg−/−mice were clearly stained positive for fibrin depositions, and the presence of these depositions seemed to be directly associated with an inflammatory response (Fig. 3A–D). Surprisingly, fibrin depositions were not a general feature of the obstructing inflammatory mass lesion found in the older mice, although a few scattered areas in the conspicuous lamina propria did stain positive for fibrin (Fig. 3E,F).
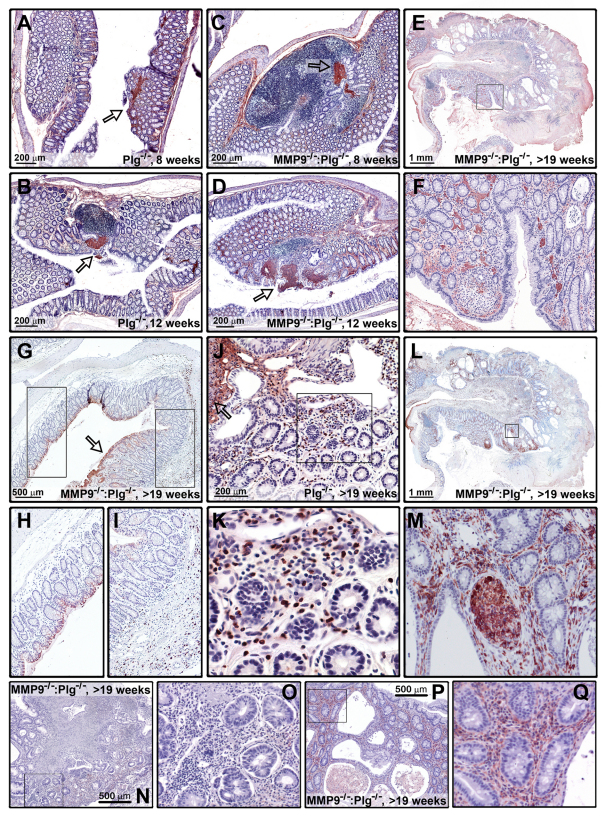
Fig. 3.
Fibrin depositions trigger the development of inflammatory mass lesions in MMP9−/−:Plg−/− mice. (A,B) Microscopic lesions in 8- and 12-week-old Plg−/− mice, showing that lesions in these mice also grow with increasing age and are associated with fibrin depositions (arrows indicate fibrin depositions). (C,D) Similar to Plg−/− mice, MMP9−/−:Plg−/− mice develop lesions in association with fibrin depositions (arrows) but, by contrast, these lesions are several-fold larger and the inflammatory reactions expand laterally away from the deposited fibrin for a considerable distance. Notice the large inflammatory follicular structure already present at 8 weeks of age. (E,F) Fibrin depositions were not a general feature of the inflammatory mass lesions in MMP9−/−:Plg−/− mice older than 19 weeks, although small restricted areas with accumulated fibrin in the lamina propria could be detected. (G–I) Staining for granulocytes using anti-Gr1 antibodies showed that the healthy part of the colon mucosa (H) contained very few granulocytes, whereas, in the inflammatory mass lesion (G,I), an abundant presence of granulocytes was found both in the submucosa and the lamina propria (arrow indicates beginning of a mass lesion). (J,K) A high level of MMP9 expression was found in the inflamed mucosa surrounding the microscopic lesions in Plg−/− mice. The expression was confined to inflammatory cells in the lamina propria, whereas epithelial cells were devoid of MMP9 staining (the arrow indicates the lesion site). (L,M) Macrophage staining with anti-CD204 antibodies revealed that some of the inflammatory mass lesions in MMP9−/−:Plg−/− mice contain macrophages in the superficial portion of the mucosa as well as in some of the expanded crypts. (N–Q) Another macrophage marker, F4/80, confirmed that the number of macrophages varied between mass lesions in different MMP9−/−:Plg−/− mice and also within each lesion. An example of a low-F4/80-expressing lesion is provided in N and O, and a representative staining of a high-F4/80-expressing lesion is provided in P and Q.
The cell-specific stainings revealed abundant levels of granulocytes in and near the inflamed lesions, whereas they were undetectable in the adjacent normal mucosal tissue in MMP9−/−:Plg−/− mice. Colons from WT and MMP9−/− mice did not contain noticeable amounts of granulocytes (Fig. 3G–I).
The MMP9 expression pattern in Plg−/− colon lesions seemed to mimic the distribution of Gr1-positive cells recruited to the deposited fibrin. Furthermore, we found that the normal colon epithelium was devoid of any staining. Although, the necrotic area of the microscopic lesions in Plg−/− mice stained positive for MMP9, we cannot exclude that at least part of this staining was due to unspecific antibody binding or excessive endogenous peroxidase activity, because similar necrotic areas in MMP9−/−:Plg−/− mice also stained positive (Fig. 3J,K). The notion that MMP9 was mainly expressed by invading granulocytes in colon lesions of Plg−/− mice was confirmed by double immunofluorescence staining of Gr1 and MMP9. All Gr1-positive cells were found to express MMP9, whereas a few (<5%) MMP9-expressing cells were in fact Gr1 negative (supplementary material Fig. S4).
In contrast to granulocytes, only scattered and varying numbers of CD204-positive macrophages were found to be present in the lamina propria and submucosa in the isolated mass lesions, although the cellular infiltrate responsible for the dilation of a portion of the crypts was indeed composed of CD204-expressing macrophages (Fig. 3L,M). Another macrophage marker, F4/80, confirmed that the number of macrophages varied between tumors isolated from different mice, ranging from zero to almost 1000 F4/80-positive cells/mm2 (Fig. 3N,O). A similar lack of pattern was found in inflamed tissue isolated from both Plg−/− and MMP9−/−:Plg−/− mice. However, normal-appearing colon tissue contained close to no F4/80-positive cells in all genotypes examined (WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice; Fig. 3N–Q).
Toluidine blue staining of the colon revealed a comparable presence of mast cells in WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/−mice at the age of 12 weeks. These cells were located in the lamina propria, often in close proximity to the mucosa muscularis, whereas no positively stained cells were detected in the submucosa. In the older mice (>19 weeks), a decreased frequency of mast cells was found in otherwise normal-appearing tissue in MMP9−/−:Plg−/− mice compared with WT mice but not compared with Plg−/− mice. In inflamed colon segments from both Plg−/− and MMP9−/−:Plg−/− mice there were significantly fewer mast cells than in WT mice, whereas there was no difference between Plg−/− and MMP9−/−:Plg−/− mice. Inflammatory mass lesions in the MMP9−/−:Plg−/− mice only contained very scarce mast cells (supplementary material Fig. S5).
Specific cell counting in areas designated as either normal, inflamed or tumorous showed that the inflamed areas of Plg−/− mice and MMP9−/−:Plg−/− mice contained comparable numbers of CD45-and Gr1-positive cells. Furthermore, the number of CD45-positive cells in the submucosa and Gr1-positive cells in the lamina propria was significantly increased in the inflammatory mass lesion of MMP9−/−:Plg−/− mice compared with inflamed areas in the same mice (supplementary material Fig. S6A-E).
Skewed epithelial cell-turnover rates do not contribute to the generation of mass lesions
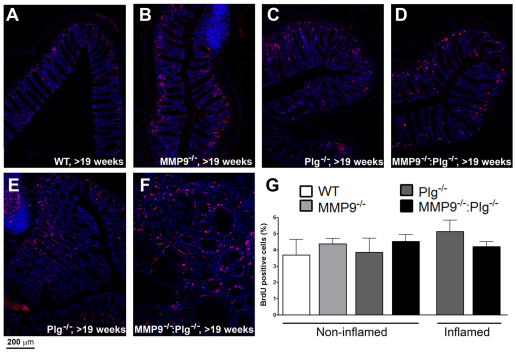
Because an elevated cell-turnover rate in the colon epithelium could in part explain the formation of the mass lesions, a bromodeoxyuracil (BrdU)-incorporation study was conducted. In normal colon epithelium no difference in cell division frequency was found between WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice, in which proliferating cells were found to be localized in the bottom of the crypts as expected (Fig. 4A,D). By contrast, the mass lesions in MMP9−/−:Plg−/− mice, as well as the inflamed areas of colon mucosa observed in Plg−/− mice, displayed an increased number of dividing cells in the lamina propria and a disordered staining pattern in the distended crypts when compared with normal colon mucosa (Fig. 4E,F). However, the fraction of all dividing cells did not differ in the inflamed tissue in comparison with the fraction in normal epithelium (Fig. 4G).
Fig. 4.
Epithelial cell proliferation in the healthy mucosa does not differ between WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/−mice. The fraction and localization of dividing cells in both normal mucosal tissue and inflamed areas were determined using BrdU-incorporation analyses (red is BrdU, blue is nuclear visualization by Hoechst). (A–D) Tangential sections of healthy colon mucosa of WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice showed a normal fraction and localization of proliferating cells, which were mainly localized in the bottom of the crypts. (E,F) Sections of inflamed and/or tumorous areas in Plg−/− and MMP9−/−:Plg−/− mice revealed that, despite the deranged mucosa of these mice, distinct areas in the mucosa still display unrestrained epithelial proliferation along with increased numbers of dividing cells in the lamina propria. (G) The percentage of BrdU-incorporated cells remained constant over large areas, although localized variations were observed. For all groups, n≥5.
In addition, the number and location of apoptotic cell nuclei in the colon epithelium, determined by TUNEL staining, were not found to differ between WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/−mice (data not shown).
Thymus, spleens and mesenteric lymph nodes show signs of chronic inflammation
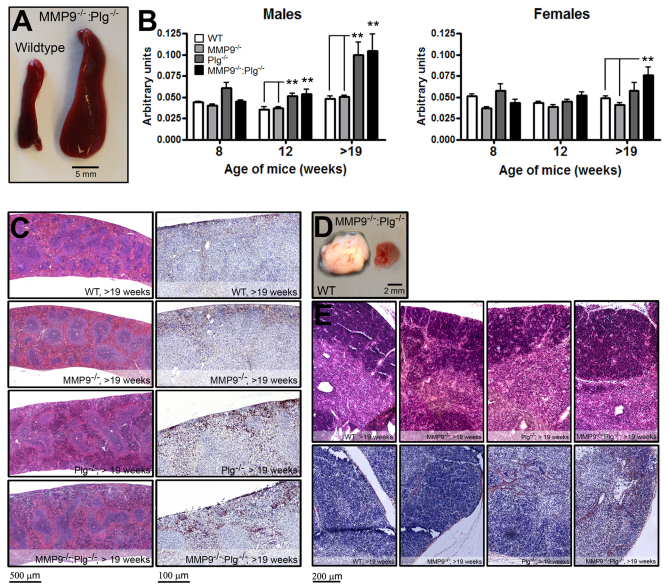
During necropsy, a substantial enlargement of the spleen was observed in male Plg−/− and MMP9−/−:Plg−/− mice and female MMP9−/−:Plg−/− mice with increasing age of the animals. This increase was evident in male mice from the age of 12 weeks, whereas, in female mice, the increase in size was first evident at an age above 19 weeks (Fig. 5A,B). No difference was observed in the spleen size between male Plg−/− and MMP9−/−:Plg−/− mice. Histological examination of the spleens revealed a normal distribution and structure of the red and white pulp despite the increased size of the spleens (Fig. 5C). It was noted that both histological examinations and flow cytometric analyses [based on light side scatter (SSC)/forward scatter (FSC) profiling] showed that the concentration of granulocytes was elevated in the enlarged spleens (WT or MMP9−/− versus Plg−/− or MMP9−/−:Plg−/− mice), whereas the relative distribution of T cells and B cells seemed unaffected (Fig. 5C; supplementary material Fig. S7A,B). Further histological analyses showed that the number of F4/80-positive macrophages/mm2 in the red pulp was the same in WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice (supplementary material Fig. S8).
Fig. 5.
Enlargement of spleens and diminution of thymus in Plg−/− and MMP9−/−:Plg−/− mice with increasing age. (A) Spleens from Plg−/− and MMP9−/−:Plg−/−mice were enlarged compared with spleens derived from WT and MMP9−/− mice. (B) A weight graph based on formalin-fixed and dehydrated spleens isolated from mice at various time points shows that the spleens of Plg−/− and MMP9−/−:Plg−/− mice increase in weight as these mice start to develop rectal lesions or the inflammatory mass lesions in the colon. **P<0.01. (C) H&E staining revealed no unexpected pathological modifications in spleens isolated from mice carrying different genotypes compared with WT spleens (left panel). However, an increased number of granulocytes were present in spleens isolated from Plg−/− and MMP9−/−:Plg−/− mice compared with WT and MMP9−/− spleens (right panel). (D) Thymus in MMP9−/−:Plg−/− mice suffering from inflammatory mass lesions in the colon (right) were generally smaller than thymus isolated from Plg−/− mice, which, in turn, were smaller than thymus isolated from age-matched WT mice (left). (E) H&E stainings of thymus did not reveal any difference between the genotypes examined (top panels). However, fibrin staining revealed increased levels of fibrin in the thymus from Plg−/− mice compared with WT, and these levels were slightly increased in MMP9−/−:Plg−/− mice (bottom panels). For granulocyte analysis in spleen, n≥5. For fibrin analysis in thymus, n≥8.
Isolation of the thymus revealed that the size of this organ was decreased in both male and female Plg−/− mice, and even more so in MMP9−/−:Plg−/− mice, in comparison with age-matched controls. Owing to the texture and small size of the thymus in these genotypes, exact quantification of size or mass was not feasible. This observation prompted us to investigate thymic fibrin depositions. Although fibrin staining varied considerably between mice, a clear increase was observed in Plg−/− mice compared with WT mice, and this increase seemed to be intensified in MMP9−/−:Plg−/− mice (Fig. 5D,E).
Similar to the spleen, the mesenteric lymph nodes (MLNs) were also found to be grossly enlarged, and were readily found and isolated, in Plg−/− and MMP9−/−:Plg−/− mice compared with WT and MMP9−/− mice. Histological investigation showed that this increase in size was not caused by fibrin depositions but that it solely reflected an increased number of inflammatory cells (data not shown).
Plg−/− and MMP9−/−:Plg−/− mice develop neutrophilia
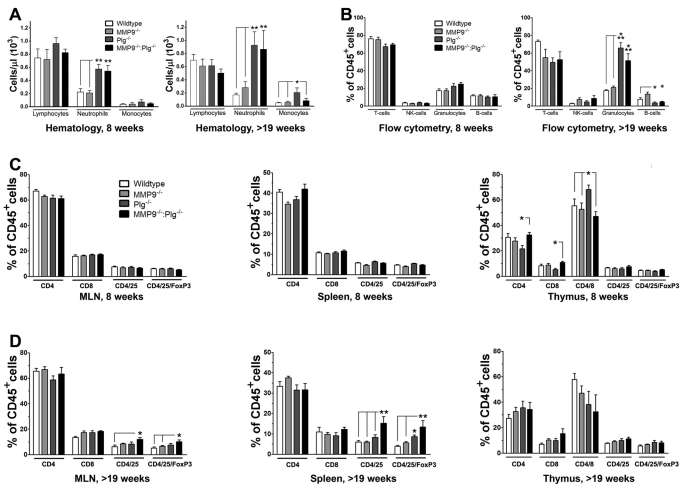
The inflammatory component of the intestinal mass lesions along with the increased size of the spleen and MLNs suggested activation of the immune system. Therefore, blood samples were collected from Plg−/− and MMP9−/−:Plg−/− mice, subjected to automated hematological analysis and compared with age-matched MMP9−/−and WT controls. We found that, in old mice (>19 weeks), the concentration of neutrophils was increased in Plg−/− and MMP9−/−:Plg−/− mice compared with age-matched controls, whereas there was no difference between Plg−/− and MMP9−/−:Plg−/−mice. The concentration of lymphocytes was unaffected by the genotype (Fig. 6A). Interestingly, the concentration of monocytes was also increased in Plg−/− mice but not in MMP9−/−:Plg−/− mice compared with the other groups. Analyses of 8-week-old mice revealed that, already at this young age, Plg−/− and MMP9−/−:Plg−/−mice showed signs of neutrophilia (Fig. 6A).
Fig. 6.
Development of inflammatory mass lesions does not result from an abnormal distribution of immune cells but is associated with an activated immune system. (A) Hematological analyses revealed an increase in the number of neutrophils in Plg−/− and MMP9−/−:Plg−/− mice of 8 weeks of age compared with WT and MMP9−/− mice. This difference was enhanced with increasing age (>19 weeks). (B) Flow cytometric analyses corroborated the hematological data, showing an increased fraction of granulocytes in old Plg−/− and MMP9−/−:Plg−/− mice (>19 weeks). Interestingly, the fraction of B cells was dramatically decreased in these mice compared with WT and MMP9−/− mice. (C,D) Flow cytometric analyses of immune cell composition of thymus, spleen and MLNs from young (age=8 weeks) and old (>19 weeks) mice. No difference in immune cell fractions was observed between the investigated genotypes at 8 weeks of age, except for a skewing of CD4+, CD8+ and CD4+CD8+ cells in the thymus of Plg−/− mice. However, with increasing age an increased number of activated T cells and TReg cells was found in MMP9−/−:Plg−/− mice compared with the other genotypes. This was most pronounced in the spleen. Data are presented as means ± s.e.m. and analyzed by ANOVA with a Newman-Keuls post-test. *P<0.05; **P<0.01; ***P<0.001. n=6–11.
Increased granulocyte and decreased B-cell fractions in blood from Plg−/− and MMP9−/−:Plg−/− mice
Owing to the abnormal hematological values in Plg−/− and MMP9−/−:Plg−/− mice, more-detailed blood analyses using flow cytometry were performed. In 8-week-old mice, we could not detect any difference in the distribution of circulating T cells, B cells, natural killer (NK) cells or granulocytes, defined by their expression of CD3, CD19, CD94 or Gr1, respectively. However, with increasing age, the fraction of granulocytes in Plg−/− and MMP9−/−:Plg−/− mice was two- to threefold increased, and, surprisingly, the fraction of B cells was several-fold decreased in Plg−/− and MMP9−/−:Plg−/− mice compared with WT. Again, no difference between Plg−/− and MMP9−/−:Plg−/− mice was observed (Fig. 6B; supplementary material Fig. S9A). This depletion of B cells did not result in reduced immunoglobulin levels, as demonstrated by Coomassie Blue staining and western blotting (supplementary material Fig. S10).
T-cell activation in Plg−/− and MMP9−/−:Plg−/− mice
The decreased size of the thymus in Plg−/− and especially in MMP9−/−:Plg−/− mice together with their enlarged spleens and MLNs prompted us to investigate the lymphocyte subpopulation in these organs. Using flow cytometry, the fractions of T-helper cells (CD4+), cytotoxic T cells (CD8+), and regulatory T (TReg) cells (CD25+/FoxP3+) in the thymus, spleens and MLNs were determined. In the spleen of MMP9−/−:Plg−/− mice (age >19 weeks), we found an increased fraction of CD4+CD25+ activated T cells compared with all other genotypes, as well as an increased fraction of CD25+FoxP3+ TReg cells compared with WT and MMP9−/− mice but not Plg−/− mice. However, Plg−/− mice also showed an increased fraction of TReg cells compared with WT and MMP9−/− mice but not to the same extent as in MMP9−/−:Plg−/− mice. Also, in MMP9−/−:Plg−/− mice, the fraction of activated T cells and TReg cells in the MLNs was significantly increased, by 89% and 97%, respectively, when compared with WT mice. However, there were no statistical significant differences in cell fractions of the MLNs between Plg−/− mice and the other genotypes. Besides these observations, an interesting decrease in CD4+CD8+ double-positive cells in the thymus of Plg−/− and MMP9−/−:Plg−/− mice was found, although statistical significance was not obtained (Fig. 6D). In 8-week-old mice there were no apparent differences in immune cell composition between the various genotypes in any of the investigated organs, besides a skewed distribution of CD4+, CD8+ and CD4+CD8+ in the thymus of Plg−/− mice (Fig. 6C). Representative flow cytometric plots are supplied in supplementary material Fig. S8B.
Retarded wound healing in MMP9−/−:Plg−/− mice
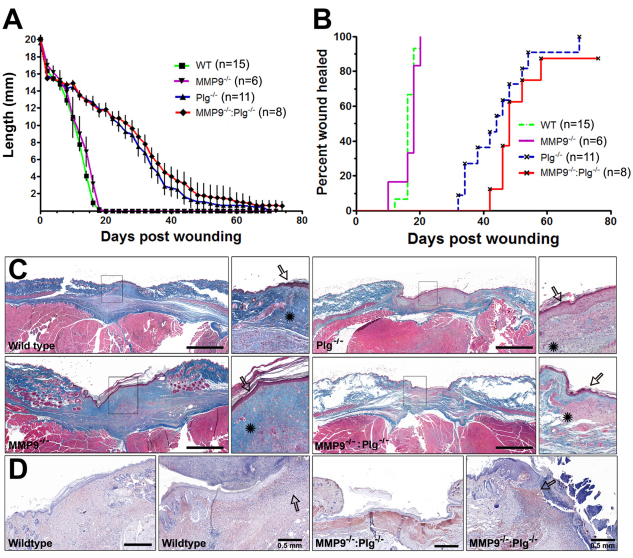
We wished to determine whether the formation of inflammatory mass lesions in MMP9−/−:Plg−/− mice reflected dysregulated tissue repair in general (Schafer and Werner, 2008) or whether the observed pathology is truly colon specific. Thus, we investigated incisional skin wound healing in WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice. MMP9 deficiency is known to have a limited impact on skin wound healing time (Mohan et al., 2002; Hattori et al., 2009), whereas Plg deficiency has been shown on several occasions to substantially delay wound healing (Rømer et al., 1996). Although we, in the present study, were able to confirm that wound healing is significantly delayed in Plg−/− mice, no overt difference was found between Plg−/− and MMP9−/−:Plg−/− mice in terms of healing kinetics or time to wound closure, as determined by average healing time and Kaplan Meier analysis, respectively (Fig. 7A,B). Trichrome staining of fully re-epithelialized wound sections from Plg−/− and MMP9−/−:Plg−/− mice revealed a severely perturbed granulation tissue with remaining provisional matrix islets dispersed underneath the epidermis. These mice also displayed a decreased cell density underneath the keratinocyte barrier compared with WT and MMP9−/− mice, in which a well organized granulation tissue was seen (Fig. 7C). In addition, immunohistological investigation showed dense fibrin staining in these sites in Plg−/− and MMP9−/−:Plg−/− mice in both fully and partially re-epithelialized wounds. By comparison, little or no fibrin staining was found in these areas in WT and MMP9−/− mice (Fig. 7D). In conclusion, we found that, although re-epithelialization is delayed in both Plg−/− and MMP9−/−:Plg−/− mice compared with WT and granulation tissue formation is severely dysregulated, incisional wound healing in MMP9−/−:Plg−/− mice did not lead to formation of skin structures that resemble the large inflammatory mass lesions observed in the colon of these mice.
Fig. 7.
Retarded skin wound healing in Plg−/− mice is not exacerbated by a lack of MMP9. (A) 20-mm-long incisional wounds were established on the back of WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice. WT and MMP9−/− mice showed similar healing kinetics, whereas both Plg−/− and MMP9−/−:Plg−/− mice showed retarded healing kinetics and a substantial delay in complete wound closure. However, no significant difference was observed between Plg−/− and MMP9−/−:Plg−/− mice. (B) A Kaplan Meier curve showing the percentage of completely closed wounds. (C) Trichrome stainings revealed that WT and MMP9−/− mice had a similar granulation tissue after being fully re-epithelialized. By contrast, Plg−/− and MMP9−/−:Plg−/− mice lacked blue-stained collagen fibers despite full epithelial recovery (arrows indicate keratinocytes; stars indicate granulation tissue). (D) Fibrin stainings of fully and partially re-epithelialized WT and MMP9−/−:Plg−/− mice shows that fibrin is mostly located beneath and in front of the protruding keratinocytes and occupies the newly generated matrix beneath the newly formed epithelium. Notice the decreased number of recruited cells in front of the protruding keratinocytes (indicated by arrows) in healing MMP9−/−:Plg−/− mice in comparison with WT mice. Scale bars: 1 mm unless otherwise noted. For all groups, n=8–15.
DISCUSSION
The present study has shown that concomitant genetic ablation of two extracellular proteases, plasmin and MMP9, results in a newly identified and unpredictable phenotype. The obtained data bring forth new information concerning the interplay between the different protease systems, and emphasize the importance of functional proteases during tissue regeneration and homeostasis. We successfully revealed the origin of the tumorous mass lesions and how they commenced their expansion by recruiting inflammatory cells and by degrading the adjacent healthy mucosa. In addition, we defined the cellular composition of the immune system before and in response to formation of the inflammatory mass lesions.
During the generation of MMP9−/−:Plg−/− mice, we observed the expected distribution of Plg-deficient alleles among the offspring as described previously (Bugge et al., 1995), whereas we found a non-expected distribution with respect to the MMP9-deficient alleles. In a similar experimental setup for generation of MMP2−/−:Plg−/− mice and MMP13−/−:Plg−/− mice, we found the expected mendelian distribution of MMP2-, MMP13- and Plg-deficient alleles among the offspring (Frøssing et al., 2010) (Anna Juncker-Jensen and L.R.L., unpublished results). On the basis of these findings, MMP9 seems more important than MMP2, MMP13 and plasmin for gestation. It has previously been shown that both MMP9 and urokinase-type plasminogen activator (uPA) are expressed by trophoblasts during implantation, although with slightly different kinetics (Teesalu et al., 1996; Teesalu et al., 1999; Sappino et al., 1989; Alexander et al., 1996). This suggests a role for MMP9 and plasmin during gestation, which is corroborated by our data showing that concomitant ablation of MMP9 and plasmin has additive negative effects on successful gestation and birth. Importantly, the non-mendelian distribution revealed in the MMP9−/−:Plg−/− F2 offspring cannot be explained by linked inheritance between MMP9 and Plg because these genes are localized to chromosome 2H3 and 17E, respectively (Degen et al., 1990; Leco et al., 1997). Thus, the observed distribution seems to be caused by insufficient proteolysis during gestation and emphasizes the pivotal role(s) of MMP9 and plasmin in this process.
The attenuation of weight gain in MMP9−/−:Plg−/− male mice at approximately 12 weeks of age, which differs with MMP9−/− mice, suggests that MMP9−/−:Plg−/− mice at this age have a decreased uptake of nutrients owing to their gastrointestinal phenotype. By contrast, this was not evident for Plg−/− mice of the same age, despite the occurrence of rectal prolapse development. Together with the skewed genetic distribution of the offspring, the increased rate of spontaneous deaths seen in MMP9−/−:Plg−/− mice compared with littermate controls and the distinctive effects of MMP9 deficiency on long-bone development, these data suggest that the phenotypic traits of MMP9−/− and Plg−/− mice in some cases interact, leading to a detrimental phenotype, whereas the characteristic phenotypic traits of both MMP−/− and Plg−/− mice are preserved in MMP9−/−:Plg−/− mice.
In addition to the well-known effect of MMP9 deficiency on bone development (Vu et al., 1998), we successfully revealed structural abnormalities, not detectable by histology, in the bones of adult MMP9−/− mice by means of μCT imaging and microarchitectural analyses. This involved a decreased trabecular thickness and increased structure model index of cancellous bone, suggesting a decreased bone strength (Ding et al., 2002). However, the microarchitecture of both the distal femur cortical bone and the midshaft cortical bone did not differ among the groups, indicating that MMP9 deficiency had a significant impact only on cancellous bone at this time point.
The development of tumorous mass lesions in the large bowels has not been reported for neither Plg−/− nor MMP9−/− mice and, in the current investigation, it was restricted to MMP9−/−:Plg−/−mice. In human pathology, an array of colorectal polypoid lesions, considered to be prolapse-related, has been described, including the redundant prolapsing mucosal folds seen in diverticular disease (Kelly, 1991), stomas, cap polyposis (Campbell et al., 1993), angiogenic polypoid proliferation (Abrahams et al., 2001), and cloacogenic polyps (Singh et al., 2007). The latter refers to prolapse of the rectal-anal transitional zone. The cap polyposis, which might be associated with diverticular disease (Campbell et al., 1993), commonly develops in the rectosigmoid colon. The angiogenic polypoid proliferation and granulation tissue polyposis (Allibone et al., 1993) described in relation to neoplastic growths are likewise taken as a form of mucosal prolapse (Abrahams et al., 2001).
Common to these prolapsing mucosal disorders is an altered crypt pattern that can include cystically dilated and angulated (diamond-shaped) crypts (Warren et al., 1990). The mucosa is commonly inflamed, and the surfaces are sometimes eroded and the lamina propria replaced by fibromuscular and elastic tissue. Granulation tissue can be prominent, particularly in the cap polyps (Cheng, 2009), and a vascular component is a key finding in the angiogenic polypoid proliferations. Additionally, distortion of the muscularis mucosa might obliterate the usual histological landmarks of mucosa and submucosa (Fenoglio et al., 2008). The surface of these polyps might be covered by a membrane of mucinous and inflammatory exudates, providing a picture similar to that of ischemic colitis. Given the common presence of a local ischemic element in the prolapsing mechanism, this latter observation is anticipated.
The presently studied murine lesions possessed several histological attributes cited above, including an altered crypt pattern with cystically dilated and angulated inflamed crypts, surface erosions, and a conspicuous pseudomembrane of mucin and inflammatory cells. Hence, motility disorders involving prolapse certainly underlie the evolution of these mass lesions despite the absence of conspicuous muscularization of the lamina propria. Given the exuberant nature of the lesions, their role as a leading point in an intussusception can be anticipated. However, histological evidence in support of this consideration was not apparent.
The presented time-course study suggested that discrete inflammatory lesions in close spatial relation to lymphoid follicles might represent the initial histologically visible event that subsequently induces lymphoid hyperplasia and reactive changes of the mucosa (similar to the so-called transitional mucosa bordering neoplastic growths), which in turn promote motility disorders, including prolapse (Allibone et al., 1993).
Concerning the mechanism responsible for the development of the observed tumorous mass lesions in MMP9−/−:Plg−/− mice, it could be speculated that their development is initiated by impeded fibrinolysis, which is also responsible for the microscopic lesions observed in Plg−/− mice (Bugge et al., 1996), and that concurrent ablation of MMP9, which also possesses fibrinolytic activity (Lelongt et al., 2001), could exacerbate the situation, leading to the observed mass lesions. However, despite substantial MMP9 expression in granulocytes in the vicinity of the microscopic lesions in Plg−/− mice, the restricted amount of fibrin in the large obstructing tumors do not support this mechanism as being solely responsible.
The time-course study showed that, in both Plg−/− and MMP9−/−:Plg−/− mice, the inflammatory mass lesions originate in close association with fibrin depositions; however, in contrast with Plg−/− mice, the inflammatory reactions associated with the fibrin depositions in MMP9−/−:Plg−/− mice seemed to expand aggressively and subsequently loose the spatial association to the areas containing the deposited fibrin. Interestingly, MMP9 has on several occasions been shown to exacerbate inflammatory reactions in colitis models (Santana et al., 2006), which is in contrast to our findings. However, Castaneda et al. showed that the source of MMP9 is of fundamental importance in this regard: loss of epithelial-derived MMP9 alleviates colitis symptoms, whereas loss of granulocyte-derived MMP9 had detrimental effects (Castaneda et al., 2005). This is in agreement with the presented data, because MMP9 expression in the lesions found in Plg−/− mice was restricted to granulocytes. Similar observations have also been made in inflammatory models not involving the intestinal tract, in which a lack of MMP9 leads to an increased influx of neutrophils and to tissue damage (Yoon et al., 2007).
Clarification of the exact functions of neutrophil-derived MMP9 during an inflammatory response still remains elusive, and a decisive role of MMP9 during ECM-degradation-dependent migration of neutrophils is not supported in the literature (Allport et al., 2002; Castaneda et al., 2005). Even though MMP9 is dispensable for this process, the activity of MMP9 might still, directly or indirectly, lead to tissue damage (Greenlee et al., 2006). One mechanism by which lack of MMP9 could exacerbate tissue damage that is related to inflammation is through diminished activity of anti-inflammatory cytokines that are dependent on proteolytic activation by MMP9, which would normally dampen the inflammatory response and rescue tissues from excessive stress (Yu and Stamenkovic, 2000). In this regard, it is likely that the formation of inflammatory mass lesions in MMP9−/−:Plg−/− mice is caused by an insufficient immunosuppressive environment resulting from a diminished proteolytic activation of anti-inflammatory cytokines. In addition, the substrate overlap of MMP9 and plasmin in relation to vital anti-inflammatory mediators, such as TGFβ (Annes et al., 2003), could explain why MMP9−/−:Plg−/− mice develop inflammatory mass lesions, whereas chemically induced colitis in mice only deficient for MMP9 does not result in these dysregulated and self-sustaining inflammatory mass lesions (Castaneda et al., 2005; Santana et al., 2006).
It is interesting to note that, whereas the colon mucosa gets inflamed in Plg−/− and MMP9−/−:Plg−/− mice, the number of mast cells, which have previously been shown to assist the development of benign neoplastic skin lesion into malignant tumors (Coussens et al., 1999), is decreased. This might reflect that mast cells in the colon were most frequently found near the base of crypts, which is a location not readily identified in the inflammatory lesions. However, the finding that mast cell numbers were already decreased in the normal-appearing colon mucosa in MMP9−/−:Plg−/− mice (age >19 weeks) might suggest an actual decrease in mast cell numbers in response to an altered inflammatory environment. Mast cells have recently been shown not to affect cancer development in the interleukin-10-deficient mouse inflammatory bowel disease model (Chichlowski et al., 2010). However, other data derived from the ApcMin/+ colon cancer model suggest a protective role of mast cells in relation to the cancer development (Sinnamon et al., 2008). Thus, the role of mast cells in colon carcinogenesis has yet to be finally clarified.
The evident contribution of a deranged inflammatory reaction in the mass lesions of MMP9−/−:Plg−/− mice could result from a general dysfunction of the immune system, an assumption that is supported by the role of these proteases in thymocyte maturation (Savino et al., 2004; Odaka et al., 2005). However, the detailed analyses of the immune system in 8-week-old mice did not support this notion. The detected splenomegaly in old Plg−/− and MMP9−/−:Plg−/− mice was not a result of red pulp hyperplasia or other structural differences as have been reported for other murine models of inflammatory bowel disease (Froicu and Cantorna, 2007). Nevertheless, we did observe an increased fraction of granulocytes in spleen and peripheral blood in both Plg−/− and MMP9−/−:Plg−/− mice. Similar findings have previously been associated with induced colitis in mice, suggesting that this skewing of cell populations is a common trait for chronic bowel inflammation (Froicu and Cantorna, 2007). Regarding the observed thymic atrophy, it could very well result from high levels of circulating pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β, which stimulate the hypothalamic-pituitary-adrenal axis, resulting in the release of glucocorticoids and subsequent depletion of cortical thymocytes (Rivier et al., 1989; Zuckerman et al., 1989; Cohen, 1992). Furthermore, the decreased relative fraction of CD4+CD8+ double-positive thymocytes was not consistent in all MMP9−/−:Plg−/− mice but rather was correlated to animals showing severe inflammatory mass lesions in the bowels, and thus could also result from the above-mentioned circulating factors. The high plasma levels of immunoglobulins despite the depletion of peripheral B-cells in old Plg−/− and MMP9−/−:Plg−/− mice might be due to long-lived plasma cells situated in the bone marrow, which are independent of repopulation by the memory B-cell compartment and can thus maintain antibody production regardless of the peripheral B-cell depletion (DiLillo et al., 2008). However, these interesting findings should be elaborated in future studies.
The dysfunctional tissue homeostasis observed as inflammatory mass lesions develop in non-challenged MMP9−/−:Plg−/− mice seems to be tissue specific during normal physiological tissue remodeling. This assumption is based on the lack of a difference in skin wound healing between Plg−/− and MMP9−/−:Plg−/− male mice. In addition, no apparent organ deviations or failure were observed in Plg−/− and MMP9−/−:Plg−/− mice besides those described or those already known to exist for MMP9−/− mice. Clearly, molecular alterations in some organs might have occurred unnoticed. However, during specific non-physiological events, decisive functional interactions might exist between the MMPs and the Plg activation system, as demonstrated by the concerted action between MMP9 and plasmin in the progression of experimental induced bullous pemphigoid (Liu et al., 2005).
It is well established that well-regulated tissue remodeling during both physiological and pathophysiological processes, including embryogenesis, wound healing and cancer progression, are required to maintain cellular homeostasis (Johnsen et al., 1998). Such successful tissue remodeling is governed by a tight spatiotemporal regulation of ECM-degrading proteases with regards to both expression and activity. To our knowledge, this is the first genetic demonstration of a vital function of a distinct protease that first becomes apparent in the absence of a second protease, and thus underlies the importance of the new emerging protective roles of proteases (Lopez-Otin and Matrisian, 2007). In addition, these findings have essential implications for our understanding of the molecular mechanism underlying the functional interactions between proteases that belong to different classes; these implications are not generally appreciated.
In conclusion, we have presented a detailed analysis and comparison of WT, MMP9−/−, Plg−/− and MMP9−/−:Plg−/− mice, and revealed a newly identified and unanticipated phenotype encompassing lethal inflammatory mass lesions in the colon of MMP9−/−:Plg−/− mice. Our data suggest that the inflammatory reactions observed in the MMP9−/−:Plg−/− mice is probably secondary to early prolapse development and lack of fibrin clearance. We propose that MMP9 expressed by granulocytes in lesions produced by inefficient fibrin degradation due to Plg deficiency rescues mice from excessive inflammation. In addition, we described the associated effects on the innate and adaptive immune system. Although the putative common target for MMP9 and plasmin remains to be finally identified, we expect that the presented data might shed light on the histological progression of idiopathic prolapse-related events observed in humans.
METHODS
Animal breeding and handling
Plg−/+ mice backcrossed into an FVB/n background for 20 generations (Panum Institute, Copenhagen, Denmark) were mated with MMP9−/+ mice that were backcrossed into an FVB/n background for 15 generations, to produce the F1 generation of MMP9−/+:Plg−/+ mice. These F1 mice were intercrossed to produce an F2 generation that included MMP9−/−:Plg−/− mice and their littermate controls (WT, MMP9−/− and Plg−/− mice). These animals were used in the cohort study. Further investigations (immune system analyses and wound healing studies) were conducted using offspring from the F2 generation. Genotyping was performed at time of weaning on tail tip DNA prepared as follows: 1–2 mm of tail was treated with proteinase K (500 μl, 100 μg/ml; Roche 3115879001) at 55°C overnight. Next, the samples were centrifuged (12,000 g, 5 minutes). The supernatant was moved to a new tube containing 500 μl of isopropanol, incubated for 5 minutes, centrifuged (12,000 g, 5 minutes) and the supernatant discarded. The pellet was dried in a HetoVac vacuum and resuspended in 50μl Tris buffer containing 1 mM EDTA by heating to 55°C for 45 minutes. The genotypes were determined using standard gel electrophoresis and PCR using the following primers: 5′-GCATACTTGTACCGCTATGG-3′ (forward MMP9), 5′-TAACCGGAGGTGCAAACTGG-3′ (reverse MMP9), 5′-GACCACCAAGCGAAACAT-3′ (insert MMP9), 5′-TGTGGGCTCTAAAGATGGAACTCC-3′ (reverse Plg), 5′-GACAAGGGGACTCGCTGGATGGCTA-3′ (forward Plg), 5′-GTGCGAGGCCAGAGGCCACTTGTGTAGCG-3′ (insert Plg). All mice were generated in SPF facilities and transferred to the experimental facility where they were kept in single ventilated cages with free access to standard food and water. The mice were re-genotyped after termination of the experiment.
To rule out the possibility of bacteriological infection of the gastrointestinal tract, which could affect the colon mucosa, the feces from mice from four randomly chosen animal cages were collected and screened for Helicobacter bilis, H. hepaticus, H. rodentium, H. muridarum and H. typhlonius by PCR (Surrey Diagnostics, UK). The mice were found not to be infected by any of these bacterial subtypes. All animals included in the cohort study were weighed weekly from 5 weeks of age and were euthanized by injection with hypnorm/dormicum (fentanyl 0.1 mg/ml, fluanison 5 mg/ml, midazolam 5 mg/ml) followed by perfusion with 10 ml PBS and 10 ml 4% PFA. At 1 hour prior to euthanization, 100 μl BrdU (20 mg/ml) were injected intraperitonally.
Tissue preparation and histochemical protocols
Tissues for histological investigations were post-fixed in 4% PFA for 24 hours and kept in 62% alcohol at 4°C until they were processed with a Shandon Patchcenter and embedded in paraffin. H&E and trichrome stainings were performed using standard protocols. Elastin was visualized using either VVG or orcein staining. For VVG staining, the slides were rinsed in water and incubated for 1 hour in a Verhoeff’s solution and rinsed in water for 10 minutes. Next, the slides were incubated in 2% iron chloride for 1 minute and washed again for 10 minutes. Next, the slides were incubated for 1 minute in 5% sodium thiosulfate, washed and finally stained with VVG solution for 4 minutes. Orcein staining was performed using an acidified 0.4% orcein solution in 70% ethanol for 30 minutes followed by staining with Borax–methyleneblue solution for 5 minutes. Mast cell staining was performed using a toluidine blue (Sigma, T3260) solution (20 ml toluidine blue stock solution + 180 ml 1% NaCl, pH 2.3) made from a stock of 1% toluidine blue in 70% ethanol.
Stereological examination of lymphoid follicles was done using a systematic random-sampling approach and Visiopharm software for picture analysis (Visiopharm, Denmark). Bones were measured using digital calipers after the bones had been cleared for soft tissues.
Fibrin, CD45, Gr1, MMP9, F4/80 and CD204 stainings were performed using the EnVision+ system (Dako, K4003) or using rabbit-anti-rat horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako, p0450) and NovaRED HRP substrate (VWR International, SK-4800) on sections pretreated with proteinase K for 15 minutes at 37°C. The following primary antibodies were used: rat-anti-mouse CD45 (BD-Biosciences, 550539) diluted 1:50; rat-anti-mouse Gr1 (eBiosciences, 14-5931-82) diluted 1:500; rat-anti-mouse CD204 (Serotec, MCA1322) diluted 1:200; rabbit-anti-mouse fibrinogen (Green et al., 2008) diluted 1:2000; rabbit-anti-mouse MMP9 (Rasch et al., 2010) diluted 1:1000; and rat-anti-mouse F4/80 diluted 1:200 (eBioscience, 14-4801-81). For Gr1 and MMP9 double immunofluorescence staining, a tyramide signal amplification (TSA) kit (Perkin Elmer, NEL701) and A594-goat-anti-rabbit antibodies including Hoechst-33342 were used for visualization. In this particular staining, the A594-goat-anti-rabbit antibodies were first added after the TSA kit had been applied and washed away thoroughly.
BrdU stainings were done using sheep anti-BrdU (Biodesign, M20107S) diluted 1:100 on sections treated with citrate buffer for 15 minutes at 98°C. Secondary antibodies conjugated to Alexa Fluor 594 (Molecular Probes, A11016) were used together with Hoechst-33342 (2 μg/ml; Invitrogen, H3570) for nuclear visualization. Apoptosis analyses were done using the TUNEL staining kit ApopTag (Chemicon, S7101) according to the manufacturer’s instructions.
Quantitative analyses of histological sections were performed on section overviews captured using a 10× objective and Visopharm software (>ten pictures per overview). Briefly, colon tissues were first characterized as either normal, inflamed or being part of an inflammatory mass lesion (only in MMP9−/−:Plg−/− mice). Next, a high-resolution picture was captured and stained cells were counted manually. Finally, the number of stained cells per mm2 was calculated. Cells contained in lymphoid follicles and areas of distended crypt structures were both omitted from the calculations.
Microarchitecture of the distal femur and midshaft femur
Bilateral distal femurs and midshaft femurs of each mouse were scanned with a high-resolution μCT scanner (vivaCT 40, Scanco Medical, Brüttisellen, Switzerland). The scanning resulted in three-dimensional (3D) reconstruction of cubic voxel sizes 10×10×10μm3. 100 slice images (covering 1000 μm) were used for the analysis of subchondral bone tissues and 150 slices for midshaft cortical bone (2048×2048 pixels). 3D microarchitectural parameters of cancellous bone, such as trabecular thickness, bone surface:volume ratio and structure model index, were computed, and meaningful 3D microarchitectural parameters of cortical bone were also calculated.
Flow cytometry and hematology
The following antibodies were used for flow cytometry: anti-CD3 (BD-Biosciences, 555274), -CD4 (BD-Biosciences, 552775), -CD19 (BD-Biosciences, 550992), -CD45 (BD-Biosciences, 550994), -CD8a (BD-Biosciences, 557654), -CD94 (eBioscience, 120941) and -Gr1 (eBioscience, 565931). All samples were analyzed using the LSRII system (BD Biosciences).
Blood was collected from the left ventricle and EDTA was added to a final concentration of 0.05 M. For flow cytometry, 20 μl blood was stained by adding 2 μl of each antibody (1:100) for 15 minutes followed by 180 μl lysis buffer (BD Biosciences, 555899). For general hematological analyses, 20 μl blood was assayed using Hemavet 950 (Drew Scientific) in accordance with the manufacturer’s instructions.
Spleen, thymus and MLNs were mashed through a 70-μm mesh, and cells were collected in PBS containing 0.5% BSA and 2 mM EDTA. Erythrocytes in spleen samples were lysed by adding 1 ml distilled water for 7 seconds followed by 20 ml PBS. 106 cells were stained using a TReg cell staining kit (eBioscience, 88–8111) using the manufacturer’s instructions.
Incisional wound healing
6-week-old mice were anaesthetized by administration of hypnorm/dormicum, and 20-mm full skin thickness incisions were made on the midline of the dorsum. Following wounding each mouse was housed separately. The wound length was measured every other day until completely healed, defined by loss of the scab and restoration of the epithelial surface of the skin. Wound samples for histology were isolated with a margin of 1–2 mm of intact but inflamed skin and down to the underlying muscle fascia to ensure that all granulation tissue was harvested. Wounds were isolated 3 days post healing or when reaching a wound length of 10 mm.
Statistical analyses
Analysis of weight data as a function of age was done using mixed modeling with each mouse entered as a random effect and stratified by gender. Age for males was fitted using a linear spline with a knot at 21 weeks in order to fit non-linear data. P-values less than 5% were considered significant. All calculations were done using SAS (v 9.1., SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
We thank Thomas H. Bugge for the Plg-deficient mice and Zena Werb for the MMP9-deficient mice. We thank Ib Jarle Christensen for statistical analysis. The expert technical assistance of Mette Musfelth Andersen, Torben Kibøl, Agnieszka Ingvorsen and Katharina Stegmann is gratefully acknowledged. Research grants supporting this work: European Union Grant 201279, MICROENVIMET, Svend Andersen Foundation, Aage Bangs Foundation, Grosserer Alfred Nielsen og Hustrus Foundation, Danish Cancer Society, the Danish Cancer Research Foundation, Agnes og Poul Friis Foundation, Danish-Chinese Centre for Proteases and Cancer, Lundbeck Foundation, and the Cancer Research Foundation of 1989.
Footnotes
COMPETING INTERESTS
The authors declare no conflicting or financial interests.
AUTHOR CONTRIBUTIONS
A.H. and B.R. conceived, designed and performed experiments, analyzed data, and prepared the manuscript. M.C.M. and M.D. performed experiments and analyzed data. S.H. analyzed data and prepared the manuscript. L.R.L. conceived and designed experiments and reviewed the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.005801/-/DC1
REFERENCES
- Abrahams N. A., Vesoulis Z., Petras R. E. (2001). Angiogenic polypoid proliferation adjacent to ileal carcinoid tumors: a nonspecific finding related to mucosal prolapse. Mod. Pathol. 14, 821–827 [DOI] [PubMed] [Google Scholar]
- Alexander C. M., Hansell E. J., Behrendtsen O., Flannery M. L., Kishnani N. S., Hawkes S. P., Werb Z. (1996). Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development 122, 1723–1736 [DOI] [PubMed] [Google Scholar]
- Allibone R. O., Hoffman J., Gosney J. R., Helliwell T. R. (1993). Granulation tissue polyposis associated with carcinoid tumours of the small intestine. Histopathology 22, 475–480 [DOI] [PubMed] [Google Scholar]
- Allport J. R., Lim Y. C., Shipley J. M., Senior R. M., Shapiro S. D., Matsuyoshi N., Vestweber D., Luscinskas F. W. (2002). Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukoc. Biol. 71, 821–828 [PubMed] [Google Scholar]
- Annes J. P., Munger J. S., Rifkin D. B. (2003). Making sense of latent TGFbeta activation. J. Cell. Sci. 116, 217–224 [DOI] [PubMed] [Google Scholar]
- Bugge T. H., Flick M. J., Daugherty C. C., Degen J. L. (1995). Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 9, 794–807 [DOI] [PubMed] [Google Scholar]
- Bugge T. H., Kombrinck K. W., Flick M. J., Daugherty C. C., Danton M. J., Degen J. L. (1996). Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell 87, 709–719 [DOI] [PubMed] [Google Scholar]
- Campbell A. P., Cobb C. A., Chapman R. W., Kettlewell M., Hoang P., Haot B. J., Jewell D. P. (1993). Cap polyposis-an unusual cause of diarrhoea. Gut 34, 562–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda F. E., Walia B., Vijay-Kumar M., Patel N. R., Roser S., Kolachala V. L., Rojas M., Wang L., Oprea G., Garg P., et al. (2005). Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology 129, 1991–2008 [DOI] [PubMed] [Google Scholar]
- Cheng M. M. (2009). Is the drugstore safe? Counterfeit diabetes products on the shelves. J. Diabetes Sci. Technol. 3, 1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowski M., Westwood G. S., Abraham S. N., Hale L. P. (2010). Role of mast cells in inflammatory bowel diseasae and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS ONE 17, e12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. (1992). Glucocorticoid-induced apoptosis in the thymus. Semin. Immunol. 4, 363–369 [PubMed] [Google Scholar]
- Coussens L. M., Raymond W. W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G. H., Hanahan D. (1999). Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13, 1382–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas S. L., Rosser J. L., Mundy G. R., Bonewald L. F. (2002). Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J. Biol. Chem. 277, 21352–21360 [DOI] [PubMed] [Google Scholar]
- Danø K., Rømer J., Nielsen B. S., Bjørn S., Pyke C., Rygaard J., Lund L. R. (1999). Cancer invasion and tissue remodeling – cooperation of preotease systems and cell types. APMIS. 107, 120–127 [DOI] [PubMed] [Google Scholar]
- Degen S. J., Bell S. M., Schaefer L. A., Elliott R. W. (1990). Characterization of the cDNA coding for mouse plasminogen and localization of the gene to mouse chromosome 17. Genomics 8, 49–61 [DOI] [PubMed] [Google Scholar]
- DiLillo D. J., Hamaguchi Y., Ueda Y., Yang K., Uchida J., Haas K. M., Kelsoe G., Tedder T. F. (2008). Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 180, 361–371 [DOI] [PubMed] [Google Scholar]
- Ding M., Odgaard A., Danielsen C. C., Hvid I. (2002). Mutual associations among microstructural, physical and mechanical properties of human cancellous bone. J. Bone Joint Surg. Br. 84, 900–907 [DOI] [PubMed] [Google Scholar]
- Dunker N., Krieglstein K. (2000). Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur. J. Biochem. 267, 6982–6988 [DOI] [PubMed] [Google Scholar]
- Engsig M. T., Chen Q. J., Vu T. H., Pedersen A. C., Therkidsen B., Lund L. R., Henriksen K., Lenhard T., Foged N. T., Werb Z., et al. (2000). Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 151, 879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio L., Cena P., Bracco C., Pomero F., Migliore E., Benedetti V., Morino M., Perin P. C. (2008). Proximalisation of colorectal carcinoma: a 10-year study in Italy. Dig. Dis. Sci. 53, 736–740 [DOI] [PubMed] [Google Scholar]
- Fingleton B. (2008). MMPs as therapeutic targets-still a viable option? Semin. Cell Dev. Biol. 19, 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M., Cantorna M. T. (2007). Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøssing S., Rønø B., Hald A., Rømer J., Lund L. R. (2010). Skin wound healing in MMP2-deficient and MMP2 / Plasminogen double-deficient mice. Exp. Dermatol. 19, e234–e240 [DOI] [PubMed] [Google Scholar]
- Garg P., Vijay-Kumar M., Wang L., Gewirtz A. T., Merlin D., Sitaraman S. V. (2009). Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G175–G184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Hart E., Shchurin A., Hoover-Plow J. (2008). Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J. Clin. Invest. 118, 3012–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. A., Almholt K., Ploug M., Rønø B., Castellino F. J., Johnsen M., Bugge T. H., Rømer J., Lund L. R. (2008). Profibrinolytic effects of metalloproteinases during skin wound healing in the absence of plasminogen. J. Invest. Dermatol. 128, 2092–2101 [DOI] [PubMed] [Google Scholar]
- Greenlee K. J., Corry D. B., Engler D. A., Matsunami R. K., Tessier P., Cook R. G., Werb Z., Kheradmand F. (2006). Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J. Immunol. 177, 7312–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N., Mochizuki S., Kishi K., Nakajima T., Takaishi H., D’Armiento J., Okada Y. (2009). MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 175, 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B., Lund L. R., Akiyama H., Ohki M., Morita Y., Rømer J., Nakauchi H., Okumura K., Ogawa H., Werb Z., et al. (2007). The plasminogen fibrinolytic pathway is required for hematopoietic regeneration. Cell Stem Cell 1, 658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen M., Lund L. R., Rømer J., Almholt K., Danø K. (1998). Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr. Opin. Cell Biol. 10, 667–671 [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Xu Z. Z., Wang X., Park J. Y., Zhuang Z. Y., Tan P. H., Gao Y. J., Roy K., Corfas G., Lo E. H., et al. (2008). Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 14, 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. K. (1991). Polypoid prolapsing mucosal folds in diverticular disease. Am. J. Surg. Pathol. 15, 871–878 [DOI] [PubMed] [Google Scholar]
- Leco K. J., Harvey M. B., Hogan A., Copeland N. G., Gilbert D. J., Jenkins N. A., Edwards D. R., Schultz G. A. (1997). Matrix metalloproteinase-9 maps to the distal end of chromosome 2 in the mouse. Dev. Genet. 21, 55–60 [DOI] [PubMed] [Google Scholar]
- Lelongt B., Bengatta S., Delauche M., Lund L. R., Werb Z., Ronco P. M. (2001). Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J. Exp. Med. 193, 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li N., Diaz L. A., Shipley M., Senior R. M., Werb Z. (2005). Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J. Clin. Invest. 115, 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C., Matrisian L. M. (2007). Emerging roles of proteases in tumour suppression. Nat. Rev. Cancer 7, 800–808 [DOI] [PubMed] [Google Scholar]
- Lund L. R., Rømer J., Bugge T. H., Nielsen B. S., Frandsen T. L., Degen J. L., Stephens R. W., Danø K. (1999). Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 18, 4645–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L. R., Green K. A., Stoop A. A., Ploug M., Almholt K., Lilla J., Nielsen B. S., Christensen I. J., Craik C. S., Werb Z., et al. (2006). Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 25, 2686–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R., Chintala S. K., Jung J. C., Villar W. V., McCabe F., Russo L. A., Lee Y., McCarthy B. E., Wollenberg K. R., Jester J. V., et al. (2002). Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 277, 2065–2072 [DOI] [PubMed] [Google Scholar]
- Odaka C., Tanioka M., Itoh T. (2005). Matrix metalloproteinase-9 in macrophages induces thymic neovascularization following thymocyte apoptosis. J. Immunol. 174, 846–853 [DOI] [PubMed] [Google Scholar]
- Page-McCaw A., Ewald A. J., Werb Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo R., Lee J. K., Shipley J. M., Curci J. A., Mao D., Ziporin S. J., Ennis T. L., Shapiro S. D., Senior R. M., Thompson R. W. (2000). Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. 105, 1641–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch M. G., Lund I. K., Illemann M., Høyer-Hansen G., Gårdsvoll H. (2010). Purification and characterization of recombinant full-length and protease domain of murine MMP-9 expressed in Drosophila S2 cells. Protein Expr. Purif. 72, 87–94 [DOI] [PubMed] [Google Scholar]
- Rivier C., Chizzonite R., Vale W. (1989). In the mouse, the activation of the hypothalamic-pituitary-adrenal axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1. Endocrinology 125, 2800–2805 [DOI] [PubMed] [Google Scholar]
- Rømer J., Bugge T. H., Pyke C., Lund L. R., Flick M. J., Degen J. L., Danø K. (1996). Plasminogen and wound healing. Nat. Med. 2, 725. [DOI] [PubMed] [Google Scholar]
- Santana A., Medina C., Paz-Cabrera M. C., Diaz-Gonzalez F., Farre E., Salas A., Radomski M. W., Quintero E. (2006). Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J. Gastroenterol. 12, 6464–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Belin D., Vassalli J. D. (1989). Plasminogen activators in tissue remodeling and invasion: mRNA localization in mouse ovaries and implanting embryos. J. Cell Biol. 109, 2471–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. (1989). Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J. Cell Biol. 109, 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W., Mendes-Da-Cruz D. A., Smaniotto S., Silva-Monteiro E., Villa-Verde D. M. (2004). Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix. J. Leukoc. Biol. 75, 951–961 [DOI] [PubMed] [Google Scholar]
- Schafer M., Werner S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9, 628–638 [DOI] [PubMed] [Google Scholar]
- Singh B., Mortensen N. J., Warren B. F. (2007). Histopathological mimicry in mucosal prolapse. Histopathology 50, 97–102 [DOI] [PubMed] [Google Scholar]
- Sinnamon M. J., Carter K. J., Sims L. P., Lafleur B., Fingleton B., Matrisian L. M. (2008). A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis 29, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg H., Rinkenberger J., Danø K., Werb Z., Lund L. R. (2003). A functional overlap of plasminogen and MMPs regulates vascularization during placental development. Development 130, 4439–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M. D., Werb Z. (2001). How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesalu T., Blasi F., Talarico D. (1996). Embryo implantation in mouse: fetomaternal coordination in the pattern of expression of uPA, uPAR, PAI-1 and alpha 2MR/LRP genes. Mech. Dev. 56, 103–116 [DOI] [PubMed] [Google Scholar]
- Teesalu T., Masson R., Basset P., Blasi F., Talarico D. (1999). Expression of matrix metalloproteinases during murine chorioallantoic placenta maturation. Dev. Dyn. 214, 248–258 [DOI] [PubMed] [Google Scholar]
- Vu T. H., Shipley J. M., Bergers G., Berger J. E., Helms J. A., Hanahan D., Shapiro S. D., Senior R. M., Werb Z. (1998). MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93, 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B. F., Dankwa E. K., Davies J. D. (1990). ‘Diamond-shaped’ crypts and mucosal elastin: helpful diagnostic features in biopsies of rectal prolapse. Histopathology 17, 129–134 [DOI] [PubMed] [Google Scholar]
- Yoon H. K., Cho H. Y., Kleeberger S. R. (2007). Protective role of matrix metalloproteinase-9 in ozone-induced airway inflammation. Environ. Health Perspect. 115, 1557–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I. (2000). Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176 [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. (1989). Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur. J. Immunol. 19, 301–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.