Abstract
This review of clinical catecholamine neurochemistry is based on the Streeten Memorial Lecture at the 19th annual meeting of the American Autonomic Society and lectures at a satellite of the 6th Congress of the International Society of Autonomic Neuroscience. Here I provide historical perspective, describe sources and meanings of plasma levels of catecholamines and their metabolites, present a model of a sympathetic noradrenergic neuron that conveys how particular aspects of sympathetic nervous function affect plasma levels of catecholamines and their metabolites, and apply the model to understand plasma neurochemical patterns associated with some drugs and disease states.
Keywords: Catecholamine, Norepinephrine, Epinephrine, Dopamine, Autonomic
Historical perspective
The chemical birth of a new era
The most famous member of the catecholamine chemical family has two names, adrenaline and epinephrine (EPI). Its precursor, the chemical messenger of the sympathetic nervous system, also has two names, noradrenaline and norepinephrine (NE). Here is how this double naming came about.
After the reports by Oliver and Schäfer about the unexpected and profoundly powerful effects of injected extracts of the adrenal gland [101], researchers worldwide were eager to identify the “active principle.” One of these was John Jacob Abel, of Johns Hopkins, who devoted about a decade of his life to the project. The first person to isolate the active principle of the adrenal gland, however, was a chemist in the laboratory of Jokichi Takamine.
Takamine visited Abel in Baltimore 1900. In a transcript of a 1927 lecture that I found in the Abel Library at the Johns Hopkins School of Medicine, Abel recalled the visit and its consequences:
After I had completed the above described investigation and while I was still endeavoring to improve my processes, I was visited one day by the Japanese chemist, J. Takamine, who examined with great interest the various compounds and salts of epinephrine that were placed before him. He inquired particularly whether I did not think it possible that my salts of epinephrine could be prepared by a simpler process than mine, more especially without the trouble and in this case wasteful process of benzoylating extracts of an animal tissue. He remarked in this connection that he loved to plant a seed and see it grow in the technical field. I told Takamine that I was quite of his opinion and that the process could no doubt be improved and simplified.
… Takamine prepared supra-renal extracts more concentrated than mine and without first attempting to separate the hormone from its numerous concomitants by benzoylating or otherwise, simply added ammonia–the reagent that I had so long employed–to his concentrated extracts, whereupon he immediately obtained the native base in the form of burr-like clusters of minute prisms in place of the amorphous base. I have often asked why I had not myself attempted to solve the problem in this very simple fashion. The truth is that I had tried to do so but always found that the dilute extract tested simply turned pink in a short time on the addition of ammonia without depositing the base either crystallized or amorphous…. Takamine’s success was due to the employment of ammonia on very highly concentrated, though impure extracts…. The efforts of years on my part in this once mysterious field of suprarenal, medullary biochemistry, marred by blunders as they were, eventuated, then, in the isolation of the hormone not in the form of the free base but in that of its monobenzoyl derivative.
William MacNider, then dean of the medical school of the University of North Carolina at Chapel Hill, commented, “This extremely important and frank statement of the chemical birth of a new era in the understanding of tissue activity portrays as no words could the industry persisted into the point of physical exhaustion, the frankness, the complete honesty untouched by jealousy or recrimination of a nobleman in the domain of science.”
After Takamine’s visit with Abel, Keizo Uenaka, whom Takamine had hired as a chemist, successfully crystallized and therefore isolated in pure form what Takamine called “adrenaline.” In 1901, Takamine reported in the Journal of Physiology this first successful crystallization of a hormone. Almost simultaneously, Thomas Aldrich, a colleague of Takamine at Parke-Davis (and possibly not coincidentally a former assistant of Abel at Johns Hopkins), correctly deduced its chemical structure. Up to that point Abel had failed in his attempts to isolate the active principle of the adrenal gland, and he never published the correct chemical structure. Medical historians give Takamine and Aldrich the credit for one of medical history’s most important scientific feats, the first identification of a hormone. In 1904, Friedrich Stolz synthesized EPI entirely chemically, so that EPI was also the first hormone to be produced artificially in a laboratory.
Takamine patented Adrenalin and went on to found three companies, one of which, Sankyo Pharmaceutical Company, merged with another drug company to become the modern day Daiichi Sankyo concern. Takamine also financed the gift of cherry trees that have graced Washington, DC’s Tidal Basin to this day.
Abel continued to pursue his career goal of identifying, isolating, and purifying hormones. He crystallized insulin in 1926. Until his death in 1938 he ran a renowned laboratory and teaching program at Johns Hopkins. He helped found the American Society for Pharmacology and Experimental Therapeutics and served as editor of the society’s official journal, the Journal of Pharmacology and Experimental Therapeutics (JPET), for 23 years. He also founded the Journal of Biological Chemistry (JBC). JPET and JBC remain prestigious journals in pharmacology and biochemistry. Abel never rivaled Takamine in monetary success, but he did acquire the wealth of a good name. Scientific reports in American journals such as JPET still use the word that Abel introduced, “epinephrine,” whereas European journals use Takamine’s “adrenaline.”
Abel always maintained that Takamine’s adrenaline was impure. In this assertion he undoubtedly was correct. The drug Parke-Davis sold for many years as Adrenalin was a purified extract of adrenal gland tissue and therefore must have contained not only EPI but a mixture of all three catecholamines of the body, EPI, NE, and dopamine (DA). NE would have been the main contaminant. DA was first synthesized in 1910 but was not identified as a normal constituent of the adrenal gland until the early 1950s. DA is present in only quite small concentrations in the adrenal gland compared with concentrations of EPI and NE. Today Adrenalin contains chemically synthesized EPI.
The Nobel chemicals
Discoveries about NE, EPI, and DA have led to many Nobel Prizes over several decades. This section presents some of these discoveries, which affirm the continuing importance of catecholamine systems in science and medicine.
After release of NE from sympathetic nerves, NE undergoes inactivation mainly by a conservative recycling process, in which sympathetic nerves take up NE from the extracellular fluid (Uptake-1). Once back inside the nerve cells, most of the NE is translocated into storage vesicles. Julius Axelrod’s studies about the disposition of catecholamines introduced the idea that termination of the actions of some neurotransmitters depends on neuronal reuptake. Axelrod shared with U.S. von Euler the 1970 Nobel Prize for Physiology or Medicine. von Euler received the Nobel Prize for identifying NE as the neurotransmitter of the mammalian sympathetic nervous system.
Ahlquist’s 1948 suggestion that there were two types of adrenoceptors led to the development of novel drugs that block or stimulate those receptors. For the development of beta-adrenoceptor blockers, Sir James Black shared the Nobel Prize for Physiology of Medicine in 1988.
Discoveries related to mechanisms of cellular activation after adrenoceptor occupation have led to three other Nobel Prizes. For the discovery of G proteins, Alfred G. Gilman and Martin Rodbell shared the Nobel Prize for Physiology or Medicine in 1994. For the discovery of cAMP, the first identified intracellular messenger, E. W. Sutherland received the 1971 Nobel Prize. For the discovery of phosphorylation as a key step in the activation or inactivation of cellular processes, Edmond H. Fischer and Edwin G. Krebs shared the 1992 Nobel Prize.
Arvid Carlsson and Paul Greengard shared in 2000 the most recent Nobel Prize for Physiology or Medicine that came from catecholamine research. Both researchers focused on the “third catecholamine,” DA. Until about the 1950s, it had been assumed that DA does not have any specific function in the body beyond serving as a chemical intermediary in the production of EPI and NE. Carlsson discovered that in the brain DA acts as a neurotransmitter in its own right. DA plays a key role in regulation of movement. Loss of DA in the nigrostriatal system produces the movement disorder that defines Parkinson disease (PD), and replenishment of DA by administration of its precursor l-DOPA rapidly improves movement in patients with PD. Carlsson also demonstrated that effective drugs to treat schizophrenia work by blocking DA receptors in the brain. Greengard discovered that communication between nerve cells mediated by DA takes place by a relatively slow, diffuse process called slow synaptic transmission, which probably underlies phenomena such as mood and vigilance and also modulates fast synaptic transmission, as in speech, movement, and sensation.
Sources and significance of plasma catecholamines and their metabolites
Catecholamines are catechols, which are chemicals that have adjacent hydroxyl groups on a benzene ring. Catechol itself does not exist in the human body, but compounds that contain catechol as part of their molecular structure are called catechols.
Human plasma normally contains six catechols. Three are the catecholamines, NE, EPI, and DA. Another is l-DOPA. Two others are metabolites of the catecholamines. Dihydroxyphenylglycol (DHPG) is the main neuronal metabolite of NE, and dihydroxyphenylacetic acid (DOPAC) is the main neuronal metabolite of DA.
Plasma norepinephrine
NE became available commercially as a drug in 1908 and is still used clinically under the brand name Levophed, such as to treat hypotensive states. Methods to measure endogenous NE in plasma were introduced more recently, by colorimetric, fluorimetric, radioenzymatic, and finally liquid chromatographic-electrochemical detection (LCED) methods. The LCED approach introduced in the late 1970s and validated in the early 1980s [46] is most common today. In LCED, the analytes of interest are separated by high-pressure liquid chromatography and quantified by amperometric or coulometric detection upon exposure of the column effluent to an oxidizing potential. The cate-cholamines in plasma usually are purified partially by batch alumina extraction before injection of the alumina eluate into the LCED apparatus.
NE in the bloodstream emanates mainly from networks of sympathetic nerves that enmesh blood vessels—especially arterioles—throughout the body and pervade organs such as the heart and kidneys. The caliber of the arterioles determines total peripheral resistance to blood flow. The sympathetic innervation of the smooth muscle cells in arteriolar walls therefore represents a focal point in neural regulation of the circulation. In the heart, sympathetic nerves form lattice-like networks around myocardial cells.
Only a small proportion of NE released from sympathetic nerves enters the bloodstream unchanged (Table 1, Table 2; Fig. 1). The main route of inactivation is by reuptake into the nerve terminals by the Uptake-1 process mediated by the cell membrane NE transporter (NET). Under resting conditions most of NE produced in sympathetic nerves is metabolized before entry of the transmitter into the interstitial fluid or plasma.
Table 1.
Sources and significance of plasma catechols and metabolites
| Compound | Determinants | Significance |
|---|---|---|
| Norepinephrine | Sympathetic nerves | Sympathetic nerve traffic prognosis (e.g., heart failure) |
| Uptake-1 activity | NET function | |
| Dopamine-β-hydroxylase | DBH deficiency | |
| LAAAD | LAAAD deficiency | |
| BH4 | DHPR deficiency | |
| GTP cyclohydrolase-I deficiency | ||
| DHPG | VMAT/Vesic. Leakage | NE turnover |
| NE reuptake | NET function | |
| MAO-A | MAO-A deficiency | |
| Familial dysautonomia | ||
| Menkes disease | ||
| MHPG | DHPG | NE turnover |
| COMT activity | Altered COMT function | |
| MHPG-sulfate | MHPG | |
| SULT1A3 | ||
| MHPG-glucuronide | MHPG | Hepatic function |
| Glucuronidase | ||
| NMN | Adrenal medulla | Pheochromcytoma diagnosis |
| Sympathetic nerves | Uptake-2 activity | |
| COMT activity | Altered COMT function | |
| NMN-sulfate | NMN | |
| SULT1A3 | ||
| VMA | MHPG | Hepatic function |
| Alcohol dehydrogenase | ||
| Aldehyde dehydrogenase | ||
| Epinephrine | Adrenal medulla | Distress |
| Shock | ||
| Glucoprivation | ||
| Calorigenesis/obesity | ||
| Asphyxia | ||
| MN | Adrenal medulla | Pheochromocytoma diagnosis |
| 21-Hydroxylase deficiency severity | ||
| MN-sulfate | MN | |
| SULT1A3 | ||
| DA | Non-neuronal gut cells | Menkes disease diagnosis |
| Circulating l-DOPA | DBH deficiency diagnosis | |
| Sympathetic nerves | ||
| DA-sulfate | Mesenteric organs | ? Gastrointestinal TH activity |
| Diet | ||
| SULT1A3 | ||
| DOPAC | Sympathetic nerves | TH activity |
| MAO-A | MAO-A activity | |
| Extraneuronal MAO | Extraneuronal MAO activity | |
| Altered COMT function | ||
| HVA | COMT activity | Altered COMT function |
| DOPAC | ||
| 3-M-tyramine | COMT activity | Altered COMT function |
| 3-M-tyramine-sulfate | 3-M-tyramine | |
| SULT1A3 | ||
| DOPA | Sympathetic nerves | TH activity |
| Diet | LAAAD deficiency | |
| DBH deficiency | ||
| DHPR deficiency | ||
| GTP cyclohydrolase deficiency | ||
| Melanocytes | Tyrosinase activity | |
| Malignant melanoma | ||
| Extraneuronal TH | Neuroblastoma diagnosis | |
| Malignant pheo. diagnosis | ||
| 3-MT | COMT | Altered COMT function |
| DOPA | ||
| 3-MT-sulfate | 3-MT | |
| SULT1A3 |
Table 2.
Plasma levels, clearances, spillovers, and half-times of catechols and their metabolites
| Compound | Level (nmol/L) |
Clearance (L/min) |
Spillover (nmol/min) |
Half-time (min) |
References |
|---|---|---|---|---|---|
| Noradrenergic | |||||
| VMA | 30 | 0.6 | 21 | 32 | Märdh et al. ([95], 1983), Kopin [85] |
| MHPG-S | 23 | 0.3a | 7.4a | Eisenhofer et al. [19] | |
| MHPG | 20 | 1.3 | 25 | 28 | Märdh et al. (1983), Eisenhofer et al. [19] |
| DHPG-S | 6.6 | 0.3a | 1.7a | Eisenhofer et al. [19] | |
| NMN-S | 6.6 | 0.1a | 0.7a | >60 | Eisenhofer et al. [19] |
| DHPG | 4.7 | 2.4 | 12 | Eisenhofer et al. [29] | |
| Goldstein et al. [41] | |||||
| NE-S | 3.4 | 0.1a | 0.5a | Goldstein et al. [63] | |
| Norepinephrine | 1.0 | 1.7 | 2.6 | 2 | Kopin et al. [86], Goldstein et al. [41, 59], Eisenhofer et al. [19] |
| NMN | 0.3 | 1.4 | 0.4 | <4 | Eisenhofer et al. [23] |
| Adrenergic | |||||
| MN-S | 3.9 | 0.1a | 0.5a | >60 | Eisenhofer et al. [19] |
| Epinephrine-S | 0.5 | 0.2a | 0.1a | Goldstein et al. [63], Eisenhofer et al. [19] | |
| MN | 0.3 | 1.4 | 0.5 | <4 | Eisenhofer et al. [23] |
| Epinephrine | 0.2 | 2.3 | 0.5 | ~3 | Eisenhofer et al. [32] Goldstein et al. [60] |
| Dopaminergic | |||||
| HVA | 70 | 0.8 | 63 | 40 | Elchisak et al. [33], Kopin [85] |
| DA-S | 9.7 | 0.1a | 1.3 | 148 | Eisenhofer et al. [18], Goldstein et al. [63] |
| DOPAC | 8.9 | 0.5a | >4.1a | Eisenhofer et al. [29] | |
| 3-MTyramine-S | 2.8 | 0.12a | 0.3a | >60 | Eisenhofer et al. [18] |
| DA | 0.1 | 2.8b | 0.3 | ~2 | Goldstein et al. [63], Levinson et al. [93] |
| 3-MTyramine | 0.03 | 0.5 | 0.02 | Buu et al. [12], Eisenhofer et al. [18] | |
| DOPA | |||||
| 3-MTyrosine | 87 | 0.005 | 0.4 | Ishimitsu and Hirose [74], Armando et al. (1991), Eisenhofer et al. [25] |
|
| DOPA | 8.9 | 0.9 | 7.4 | Wolfovitz et al. [130], Armando et al. (1991), Goldstein et al. [41] |
|
| 3-MTyrosine-S | 2.8 | 0.1 | 0.3 | >60 | Eisenhofer et al. [18] |
| DOPA-S | 2.1 | Goldstein et al. [63] |
Calculation based on renal removal or urinary excretion and therefore may underestimate total body clearance and spillover
Calculation based on one-half the published clearance from antecubital venous plasma
Fig. 1.
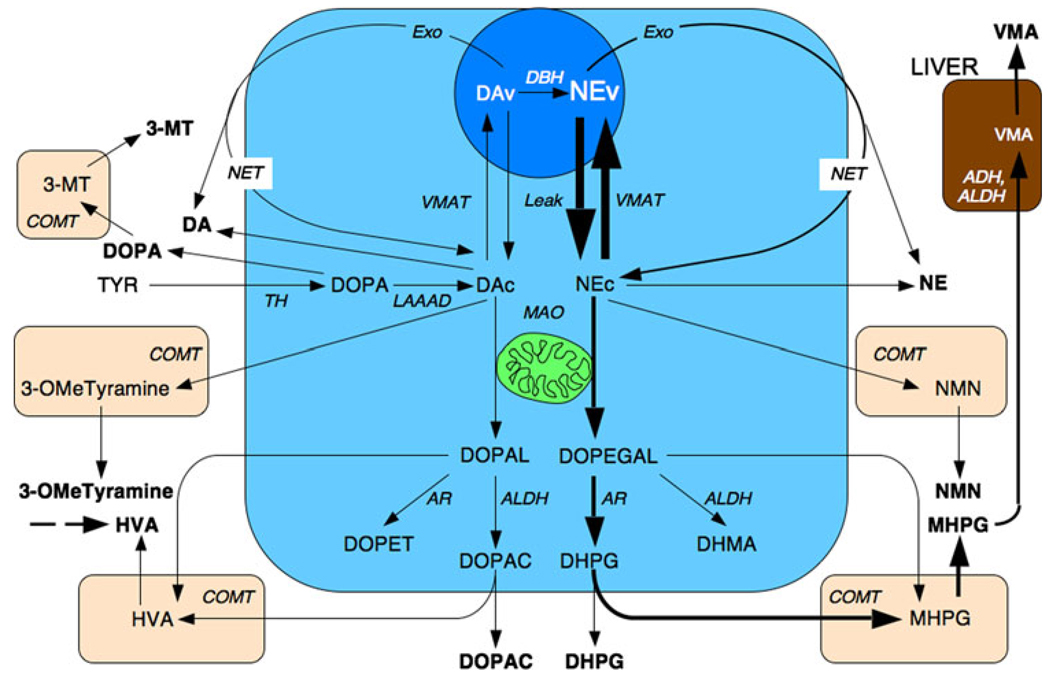
Diagram of steps in catecholamine biosynthesis, release, cellular uptake, and metabolism. ADH alcohol dehydrogenase, ALDH aldehyde dehydrogenase, AR aldose/aldehyde reductase, COMT cate-chol-O-methyltransferase, DA dopamine, DBH dopamine-β-hydroxy-lase, DHMA dihydroxymandelic acid, DHPG dihydroxyphenylglycol, DOPA dihydroxyphenylalanine, DOPAC dihydroxyphenylacetic acid, DOPEGAL dihydroxyphenylglycolaldehdye, Exo exocytosis, HVA homovanillic acid, LAAAD l-aromatic-amino-acid decarboxylase, MAO monoamine oxidase, MHPG methoxyhydroxyphenylglycol, 3-MT 3-methoxytyrosine, 3-OMdTyramine 3-methoxytyramine, NEc cytoplasmic norepinephrine, NEv vesicular norepinephrine, NET cell membrane norepinephrine transporter, NMN normetanephrine, TH tyrosine hydroxylase, TYR tyrosine, VMA vanillylmandelic acid, VMAT vesicular monoamine transporter
Since plasma NE is derived from sympathetic nerves, plasma NE levels have been used widely to indicate sympathetic nervous system activity. The relationship between plasma NE levels and sympathetic nerve traffic is complex. This complexity does not invalidate plasma NE levels in diagnosis or assessment of drug effects, but it does mean that one must interpret plasma NE levels with care, keeping in mind the purpose of the test, characteristics of the patient, possible interacting effects of medications, and conditions at the time of sampling.
The plasma NE concentration depends on both the rate of release of NE into the plasma and the rate of removal from the plasma. Thus, a high plasma NE level does not necessarily indicate a high rate of sympathetic nerve traffic. Decreased removal of NE from the plasma via the NET can also increase plasma NE levels without a change in the rate of sympathetic nerve traffic. This is relevant to clinical laboratory evaluation of chronic autonomic failure, in which an orthostatic fall in plasma NE clearance can produce false-negative neurochemical results [97].
For blood sampling from humans, most researchers use the antecubital vein. Since sympathetic nervous activity in the forearm and hand arm influences levels of NE in antecubital venous plasma, arm venous levels may not accurately reflect changes in sympathetic nervous activity elsewhere. In particular, mesenteric organs release NE into portal venous blood that is delivered to the liver, where NE is metabolized efficiently. Therefore, NE in systemic plasma does not reflect splanchnic sympathetic outflows.
Any of several endogenous biochemicals have the potential to modulate release of NE from the nerve terminals. These include NE itself by activating alpha-2 adrenoceptors.
In some pathological states and in response to a variety of sympathomimetic amines NE may be released from sympathetic nerve terminals by a non-exocytotic mechanism. Cardiac ischemic anoxia exemplifies such a pathologic state [87].
In virtually all organs some of released NE enters the venous drainage. The rate of entry of NE into the arterial plasma (“total body spillover”) can be measured using a tracer kinetic method, based on dilution of infused 3H–NE by endogenous NE [34]. By applying the tracer dilution principle one can also calculate NE spillover in particular organs such as the heart, kidneys, mesenteric organs, forearm, and brain [35]. The measurement of regional NE spillover has an important limitation. Without other neurochemical information one cannot distinguish NE release from neuronal reuptake as determinants of NE spillover, in the whole body or in specific organs. A modification based on dilution not only of 3H–NE but also of 3H-normetanephrine (NMN) by the corresponding endogenous compounds enables such a distinction [86]. In the kidneys, NE release into interstitial fluid averages 3 times NE spillover, in skeletal muscle 12 times NE spillover, and in the heart more than 20 times NE spillover, due to efficient local neuronal reuptake of NE from the interstitial fluid.
Release of 3H–NE from sympathetic nerves after neuronal uptake would complicate the tracer kinetic approach. Vesicular sequestration of cytosolic 3H–NE is very efficient, however, so that release of 3H–NE back into the bloodstream is negligible [21].
Many studies have noted that both plasma NE concentrations and directly recorded skeletal muscle sympathetic activity increase with subject age [110]. The increased plasma NE concentrations appear to reflect both increased spillover and decreased clearance [36].
Plasma dihydroxyphenylglycol
Dihydroxyphenylglycol is formed from NE in the sympathoneural cytosol by sequential deamination of NE to form dihydroxyphenylglycolaldehyde (DOPEGAL) and reduction of the aldehyde by aldehyde reductase/aldose reductase to form the glycol DHPG. DHPG diffuses rapidly across cell membranes into the extracellular fluid and from there into the bloodstream and into extraneuronal cells, where it is metabolized by COMT to form methoxyhydroxyphenylglycol (MHPG).
Cytosolic NE has two sources. Most comes from continuous vesicular leakage; a small, variable amount comes from uptake of NE from the extracellular fluid. Plasma DHPG has in essence the same sources [43]. Since vesicular leakage and axoplasmic deamination of NE are the main determinants of NE turnover, plasma DHPG offers a biochemical index of NE turnover, a parameter distinct from NE release.
Combined measurements of plasma NE and DHPG levels provide additional information about sympathetic nervous function that levels of neither compound alone provide. When sympathetically-mediated exocytosis increases, plasma levels of both NE and DHPG increase, the former because a small proportion of released NE spills over into the bloodstream and the latter because a portion of the released NE is taken up into the nerve terminals and deaminated.
Increases in plasma NE levels from diminished reuptake of NE are not attended by increases in plasma DHPG levels, and the ratio of NE:DHPG increases in this setting. Conversely, an elevated plasma NE:DHPG ratio can help identify dysautonomia from NET hypofunction [112]; however, there are several other potential determinants of an elevated NE:DHPG ratio in a given patient, including inhibition of MAO or of aldehyde/aldose reductase. Sustained sympathetic activation, by increasing the size of the readily releasable pool of vesicles, would also be expected to increase the NE:DHPG ratio [94].
Measurements of 3H-labeled and endogenous NE and DHPG enable estimation of rates of vesicular leakage, intraneuronal deamination of NE, and the proportion of released NE that undergoes reuptake into the nerve terminals. These estimates indicate a tremendously high exchange rate of amines between the axoplasm and the vesicles [25], turnover of NE as a result of intraneuronal deamination mainly after leakage from vesicles into the axoplasm, and reuptake of endogenously released NE that varies from organ to organ and is especially prominent in the heart [22].
Plasma normetanephrine
Catechol-O-methyltransferase (COMT) catalyzes the O-methylation of the 3-hydroxyl group of catechols. The O-methylated derivative of l-DOPA is 3-methoxytyrosine (3-MT), of DA 3-methoxytyramine, of NE normetanephrine, and of EPI metanephrine (MN). The term “metanephrines” is used to refer to the latter two compounds.
In most cells the O-methylated compounds that contain amine groups undergo further metabolic breakdown by MAO. Deamination of 3-methoxytyramine yields homovanillic acid (HVA) and of NMN and MN yields MHPG. In cells such as in the gut that express monoamine-preferring phenolsulfotransferase (SULT1A3) activity, the non-acidic metabolites, methoxytyramine, NMN, MN, and MHPG, undergo extensive sulfate-conjugation. Catecholamine metabolites can also be excreted as glucuronides in the bile or urine.
High levels of COMT are found in the liver, kidneys and other extraneuronal cells as well as in adrenomedullary chromaffin cells. Formation of NMN in the body therefore occurs from extraneuronal uptake and metabolism of NE released from sympathetic terminals and from O-methylation within the adrenal gland [30]. Because of the importance of reuptake and intraneuronal deamination of endogenously released NE, plasma NMN levels are lower than those of DHPG, despite similar clearances of the compounds from the plasma. The rate of extra-adrenal production of NMN provides a unique marker of extra-neuronal metabolism of NE.
The common painkiller acetaminophen (Tylenol) interferes with the liquid chromatographic-electrochemical assay for plasma NMN. Patients undergoing blood sampling for assays of plasma levels of MNs should not take any medications containing acetaminophen for at least 3 days before the test.
Plasma methoxyhydroxyphenylglycol
Methoxyhydroxyphenylglycol in human plasma is derived from multiple sources, including (a) deamination of NMN after its cellular uptake; (b) deamination of NMN after cellular uptake and intracellular O-methylation of NE; (c) O-methylation of DHPG after its uptake from the circulation; and (d) O-methylation of DHPG after its uptake from the interstitial fluid but before its entry into the circulation. Of these sources the most prominent is the last [26].
The metabolic fate of circulating MHPG is also complex and includes sulfation, glucuronidation, urinary excretion, and especially conversion by alcohol dehydrogenase and then aldehyde dehydrogenase (ALDH) to VMA in the liver [8]. Although earlier work suggested that plasma MHPG or MHPG-sulfate might reflect release of NE in the brain, plasma levels of these metabolites are derived mainly from NE turnover in the periphery.
Plasma vanillylmandelic acid
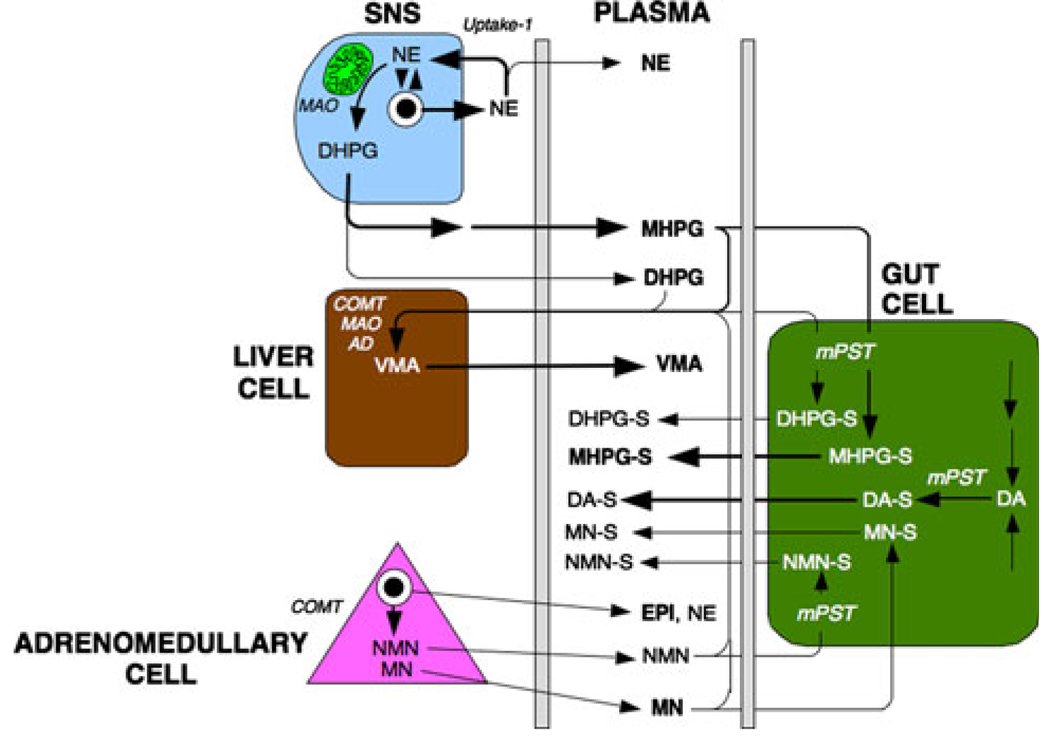
MHPG is converted to vanillylmandelic acid (VMA) by oxidation of the glycol catalyzed via alcohol dehydrogenase and oxidation of the resulting aldehyde via ALDH. Most of VMA production in humans comes from conversion from MHPG in the liver (Fig. 2).
Fig. 2.
Diagram of sympathoneural, hepatic, adrenomedullary, and gut contributions to plasma levels of catecholamines and their metabolites. DA-S DA sulfate, DHPG-S DHPG sulfate, MHPG-S MHPG sulfate, MN-S MN sulfate, mPST monoamine-preferring phenolsulfotransferase, NMN-S NMN sulfate
Only small amounts of VMA are formed from O-methylation of dihydroxymandelic acid (DHMA), which is a minor metabolite of NE in humans. Circulating VMA and MHPG therefore are derived mainly from DHPG [29] and consequently from oxidative deamination of NE in sympathetic nerves by MAO. Accordingly, MAO plays a key role in the metabolic fate of NE (Fig. 1).
Plasma epinephrine
Since cells of the adrenal medulla secrete their contents directly into the bloodstream, plasma EPI levels generally reflect neural outflow to the adrenal medulla. Plasma EPI levels are very low in antecubital venous plasma of healthy volunteers at rest—as little as 30 pmol/L—lower than plasma levels of NE, which normally average about 1 nmol/ L. Whereas plasma levels of NE increase with advancing age, plasma EPI levels tend to decrease. Plasma EPI levels and urinary EPI excretion also tend to be lower in obese than in lean women and lower in women than in men [66, 69].
Plasma EPI concentrations increase markedly and to a greater extent than do NE concentrations in response to hypoglycemia, hemorrhagic hypotension, asphyxia, circulatory collapse, and distress, presumably reflecting relatively greater adrenomedullary hormonal than sympathetic noradrenergic system activation. Even mild, asymptomatic hypoglycemia elicits larger increases in EPI than NE levels, and in the relatively benign form of circulatory failure represented by fainting, plasma EPI concentrations increase with smaller increases in plasma NE concentrations [42]. Across a variety of stressors, EPI responses are more closely related to corticotropin responses than to NE responses, indicating a close association of hypothalamic– pituitary–adrenocortical (HPA) with adrenomedullary activation [58].
Tracer kinetic studies have demonstrated EPI spillover in the heart during severe exercise and in some patients with essential hypertension [3]. Although extra-adrenal EPI synthesis and phenylethanolamine-N-methyltransferase (PNMT) have been reported in the heart, it is likely that the EPI released in the heart is derived from uptake from the circulation.
Plasma metanephrine
Under resting conditions most of the metabolism of EPI takes place before the hormone enters the bloodstream (Fig. 2). Since adrenomedullary chromaffin cells possess COMT, MN constitutes a major metabolite of EPI before release of EPI into the extracellular fluid, whereas in sympathetic nerves, which contain MAO-A but not COMT, DHPG is the main metabolite of NE before release of NE into the extracellular fluid.
The fate of EPI that enters the bloodstream differs quantitatively from that of NE. EPI is a poorer substrate than is NE for Uptake-1 via the NET and a better substrate than is NE for extraneuronal uptake (Uptake-2). EPI is also a better substrate than NE for COMT. Because of these differences, more of circulating EPI than of circulating NE is metabolized by extraneuronal uptake and O-methylation.
Plasma MN levels are roughly the same as plasma NMN levels, even though plasma NE levels exceed EPI levels by about five- to tenfold. The relatively high MN concentration results from a much greater rate of production of EPI than of NE in adrenomedullary chromaffin cells, metabolism of adrenomedullary catecholamines by COMT, and a relatively high proportion of metabolism of circulating EPI by the same enzyme.
Plasma dopamine
Plasma DA concentrations are similar to those of EPI, but because of the much lower potency of DA than of EPI circulating DA does not act as a hormone. Stressors that elicit release of NE from sympathetic nerves produce much larger increases in plasma NE levels than in plasma DA levels.
The finding of surprisingly high plasma levels of DA in humans undergoing tyramine infusion as part of autonomic function testing led to the proposal that infused tyramine releases endogenous DA or is converted to DA after cellular uptake of tyramine [77]. It is now known that tyramine stored in aqueous solution at 4°C undergoes slow spontaneous auto-oxidation to form DA [71]. Tyramine testing of autonomic function therefore should be done using either freshly prepared infusate or solution that has been stored at −70°C or colder.
The results of a recent study support the view that in humans, free (unconjugated) DA in plasma is derived substantially from sympathetic noradrenergic nerves [49]. DA is estimated to constitute only a very small proportion (about 2–4%) of the catecholamine released by sympathetic stimulation. The vesicles undergoing exocytosis from sympathetic nerves are estimated to contain about 25–50 times as much NE as DA.
DA outside the brain can function as an autocrine–paracrine substance. This role is understood best in the case of the kidneys. Exogenously administered DA dilates renal blood vessels, increases glomerular filtration, and increases sodium excretion via specific receptors in the kidneys and also via inhibition of aldosterone secretion from the adrenal cortex. Proximal tubular cells express DA receptors and produce DA after uptake of l-DOPA from the circulation and decarboxylation catalyzed by l-aromatic-amino-acid decarboxylase (LA-AAD). In humans, virtually all of DA in urine comes from renal uptake and decarboxylation of l-DOPA [130]. DA produced in the adrenal cortex also appears to be derived from uptake and decarboxylation of circulating l-DOPA [11].
Plasma dihydroxyphenylacetic acid
Dihydroxyphenylacetic acid is the product of oxidation of the catecholaldehyde resulting from deamination of DA, dihydroxyphenylacetaldehyde (DOPAL). Whereas the aldehyde intermediate produced upon oxidative deamination of NE (DOPEGAL) undergoes metabolism mainly by aldehyde reductase/aldose reductase to form DHPG, DOPAL is metabolized mainly by ALDH to form DOPAC. This difference helps explain why the main end-product of DA metabolism is HVA, whereas MHPG and VMA formed in the liver from MHPG are the main end-products of NE metabolism.
Plasma DOPAC levels average about 50 times those of DA, due to much slower clearance of DOPAC than of DA from the circulation and due to neuronal uptake of DA. At least some of plasma DOPAC is derived from metabolism of DA in the cytoplasm of sympathetic nerves. Thus, VMAT blockade by reserpine increases plasma DOPAC levels. Blockade of tyrosine hydroxylase (TH) by α-methyl-para-tyrosine treatment decreases plasma DOPAC levels, and patients with pure autonomic failure (PAF) associated with diffuse loss of sympathetic nerves have low plasma DOPAC levels [61]. Immobilization in rats rapidly increases plasma DOPAC levels [89], and blockade of catecholamine biosynthesis by α-methyl-para-tyrosine prevents stress-induced increases in plasma DOPAC [88].
Plasma DOPAC is also formed from metabolism of DA in non-neuronal cells of the gastrointestinal tract. Meal ingestion increases plasma DOPAC levels [63], although the determinants of the dietary influences remain unknown.
Plasma dopamine sulfate
With the exceptions of acidic end-products of catecholamine metabolism (HVA and VMA), catecholamines and their metabolites are susceptible to sulfate conjugation by a specific sulfotransferase isoenzyme (monoamine-preferring phenolsulfotransferase, mPST, SULT1A3). In humans, a single amino acid substitution confers the enzyme with particularly high affinity for DA and the O-methylated metabolites of catecholamines, including NMN, MN, and methoxytyramine. SULT1A3 is found in high concentrations in mesenteric organs, which are the main source of the sulfate-conjugated metabolites [20].
In humans at least 95% of DA in plasma circulates in sulfoconjugated form. Plasma DA sulfate results importantly from ordinary dietary constituents. In fasting normal volunteers, ingestion of a standard meal increases plasma DA sulfate levels more than 50-fold, with proportionately smaller increases in plasma levels of DA [20, 63].
Most organs produce little DA sulfate as judged from increments in plasma levels of the compound between the arterial inflow and venous outflow. In the body as a whole DA sulfate production appears to come mainly from conjugation of DA in mesenteric organs [20].
DA infusion into patients with deficiency of LAAAD markedly increases plasma DA sulfate levels [63]. Therefore, plasma DA sulfate derives at least partly from circulating DA; however, at least 90% of the sulfoconjugation of DA normally takes place before the DA enters the bloodstream, with little of plasma DA sulfate forming from circulating DA.
Plasma DA sulfate is not derived to any important extent from DA in sympathetic nerves. Thus, patients with PAF or multiple system atrophy (MSA) have normal plasma levels of DA sulfate [131], and DA sulfate levels respond relatively little to acute exposure to various stressors such as exercise.
In summary, meal ingestion markedly increases plasma DA sulfate levels. This could result from actual ingestion of l-DOPA, DA, or DA sulfate, from conversion of ingested tyramine to DA, from actions of tyrosinase to generate l-DOPA in the gastrointestinal lumen, or from increased release and metabolism of endogenous DA in gastrointestinal lining cells. Tyrosine generated from breakdown of dietary protein can enter sympathetic nerves or other cells containing TH, resulting in production of l-DOPA outside the gastrointestinal tract. Some of this l-DOPA enters the bloodstream, and uptake and decarboxylation of circulating l-DOPA provides a means to generate DA sulfate continuously from endogenous DA. Since DA sulfate derives to a relatively small extent from circulating DA, in fasting subjects the rate of entry of DA sulfate into plasma might reflect DA production and turnover in mesenteric organs.
Plasma homovanillic acid
Plasma HVA levels are derived mainly from O-methylation of DOPAC in non-neuronal cells. This explains why COMT inhibition increases plasma DOPAC levels as HVA levels fall. The liver and kidneys possess high levels of COMT activity; however, in humans, a substantial proportion of HVA production takes place in mesenteric organs, from metabolism of DA formed locally from the actions of TH and LAAAD on tyrosine and from uptake and decarboxylation of circulating DOPA [18].
Plasma DOPA
l-DOPA is the precursor of the catecholamines and the immediate product of the rate-limiting step in catecholamine biosynthesis, conversion of tyrosine to l-DOPA by TH. l-DOPA therefore occupies a pivotal position in the function of effector systems that use catecholamines.
In humans plasma levels of l-DOPA exceed those of NE by about tenfold, due to much more rapid clearance of NE than of l-DOPA from the plasma. It was thought that all the l-DOPA synthesized in sympathetic nerve endings was converted rapidly to DA. Release of l-DOPA from sympathetic nerve endings into the bloodstream would not be expected; however, in humans there virtually always are increments of plasma l-DOPA levels between the arterial inflow and venous outflow in the limbs, heart, head, leg, adrenal gland, and gut [41, 64].
Patients with sympathectomized limbs have no or reduced regional arteriovenous increments in l-DOPA levels [64]. Patients who have diseases associated with loss of sympathetic terminals in the heart have an analogous absence of the increment in plasma l-DOPA levels between the arterial inflow and coronary sinus outflow [55]. In laboratory animals chemical destruction of sympathetic nerve terminals eliminates regional arteriovenous increments in plasma l-DOPA levels in the hind limb, gut, and kidneys. These findings indicate a sympathoneural contribution to plasma l-DOPA levels.
Acute changes in arterial plasma l-DOPA levels probably reflect altered catecholamine biosynthesis in sympathetic nerves. Thus, in rats, immobilization increases l-DOPA levels in arterial plasma within a few minutes, and blockade of catecholamine biosynthesis or of sympathetic nerve traffic prevents these increases [88]. Nevertheless, in rats, chemical sympathectomy does not completely eliminate arterial plasma l-DOPA, and in dogs, chemical sympathectomy does not reduce arterial plasma l-DOPA levels at all. In humans, PAF is associated with decreased—but by no means absent—plasma l-DOPA levels [61]. These findings suggest important additional non-neuronal sources of l-DOPA in arterial plasma. The source of this residual l-DOPA is unknown. In normal volunteers, meal ingestion increases plasma l-DOPA levels [63].
In order to maintain NE stores, the rate of synthesis of NE must balance the rate of turnover. This explains why the regional rate of entry of l-DOPA into the circulation correlates better with regional spillover of DHPG, an index of NE turnover, than with indices of NE release [29].
Model of a sympathetic noradrenergic nerve
From a model of a sympathetic noradrenergic neuron (Fig. 1) one can appreciate how particular aspects of sympathetic nervous function affect levels of catechol-amines and their metabolites.
NE synthesis
NE synthesis begins with hydroxylation of tyrosine by TH to form the catechol amino acid l-DOPA in the neuronal cytoplasm. The reaction requires oxygen, iron, and tetra-hydrobiopterin co-factor.
l-DOPA undergoes enzymatic decarboxylation catalyzed by LAAAD to form DA. LAAAD requires pyridoxal phosphate (vitamin B-6) as a co-factor. One can monitor the reaction by the formation of carbon dioxide and auto-oxidation of DA to chromes that render the solution tannish in color and black pigmented precipitates.
DA conversion to NE is catalyzed by dopamine-beta-hydroxylase (DBH). DBH is a copper enzyme, and as discussed below diseases associated with abnormal copper transport can be detected by neurochemical indices of decreased DBH activity. Since DBH is localized to the storage vesicles, NE synthesis also requires the vesicular monoamine transporters (VMAT-1 and VMAT-2 are expressed in sympathetic neurons [17]) and a proton pump that drives down intravesicular pH. DBH activity also depends on availability of ascorbic acid and oxygen.
Cells and neurons that express PNMT can convert NE to EPI. The reaction requires S-adenosyl methionine (SAMe) as the methyl donor. Importantly, cortisol is trophic for PNMT, so that disorders or drug treatments associated with decreased activity of the HPA axis involve decreased adrenomedullary EPI synthesis and turnover.
NE release and reuptake
Only a small percentage of stored NE is released during sympathetic stimulation. Of the released NE, most is taken back up into the neuronal cytoplasm via the Uptake-1 process mediated by the cell membrane NET. Because of the high efficiency of the NET, only a small proportion of released NE makes its way unchanged to the circulation.
Fate of cytoplasmic NE
Norepinephrine in vesicular stores leaks passively into the cytoplasm but under normal conditions is recycled efficiently by the VMAT. A small proportion of the NE in the cytoplasm undergoes enzymatic oxidative deamination catalyzed by monoamine oxidase (MAO), localized to the outer mitochondrial membrane, to form the catecholaldehyde, DOPEGAL. This extremely reactive compound undergoes conversion by aldose/aldehyde reductase (AR) to form DHPG, the main neuronal metabolite of NE.
Because of TH and LAAAD in the neuronal cytoplasm, DA is synthesized continuously in sympathetic nerves. Some of the cytoplasmic DA is also deaminated by MAO to form the catecholaldehyde, DOPAL. DOPAL is converted by ALDH to DOPAC. It is generally accepted that whereas DOPEGAL is metabolized mainly by AR to the glycol DHPG, DOPAL is metabolized mainly by ALDH to the acid DOPAC. Plasma levels of the acid metabolite of DOPEGAL, DHMA, and the alcohol metabolite of DOPAL, dihydroxyphenylethanol (DOPET), are normally below the detection limits of available assays.
Under resting conditions, most of the loss of NE from innervated tissues (turnover) results from net leakage and oxidative deamination of NE rather than reuptake of released NE.
Extraneuronal uptake and metabolism
Norepinephrine in the extracellular fluid is subject to extraneuronal uptake mediated by a transporter different from the NET (Uptake-2). Non-neuronal cells and adrenomedullary chromaffin cells contain COMT, which converts NE to NMN by 3-methoxylation, using SAMe as the methyl donor. Thus, NMN is not a catechol. Analogously, EPI taken up into non-neuronal cells is subject to enzymatic O-methylation to form MN. Because adrenomedullary chromaffin cells express COMT, plasma MN is derived mainly from net leakage of EPI from vesicular stores and thereby provides a measure of EPI turnover [30].
Extraneuronal cells contain both MAO-A and MAO-B, whereas sympathetic nerves are thought to contain only MAO-A. In non-neuronal cells, NMN is deaminated and DHPG O-methylated to form MHPG, a major end product of NE metabolism. In the liver, MHPG is converted to VMA.
Especially in the gastrointestinal tract, DA is extensively sulfate-conjugated by monoamine-preferring sulfotransferase to form DA sulfate. About 99% of circulating DA is in the form of the sulfate conjugate. Catecholamines and their glycol metabolites also undergo enzymatic sulfoconjugation in the gut.
Most of DA and NE synthesis and metabolism in the body as a whole takes place not in the brain or in sympathetic nerves but in the gut [18, 19]. The functional significance of this high rate of synthesis and metabolism remain poorly understood. Because the venous drainage of the gut is directed to the liver via the portal vein, levels of catecholamines and other catechols in systemic plasma do not reflect the splanchnic contribution to overall catecholamine synthesis and metabolism in the body.
Drug effects on plasma catecholamines and their metabolites
In this section the model of the sympathetic noradrenergic neuron is used to predict neurochemical patterns associated with drugs.
Alpha-methyl-para-tyrosine
Alpha-methyl-para-tyrosine (metyrosine) competitively antagonizes TH. Plasma levels of DOPA, NE, DOPAC, and DHPG therefore decrease [64]. Alpha-methyl-para-tyrosine is used to decrease catecholamine biosynthesis in patients with pheochromocytoma prior to surgery [117]. By inhibiting catecholamine synthesis, the drug produces depressed mood and Parkinsonism as long-term side effects [38].
Carbidopa
Carbidopa, which is combined with levodopa in Sinemet, inhibits decarboxylation of levodopa to DA outside the brain. Plasma DOPA levels therefore increase [128]. Although carbidopa effectively inhibits LAAAD, the attained plasma l-DOPA concentration is so high (about 10,000 nmol/L) that plasma DOPAC levels typically increase by more than 20-fold (from about 7 to about 180 nmol/L), implying that patients taking Sinemet actually have substantially increased production and metabolism of DA outside the brain.
Clonidine
Clonidine is an alpha-2 adrenoceptor agonist that acts in the central nervous system to decrease sympathetic nervous system outflows and in the periphery at presynaptic receptors to decrease NE release from sympathetic nerve terminals [1]. By both effects clonidine decreases plasma NE levels. In patients with pheochromocytoma plasma NE levels can be increased because of release of NE into the bloodstream independently of the sympathetic nervous system. In such patients failure of clonidine to reduce plasma NE constitutes a positive diagnostic test result [28, 67]. Conversely, the combination of a high plasma NE level and a large fall in blood pressure in response to clonidine may identify patients with “hypernoradrenergic hypertension” [59].
Yohimbine
Yohimbine exerts effects opposite to those of clonidine. Intravenous infusion of yohimbine increases sympathetic neural outflows and blocks alpha-2 adrenoceptors on sympathetic nerve terminals, thereby increasing plasma NE levels [68]. Yohimbine challenge testing can assess whether a patient with neurogenic orthostatic hypotension has releasable NE stores [107], which can be a target for treatment. Yohimbine challenge testing can also reveal excessive NE release in patients with anxiety or panic disorder.
Ganglion blockade
Ganglion blockers such as trimethaphan (TRI) and pentolinium (PEN) interfere with ganglionic transmission by blocking neuronal acetylcholine receptors (nAChRs). These drugs produce symptoms and signs of both parasympathetic and sympathetic failure, including dry mouth, constant pulse rate, decreased sweating, fixed pupils, and orthostatic hypotension. Plasma NE levels fall [80] to a greater extent than do plasma DHPG levels [10], consistent with ongoing NE turnover due to net leakage from vesicles into the neuronal cytoplasm. Plasma DOPA does not change, as expected due to unchanged NE synthesis [64].
Tyramine
Indirectly acting sympathomimetic amines such as dextroamphetamine and tyramine release NE from sympathetic nerve endings and increase plasma NE levels. These drugs are substrates for both the NET and VMAT. Probably by intravesicular alkalinization they enhance NE leakage from storage vesicles into the axoplasm. They also interfere with the efficiency of the NET, resulting in transport of the axoplasmic NE into the extracellular fluid. In humans, infusion of tyramine or dextroamphetamine therefore increases plasma NE levels [60, 109]. During tyramine infusion plasma DHPG levels increase more than do plasma NE levels [48], probably because of buildup of NE in the axoplasm.
Foodstuffs such as hard cheeses and red wines contain large amounts of tyramine. Normally dietary tyramine is metabolized in the gastrointestinal tract and liver before the amine can enter the systemic circulation. In patients taking an MAO inhibitor, tyramine is able to reach the sympathetic nerve terminals, and after neuronal and vesicular uptakes of tyramine paroxysmal hypertension can result from release of vesicular NE—a phenomenon termed the “cheese effect” [115]. Because of the susceptibility to severe hypertension due to the cheese effect MAO inhibitors have not had wide usage as antidepressants, despite their clinical efficacy.
Contamination of TYR infusates with DA can increase plasma DA levels artifactually [71, 78]. During infusion of relatively uncontaminated TYR, individual values for changes in plasma DA concentrations are positively correlated with those in NE [49], consistent with a neuronal source of plasma DA.
Isoproterenol
Infusion of the beta-adrenoceptor agonist isoproterenol (ISO) increases plasma NE levels [65, 124]. The increases probably reflect a combination of agonist occupation of beta-2 adrenoceptors on sympathetic nerves [13] and reflexive stimulation of sympathetic outflows as a response to systemic vasodilation. Patients with chronic autonomic failure associated with generalized sympathetic noradrenergic denervation have attenuated plasma NE responses to infused ISO [113].
Reserpine
Reserpine (RES) is a classical drug derived from the root of the Rauwolfia serpentina (Indian snakeroot) plant. A highly lipophilic drug, RES enters monoaminergic neurons and chromaffin cells and irreversibly blocks the type-1 and type-2 vesicular monoamine transporters (VMAT-1 and VMAT-2). RES administration rapidly increases net leakage of vesicular NE stores into the cytoplasm of sympathetic nerves and depletes NE stores [14]. Increased oxidative deamination of cytoplasmic NE catalyzed by MAO results in increased formation of DHPG, and plasma DHPG levels increase [26]. Subsequently, as vesicular NE stores become depleted, plasma DHPG decreases to low levels.
COMT inhibitors (entacapone)
Entacapone (ENT, brand name Comtan), an inhibitor of COMT, does not penetrate the blood–brain barrier. Since levodopa is a catechol, ENT treatment decreases plasma levels of 3-MT (3-methoxydopa) [7]. Combined treatment of PD with levodopa/carbidopa and ENT (brand name Stalevo) is thought to augment delivery of therapeutic levodopa to the brain. In humans, ENT does not increase peak plasma DOPA concentrations; however, the area under the curve for DOPA concentrations versus time is increased [84, 100].
ENT does not appreciably affect plasma levels of catecholamines [118], likely reflecting relatively minor effects of non-neuronal uptake and O-methylation on the fate of NE released from sympathetic nerves. On the other hand, ENT augments cardiac responses to infused EPI and ISO, and the drug combination can precipitate ventricular arrhythmias [73].
MAO inhibitors
Monoamine oxidase figures much more prominently in the metabolic fate of DA and NE than does COMT, because sympathetic nerves do not express COMT. Plasma levels of DOPAC, the main deaminated metabolite of DA, and of DHPG, the main deaminated metabolite of NE, exceed by far those of the corresponding O-methylated metabolites, 3-MT and NMN.
There are two isoforms of MAO, MAO-A and MAO-B. The genes encoding MAO-A and MAO-B are located close to each other on the X-chromosome. Sympathetic nerves express MAO-A, whereas most extraneuronal cells express both MAO-A and MAO-B. Clorgyline selectively inhibits MAO-A, and selegiline (Deprenyl, Eldepryl) and rasagiline selectively inhibit MAO-B. It is because of the MAO-B selectivity that selegiline and rasagiline are not associated with the “cheese effect.” Unexpectedly, selegiline treatment decreases plasma DHPG levels in humans [27], whereas it does not do so in rats [72].
NET inhibitors
Tricyclic anti-depressants, cocaine, and amphetamines inhibit the cell membrane NET. The effects of these agents on plasma levels of NE and its metabolites are complex, because these drugs all enter the brain and can affect sympathetic neuronal outflows. Desipramine, a classical tricyclic anti-depressant, inhibits skeletal muscle sympathetic nerve traffic and decreases NE spillover in the body as a whole, the forearm, and the kidneys [37]. In contrast, desipramine increases plasma EPI levels, indicating differential effects on sympathetic noradrenergic outflows adrenomedullary outflows [23]. NET inhibition also decreases plasma DHPG levels, an effect that has been attributed to decreased NE turnover [132]. Desipramine abolishes plasma DHPG responses and augments plasma NE responses to yohimbine [132].
In humans, intranasal cocaine increases blood pressure substantially. After taking into account baroreflexive sympathoinhibition, skeletal muscle sympathetic outflow is increased [79]. In cocaine addicts, i.v., cocaine increases plasma NE and EPI levels [116].
Methylphenidate increases plasma EPI but not NE levels [126]. Because of an association of plasma EPI responses with neuroimaging evidence of striatal DA release, it has been suggested that striatal DA affects adrenomedullary outflow. Amphetamine increases plasma NE levels [60].
Duloxetine, atomoxetine, and reboxetine are non-tricyclic antidepressants that block the NET. In humans, dulxetine increases plasma NE levels and reduces the plasma DHPG:NE ratio [125]. Reboxetine does not affect plasma NE levels during supine rest [96], possibly because of counter-balancing effects of reuptake inhibition and decreased sympathetic neuronal outflow [120]. Reboxetine decreases plasma DHPG levels [105]. Amitriptyline also tends to increase plasma NE and decrease plasma DHPG levels, consistent with some NET inhibition [105].
l-DOPS
l-threo-3,4-dihydroxyphenylserine (l-DOPS), a NE prodrug, is an investigational agent for neurogenic orthostatic hypotension.
After l-DOPS administration plasma NE levels increase [83] but by surprisingly little and insufficiently to increase of themselves to blood pressure [56]. In contrast with small increases in plasma NE levels there are robust increases in plasma DHPG and DHMA levels. The pressor response to l-DOPS therefore seems mainly to reflect actions on adrenoceptors within tissues by NE that has escaped extensive metabolic breakdown by MAO and COMT and has not yet reached the systemic circulation (Fig. 3).
Fig. 3.
Diagram of the fate of l-threo-dihydroxyphenylserine (l-DOPS). CAR carbidopa, ENT entacapone, NAAT neutral amino acid transporter, O-Me-DOPS O-methyl DOPS
Plasma catecholamines and their metabolites in disease states
This section applies the model of the sympathetic noradrenergic neuron (Fig. 1) to understand catecholamine neurochemical patterns associated with some disease states.
Disorders of catecholamine biosynthesis
Tyrosine hydroxylase deficiency
Considering that TH is the rate-limiting enzyme in catecholamine biosynthesis it is not surprising that TH deficiency is an extremely rare pediatric disease. One would predict low plasma DOPA levels in this disease, but this has not been reported.
Disorders of tetrahydrobiopterin synthesis or regeneration
Tetrahydrobiopterin (BH4) is a required co-factor for TH, phenylalanine hydroxylase, tryptophan hydroxylase, and nitric oxide synthase. Several distinct genetic disorders attenuate BH4 synthesis or regeneration because of deficiencies of GTP cyclohydrolase I (GTPCH 1), 6-pyruvoyl tetrahydropterin synthase, sepiapterin reductase, dihydropteridine reductase (DHPR), or pterin-4′-carbinolamine dehydratase.
Autosomal dominant mutation of the gene encoding GTPCH 1, the rate-limiting enzyme in biosynthesis of BH4, produces DOPA-responsive dystonia (also called hereditary progressive dystonia with marked diurnal fluctuation and Segawa disease). Autosomal recessive GTPCH 1 deficiency with complete loss of enzyme activity produces severe, progressive neurodegeneration.
In DHPR deficiency, failure to regenerate BH4, which is absolutely required for tyrosine hydroxylation, results in low plasma DOPAC levels [47]. In contrast, in DBH deficiency, failure to convert DA to NE leads to high plasma DOPAC levels and low DHPG levels [5].
l-Aromatic-amino-acid decarboxylase deficiency
Patients with LAAAD deficiency have high DOPA levels, whereas levels of DOPAC, DHPG, and DA sulfate are low, consistent with decreased conversion of DOPA to DA [119].
Dopamine-beta-hydroxylase deficiency
Dopamine-beta-hydroxylase deficiency produces a distinctive biochemical pattern including elevated plasma l-DOPA levels and low or absent levels of NE [122] and the NE metabolite DHPG [5]. The buildup of plasma l-DOPA probably results from compensatorily increased tyrosine hydroxylation in sympathetic nerves. A high ratio of plasma l-DOPA:DHPG occurs also in Menkes disease and familial dysautonomia (FD), but the severity of decrease in plasma DHPG is worse in DBH deficiency.
In patients with DBH deficiency, treatment with the NE pro-drug l-threo-dihydroxyphenylserine (l-DOPS) is remarkably effective in ameliorating orthostatic hypotension. The extent of increase in blood pressure is correlated with the extent of increase in plasma NE [6].
Menkes disease
Menkes disease is an X-linked recessive neurodevelopmental disorder resulting from mutation in a coppertransporting ATPase gene. Neonatal diagnosis is crucial for instituting treatment early enough to improve outcome. Since DBH requires copper as a co-factor, Menkes disease can be detected by relatively high concentrations of DA and its metabolites relative to those of NE and its metabolites. All patients with Menkes disease have high plasma DOPAC:DHPG and high DA:NE ratios [81]. Plasma DA:NE and DOPAC:DHPG ratios are remarkably sensitive and specific for diagnosing Menkes disease in at-risk newborns, introducing the possibility of newborn screening for this otherwise lethal disease [82].
Familial dysautonomia
Familial dysautonomia, also known as Riley–Day syndrome and hereditary sensory and autonomic neuropathy type III, is a rare inherited disorder transmitted as an autosomal recessive trait in individuals of Ashkenazic extraction. FD features extensive sensory and autonomic dysfunction. The etiologic basis is mutation of the gene, IKBKAP. The most common mutation causes a splicing alteration that leads to tissue-specific decreased expression of IkappaB kinase-associated protein—especially in neuronal tissue [114]. Several target genes are down-regulated, and many of the down-regulated genes participate in cell migration and survival. This may explain neuropathological findings such as diminished numbers of small nerve fibers and neuronal loss in dorsal root ganglia [104]. A decrease in sympathetic neuronal innervation in FD seems to be pervasive, involving the skin [70], renal blood vessels [103], and heart [45].
FD patients have deficient orthostatic increments in plasma NE levels [133] and low plasma ratios of DOPA:DHPG [4]. The elevated DOPA:DHPG ratios probably reflect compensatorily increased NE production in a decreased complement of sympathetic nerves, in line with the post-mortem histopathologic finding of increased TH immunoreactivity in sympathetic ganglionic neurons [102].
In a long-term follow-up study there were no changes in plasma levels of individual catechols in FD; however, there were further increases in DOPA:DHPG ratios [50]. In FD, plasma catechol profiles seem sufficiently stable at least over a decade to be used as a biomarker of disease involvement. An increasing DOPA:DHPG ratio suggests slight but consistent further loss of noradrenergic terminals.
Disorders of catecholamine metabolism
Monoamine oxidase deficiency
The genes encoding the two subtypes of MAO exist very close to each other on the X-chromosome. Deficiency of MAO-A manifests clinically and neurochemically entirely differently from that of MAO-B. Whereas MAO-B deficiency produces few if any neurobehavioral consequences, MAO-A deficiency produces an inherited tendency to violent anti-social behavior. Patients with MAO-A deficiency have very low plasma DOPAC levels [91], whereas patients with MAO-B deficiency have normal plasma DOPAC levels, consistent with the intraneuronal site of MAO-A.
Neoplastic disorders of catecholaminergic cells
Pheochromocytoma
Patients with symptoms or signs from pheochromocytoma virtually always have high plasma NMN or MN levels, reflecting metabolism of NE or EPI in the tumor before release of the catecholamines into the circulation. Plasma levels of MNs (NMN and MN) constitute the most sensitive blood test to detect pheochromocytoma devised so far [90]. The sensitivity exceeds that of plasma NE and EPI levels, because catecholamines produced in the tumor undergo metabolism continuously by COMT.
Most pheochromocytomas secrete predominantly NE, many produce both NE and EPI, and more rarely others secrete predominantly EPI. The differences in catecholamine secretion reflect differences in expression of catecholamine biosynthetic enzymes and can explain differences in presenting symptoms. Paroxysmal hypertension and symptoms such as palpitations, anxiety, dyspnea and hyperglycemia are more common in patients with pheochromocytomas producing EPI than producing NE. Pheochromocytomas in patients with multiple endocrine neoplasia, Type 2 (MEN 2) can produce EPI and have an adrenergic phenotype, while those from patients with von Hippel-Lindau (VHL) disease have a noradrenergic phenotype [31]. Thus, differences in biochemical and clinical presentation of pheochromocytoma can reflect the different underlying mutations.
Patients with malignant pheochromocytoma also have elevated plasma l-DOPA levels [62]. Malignant pheochromocytoma cells appear to be so undifferentiated that although they can hydroxylate tyrosine to form l-DOPA they often do not decarboxylate l-DOPA efficiently to form DA or hydroxylate DA to form NE.
Neuroblastoma
Neuroblastoma constitutes one of the most common solid cancers of children. As the name of the tumor suggests, neuroblastoma cells derive from the neural crest in embryological development, and they contain TH. Patients harboring a neuroblastoma have high—sometimes spectacularly high—plasma l-DOPA levels [62]. Cultured human neuroblastoma cell lines contain little if any catecholamine.
Melanoma
High plasma l-DOPA levels occur in malignant melanoma [39, 92]. The tumor cells do not contain TH but do contain high levels of tyrosinase, and l-DOPA is produced in phase I melanogenesis, either from direct oxidation of tyrosine or from dopaquinone.
Adrenocortical-adrenomedullary disorders
Addison’s disease
Addison’s disease is usually due to an autoimmune adrenalitis of the adrenal cortex. The disease involves impaired adrenal medullary secretion of EPI. The medulla is intact, but plasma levels of EPI are decreased [9]. This occurs despite glucocorticoid replacement, indicating that the normal high intra-adrenal steroid levels are required for adequate production of catecholamines in the human adrenal medulla. EPI secretion is also impaired in secondary adrenocortical insufficiency in children with hypocorticotropic hypopituitarism, further supporting the importance of a local source of steroids for adrenal medullary release of catecholamines.
21-Hydroxylase deficiency
Patients with severe 21-hydroxylase deficiency have markedly decreased plasma concentrations of EPI associated with incomplete formation of the adrenal medulla [98]. These patients have low plasma concentrations of MN, consistent with decreased adrenal medullary stores of EPI.
Chronic autonomic failure syndromes
Primary chronic autonomic failure syndromes have been classified into PAF, MSA, and PD with autonomic failure. The three conditions differ clearly in patterns of plasma catechols and MNs, which generally fit with the concept of generalized loss of sympathoadrenomedullary cells in PAF, intact sympathoadrenomedullary cells in MSA, and intact adrenomedullary cells but organ-selective sympathetic denervation, especially in the heart, in PD. Because of severe baroreflex-sympathoneural failure that is characteristic of chronic autonomic failure syndromes, they all involve subnormal orthostatic increments in plasma NE levels [134].
Pure autonomic failure
Pure autonomic failure is associated with generalized noradrenergic denervation. Patients with PAF therefore have low NE, DHPG, and NMN levels [61]. Based on low EPI and MN levels, PAF also entails decreased adrenomedullary turnover and release of EPI.
Multiple system atrophy
Patients with MSA generally have plasma normal levels of catecholamines and their metabolites, consistent with intact sympathetic innervation [61].
Parkinson disease with orthostatic hypotension (PD + OH)
About 40% of patients with PD have orthostatic hypotension (OH). Patients with PD + OH have low cardiac NE spillover [55], low NMN levels, and low DHPG for given NE levels but have normal mean NE, EPI, and MN levels [57]. PD + OH patients have lower plasma NE levels than do PD patients without OH [111]. Microdialysate DHPG concentrations are similarly low in PD + OH and PAF, and the two groups also have similarly small plasma DHPG responses to tyramine and NE responses to yohimbine and virtually absent NE responses to ISO [113]. Taken together, the results support the concept of not only cardiac but also extracardiac noradrenergic denervation in PD + OH.
After uptake into cells, l-DOPA can be metabolized by LAAAD or COMT. LAAAD converts l-DOPA to DA, and COMT converts l-DOPA to 3-MT. Both enzymes figure prominently in the clinical use of l-DOPA to treat PD. Not only do they metabolize circulating l-DOPA but they also are thought to constitute an enzymatic blood–brain barrier. The catechol hydrazide drugs, carbidopa and benserazide, inhibit LAAAD outside the brain. They are used in combination with l-DOPA to increase delivery of l-DOPA to the brain while decreasing nausea from occupation of DA receptors in the area postrema, a circumventricular posterior brainstem region that has an imperfect blood–brain barrier (This is why a brand name for a levodopa/carbidopa combination is Sinemet, from the Latin words for “without vomiting”). COMT inhibitors (e.g., tolcapone, ENT) increase the smoothness and duration of l-DOPA effects without increasing plasma l-DOPA levels.
Autoimmune autonomic ganglionopathy
Autoimmune autonomic ganglionopathy (AAG) is a rare disease that manifests as pandysautonomia. AAG is thought to result from decreased ganglionic neurotransmission due to circulating antibodies to the neuronal nicotinic receptor [51, 123]. Preliminary data suggest that AAG differs from PAF in terms of clinical laboratory findings indicating post-ganglionic noradrenergic denervation. Both disorders feature low plasma levels of catecholamines during supine rest, but plasma levels of other endogenous catechols, DOPA, DOPAC, and DHPG, seem to be lower in PAF than in AAG, probably reflecting more severely decreased NE synthesis and turnover in PAF due to diffuse sympathetic noradrenergic denervation [54].
Functional dysautonomias
Postural tachycardia syndrome
Postural tachycardia syndrome (POTS) is characterized by orthostatic tachycardia accompanied by multi-system complaints including chronic fatigue, exercise intolerance, and pain mainly in Caucasian pre-menopausal women. The disorder seems increasingly common [106]. Consistent with recruitment of sympathetic outflows during orthostasis, POTS patients have high plasma NE levels when upright [40].
POTS also entails neurocirculatory abnormalities during supine rest. These include relatively fast mean heart rates and increased NE, DA, and EPI concentrations and increased cardiac NE spillover [40, 44, 52], indicating increased sympathetically mediated exocytosis and adrenomedullary hormonal system activation in POTS patients even while they are supine.
A sub-group of POTS patients are thought to have partial sympathetic denervation and compensatorily increased cardiac sympathetic outflow [76]. A family with inherited POTS was found to have a hypofunctional mutation of the gene encoding the cell membrane NET [112]. These findings have the common feature of decreased NET activity. Although acute administration of NET inhibitors produces hemodynamic and neurochemical alterations reminiscent of POTS [108], most POTS patients seem to have normal NET function [44].
Neurally mediated hypotension (reflex syncope)
Neurocardiogenic syncope (also called reflex syncope, vasovagal syncope, and fainting) is the most common cause of acute loss of consciousness in adults. Between episodes, plasma levels of catecholamines and their metabolites are normal. Patients with blood sampled sequentially before tilt-induced syncope have progressive, marked increases in plasma EPI levels before syncope [53]. Simultaneously obtained NE levels increase to a smaller extent than do EPI levels (“sympathoadrenal imbalance”), while forearm vascular resistance decreases. Directly recorded skeletal muscle sympathetic nerve traffic often decreases markedly prior to or at the time of syncope [16, 99, 127], also consistent with sympathoad-renal imbalance.
Congestive heart failure
Rates of synthesis, vesicular uptake, release, neuronal reuptake, and intraneuronal metabolism of NE have been assessed comprehensively in patients with congestive heart failure [24]. NE release and neuronal reuptake are both increased in the failing heart; however, the efficiency of NE reuptake is reduced, so that cardiac NE spillover is increased more than its estimated neuronal NE release. Cardiac NE stores are lower [14] and the rate of vesicular leakage is accordingly also lower in the failing than in the normal heart. Cardiac spillover of DOPA and NE turnover are increased. Thus, in congestive heart failure, increased neuronal release of NE and decreased efficiency of neuronal NE reuptake both contribute to increased delivery of NE to its receptors. Decreased vesicular leakage of NE secondary to decreased myocardial NE stores limits the increase in cardiac NE turnover. Decreased NE store size in the failing heart appears to result not from insufficient TH activity but from chronically increased NE turnover and reduced efficiency of NE reuptake and storage.
Among patients with congestive heart failure those with high plasma NE levels have a worse prognosis [15, 75, 121]. This may reflect a positive feedback loop involving greater recruitment of sympathetic noradrenergic outflows to maintain cardiac performance and deleterious long-term effects of cardiac sympathetic stimulation.
Stress cardiopathy
The term takotsubo cardiomyopathy refers to a relatively recently described form of acute, reversible cardiomyopathy, in which apical akinesia gives the heart the shape of a takotsubo, a Japanese fishing pot for trapping octopus. Takotsubo cardiomyopathy occurs mainly in elderly women soon after exposure to severe emotional distress [2]. Symptoms mimic acute myocardial infarction, but coronary angiography fails to demonstrate coronary occlusion. The condition can trigger sudden cardiac failure or death, yet in survivors cardiac function typically normalizes within a few weeks. Takotsubo cardiomyopathy features remarkably elevated plasma levels of NE, EPI, and DHPG [129], consistent with extreme sympathetic nervous and adrenomedullary hormonal activation that could produce catecholaminergic cardiotoxicity.
Surgical sympathectomies
Patients with surgical sympathectomies have low plasma levels of DA and NE [49], whereas EPI:NE ratios are increased (unpublished observations), suggesting decreased sympathetically mediated exocytosis and compensatory adrenomedullary activation.
Conclusions
Much of the history of scientific medicine over the past century can be written in terms of milestone discoveries based on catecholamine research. There are several reasons for this remarkable history. Catecholamines constitute the only neurochemical messengers where virtually all steps in an entire functional cycle are amenable to detailed scientific study, from central neural changes to nerve impulses to transmitter release to transmitter deactivation to receptor function to cellular activation to afferent information back to the central nervous system. Genes encoding catecholamine synthesizing and metabolizing enzymes, transporters, and receptors have been identified, enabling studies of pathogenetic mechanisms linking genotypic changes with specific neurochemical phenotypes. The adrenomedullary hormonal and sympathetic noradrenergic systems, two of the most powerful and rapidly-acting of the body’s “stress” systems, use the catecholamines EPI and NE as the main effector biochemicals, and NE and DA are classical central neurotransmitters that participate importantly in movement, attention, memory, mood, and responses to stressors. Catecholaminergic systems provide models for three ways by which the body regulates the internal environment, via neurotransmitters, hormones, and autocrine/paracrine factors. Finally, as highlighted in this review, measurement of plasma levels of l-DOPA, catecholamines, and catecholamine metabolites help elucidate effects and mechanisms of action of drugs and enhance diagnosis and understanding of pathophysiology of many clinical disorders.
References
- 1.Aggarwal A, Esler MD, Socratous F, Kaye DM. Evidence for functional presynaptic alpha-2 adrenoceptors and their down-regulation in human heart failure. J Am Coll Cardiol. 2001;37:1246–1251. doi: 10.1016/s0735-1097(01)01121-4. [DOI] [PubMed] [Google Scholar]
- 2.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med. 2006;68:8–16. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- 4.Axelrod FB, Goldstein DS, Holmes C, Berlin D, Kopin IJ. Pattern of plasma levels of catecholamines in familial dysautonomia. Clin Auton Res. 1996;6:205–209. doi: 10.1007/BF02291135. [DOI] [PubMed] [Google Scholar]
- 5.Biaggioni I, Goldstein DS, Atkinson T, Robertson D. Dopamine-beta-hydroxylase deficiency in humans. Neurology. 1990;40:370–373. doi: 10.1212/wnl.40.2.370. [DOI] [PubMed] [Google Scholar]
- 6.Biaggioni I, Robertson D. Endogenous restoration of noradrenaline by precursor therapy in dopamine-beta-hydroxylase deficiency. Lancet. 1987;2:1170–1172. doi: 10.1016/s0140-6736(87)91317-1. [DOI] [PubMed] [Google Scholar]
- 7.Blandini F, Nappi G, Fancellu R, Mangiagalli A, Samuele A, Riboldazzi G, Calandrella D, Pacchetti C, Bono G, Martignoni E. Modifications of plasma and platelet levels of l-DOPA and its direct metabolites during treatment with tolcapone or entacapone in patients with Parkinson’s disease. J Neural Transm. 2003;110:911–922. doi: 10.1007/s00702-003-0004-z. [DOI] [PubMed] [Google Scholar]
- 8.Blombery PA, Kopin IJ, Gordon EK, Markey SP, Ebert MH. Conversion of MHPG to vanillylmandelic acid. Implications for the importance of urinary MHPG. Arch Gen Psychiatry. 1980;37:1095–1098. doi: 10.1001/archpsyc.1980.01780230013001. [DOI] [PubMed] [Google Scholar]
- 9.Bornstein S, Breidert M, Ehrhart-Bornstein M, Kloos B, Scherbaum W. Plasma catecholamines in patients with Addison’s disease. Clin Endocrinol. 1995;42:215–218. doi: 10.1111/j.1365-2265.1995.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruce S, Tack C, Patel J, Pacak K, Goldstein DS. Local sympathetic function in human skeletal muscle and adipose tissue assessed by microdialysis. Clin Auton Res. 2002;12:13–19. doi: 10.1007/s102860200005. [DOI] [PubMed] [Google Scholar]
- 11.Buu NT, Lussier C. Origin of dopamine in the rat adrenal cortex. Am J Physiol. 1990;258:F287–F291. doi: 10.1152/ajprenal.1990.258.2.F287. [DOI] [PubMed] [Google Scholar]
- 12.Buu NT, Angers M, Chevalier D, Kuchel O. A new method for the simultaneous analysis of free and sulfoconjugated normetanephrine, metanephrine, and 3-methoxytyramine in human urine by HPLC with electrochemical detection. J Lab Clin Med. 1984;104:425–432. [PubMed] [Google Scholar]
- 13.Chang PC, Grossman E, Kopin IJ, Goldstein DS. On the existence of functional beta-adrenoceptors on vascular sympathetic nerve endings in the human forearm. J Hypertens. 1994;12:681–690. [PubMed] [Google Scholar]
- 14.Chidsey CA, Braunwald E, Morow AG, Mason DT. Myocardial norepinephrine concentration in man. Effects of reserpine and congestive heart failure. N Engl J Med. 1963;269:653–658. doi: 10.1056/NEJM196309262691302. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JN, Levine BT, Olivari MT. Plasma norepinephrine as a guide to prognosis in patients with congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 16.Dietz NM, Halliwill JR, Spielmann JM, Lawler LA, Papouchado BG, Eickhoff TJ, Joyner MJ. Sympathetic withdrawal and forearm vasodilation during vasovagal syncope in humans. J Appl Physiol. 1997;82:1785–1793. doi: 10.1152/jappl.1997.82.6.1785. [DOI] [PubMed] [Google Scholar]
- 17.Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447:636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Aneman A, Friberg P, Hooper D, Fandriks L, Lonroth H, Hunyady B, Mezey E. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg P. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. J Neurochem. 1996;66:1565–1573. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhofer G, Coughtrie MWH, Goldstein DS. Dopamine sulfate: an enigma resolved. Clin Exp Pharmacol Physiol. 1999;26:S41–S53. [PubMed] [Google Scholar]
- 21.Eisenhofer G, Esler MD, Goldstein DS, Kopin IJ. Neuronal uptake, metabolism, and release of tritium-labeled norepinephrine during assessment of its plasma kinetics. Am J Physiol. 1991;261:E505–E515. doi: 10.1152/ajpendo.1991.261.4.E505. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhofer G, Esler MD, Meredith IT, Dart A, Cannon RO, 3rd, Quyyumi AA, Lambert G, Chin J, Jennings GL, Goldstein DS. Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation. 1992;85:1775–1785. doi: 10.1161/01.cir.85.5.1775. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhofer G, Friberg P, Goldstein DS, Esler M. Differential actions of desipramine on sympathoadrenal release of noradrenaline and adrenaline. Br J Clin Pharmacol. 1995;40:263–265. doi: 10.1111/j.1365-2125.1995.tb05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Goldstein DS, Kopin IJ. Plasma dihydroxyphenylglycol for estimation of noradrenaline neuronal reuptake in the sympathetic nervous system in vivo. Clin Sci. 1989;76:171–182. doi: 10.1042/cs0760171. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhofer G, Goldstein DS, Ropchak TG, Nguyen HQ, Keiser HR, Kopin IJ. Source and physiological significance of plasma 3,4-dihydroxyphenylglycol and 3-methoxy-4-hydroxyphenylglycol. J Auton Nerv Syst. 1988;24:1–14. doi: 10.1016/0165-1838(88)90130-0. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 28.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656–2666. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 30.Eisenhofer G, Rundqvist B, Aneman A, Friberg P, Dakak N, Kopin IJ, Jacobs MC, Lenders JW. Regional release and removal of catecholamines and extraneuronal metabolism to metanephrines. J Clin Endocrinol Metab. 1995;80:3009–3017. doi: 10.1210/jcem.80.10.7559889. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhofer G, Walther M, Huynh T-T, et al. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86:1999–2008. doi: 10.1210/jcem.86.5.7496. [DOI] [PubMed] [Google Scholar]
- 32.Eisenhofer G, Esler MD, Cox HS, et al. Differences in the neuronal removal of circulating epinephrine and norepinephrine. J Clin Endocrinol Metab. 1990;70:1710–1720. doi: 10.1210/jcem-70-6-1710. [DOI] [PubMed] [Google Scholar]
- 33.Elchisak MA, Polinsky RJ, Ebert MH, Kopin IJ. Kinetics of homovanillic acid and determination of its production rate in humans. J Neurochem. 1982;38:380–385. doi: 10.1111/j.1471-4159.1982.tb08640.x. [DOI] [PubMed] [Google Scholar]
- 34.Esler M. Assessment of sympathetic nervous function in humans from noradrenaline plasma kinetics. Clin Sci. 1982;62:247–254. doi: 10.1042/cs0620247. [DOI] [PubMed] [Google Scholar]
- 35.Esler M. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 36.Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Cox HS, Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- 37.Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, Lambert G, Cox HS, Jennings G. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol. 1991;260:R817–R823. doi: 10.1152/ajpregu.1991.260.4.R817. [DOI] [PubMed] [Google Scholar]
- 38.Fahn S. Long-term treatment of tardive dyskinesia with presynaptically acting dopamine-depleting agents. Adv Neurol. 1983;37:267–276. [PubMed] [Google Scholar]
- 39.Faraj BA, Camp VM, Murray DR, Kutner M, Hearn J, Nixon D. Plasma l-DOPA in the diagnosis of malignant melanoma. Clin Chem. 1986;32:159–161. [PubMed] [Google Scholar]
- 40.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein DS, Cannon RO, Quyyumi A, Chang P, Duncan M, Brush JE, Jr, Eisenhofer G. Regional extraction of circulating norepinephrine, DOPA, and dihydroxyphenylglycol in humans. J Auton Nerv Syst. 1991;34:17–35. doi: 10.1016/0165-1838(91)90005-n. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J Clin Investig. 1988;81:213–220. doi: 10.1172/JCI113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein DS, Eldadah B, Holmes C, Pechnik S, Moak J, Sharabi Y. Neurocirculatory abnormalities in chronic orthostatic intolerance. Circulation. 2005;111:839–845. doi: 10.1161/01.CIR.0000155613.20376.CA. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein DS, Eldadah B, Sharabi Y, Axelrod FB. Cardiac sympathetic hypo-innervation in familial dysautonomia. Clin Auton Res. 2008;18:115–119. doi: 10.1007/s10286-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein DS, Feuerstein G, Izzo JL, Jr, Kopin IJ, Keiser HR. Validity and reliability of liquid chromatography with electrochemical detection for measuring plasma levels of norepinephrine and epinephrine in man. Life Sci. 1981;28:467–475. doi: 10.1016/0024-3205(81)90139-9. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein DS, Hahn S-H, Holmes C, Tifft C, Harvey-White J, Milstien S, Kaufman S. Monoaminergic effects of folinic acid, l-DOPA, and 5-hydroxytryptophan in dihydropteridine reductase deficiency. J Neurochem. 1995;64:2810–2813. doi: 10.1046/j.1471-4159.1995.64062810.x. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein DS, Holmes C. Metabolic fate of the sympathoneural imaging agent 6-[18F]fluorodopamine in humans. Clin Exp Hypertens. 1997;19:155–161. doi: 10.3109/10641969709080812. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein DS, Holmes C. Neuronal source of plasma dopamine. Clin Chem. 2008;54:1864–1871. doi: 10.1373/clinchem.2008.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldstein DS, Holmes C, Axelrod FB. Plasma catechols in familial dysautonomia: a long-term follow-up study. Neurochem Res. 2008;33:1889–1893. doi: 10.1007/s11064-008-9662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin Auton Res. 2002;12:281–285. doi: 10.1007/s10286-002-0020-3. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO, 3rd, Sharabi Y, Esler MD, Eisenhofer G. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002;106:2358–2365. doi: 10.1161/01.cir.0000036015.54619.b6. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H. Sympathoadrenal imbalance before neurocardiogenic syncope. Am J Cardiol. 2003;91:53–58. doi: 10.1016/s0002-9149(02)02997-1. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein DS, Holmes C, Imrich R. Clinical laboratory evaluation of autoimmune autonomic ganglionopathy: preliminary observations. Auton Neurosci. 2009;146(1–2):18–21. doi: 10.1016/j.autneu.2008.12.004. (Special Edition) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein DS, Holmes C, Sewell L, Pechnik S, Kopin IJ. Effects of carbidopa and entacapone on the metabolic fate of the norepinephrine pro-drug l-DOPS. J Clin Pharmacol. 2010 doi: 10.1177/0091270010363476. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003;60:1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- 59.Goldstein DS, Levinson PD, Zimlichman R, Pitterman A, Stull R, Keiser HR. Clonidine suppression testing in essential hypertension. Ann Intern Med. 1985;102:42–49. doi: 10.7326/0003-4819-102-1-42. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein DS, Nurnberger J, Jr, Simmons S, Gershon ES, Polinsky R, Keiser HR. Effects of injected sympathomimetic amines on plasma catecholamines and circulatory variables in man. Life Sci. 1983;32:1057–1063. doi: 10.1016/0024-3205(83)90110-8. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein DS, Polinsky RJ, Garty M, Robertson D, Brown RT, Biaggioni I, Stull R, Kopin IJ. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Ann Neurol. 1989;26:558–563. doi: 10.1002/ana.410260410. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein DS, Stull R, Eisenhofer G, Sisson JC, Weder A, Averbuch SD, Keiser HR. Plasma 3,4-dihydroxyphenylalanine (dopa) and catecholamines in neuroblastoma or pheochromocytoma. Ann Intern Med. 1986;105:887–888. doi: 10.7326/0003-4819-105-6-887. [DOI] [PubMed] [Google Scholar]
- 63.Goldstein DS, Swoboda KJ, Miles JM, Coppack SW, Aneman A, Holmes C, Lamensdorf I, Eisenhofer G. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84:2523–2531. doi: 10.1210/jcem.84.7.5864. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein DS, Udelsman R, Eisenhofer G, Stull R, Keiser HR, Kopin IJ. Neuronal source of plasma dihydroxyphenylalanine. J Clin Endocrinol Metab. 1987;64:856–861. doi: 10.1210/jcem-64-4-856. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein DS, Zimlichman R, Stull R, Keiser HR. Plasma catecholamine and hemodynamic responses during isoproterenol infusions in humans. Clin Pharmacol Ther. 1986;40:233–238. doi: 10.1038/clpt.1986.168. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Trapaga JL, Nelesen RA, Dimsdale JE, Mills PJ, Kennedy B, Parmer RJ, Ziegler MG. Plasma epinephrine levels in hypertension and across gender and ethnicity. Life Sci. 2000;66:2383–2392. doi: 10.1016/s0024-3205(00)00568-3. [DOI] [PubMed] [Google Scholar]
- 67.Grossman E, Goldstein DS, Hoffman A, Keiser HR. Glucagon and clonidine testing in the diagnosis of pheochromocytoma. Hypertension. 1991;17:733–741. doi: 10.1161/01.hyp.17.6.733. [DOI] [PubMed] [Google Scholar]
- 68.Grossman E, Rea RF, Hoffman A, Goldstein DS. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am J Physiol. 1991;260:R142–R147. doi: 10.1152/ajpregu.1991.260.1.R142. [DOI] [PubMed] [Google Scholar]
- 69.Gustafson AB, Kalkhoff RK. Influence of sex and obesity on plasma catecholamine response to isometric exercise. J Clin Endocrinol Metab. 1982;55:703–708. doi: 10.1210/jcem-55-4-703. [DOI] [PubMed] [Google Scholar]
- 70.Hilz MJ, Axelrod FB, Bickel A, Stemper B, Brys M, Wendelschafer-Crabb G, Kennedy WR. Assessing function and pathology in familial dysautonomia: assessment of temperature perception, sweating and cutaneous innervation. Brain. 2004;127:2090–2098. doi: 10.1093/brain/awh235. [DOI] [PubMed] [Google Scholar]
- 71.Holmes C, Moak J, Eldadah B, Zimmerly E, Sharabi Y, Goldstein DS. Dopamine contamination of infused tyramine. Clin Chem. 2005;51:1733–1735. doi: 10.1373/clinchem.2005.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hovevey-Sion D, Kopin IJ, Stull RW, Goldstein DS. Effects of monoamine oxidase inhibitors on levels of catechols and homovanillic acid in striatum and plasma. Neuropharmacology. 1989;28:791–797. doi: 10.1016/0028-3908(89)90169-x. [DOI] [PubMed] [Google Scholar]
- 73.Illi A, Sundberg S, Ojala-Karlsson P, Korhonen P, Scheinin M, Gordin A. The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine. Clin Pharmacol Ther. 1995;58:221–227. doi: 10.1016/0009-9236(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 74.Ishimitsu T, Hirose S. Determination of m- and p-O-methylated products of L-3,4-dihydroxyphenylalanine using high-performance liquid chromatography and electrochemical detection. Anal Biochem. 1985;150:300–308. doi: 10.1016/0003-2697(85)90514-7. [DOI] [PubMed] [Google Scholar]
- 75.Isnard R, Pousset F, Trochu J, Chafirovskaia O, Carayon A, Golmard J, Lechat P, Thomas D, Bouhour J, Komajda M. Prognostic value of neurohormonal activation and cardiopulmonary exercise testing in patients with chronic heart failure. Am J Cardiol. 2000;86:417–421. doi: 10.1016/s0002-9149(00)00957-7. [DOI] [PubMed] [Google Scholar]
- 76.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 77.Jacob G, Costa F, Vincent S, Robertson D, Biaggioni I. Neurovascular dissociation with paradoxical forearm vasodilation during systemic tyramine administration. Circulation. 2003;107:2475–2479. doi: 10.1161/01.CIR.0000065605.37863.C0. [DOI] [PubMed] [Google Scholar]
- 78.Jacob G, Gamboa A, Diedrich A, Shibao C, Robertson D, Biaggioni I. Tyramine-induced vasodilation mediated by dopamine contamination: a paradox resolved. Hypertension. 2005;46:355–359. doi: 10.1161/01.HYP.0000172353.62657.8b. [DOI] [PubMed] [Google Scholar]
- 79.Jacobsen TN, Grayburn PA, Snyder RW, 2nd, Hansen J, Chavoshan B, Landau C, Lange RA, Hillis LD, Victor RG. Effects of intranasal cocaine on sympathetic nerve discharge in humans. J Clin Investig. 1997;99:628–634. doi: 10.1172/JCI119205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jordan J, Shannon JR, Black BK, Lance RH, Squillante MD, Costa F, Robertson D. N(N)-nicotinic blockade as an acute human model of autonomic failure. Hypertension. 1998;31:1178–1184. doi: 10.1161/01.hyp.31.5.1178. [DOI] [PubMed] [Google Scholar]
- 81.Kaler SG, Goldstein DS, Holmes C, Salerno JA, Gahl WA. Plasma and cerebrospinal fluid neurochemical pattern in Menkes disease. Ann Neurol. 1993;33:171–175. doi: 10.1002/ana.410330206. [DOI] [PubMed] [Google Scholar]
- 82.Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Donsante A, Liew CJ, Sato S, Patronas N. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, Nardin R, Freeman R. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–728. doi: 10.1161/01.CIR.0000083721.49847.D7. [DOI] [PubMed] [Google Scholar]
- 84.Keranen T, Gordin A, Harjola VP, Karlsson M, Korpela K, Pentikainen PJ, Rita H, Seppala L, Wikberg T. The effect of catechol-O-methyl transferase inhibition by entacapone on the pharmacokinetics and metabolism of levodopa in healthy volunteers. Clin Neuropharmacol. 1993;16:145–156. doi: 10.1097/00002826-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37:333–364. [PubMed] [Google Scholar]
- 86.Kopin IJ, Rundqvist B, Friberg P, Lenders J, Goldstein DS, Eisenhofer G. Different relationships of spillover to release of norepinephrine in human heart, kidneys, and forearm. Am J Physiol. 1998;275:R165–R173. doi: 10.1152/ajpregu.1998.275.1.R165. [DOI] [PubMed] [Google Scholar]
- 87.Kurz T, Richardt G, Seyfarth M, Schomig A. Nonexocytotic noradrenaline release induced by pharmacological agents or anoxia in human cardiac tissue. Naunyn Schmiedeberg’s Arch Pharmacol. 1996;354:7–16. doi: 10.1007/BF00168700. [DOI] [PubMed] [Google Scholar]
- 88.Kvetnansky R, Armando I, Weise VK, Holmes C, Fukuhara K, Deka-Starosta A, Kopin IJ, Goldstein DS. Plasma dopa responses during stress: dependence on sympathoneural activity and tyrosine hydroxylation. J Pharmacol Exp Ther. 1992;261:899–909. [PubMed] [Google Scholar]
- 89.Kvetnansky R, Goldstein DS, Weise VK, Holmes C, Szemeredi K, Bagdy G, Kopin IJ. Effects of handling or immobilization on plasma levels of 3, 4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J Neurochem. 1992;58:2296–2302. doi: 10.1111/j.1471-4159.1992.tb10977.x. [DOI] [PubMed] [Google Scholar]
- 90.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 91.Lenders JWM, Eisenhofer G, Abeling NGGM, Berger W, Murphy DL, Konings CH, Wagemakers LMB, Kopin IJ, Karoum F, van Gennip AH, Brunner HG. Specific genetic deficiencies of the A and B isozymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J Clin Investig. 1996;97:1010–1019. doi: 10.1172/JCI118492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Letellier S, Garnier JP, Spy J, Bousquet B. Determination of the l-DOPA/l-tyrosine ratio in human plasma by high-performance liquid chromatography. Usefulness as a marker in metastatic malignant melanoma. J Chromatogr B Biomed Appl. 1997;696:9–17. doi: 10.1016/s0378-4347(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 93.Levinson PD, Goldstein DS, Munson PJ, Gill JR, Jr, Keiser HR. Endocrine, renal, and hemodynamic responses to graded dopamine infusions in normal men. J Clin Endocrinol Metab. 1985;60:821–826. doi: 10.1210/jcem-60-5-821. [DOI] [PubMed] [Google Scholar]
- 94.Lonart G, Sudhof TC. Assembly of SNARE core complexes prior to neurotransmitter release sets the readily releasable pool of synaptic vesicles. J Biol Chem. 2000;275:27703–27707. [PubMed] [Google Scholar]
- 95.Mardh G, Sjoquist B, Anggard E. Norepinephrine metabolism in man using deuterium labeling: turnover 4-hydroxy-3-methoxymandelic acid. J Neurochem. 1982;38:1582–1587. doi: 10.1111/j.1471-4159.1982.tb06636.x. [DOI] [PubMed] [Google Scholar]
- 96.Mayer AF, Schroeder C, Heusser K, Tank J, Diedrich A, Schmieder RE, Luft FC, Jordan J. Influences of norepinephrine transporter function on the distribution of sympathetic activity in humans. Hypertension. 2006;48:120–126. doi: 10.1161/01.HYP.0000225424.13138.5d. [DOI] [PubMed] [Google Scholar]
- 97.Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension. 1992;19:628–633. doi: 10.1161/01.hyp.19.6.628. [DOI] [PubMed] [Google Scholar]
- 98.Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, Van Wyk JJ, Bornstein SR. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 99.Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, Kuusela TA, Diedrich AM. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509–2513. doi: 10.1161/01.cir.96.8.2509. [DOI] [PubMed] [Google Scholar]
- 100.Nutt JG, Woodward WR, Beckner RM, Stone CK, Berggren K, Carter JH, Gancher ST, Hammerstad JP, Gordin A. Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in parkinsonian patients. Neurology. 1994;44:913–919. doi: 10.1212/wnl.44.5.913. [DOI] [PubMed] [Google Scholar]
- 101.Oliver G, Schafer EA. On the physiological action of extract of the suprarenal capsules. J Physiol. 1895;17:x–xiv. doi: 10.1113/jphysiol.1895.sp000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearson J, Brandeis L, Goldstein M. Tyrosine hydroxylase immunoreactivity in familial dysautonomia. Science. 1979;206:71–72. doi: 10.1126/science.39339. [DOI] [PubMed] [Google Scholar]
- 103.Pearson J, Gallo G, Gluck M, Axelrod F. Renal disease in familial dysautonomia. Kidney Int. 1980;17:102–112. doi: 10.1038/ki.1980.12. [DOI] [PubMed] [Google Scholar]
- 104.Pearson J, Pytel BA. Quantitative studies of sympathetic ganglia and spinal cord intermedio-lateral gray columns in familial dysautonomia. J Neurol Sci. 1978;39:47–59. doi: 10.1016/0022-510x(78)90187-9. [DOI] [PubMed] [Google Scholar]
- 105.Penttila J, Syvalahti E, Hinkka S, Kuusela T, Scheinin H. The effects of amitriptyline, citalopram and reboxetine on autonomic nervous system. A randomised placebo-controlled study on healthy volunteers. Psychopharmacology (Berl) 2001;154:343–349. doi: 10.1007/s002130000664. [DOI] [PubMed] [Google Scholar]
- 106.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 107.Robertson D, Goldberg MR, Tung CS, Hollister AS, Robertson RM. Use of alpha 2 adrenoreceptor agonists and antagonists in the functional assessment of the sympathetic nervous system. J Clin Investig. 1986;78:576–581. doi: 10.1172/JCI112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schroeder C, Tank J, Boschmann M, Diedrich A, Sharma AM, Biaggioni I, Luft FC, Jordan J. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105:347–353. doi: 10.1161/hc0302.102597. [DOI] [PubMed] [Google Scholar]
- 109.Scriven AJI, Dollery CT, Murphy MB, Macquin I, Brown MJ. Blood pressure and plasma norepinephrine concentrations after endogenous norepinephrine release by tyramine. Clin Pharmacol Ther. 1983;33:710–716. doi: 10.1038/clpt.1983.97. [DOI] [PubMed] [Google Scholar]
- 110.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Senard JM, Valet P, Durrieu G, Berlan M, Tran MA, Montastruc JL, Rascol A, Montastruc P. Adrenergic supersensitivity in parkinsonians with orthostatic hypotension. Eur J Clin Investig. 1990;20:613–619. doi: 10.1111/j.1365-2362.1990.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 112.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 113.Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS. Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson’s disease with orthostatic hypotension. Mov Disord. 2008;23:1725–1732. doi: 10.1002/mds.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, Liebert CB, Chadwick B, Idelson M, Reznik L, Robbins C, Makalowska I, Brownstein M, Krappmann D, Scheidereit C, Maayan C, Axelrod FB, Gusella JF. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith SE. Cheese reaction and tyramine. Lancet. 1971;1:130–131. doi: 10.1016/s0140-6736(71)90858-0. [DOI] [PubMed] [Google Scholar]
- 116.Sofuoglu M, Nelson D, Babb DA, Hatsukami DK. Intravenous cocaine increases plasma epinephrine and norepinephrine in humans. Pharmacol Biochem Behav. 2001;68:455–459. doi: 10.1016/s0091-3057(01)00482-8. [DOI] [PubMed] [Google Scholar]
- 117.Steinsapir J, Carr AA, Prisant LM, Bransome ED., Jr Metyrosine and pheochromocytoma. Arch Intern Med. 1997;157:901–906. [PubMed] [Google Scholar]
- 118.Sundberg S, Scheinin M, Illi A, Akkila J, Gordin A, Keranen T. The effects of the COMT inhibitor entacapone on haemodynamics and peripheral catecholamine metabolism during exercise. Br J Clin Pharmacol. 1993;36:451–456. doi: 10.1111/j.1365-2125.1993.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swoboda KJ, Hyland K, Goldstein DS, Kuban KC, Arnold LA, Holmes CS, Levy HL. Clinical and therapeutic observations in aromatic l-amino acid decarboxylase deficiency. Neurology. 1999;53:1205–1211. doi: 10.1212/wnl.53.6.1205. [DOI] [PubMed] [Google Scholar]
- 120.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003;107:2949–2954. doi: 10.1161/01.CIR.0000072786.99163.FE. [DOI] [PubMed] [Google Scholar]
- 121.Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- 122.Thompson JM, O’Callaghan CJ, Kingwell BA, Lambert GW, Jennings GL, Esler MD. Total norepinephrine spillover, muscle sympathetic nerve activity and heart-rate spectral analysis in a patient with dopamine beta-hydroxylase deficiency. J Auton Nerv Syst. 1995;55:198–206. doi: 10.1016/0165-1838(95)00048-3. [DOI] [PubMed] [Google Scholar]
- 123.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 124.Vincent HH, Man in’t Veld AJ, Boomsma F, Wenting GJ, Schalekamp MADH. Elevated plasma noradrenaline in response to beta-adrenoceptor stimulation in man. Br J Clin Pharmacol. 1982;13:717–721. doi: 10.1111/j.1365-2125.1982.tb01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vincent S, Bieck PR, Garland EM, Loghin C, Bymaster FP, Black BK, Gonzales C, Potter WZ, Robertson D. Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation. 2004;109:3202–3207. doi: 10.1161/01.CIR.0000130847.18666.39. [DOI] [PubMed] [Google Scholar]
- 126.Volkow ND, Wang GJ, Fowler JS, Molina PE, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas NR, Zhu W, Swanson JM. Cardiovascular effects of methylphenidate in humans are associated with increases of dopamine in brain and of epinephrine in plasma. Psychopharmacology (Berl) 2003;166:264–270. doi: 10.1007/s00213-002-1340-7. [DOI] [PubMed] [Google Scholar]
- 127.Wallin BG, Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- 128.Williams M, Young JB, Rosa RM, Gunn S, Epstein FH, Landsberg L. Effect of protein ingestion on urinary dopamine excretion: evidence for the functional importance of renal decarboxylation of circulating 3,4-dihydroxyphenylalanine in man. J Clin Investig. 1986;78:1687–1693. doi: 10.1172/JCI112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 130.Wolfovitz E, Grossman E, Folio CJ, Keiser HR, Kopin IJ, Goldstein DS. Derivation of urinary dopamine from plasma dihydroxyphenylalanine in humans. Clin Sci. 1993;84:549–557. doi: 10.1042/cs0840549. [DOI] [PubMed] [Google Scholar]
- 131.Yamamoto T, Polinsky RJ, Goldstein DS, Baucom CE, Kopin IJ. Plasma sulfoconjugated dopamine levels are normal in patients with autonomic failure. J Lab Clin Med. 1996;128:488–491. doi: 10.1016/s0022-2143(96)90045-1. [DOI] [PubMed] [Google Scholar]
- 132.Zavadil AP, Ross RJ, Calil HM, Linnoila M, Blombery P, Jimerson DC, Kopin IJ, Potter WZ. The effect of desmethylimipramine on the metabolism of norepinephrine. Life Sci. 1984;35:1061–1068. doi: 10.1016/0024-3205(84)90070-5. [DOI] [PubMed] [Google Scholar]
- 133.Ziegler MG, Lake CR, Kopin IJ. Deficient sympathetic nervous response in familial dysautonomia. N Engl J Med. 1976;294:630–633. doi: 10.1056/NEJM197603182941202. [DOI] [PubMed] [Google Scholar]
- 134.Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med. 1977;296:293–297. doi: 10.1056/NEJM197702102960601. [DOI] [PubMed] [Google Scholar]