SUMMARY
SHP-2 (encoded by PTPN11) is a ubiquitously expressed protein tyrosine phosphatase required for signal transduction by multiple different cell surface receptors. Humans with germline SHP-2 mutations develop Noonan syndrome or LEOPARD syndrome, which are characterized by cardiovascular, neurological and skeletal abnormalities. To study how SHP-2 regulates tissue homeostasis in normal adults, we used a conditional SHP-2 mouse mutant in which loss of expression of SHP-2 was induced in multiple tissues in response to drug administration. Induced deletion of SHP-2 resulted in impaired hematopoiesis, weight loss and lethality. Most strikingly, induced SHP-2-deficient mice developed severe skeletal abnormalities, including kyphoses and scolioses of the spine. Skeletal malformations were associated with alterations in cartilage and a marked increase in trabecular bone mass. Osteoclasts were essentially absent from the bones of SHP-2-deficient mice, thus accounting for the osteopetrotic phenotype. Studies in vitro revealed that osteoclastogenesis that was stimulated by macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) was defective in SHP-2-deficient mice. At least in part, this was explained by a requirement for SHP-2 in M-CSF-induced activation of the pro-survival protein kinase AKT in hematopoietic precursor cells. These findings illustrate an essential role for SHP-2 in skeletal growth and remodeling in adults, and reveal some of the cellular and molecular mechanisms involved. The model is predicted to be of further use in understanding how SHP-2 regulates skeletal morphogenesis, which could lead to the development of novel therapies for the treatment of skeletal malformations in human patients with SHP-2 mutations.
INTRODUCTION
SHP-2 (encoded by PTPN11) is a broadly expressed Src homology-2 domain (SH2)-containing protein tyrosine phosphatase (PTP) that has been implicated in signal transduction initiated by multiple growth factor and cytokine receptors (Chong and Maiese, 2007; Matozaki et al., 2009). The exact mechanism by which SHP-2 participates in receptor signal transduction is uncertain and is likely to vary from receptor to receptor. However, commonly, SHP-2 seems to be required for activation of the Ras signaling pathway, which drives several different cell responses, including growth, survival, proliferation and differentiation (Chong and Maiese, 2007; Dance et al., 2008; Matozaki et al., 2009). In addition, SHP-2 might also function as a negative regulator of receptor-mediated signal transduction, e.g. during cytokine receptor signaling, acting to dephosphorylate STAT transcription factors (Chong and Maiese, 2007).
In humans, autosomal dominant germline mutations of the PTPN11 gene result in different clinical syndromes with many overlapping features. Noonan syndrome type I (NS) is caused by gain-of-function PTPN11 mutations that disrupt intramolecular fold-mediated inhibition of the PTP domain of SHP-2, resulting in increased PTP activity (Fragale et al., 2004; Keilhack et al., 2005; Noonan, 1968; Tartaglia et al., 2001). LEOPARD syndrome (LS), by contrast, is caused by PTPN11 mutations that result in reduced PTP activity. The mutant PTP is then thought to act in a dominant-negative fashion to inhibit SHP-2 expressed from the normal PTPN11 allele (Digilio et al., 2002; Gorlin et al., 1969; Hanna et al., 2006; Kontaridis et al., 2006; Tartaglia et al., 2006). Clinical features of these syndromes that have been recognized include facial dysmorphia, short stature, cardiovascular defects, pulmonary stenosis, lentigines and skeletal abnormalities including lateral curvature (scoliosis) and dorsal curvature (kyphosis) of the spine. How mutations that have opposing effects upon SHP-2 activity result in essentially identical diseases is currently unknown, although it has been proposed that dysregulated activation of the Ras-mitogen-activated protein kinase (MAPK) signaling pathway is the underlying cause (Edouard et al., 2007; Gelb and Tartaglia, 2006). In support of this, it has recently been demonstrated that Costello syndrome and cardio-facio-cutaneous syndrome, both of which share clinical features with NS and LS, are caused by germline mutations in genes that encode components of this pathway (Estep et al., 2006; Gripp et al., 2006; Niihori et al., 2006; Rodriguez-Viciana et al., 2006).
Studies of genetically engineered SHP-2-deficient mice have the potential to shed light upon the molecular and cellular basis for the clinical abnormalities observed in humans with PTPN11 mutations. Mice that are homozygous for a ptpn11-null allele die at day 10 of embryonic development as a result of defective gastrulation, which, at least in part, can be accounted for by suboptimal fibroblast growth factor (FGF) receptor signaling (Saxton et al., 1997). To circumvent this early lethality, different groups generated mice that harbor conditional loxP-flanked (floxed) ptpn11 alleles that were then crossed with tissue-specific Cre recombinase transgenic mice (Fornaro et al., 2006; Zhang et al., 2004). Accordingly, mice that lack SHP-2 specifically in neuronal cells, neuronal progenitors, liver, pancreas, striated and cardiac muscle, mammary gland and T cells have now been generated (Bard-Chapeau et al., 2006; Fornaro et al., 2006; Hagihara et al., 2009; Ke et al., 2006; Ke et al., 2007; Kontaridis et al., 2008; Krajewska et al., 2008; Nguyen et al., 2006; Princen et al., 2009; Zhang et al., 2004; Zhang et al., 2009). Studies of these mice have confirmed an essential role for SHP-2 in signal transduction through a number of cell surface receptors in these tissues that is necessary for normal tissue functioning.
To further understand the role of SHP-2 in tissue homeostasis, we generated homozygote ptpn11 floxed mice that carry a ubiquitously expressed tamoxifen-inducible estrogen receptor-2 (ert2)-cre transgene (Ruzankina et al., 2007; Zhang et al., 2004). Induced loss of SHP-2 expression in this model resulted in multiple abnormalities, foremost amongst which was the development of severe skeletal malformations. Therefore, the model is expected to be useful in understanding the cellular basis and molecular basis for the role of SHP-2 in skeletal growth and remodeling, and the etiology of skeletal abnormalities in humans with PTPN11 mutations.
RESULTS
Induced deletion of SHP-2 from adult mice results in early lethality
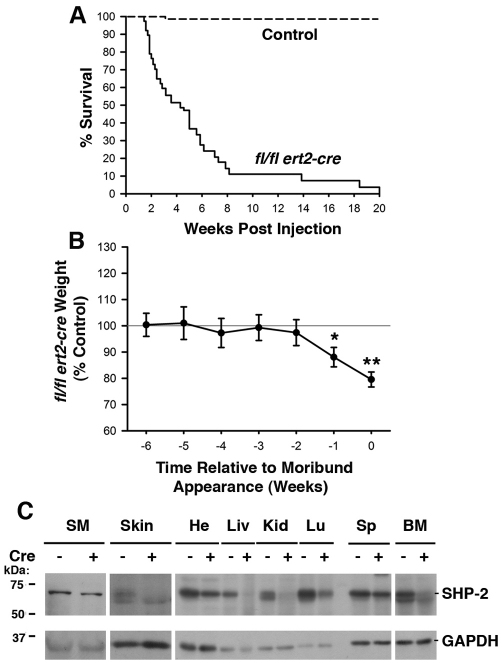
To examine the effect of global deletion of SHP-2 in adults, we generated ptpn11 exon 4 floxed (ptpn11fl/fl) mice that carry an ubiquitin-promoter-driven ert2-cre transgene. Administration of the estrogen antagonist tamoxifen to these mice was predicted to result in nuclear translocation of the ERT2-Cre fusion protein and recombination at the floxed ptpn11 locus to yield a null ptpn11 allele in all tissues. 6- to 8-week-old ptpn11fl/fl ert2-cre mice were administered tamoxifen via intraperitoneal injection on two consecutive days. By 4 weeks post-injection, we observed 50% mortality of ptpn11fl/fl ert2-cre mice, with 100% mortality by 20 weeks post-injection (Fig. 1A). By contrast, essentially no death was observed in any of the tamoxifen-injected ptpn11+/+, ptpn11fl/+, ptpn11fl/+ ert2-cre and ptpn11fl/fl littermate controls over the course of several months.
Fig. 1.
Induced SHP-2 deficiency in adult mice results in weight loss and rapid mortality. (A) Kaplan-Meier plot depicting the survival of ptpn11fl/fl ert2-cre mice (n=38) and pooled ptpn11+/+, ptpn11fl/+, ptpn11fl/+ ert2-cre and ptpn11fl/fl littermate control mice (n=75) following tamoxifen injection at 6–8 weeks of age. (B) Weight of tamoxifen-injected ptpn11fl/fl ert2-cre mice (n=19) expressed as a mean percentage ± 1 s.e.m. of the weight of tamoxifen-injected littermate controls (n=33) at time points at and prior to the time that ptpn11fl/fl ert2-cre mice appear moribund. Mice were injected with tamoxifen at 6–8 weeks of age. Statistical significance was determined using a one sample Student’s t-test. In A and B, all control mice were similar with regards to survival and weight following tamoxifen injection and no effect of haploinsufficiency of SHP-2 in ptpn11fl/+ ert2-cre mice was observed. For ptpn11fl/fl ert2-cre mice, no influence of mouse gender upon survival or weight loss was apparent. *P<0.05; **P<0.005. (C) Western blots showing expression of SHP-2 in the indicated organs from ptpn11fl/fl ert2-cre (+) and littermate control ptpn11fl/fl mice, both injected with tamoxifen 15 days previously (at 6–8 weeks of age). SM, skeletal muscle; He, heart; Liv, liver; Kid, kidney; Lu, lung; Sp, spleen; BM, bone marrow. Blots were stripped and reprobed with an anti-GAPDH antibody to demonstrate equivalent protein loading. Shown are representative experiments of three repeats.
ptpn11fl/fl ert2-cre mice exhibited marked weight loss prior to death (Fig. 1B). Weight loss was precipitous and was evident 1 week before death. To determine the efficiency of SHP-2 deletion, organ lysates from tamoxifen-injected ptpn11fl/fl ert2-cre mice and controls were prepared and examined for expression of SHP-2 by western blotting. In the majority of tissues examined a substantial reduction in SHP-2 protein was observed (Fig. 1C).
Pathology of induced SHP-2-deficient mice
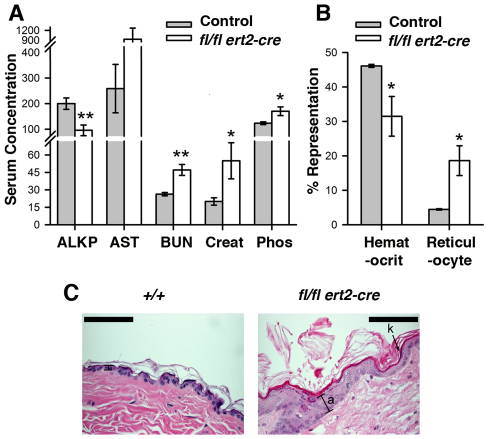
Postmortem analysis did not identify an obvious cause of death of tamoxifen-injected ptpn11fl/fl ert2-cre mice. Elevated serum levels of blood urea nitrogen (BUN), creatinine and phosphorous (Fig. 2A) suggested kidney dysfunction, although upon histological analysis no obvious lesions in the kidneys were noted (not shown). Furthermore, although mice showed reduced alkaline phosphatase (ALKP) and a trend towards increased aspartate transaminase (AST) in serum (Fig. 2A), no liver abnormalities were detected upon histological analysis of ptpn11fl/fl ert2-cre mice. Considering the high frequency of cardiac defects in NS and LS patients as well as in mice with cardiac- or muscle-specific deletion of SHP-2, hearts from ptpn11fl/fl ert2-cre mice were also examined for lesions. No lesions in cardiac valves or walls were detected. This might be explained by a requirement for SHP-2 in cardiac development but not in continued cardiac function in adults. Alternatively, the absence of heart abnormalities in this model might be explained by the relatively poor deletion of SHP-2 in this tissue (Fig. 1C).
Fig. 2.
Altered levels of serum biomarkers, anemia and skin abnormalities in induced SHP-2-deficient mice. (A) Shown are the mean levels of the indicated biomarkers in serum ± 1 s.e.m. of tamoxifen-injected (at 6–8 weeks) moribund ptpn11fl/fl ert2-cre mice and littermate control mice (n=6 each genotype). Age range of mice at the time of analysis was 9–12 weeks. ALKP and AST concentrations are shown in U/l; BUN, mg/dl; phosphorous, μg/ml; creatinine, μg/cl. Statistical significance was determined using a two sample Student’s t-test. *P<0.05; **P<0.005. (B) Depicted are mean hematocrit (percent blood volume) and reticulocyte counts (percent blood cells) ± 1 s.e.m. performed on heparinized whole blood from mice in A. Statistical significance was determined using a two sample Student’s t-test. (C) Representative H&E-stained skin section (n=6) of a tamoxifen-injected (at 6 weeks of age) moribund ptpn11fl/fl ert2-cre mouse and a littermate ptpn11+/+ control showing hyperkeratosis (k) and acanthosis (a) in the former. Analysis was performed 4 weeks after tamoxifen injection. Scale bars: 200 μm.
Further postmortem analysis of ptpn11fl/fl ert2-cre mice did not reveal lesions in any tissues that might account for death. However, in skin, a marked epidermal hyperplasia (acanthosis) and thickening of the outermost layer of dead keratinocytes (hyperkeratosis) was observed (Fig. 2C). Furthermore, moribund mice exhibited anemia as evidenced by a reduced peripheral blood hematocrit (Fig. 2B). However, reticulocytogenesis is apparently intact in these mice. The percentage representation of reticulocytes in peripheral blood was elevated, as was the reticulocyte index (Fig. 2C and data not shown). Other abnormalities in ptpn11fl/fl ert2-cre mice included impaired hematopoiesis and skeletal malformations. Both of these are described in detail below.
Impaired hematopoiesis in induced SHP-2-deficient mice
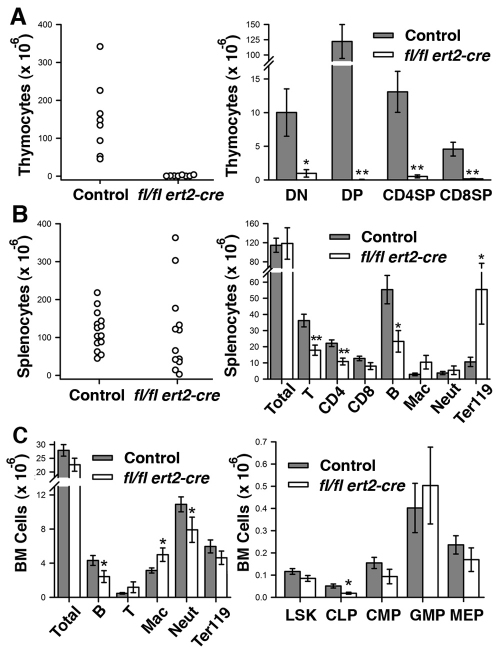
Thymi isolated from tamoxifen-injected moribund ptpn11fl/fl ert2-cre mice were exceptionally small and showed much-reduced cellularity (Fig. 3A). Numbers of each of CD4–CD8– double-negative (DN), CD4+CD8+ double-positive (DP) and CD4+CD8–and CD4–CD8+ single-positive (SP) T cells were dramatically reduced in the thymi of ptpn11fl/fl ert2-cre mice (Fig. 3A). Spleens showed considerable variability in size and cellularity (Fig. 3B). In mice in which splenomegaly was apparent, this could be accounted for by a large increase in the number of cells that express Ter119, which is a marker of late-stage erythroid precursors. This increase is indicative of extra-medullary hematopoiesis. Consistent with reduced thymic cellularity, the total number of T cells in spleens was reduced. Likewise, numbers of B220+ B cells in spleen were substantially reduced. By contrast, normal numbers of macrophages and neutrophils were found in spleens of ptpn11fl/fl ert2-cre mice (Fig. 3B).
Fig. 3.
Altered hematopoiesis in induced SHP-2-deficient mice. (A) Numbers of total thymocytes (left) and indicated thymocyte subpopulations (right) in thymi isolated from tamoxifen-injected moribund ptpn11fl/fl ert2-cre mice (n=8) and littermate controls (n=8). DN, CD4–CD8–double negative; DP, CD4+CD8+ double positive; SP, CD4+CD8– and CD4–CD8+ single positive T cells. (B) Numbers of total splenocytes (left) and indicated splenocyte subpopulations (right) in spleens isolated from tamoxifen-injected moribund ptpn11fl/fl ert2-cre mice (n=12) and littermate controls (n=14). T cells (T), B cells (B), macrophages (Mac) and neutrophils (Neut) were identified as TCRβ+, B220+, CD11bint/GR1int and CD11bhi/GR1hi, respectively. Ter119+ cells represent erythrocyte precursors. (C) Total numbers and numbers of indicated lineage-positive cells (left) and numbers of indicated lineage-negative progenitor cells (right) in bone marrow of tamoxifen-injected moribund ptpn11fl/fl ert2-cre mice and littermate controls (for each genotype, n=11 for lineage-positive and n=5 for lineage-negative). LSK, Lin−Sca-1+c-Kit+ cells, which are enriched for HSCs; CLP, Lin−Sca-1loc-KitloCD127+; CMP, Lin−Sca-1−c-Kit+CD34+CD16/32lo; GMP, Lin−Sca-1−c-Kit+CD34+CD16/32hi; MEP, Lin−Sca-1−c-Kit+CD34−CD16/32–/lo. In scatter plots, each symbol represents an individual mouse. In bar graphs, the mean ± 1 s.e.m. is depicted. All mice were injected with tamoxifen at 6–8 weeks of age. Age range of mice at time of analysis was 10–16 weeks. Statistical significance was determined by two sample Student’s t-test. *P<0.05; **P<0.005.
We also examined the number of hematopoietic cells in bone marrow of ptpn11fl/fl ert2-cre mice (Fig. 3C). A significant decrease in the number of B220+ B cells and neutrophils, and a significant increase in the number of macrophages, was apparent. By contrast, there were normal numbers of T cells and Ter119+ cells. Regarding hematopoietic precursor cells, total numbers of Lin−Sca-1+c-kit+ (LSK) cells, a population enriched in hematopoietic stem cells (HSCs), remained unchanged (Fig. 3C). However, numbers of common lymphoid progenitor cells (CLPs) were significantly reduced. Therefore, this reduced number of CLP might, at least in part, account for the reduced number of T cells in thymus and spleen, and also the reduced number of B cells in bone marrow and spleen. Notably, numbers of common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythroid progenitors (MEPs) were found to be normal in ptpn11fl/fl ert2-cre mice (Fig. 3C).
Skeletal abnormalities in induced SHP-2-deficient mice
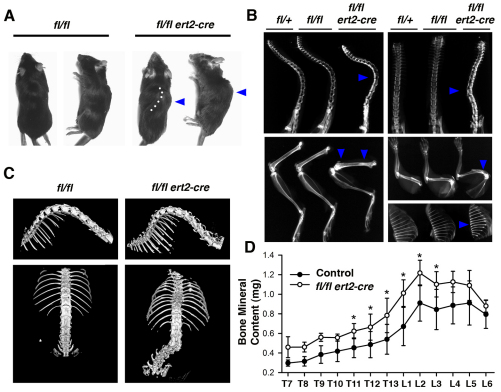
One of the most striking features of tamoxifen-injected ptpn11fl/fl ert2-cre mice is the development of skeletal abnormalities. Visual inspection of live and deceased mice revealed pronounced kyphosis and scoliosis (Fig. 4A). These skeletal abnormalities were apparent as soon as 2 weeks after tamoxifen injection and were observed in all mice to greater or lesser degrees prior to euthanasia or natural death. Kyphosis and scoliosis were confirmed by X-ray analysis of spines (Fig. 4B). Furthermore, curvature of humeri was detected (Fig. 4B). X-ray analysis also revealed an increased radiodensity of all vertebral bodies in the spine of ptpn11fl/fl ert2-cre mice. In addition, increased radiodensity was apparent in metaphyses of humeri and femora and in all rib bones of ptpn11fl/fl ert2-cre mice (Fig. 4B). These findings suggest that bone malformations and osteopetrosis affect the entire skeleton of ptpn11fl/fl ert2-cre mice.
Fig. 4.
Spinal curvature and increased bone mineral content in induced SHP-2-deficient mice. (A) Gross morphology of moribund ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate control mice. Note lateral spinal curvature (dotted line) and hump (arrowhead) in the ptpn11fl/fl ert2-cre mouse. (B) X-rays of tamoxifen-injected moribund ptpn11fl/fl ert2-cre mice showing kyphosis (top left) and scoliosis (top right), compared with the indicated littermate controls. Note also increased radiodensity of vertebral bodies of spines, metaphyses of femur (bottom left) and humerus (middle right), and entire rib bones (bottom right) of the ptpn11fl/fl ert2-cre mice (all indicated with arrowheads). In A and B, mice were treated with tamoxifen 5 weeks previously, at 6–8 weeks of age. (C) μCT images of the isosurfaces of spines of ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate control mice injected with tamoxifen 5 weeks previously, at 7 weeks of age. Images show scoliosis with rotated vertebral bodies. (D) Mean bone mineral content ± 1 s.e.m. of individual thoracic (T) and lumbar (L) vertebrae from ptpn11fl/fl ert2-cre mice and littermate controls determined by μCT scanning (n=4 for each mouse strain). All mice were treated with tamoxifen 5 weeks previously, at 7 weeks of age. Statistical significance was determined by paired Student’s t-test. *P<0.05.
To understand spinal curvature in three dimensions and to quantitate the increased bone radiodensity in ptpn11fl/fl ert2-cre spines, we scanned thoracic and lumbar vertebral bones using micro-computed tomography (μCT). In these experiments, we compared spines from a cohort of ptpn11fl/fl ert2-cre and littermate control mice that had been injected with tamoxifen 5 weeks previously (Fig. 4C,D). Results of μCT analyses demonstrated severe scoliosis with a rotation of vertebral bodies and osteophyte-like ectopic calcifications on the spinal column (Fig. 4C). In addition, increased bone mineral content in vertebrae of induced SHP-2-deficient mice was confirmed (Fig. 4D). Increased bone mineral content was especially apparent in lower thoracic and upper lumbar vertebrae, i.e. in the region where kyphosis and scoliosis is most frequently observed. μCT scanning also revealed several other skeletal abnormalities in ptpn11fl/fl ert2-cre mice, including increased rib thickness, evidence of previous rib fractures and the presence of ectopic calcified growths on ribs (Fig. 4C and data not shown).
Bone histology
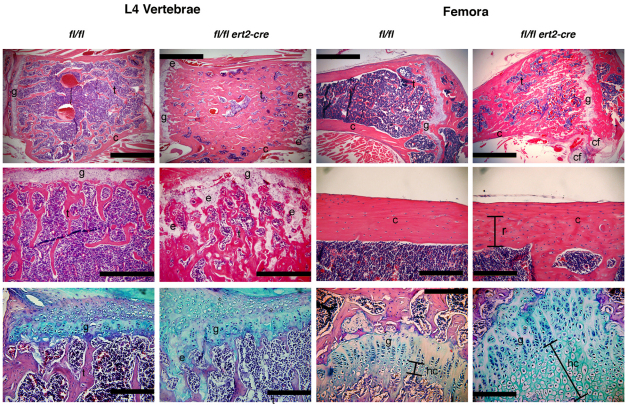
Histological analysis confirmed that bones from tamoxifen-injected ptpn11fl/fl ert2-cre mice were osteopetrotic compared with littermate control mice (Fig. 5). Vertebrae and metaphyses of femora and humeri showed marked increases in the amount of trabecular bone (Fig. 5, top panels, and data not shown). Changes in the amount of cortical bone were less apparent. However, the uniform organization of remodeled cortical bone layers was clearly disrupted in SHP-2-deficient long bones, with regions of remodeled cortical bone showing ultrastructural features of trabecular bone (Fig. 5, right middle panels). Presumably, this trabecular-like cortical bone is formed after the time of tamoxifen administration.
Fig. 5.
Increased and disorganized bone and cartilage in induced SHP-2-deficient mice. Shown are representative images of L4 vertebrae and femora of moribund tamoxifen-injected ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate controls. Mice were injected with tamoxifen at 7 weeks of age and analysis was performed at 12 weeks of age. Top and middle panels are stained with H&E, and bottom panels are stained with Alcian blue to highlight cartilage. Scale bars: 1 mm in top panels, 500 μm in L4 vertebrae middle panels, and 200 μm in femora middle panels and all bottom panels. The amount of trabecular bone (t) is dramatically increased in ptpn11fl/fl ert2-cre vertebrae and femora. A region of remodeled (r) cortical bone (c) in femora of ptpn11fl/fl ert2-cre mice shows disorganized bone in this region compared with the same region in the control. Remains of ectopic cartilaginous elements (e) were identified in the trabecular bone region and ectopic cartilage formation (cf) was identified next to growth plates (g) in ptpn11fl/fl ert2-cre mice. The columnar formation of growth plates was disorganized in ptpn11fl/fl ert2-cre mice and the area of hypertrophic chondrocytes (hc) was elongated.
In addition to abnormalities in bone, cartilage abnormalities were noted in ptpn11fl/fl ert2-cre mice. Cartilaginous growth plates of vertebrae, femora and humeri were disorganized (Fig. 5, top and left middle panels, and data not shown). Alcian blue staining confirmed the disorganization of the columnar growth plate and the absence of a columnar morphology of differentiating chondrocytes in ptpn11fl/fl ert2-cre mice (Fig. 5, bottom panels). In addition, the remains of ectopic cartilaginous elements in trabecular bone were identified in ptpn11fl/fl ert2-cre mice (Fig. 5, top left, middle left and bottom left panels). Interestingly, ectopic cartilage was also identified next to growth plates in vertebrae and long bones (Fig. 5, top right panels, and data not shown). Lastly, the area of hypertrophic chondrocytes was much expanded in ptpn11fl/fl ert2-cre mice (Fig. 5, bottom right panels). These findings suggest that the process of bone remodeling through endochondral bone formation was largely disrupted by loss of SHP-2.
Induced loss of SHP-2 results in impaired osteoclastogenesis
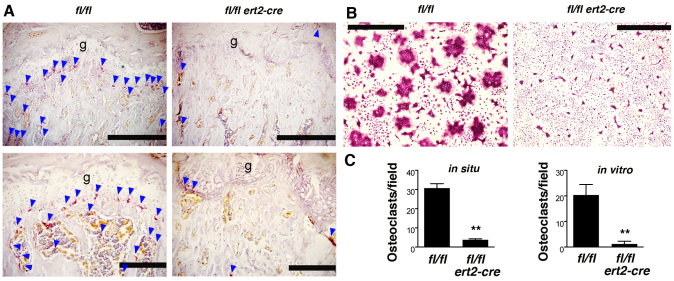
To further investigate the basis for the skeletal phenotype observed in tamoxifen-injected ptpn11fl/fl ert2-cre mice, we enumerated bone osteoclasts, the cell type that is responsible for resorption of bone and for maintenance of bone homeostasis. To identify osteoclasts, bone sections were stained for tartrate-resistant acid phosphatase (TRAP), an osteoclast-specific marker. Osteoclasts were readily identified in the metaphyses of ptpn11fl/fl control bone as well as in the secondary spongiosa (Fig. 6A,C). By contrast, much fewer osteoclasts were found in the same regions of ptpn11fl/fl ert2-cre bones.
Fig. 6.
Impaired osteoclastogenesis in induced SHP-2-deficient mice. (A) Femur sections of tamoxifen-injected moribund ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate controls were stained for TRAP to visualize osteoclasts (red color). Images are from 11-week-old mice injected with tamoxifen 5 weeks previously. The location of osteoclasts beneath growth plates (g) and within the secondary spongiosa are indicated with blue arrowheads. Note the paucity of osteoclasts in ptpn11fl/fl ert2-cre mice. Scale bars: top panels, 500 μm; bottom panels, 200 μm. (B) Bone marrow cells from ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate control mice, both treated with tamoxifen 3 weeks previously (at 7 weeks of age), were cultured with M-CSF and RANKL for 7 days on glass coverslips. Osteoclasts were identified by TRAP staining. Note the abundance of multinucleated osteoclasts in control cultures and absence from ptpn11fl/fl ert2-cre cultures. Scale bars: 100 μm. (C) Shown are the mean numbers of osteoclasts + 1 s.e.m. per field identified in bone sections (in situ) and in vitro osteoclast differentiation experiments described in A and B. For in situ analysis, the field size was as shown in the top panels of A and encompassed the growth plate and secondary spongiosa regions. Data are derived from randomly selected fields of femur heads from moribund ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate controls injected with tamoxifen at 6 weeks of age (n=3 in each genotype). Mice were 10–11 weeks of age at the time of analysis. Size of fields in in vitro experiments are as indicated in B and were selected randomly on coverslips. Bone marrow was derived from ptpn11fl/fl ert2-cre mice and ptpn11fl/fl littermate mice that were injected with tamoxifen at 6–8 weeks of age (n=6 in each genotype). Osteoclast differentiation experiments were initiated 1–3 weeks thereafter. Statistical significance was determined by paired Student’s t-test. **P<0.005.
Osteoclasts are of hematopoietic origin and are derived principally from GMPs (Asagiri and Takayanagi, 2007; Menaa et al., 2000; Takayanagi, 2007; Yavropoulou and Yovos, 2008). Culture of whole bone marrow cells in vitro with the cytokines macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) leads to selective outgrowth of this cell type (Asagiri and Takayanagi, 2007). Therefore, to determine whether the paucity of osteoclasts in bones of ptpn11fl/fl ert2-cre mice could be explained by defective osteoclastogenesis, and at the same time understand whether any defective osteoclastogenesis were intrinsic to the hematopoietic compartment, we examined osteoclastogenesis in vitro. Whole bone marrow cells from ptpn11fl/fl ert2-cre and control mice were cultured with M-CSF and RANKL for 7 days, after which time osteoclasts were identified by TRAP staining (Fig. 6B,C). Under these conditions, multinucleated TRAP+ osteoclasts were readily identified in control cultures. By contrast, much fewer osteoclasts were identified in cultures from ptpn11fl/fl ert2-cre mice. Therefore, osteoclastogenesis is impaired in ptpn11fl/fl ert2-cre mice. Furthermore, this seems to be an intrinsic property of hematopoietic cells and not secondary to other factors such as reduced bone marrow volume.
Impaired M-CSF signal transduction in the absence of SHP-2
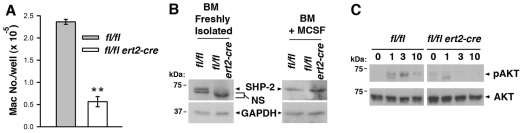
Defective osteoclastogenesis in tamoxifen-treated ptpn11fl/fl ert2-cre mice could be explained by impaired M-CSF or RANKL signaling, or both. Evidence that at least M-CSF signaling is blocked in the absence of SHP-2 was provided by experiments in which bone marrow cells were treated with this cytokine alone (Fig. 7). Treatment of control bone marrow cells with M-CSF for 5 days promoted the development of abundant numbers of macrophages, as expected. By contrast, considerably fewer macrophages grew out from cultures of ptpn11fl/fl ert2-cre bone marrow treated with M-CSF for the same time (Fig. 7A). Furthermore, of those macrophages that did develop in ptpn11fl/fl ert2-cre bone marrow cultures, these were found to express the same levels of SHP-2 as macrophages that had grown out from control cultures (Fig. 7B). These findings suggest that SHP-2 is essential for M-CSF signal transduction in macrophage-osteoclast precursor cells, i.e. only those small number of precursors that retain expression of SHP-2 in tamoxifen-treated ptpn11fl/fl ert2-cre mice are able to respond to M-CSF. In itself, this impaired response to M-CSF would account for the defective osteoclastogenesis in induced SHP-2-deficient mice.
Fig. 7.
Blocked M-CSF signal transduction in induced SHP-2-deficient mice. (A) Bone marrow cells from a ptpn11fl/fl ert2-cre mouse and a littermate ptpn11fl/fl mouse (both treated with tamoxifen 10 days beforehand at 7 weeks of age) were cultured in wells of a 24-well plate (1×106 cells/well) in the presence of M-CSF. After 5 days, macrophages in wells were harvested and counted. Depicted is the mean number of macrophages ± 1 s.e.m. (n=4). Results are representative of four repeat experiments. Statistical significance was determined by two sample Student’s t-test. **P<0.005. (B) Western blots showing expression of SHP-2 in bone marrow cells (freshly isolated or after culture in M-CSF for 5 days) from a ptpn11fl/fl ert2-cre mouse and a littermate ptpn11fl/fl mouse (both treated with tamoxifen 3 weeks beforehand at 6 weeks of age). NS, non-specific band. Blots were stripped and reprobed with an anti-GAPDH antibody to verify equivalent protein loading. Similar results were obtained in five independent experiments. (C) Lineage-negative bone marrow cells from a ptpn11fl/fl ert2-cre mouse and a littermate ptpn11fl/fl mouse (both treated with tamoxifen 10 days beforehand at 6 weeks of age) were stimulated with M-CSF for the indicated times (in minutes). Activation of AKT was determined by western blotting of whole-cell lysates using a phospho-specific anti-AKT antibody. Blots were reprobed with an anti-AKT antibody to verify equal loading. Similar results were obtained in three repeat experiments.
The two principal ways in which M-CSF is thought to promote the proliferation and survival of macrophage-osteoclast precursors is through activation of MAPK and the antiapoptotic protein kinase AKT (Ross and Teitelbaum, 2005; Takayanagi, 2007). SHP-2 has been previously implicated in receptor-mediated activation of both these protein kinases (Dance et al., 2008; Hakak et al., 2000; Ivins Zito et al., 2004). Therefore, we investigated whether M-CSF-induced activation of MAPK or AKT was impaired in macrophage-osteoclast precursors in the absence of SHP-2. For this purpose, we examined M-CSF-induced responses in lineage-negative bone marrow cells, a relatively large percentage of which are GMP (Fig. 3C). As determined in western blot experiments, M-CSF induced only weak and inconsistent activation of MAPK in lineage-negative cells, even in control mice (not shown). Therefore, it was not possible to reliably determine whether activation of MAPK was impaired in lineage-negative cells in the absence of SHP-2. By contrast, activation of AKT in lineage-negative cells was readily detected in control mice after stimulation with M-CSF (Fig. 7C). However, in tamoxifen-treated ptpn11fl/fl ert2-cre mice, M-CSF essentially failed to induce activation of AKT in lineage-negative cells (Fig. 7C). Thus, these findings provide a molecular basis for blocked osteoclastogenesis in ptpn11fl/fl ert2-cre mice.
Discussion
Previous studies of conditional SHP-2-deficient mice have revealed an important physiological role for this PTP in multiple different tissues, including the central nervous system, cardiac and striated muscle, liver, pancreas, mammary gland, and thymus (Bard-Chapeau et al., 2006; Fornaro et al., 2006; Hagihara et al., 2009; Ke et al., 2006; Ke et al., 2007; Kontaridis et al., 2008; Krajewska et al., 2008; Nguyen et al., 2006; Princen et al., 2009; Zhang et al., 2004; Zhang et al., 2009). These mice serve as models with which to understand the role of SHP-2 in tissue homeostasis that would be relevant to an understanding of the etiology of certain clinical manifestations in humans with SHP-2 mutations. However, not all features of NS and LS are recapitulated in these mouse models, in part owing to the tissue-restricted nature of SHP-2 deletion. In the current studies, therefore, we have attempted to address this issue by examining the effect of global deletion of SHP-2 de novo in adult mice.
Systemic loss of SHP-2 resulted in the early demise of mice that was associated with abnormalities of liver and kidney function, and metabolic abnormalities suggested by precipitous weight loss. However, a definitive cause of death was not identified. One possibility is that death results from lung or cardiac compression secondary to skeletal alterations in this model (see below). One novel finding associated with induced SHP-2 loss in this model was skin epidermal acanthosis and hyperkeratosis. This finding points to a role for SHP-2 as a regulator of keratinocyte proliferation and differentiation. It is tempting to speculate that SHP-2 regulates keratinocyte proliferation and differentiation through its ability to promote activation of the Ras-MAPK signaling pathway. In this regard, augmented Ras-MAPK signaling has been shown previously to result in terminal differentiation and inhibition of proliferation of keratinocytes (Lin and Lowe, 2001; Roper et al., 2001). However, published literature on the role of the Ras-MAPK signaling pathway in the regulation of keratinocyte proliferation is conflicting (Cai et al., 2002). Nonetheless, the finding here of skin abnormalities in induced SHP-2-deficient mice is consistent with the occurrence of cutaneous lentigines in LS patients (Digilio et al., 2002; Gorlin et al., 1969).
Induced systemic deletion of SHP-2 was also found to result in disorders of hematopoiesis. The role of SHP-2 in hematopoiesis in vivo in mice has not been properly examined before. Most studies that have addressed this issue have used SHP-2-deficient embryonic stem (ES) cells (Chan et al., 2003; Feng, 2007; Qu et al., 2001; Zou et al., 2006). SHP-2 is required for ES cell differentiation to mesoderm and mesoderm differentiation to hemangioblasts (Qu et al., 2001; Chan et al., 2003). Therefore, it has not been possible to examine the effect of loss of SHP-2 upon HSC renewal or differentiation of HSCs into hematopoietic lineages by using SHP-2-deficient ES cells. As an alternative approach, Chan et al. examined the influence of SHP-2 haploinsufficiency upon HSC function in competitive repopulation studies wherein heterozygote ptpn11+/− and wild-type HSCs were co-transferred into irradiated wild-type recipients (Chan et al., 2006). Despite the finding that the number of HSCs was not different in the bone marrow of ptpn11+/− and wild-type mice, these studies showed a clear reduced repopulating activity of the ptpn11+/− HSCs that was associated with diminished HSC self renewal. In the current studies, in which deletion of SHP-2 was induced de novo in adult mice, we also did not observe a reduction in HSC number. However, this does not necessarily challenge the view that SHP-2 is required for HSC self-renewal, and this might be revealed in transplantation experiments. In addition to normal HSC numbers being detected, the number of CMPs, MEPs and GMPs were found to be normal in bone marrow following induced loss of SHP-2. This suggests that SHP-2 is not essential for differentiation of HSCs into these committed precursors. In contrast to this, the number of CLPs was substantially reduced in induced SHP-2-deficient mice, thus demonstrating a role for SHP-2 in the differentiation of HSCs into CLPs and/or CLP survival.
A reduced number of CLPs is consistent with the finding of reduced numbers of thymocytes and of T and B cells in peripheral lymphoid organs. However, the severe thymic atrophy that is observed upon loss of SHP-2 points to additional roles for SHP-2 in the generation of T cells. As revealed with the use of a (LCK)-promoter-driven Cre transgene that is active at the late DN stage of T cell development, SHP-2 is required for normal pre-T-cell-receptor (pre-TCR) signaling in developing thymocytes (Nguyen et al., 2006). Signal transduction through the pre-TCR triggers a proliferative burst in DN cells, culminating in the development of DP cells. Therefore, a reduced number of CLPs together with factors such as impaired pre-TCR signal transduction probably account for the observed near complete block in T cell development. Presumably, T cells that exist in peripheral lymphoid organs in induced SHP-2-deficient mice exited the thymus prior to the time of SHP-2 loss. In contrast to bone marrow, loss of SHP-2 protein expression in whole spleen was only modest in induced SHP-2-deficient mice (Fig. 1C). Therefore, changes in the cellular composition of spleen in these animals can most likely be explained solely by altered hematopoiesis. Alternatively, SHP-2 might initially be deleted in a more substantial fraction of mature splenocytes but confer a survival disadvantage to deleted cells that results in their rapid clearance.
Aside from lymphopenia, induced SHP-2-deficient mice also developed anemia. This finding is consistent with previous reports that physical association of SHP-2 with the erythropoietin receptor is required for full proliferative activity of erythrocyte precursors in vitro (Wojchowski et al., 1999). However, the number of Ter119+ erythrocyte precursors were found to be normal in bone marrow of induced SHP-2-deficient mice and were elevated in spleen of these animals, which is indicative of extramedullary hematopoiesis. Furthermore, numbers of reticulocytes were elevated in the peripheral blood. Therefore, only the terminal stages of erythrocyte development may be affected by SHP-2 loss in vivo. Alternatively, the anemia that is observed might not be intrinsic to the hematopoietic compartment but instead be caused by extrinsic, yet-to-be-determined, factors.
Despite the hematological and immunological abnormalities found in this model, similar disorders have not been reported in NS and LS patients as far as we are aware. In NS, there is increased susceptibility to juvenile myelomonocytic leukemia (JMML) and, in non-NS patients with JMML, PTPN11 somatic missense mutations are frequent (Tartaglia et al., 2003). Furthermore, genome-wide association studies have revealed strong linkage of PTPN11 polymorphisms to autoimmune and inflammatory disorders in humans, such as type I diabetes and Crohn’s disease (Todd et al., 2007). The fact that there is discordance with regards these actual or implied phenotypes in humans and the mouse phenotype described here most probably reflects differences in the nature of the mutations involved.
Skeletal malformation is the most striking phenotype resulting from induced loss of SHP-2 in adult animals. The majority of mice developed kyphosis and/or scoliosis within weeks of SHP-2 loss. This is clinically significant because similar skeletal malformations have been documented in NS patients (Noonan, 2006). In one study of Korean NS patients, as many as 30% of sufferers were found to develop kyphoses or scoliosis (Lee et al., 2001). Skeletal defects in humans with SHP-2 mutations, however, are not limited to the spine: chest deformity in the form of pectus carinatum and pectus excavatum are observed in both NS and LS patients (Sarkozy et al., 2008; Sharland et al., 1992). Thus, a normal range of Ras signaling seems to be required for normal skeletal structure in humans because excessive Ras signaling, as in NS, and reduced Ras signaling, as in LS, both cause skeletal abnormalities. Consistent with this, patients with neurofibromatosis, which is caused by germline mutations in the neurofibromin 1 (NF1) gene, which encodes a negative regulator of Ras, also develop scoliosis (Schindeler and Little, 2008).
In induced SHP-2-deficient mice, skeletal abnormalities are associated with osteopetrosis and cartilage alterations including accumulation of collagenous matrix (osteoid) in bone. As documented by X-ray analysis, μCT scanning and histology, osteopetrosis affects the entire skeleton, including vertebrae, long bones and ribs. Also, ectopic calcified growths were frequently observed on the spine and ribs. Histological analysis revealed a paucity of osteoclasts in vertebrae and long bones of induced SHP-2-deficient mice and in vitro M-CSF- and RANKL-induced differentiation of osteoclasts from bone marrow precursor cells was shown to be severely impaired. Therefore, a defect in osteoclastogenesis that is intrinsic to osteoclast precursor cells seems to be a major cause of the osteopetrosis in induced SHP-2-deficient mice. In addition, osteoid formation in induced SHP-2-deficient mice could contribute to low osteoclast number and interruption of the normal process of bone resorption in vivo.
Another possible cause of osteopetrosis in induced SHP-2-deficient mice is increased osteoblast activity. The function of the Ras-MAPK pathway in osteogenesis is controversial, with some studies showing a role for this pathway in the promotion of osteoblast development and others showing an inhibitory role (reviewed by Schindeler and Little, 2006). Nonetheless, increased osteoblast activity in induced SHP-2-deficient mice would be consistent with the finding that NF1 is required for the proper differentiation and function of osteoblasts (Kolanczyk et al., 2007; Yu et al., 2005). Indeed, we determined that expression of the bone formation markers alkaline phosphatase, integrin-binding sialoprotein and Runx2 was increased in induced SHP-2-deficient mice as soon as 1 week after tamoxifen administration (supplementary material Fig. S1). Increased activity of osteoblasts, therefore, together with defective osteoclastogenesis, would accelerate the increase in bone mass in this model.
The ability of M-CSF to drive differentiation of macrophage-osteoclast precursor cells is blocked following induced loss of SHP-2. This finding, therefore, accounts for the defective osteoclastogenesis, although we cannot rule out the possibility that RANKL signaling is also impaired in these precursors. Mechanistically, we determined that AKT is not activated properly in response to M-CSF stimulation. This is significant because M-CSF-induced activation of AKT provides essential survival signals to macrophage-osteoclast precursors necessary for their expansion and differentiation (Ross and Teitelbaum, 2005; Takayanagi, 2007). During the course of M-CSF signal transduction, SHP-2 has the potential to promote activation of AKT directly or indirectly through the regulation of Ras. Regardless, the notion that impaired activation of AKT in osteoclast precursors contributes to the osteopetrotic phenotype of induced SHP-2-deficient mice is supported by findings in ovariectomized female NF1+/− mice. Parallel to the phenotype described here, ovariectomized NF1+/−mice develop osteopenia that is at least in part a consequence of increased generation of osteoclasts. This increased osteoclast generation represents an intrinsic osteoclast defect that is coincident with augmented activation of AKT (Yan et al., 2008; Yang et al., 2006).
Despite the finding that M-CSF signaling is blocked in the absence of SHP-2, the number of macrophages in bone marrow and peripheral lymphoid organs of induced SHP-2-deficient mice were not reduced. There are two potential explanations for this finding. First, macrophage differentiation might proceed independently of M-CSF in whole animals. For instance, M-CSF-deficient and M-CSFR-deficient mice, although both severely osteopetrotic, show varying degrees of macrophage depletion, depending upon the tissue examined and age (Dai et al., 2002; Wiktor-Jedrzejczak et al., 1982; Yoshida et al., 1990). In some tissues, macrophage numbers might be reduced by less than 50% of those found in corresponding tissues of littermate wild-type mice (Dai et al., 2002). Presumably, this reflects the action of other macrophage-differentiation-promoting cytokines such as IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Second, the longevity of tissue macrophages is considerably greater than that of osteoclasts. The estimated half-life of murine tissue macrophages ranges from weeks to months, again depending upon tissue (Murphy et al., 2008; Papadimitriou and Ashman, 1989). By contrast, the estimated half-life of murine osteoclasts is around 1.3 days (Marshall and Davie, 1991). In the model described herein, the majority of induced SHP-2-deficient mice succumbed within weeks of gene deletion, at which point macrophage numbers were determined. Within this time frame, substantial numbers of macrophages that had developed prior to the time of SHP-2 deletion would remain in tissues. Thus, the relatively long half-life of tissue macrophages and M-CSF-independent-driven macrophage differentiation are probably significant contributing factors that explain the lack of diminution of macrophage numbers following induced loss of SHP-2.
In summary, we report here a model in which induced systemic deletion of SHP-2 in adult mice results in early lethality, metabolic, skin and hematologic disorders, and, most strikingly, the development of severe skeletal abnormalities. We anticipate that the model will be of further use in dissecting the role of SHP-2 as a regulator of skeletal morphogenesis. Knowledge gained is likely to yield insight into the etiology of skeletal malformations in humans with SHP-2 mutations and provide a rational basis for the development of therapies for the prevention and treatment of this condition.
METHODS
Mice
ptpn11fl/fl mice and ubiquitin-promoter-driven ert2-cre transgenic mice have been described (Ruzankina et al., 2007; Zhang et al., 2004). Mice were intercrossed to generate ptpn11fl/fl ert2-cre mice and littermate ptpn11+/+, ptpn11fl/+, ptpn11fl/+ ert2-cre and ptpn11fl/fl control mice. Mice were injected twice with tamoxifen (Sigma; 200 μg/g body weight in corn oil on 2 consecutive days) at 6–8 weeks of age. Animal health was monitored daily. Initial observations established that, once ptpn11fl/fl ert2-cre mice became moribund, they died within 24 hours. Thereafter, moribund mice were euthanized immediately upon identification. Moribund euthanized ptpn11fl/fl ert2-cre mice are included in survival and weight loss analyses. Unless otherwise stated all other analyses were performed upon ptpn11fl/fl ert2-cre mice and control mice at the time that the former became moribund. All research was performed in compliance with University of Michigan guidelines and was approved by the University Committee on the Use and Care of Animals.
Clinical chemistry
Spin hematocrits and methylene blue reticulocyte counts were performed on heparinized whole blood. Levels of biomarkers in serum were analyzed on a Vettest Chemistry Analyzer Model 8008 (Idexx).
Histology
Tissues were fixed in 10% buffered formalin, transferred to 70% ethanol and embedded in paraffin. Bone samples were decalcified over a period of 2 weeks in 10% EDTA-ammonium hydroxide, pH 7.2, prior to embedding. Sections (8 μm for bone, 5 μm for all other tissues) were stained with H&E, Alcian blue with an eosin-orange G counterstain, or for TRAP using a TRAP staining kit (Sigma).
Flow cytometry
Single cell suspensions of thymocytes, splenocytes and hind-limb-derived bone marrow cells were stained with fluorochrome-labeled monoclonal antibodies to TCRβ, CD3, CD19, CD4, CD8, CD11c, NK1.1, B220, CD11b, GR-1 and Ter119 (all BD Biosciences) and analyzed by flow cytometry on a FacsCanto (BD Biosciences) to enumerate different leukocyte subpopulations. In addition, bone marrow cells were additionally stained with labeled antibodies to Sca-1, c-Kit, CD127, CD34 and CD16/32 (BD Biosciences) to enumerate lineage-negative precursor populations.
X-ray and μCT
X-ray images were acquired using a microradiography machine (Faxitron Corporation). μCT scanning was performed using a cone beam μCT system (GE Healthcare Biosciences). Image reconstruction was performed on 25-μm voxels, a threshold was generated to define mineralized tissue and regions of interest were analyzed using the MicroView Analysis program (GE Healthcare Biosciences).
Osteoclastogenesis
Bone marrow cells were cultured overnight on plastic Petri dishes in minimal essential medium containing 10% fetal calf serum, antibiotics, 25 ng/ml recombinant murine M-CSF and 100 ng/ml recombinant murine RANKL (R&D Systems). Nonadherent cells were then replated into wells of 24-well plastic plates containing glass coverslips. After 7 days, cells on coverslips were fixed and stained for TRAP.
SHP-2 expression
To prepare tissue lysates, organs were crushed and lysed in 1% NP-40 lysis buffer. Lysates were run on 10% SDS-PAGE gels and analyzed by western blotting using an anti-SHP-2 rabbit polyclonal antibody (Cell Signaling) before stripping and reprobing with a GAPDH antibody (Santa Cruz) as a loading control.
In other experiments, bone marrow cells were cultured in wells of 24-well plastic plates in the same medium used for in vitro osteoclastogenesis minus RANKL. After 5 days, macrophages were lysed in wells and analyzed for expression of SHP-2 and GAPDH as above. Comparisons were made with freshly isolated bone marrow cells.
AKT activation
Bone marrow cells were depleted of TCRβ-, CD3-, CD19-, CD4-, CD8-, CD11c-, NK1.1-, B220-, GR-1- and Ter119-expressing lineage-positive cells by negative selection using MACS columns (Miltenyi). Cells were then stimulated with M-CSF (100 ng/ml) for the indicated times before lysis. Activation of AKT was determined by western blotting using a phospho-specific (T308) anti-AKT antibody (Cell Signaling). Blots were stripped and reprobed with an anti-AKT antibody to ascertain equal loading (Cell Signaling).
Statistical analysis
Statistical significance was determined using Student’s one sample, two sample or paired sample t-tests as indicated. In one sample t-tests, in the case that more than one control mouse was available for each test mouse, a mean control value was calculated for use in statistical calculations. *P<0.05; **P<0.005.
Supplementary Material
Acknowledgments
We thank Eric Brown (University of Pennsylvania) for providing ubiquitin ert2-cre mice. This work was supported by National Institutes of Health grant AI050699 to P.D.K.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
T.J.B., N.K., Y.M. and P.D.K. designed experiments, T.J.B., N.K., P.E.L., E.L., J.E.W. and P.D.K. performed experiments, G.-S.F. contributed SHP-2 floxed mice, and T.J.B., N.K. and P.D.K. analyzed data and wrote the paper.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.006130/-/DC1
REFERENCES
- Asagiri M., Takayanagi H. (2007). The molecular understanding of osteoclast differentiation. Bone 40, 251–264 [DOI] [PubMed] [Google Scholar]
- Bard-Chapeau E. A., Yuan J., Droin N., Long S., Zhang E. E., Nguyen T. V., Feng G. S. (2006). Concerted functions of Gab1 and Shp2 in liver regeneration and hepatoprotection. Mol. Cell. Biol. 26, 4664–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T., Nishida K., Hirano T., Khavari P. A. (2002). Gab1 and SHP-2 promote Ras/MAPK regulation of epidermal growth and differentiation. J. Cell Biol. 159, 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. J., Johnson S. A., Li Y., Yoder M. C., Feng G. S. (2003). A definitive role of Shp-2 tyrosine phosphatase in mediating embryonic stem cell differentiation and hematopoiesis. Blood 102, 2074–2080 [DOI] [PubMed] [Google Scholar]
- Chan R. J., Li Y., Hass M. N., Walter A., Voorhorst C. S., Shelley W. C., Yang Z., Orschell C. M., Yoder M. C. (2006). Shp-2 heterozygous hematopoietic stem cells have deficient repopulating ability due to diminished self-renewal. Exp. Hematol. 34, 1230–1239 [DOI] [PubMed] [Google Scholar]
- Chong Z. Z., Maiese K. (2007). The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol. Histopathol. 22, 1251–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X. M., Ryan G. R., Hapel A. J., Dominguez M. G., Russell R. G., Kapp S., Sylvestre V., Stanley E. R. (2002). Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 [DOI] [PubMed] [Google Scholar]
- Dance M., Montagner A., Salles J. P., Yart A., Raynal P. (2008). The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 20, 453–459 [DOI] [PubMed] [Google Scholar]
- Digilio M. C., Conti E., Sarkozy A., Mingarelli R., Dottorini T., Marino B., Pizzuti A., Dallapiccola B. (2002). Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am. J. Hum. Genet. 71, 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard T., Montagner A., Dance M., Conte F., Yart A., Parfait B., Tauber M., Salles J. P., Raynal P. (2007). How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms? Cell. Mol. Life Sci. 64, 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep A. L., Tidyman W. E., Teitell M. A., Cotter P. D., Rauen K. A. (2006). HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am. J. Med. Genet A 140, 8–16 [DOI] [PubMed] [Google Scholar]
- Feng G. S. (2007). Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell Res. 17, 37–41 [DOI] [PubMed] [Google Scholar]
- Fornaro M., Burch P. M., Yang W., Zhang L., Hamilton C. E., Kim J. H., Neel B. G., Bennett A. M. (2006). SHP-2 activates signaling of the nuclear factor of activated T cells to promote skeletal muscle growth. J. Cell Biol. 175, 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale A., Tartaglia M., Wu J., Gelb B. D. (2004). Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum. Mutat. 23, 267–277 [DOI] [PubMed] [Google Scholar]
- Gelb B. D., Tartaglia M. (2006). Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Hum. Mol. Genet. 15, R220–R226 [DOI] [PubMed] [Google Scholar]
- Gorlin R. J., Anderson R. C., Blaw M. (1969). Multiple lentigenes syndrome. Am. J. Dis. Child 117, 652–662 [DOI] [PubMed] [Google Scholar]
- Gripp K. W., Lin A. E., Stabley D. L., Nicholson L., Scott C. I., Jr, Doyle D., Aoki Y., Matsubara Y., Zackai E. H., Lapunzina P., et al. (2006). HRAS mutation analysis in Costello syndrome: genotype and phenotype correlation. Am. J. Med. Genet. A 140, 1–7 [DOI] [PubMed] [Google Scholar]
- Hagihara K., Zhang E. E., Ke Y. H., Liu G., Liu J. J., Rao Y., Feng G. S. (2009). Shp2 acts downstream of SDF-1alpha/CXCR4 in guiding granule cell migration during cerebellar development. Dev. Biol. 334, 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y., Hsu Y. S., Martin G. S. (2000). Shp-2 mediates v-Src-induced morphological changes and activation of the anti-apoptotic protein kinase Akt. Oncogene 19, 3164–3171 [DOI] [PubMed] [Google Scholar]
- Hanna N., Montagner A., Lee W. H., Miteva M., Vidal M., Vidaud M., Parfait B., Raynal P. (2006). Reduced phosphatase activity of SHP-2 in LEOPARD syndrome: consequences for PI3K binding on Gab1. FEBS Lett. 580, 2477–2482 [DOI] [PubMed] [Google Scholar]
- Ivins Zito C., Kontaridis M. I., Fornaro M., Feng G. S., Bennett A. M. (2004). SHP-2 regulates the phosphatidylinositide 3′-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J. Cell Physiol. 199, 227–236 [DOI] [PubMed] [Google Scholar]
- Ke Y., Lesperance J., Zhang E. E., Bard-Chapeau E. A., Oshima R. G., Muller W. J., Feng G. S. (2006). Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J. Biol. Chem. 281, 34374–34380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y., Zhang E. E., Hagihara K., Wu D., Pang Y., Klein R., Curran T., Ranscht B., Feng G. S. (2007). Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol. Cell. Biol. 27, 6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhack H., David F. S., McGregor M., Cantley L. C., Neel B. G. (2005). Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. J. Biol. Chem. 280, 30984–30993 [DOI] [PubMed] [Google Scholar]
- Kolanczyk M., Kossler N., Kuhnisch J., Lavitas L., Stricker S., Wilkening U., Manjubala I., Fratzl P., Sporle R., Herrmann B. G., et al. (2007). Multiple roles for neurofibromin in skeletal development and growth. Hum. Mol. Genet. 16, 874–886 [DOI] [PubMed] [Google Scholar]
- Kontaridis M. I., Swanson K. D., David F. S., Barford D., Neel B. G. (2006). PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J. Biol. Chem. 281, 6785–6792 [DOI] [PubMed] [Google Scholar]
- Kontaridis M. I., Yang W., Bence K. K., Cullen D., Wang B., Bodyak N., Ke Q., Hinek A., Kang P. M., Liao R., et al. (2008). Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation 117, 1423–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska M., Banares S., Zhang E. E., Huang X., Scadeng M., Jhala U. S., Feng G. S., Krajewski S. (2008). Development of diabesity in mice with neuronal deletion of Shp2 tyrosine phosphatase. Am. J. Pathol. 172, 1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Chang B. S., Hong Y. M., Yang S. W., Lee C. S., Seo J. B. (2001). Spinal deformities in Noonan syndrome: a clinical review of sixty cases. J. Bone Joint Surg. Am. 83-A, 1495–1502 [PubMed] [Google Scholar]
- Lin A. W., Lowe S. W. (2001). Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl. Acad. Sci. USA 98, 5025–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. J., Davie M. W. (1991). An immunocytochemical method for studying the kinetics of osteoclast nuclei on intact mouse parietal bone. Histochem. J. 23, 402–408 [DOI] [PubMed] [Google Scholar]
- Matozaki T., Murata Y., Saito Y., Okazawa H., Ohnishi H. (2009). Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation. Cancer Sci. 100, 1786–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaa C., Kurihara N., Roodman G. D. (2000). CFU-GM-derived cells form osteoclasts at a very high efficiency. Biochem. Biophys. Res. Commun. 267, 943–946 [DOI] [PubMed] [Google Scholar]
- Murphy J., Summer R., Wilson A. A., Kotton D. N., Fine A. (2008). The prolonged life-span of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 38, 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. V., Ke Y., Zhang E. E., Feng G. S. (2006). Conditional deletion of Shp2 tyrosine phosphatase in thymocytes suppresses both pre-TCR and TCR signals. J. Immunol. 177, 5990–5996 [DOI] [PubMed] [Google Scholar]
- Niihori T., Aoki Y., Narumi Y., Neri G., Cave H., Verloes A., Okamoto N., Hennekam R. C., Gillessen-Kaesbach G., Wieczorek D., et al. (2006). Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat. Genet. 38, 294–296 [DOI] [PubMed] [Google Scholar]
- Noonan J. A. (1968). Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am. J. Dis. Child 116, 373–380 [DOI] [PubMed] [Google Scholar]
- Noonan J. A. (2006). Noonan syndrome and related disorders: alterations in growth and puberty. Rev. Endocr. Metab. Disord. 7, 251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Ashman R. B. (1989). Macrophages: current views on their differentiation, structure, and function. Ultrastruct. Pathol. 13, 343–372 [DOI] [PubMed] [Google Scholar]
- Princen F., Bard E., Sheikh F., Zhang S. S., Wang J., Zago W. M., Wu D., Trelles R. D., Bailly-Maitre B., Kahn C. R., et al. (2009). Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol. Cell. Biol. 29, 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C. K., Nguyen S., Chen J., Feng G. S. (2001). Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood 97, 911–914 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Tetsu O., Tidyman W. E., Estep A. L., Conger B. A., Cruz M. S., McCormick F., Rauen K. A. (2006). Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science 311, 1287–1290 [DOI] [PubMed] [Google Scholar]
- Roper E., Weinberg W., Watt F. M., Land H. (2001). p19ARF-independent induction of p53 and cell cycle arrest by Raf in murine keratinocytes. EMBO Rep. 2, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross F. P., Teitelbaum S. L. (2005). alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 208, 88–105 [DOI] [PubMed] [Google Scholar]
- Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V. P., Velez M., Bhandoola A., Brown E. J. (2007). Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkozy A., Digilio M. C., Dallapiccola B. (2008). Leopard syndrome. Orphanet. J. Rare Dis. 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton T. M., Henkemeyer M., Gasca S., Shen R., Rossi D. J., Shalaby F., Feng G. S., Pawson T. (1997). Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 16, 2352–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindeler A., Little D. G. (2006). Ras-MAPK signaling in osteogenic differentiation: friend or foe? J. Bone Miner. Res. 21, 1331–1338 [DOI] [PubMed] [Google Scholar]
- Schindeler A., Little D. G. (2008). Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NF1). Bone 42, 616–622 [DOI] [PubMed] [Google Scholar]
- Sharland M., Burch M., McKenna W. M., Paton M. A. (1992). A clinical study of Noonan syndrome. Arch. Dis. Child 67, 178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H. (2007). Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 [DOI] [PubMed] [Google Scholar]
- Tartaglia M., Mehler E. L., Goldberg R., Zampino G., Brunner H. G., Kremer H., van der Burgt I., Crosby A. H., Ion A., Jeffery S., et al. (2001). Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 29, 465–468 [DOI] [PubMed] [Google Scholar]
- Tartaglia M., Niemeyer C. M., Fragale A., Song X., Buechner J., Jung A., Hahlen K., Hasle H., Licht J. D., Gelb B. D. (2003). Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 34, 148–150 [DOI] [PubMed] [Google Scholar]
- Tartaglia M., Martinelli S., Stella L., Bocchinfuso G., Flex E., Cordeddu V., Zampino G., Burgt I., Palleschi A., Petrucci T. C., et al. (2006). Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am. J. Hum. Genet. 78, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Walker N. M., Cooper J. D., Smyth D. J., Downes K., Plagnol V., Bailey R., Nejentsev S., Field S. F., Payne F., et al. (2007). Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 39, 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. (1982). Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J. Exp. Med. 156, 1516–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojchowski D. M., Gregory R. C., Miller C. P., Pandit A. K., Pircher T. J. (1999). Signal transduction in the erythropoietin receptor system. Exp. Cell. Res. 253, 143–156 [DOI] [PubMed] [Google Scholar]
- Yan J., Chen S., Zhang Y., Li X., Li Y., Wu X., Yuan J., Robling A. G., Kapur R., Chan R. J., et al. (2008). Rac1 mediates the osteoclast gains-in-function induced by haploinsufficiency of Nf1. Hum. Mol. Genet. 17, 936–948 [DOI] [PubMed] [Google Scholar]
- Yang F. C., Chen S., Robling A. G., Yu X., Nebesio T. D., Yan J., Morgan T., Li X., Yuan J., Hock J., et al. (2006). Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J. Clin. Invest. 116, 2880–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavropoulou M. P., Yovos J. G. (2008). Osteoclastogenesis-current knowledge and future perspectives. J. Musculoskelet. Neuronal. Interact. 8, 204–216 [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D. (1990). The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 [DOI] [PubMed] [Google Scholar]
- Yu X., Chen S., Potter O. L., Murthy S. M., Li J., Pulcini J. M., Ohashi N., Winata T., Everett E. T., Ingram D., et al. (2005). Neurofibromin and its inactivation of Ras are prerequisites for osteoblast functioning. Bone 36, 793–802 [DOI] [PubMed] [Google Scholar]
- Zhang E. E., Chapeau E., Hagihara K., Feng G. S. (2004). Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. USA 101, 16064–16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. S., Hao E., Yu J., Liu W., Wang J., Levine F., Feng G. S. (2009). Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proc. Natl. Acad. Sci. USA 106, 7531–7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G. M., Chan R. J., Shelley W. C., Yoder M. C. (2006). Reduction of Shp-2 expression by small interfering RNA reduces murine embryonic stem cell-derived in vitro hematopoietic differentiation. Stem Cells 24, 587–594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.