Abstract
Background
GTI-2040 is a 20-mer antisense oligonucleotide targeting the mRNA of ribonucleotide reductase M2. It was combined with oxaliplatin and capecitabine in a phase I trial in patients with advance solid tumors based on previous studies demonstrating potentiation of chemotherapy with ribonucleotide reductase inhibitors.
Methods
Patients at least 18 years of age with advanced incurable solid tumors and normal organ function as well as a Karnofsky performance status of ≥60% were eligible. One prior chemotherapy regimen for advanced disease or relapse within 12 months of adjuvant chemotherapy was required. Patients could have received prior fluoropyrimidines, including capecitabine, but not oxaliplatin. Treatment cycles were 21 days. In each cycle, GTI-2040 was given as a continuous intravenous infusion over 14 days, oxaliplatin as a 2-h intravenous infusion on day 1, and capecitabine orally twice a day for 14 days. In cycle 1 only, oxaliplatin and capecitabine were started on day 2 to allow ribonucleotide reductase mRNA levels to be measured with and without oxaliplatin and capecitabine. Doses were escalated in cohorts of three patients using a standard 3 + 3 design until the maximum tolerated dose was established, defned as no more than one first-cycle dose-limiting toxicity among six patients treated at a given dose level.
Results
The maximum tolerated dose was estimated to be the combination of GTI-2040 3 mg/kg per day for 14 days, capecitabine 600 mg/m2 twice daily for 14 days, and oxaliplatin 100 mg/m2 every 21 days. Dose-limiting toxicities were hematologic. GTI-2040 pharmacokinetics, obtained at steady-state on days 7 and 14, showed the high inter-patient variability previously reported. Two of six patients had stable disease at the maximum tolerated dose and one patient, with heavily pre-treated non-small cell lung cancer, had a partial response at a higher dose level. In samples from a limited number of patients, there was no clear decrease in ribonucleotide reductase expression in peripheral blood mononuclear cells during treatment.
Conclusion
A combination of GTI-2040, capecitabine and oxaliplatin is feasible in patients with advanced solid tumors.
Keywords: GTI-2040, Oxaliplatin, Capecitabine, Ribonucleotide reductase, Pharmacokinetics
Introduction
The flexibility of drug design offered by antisense oligonucleotides (ASO) targeting specific mRNA molecules offers the promise of selectively blocking key pathways important to neoplastic biology. This class of agent has shown evidence of clinical activity in several clinical trials. Phase III trials using the ASO Gi3139 targeting Bcl-2, while not demonstrating significantly improved survival, have demonstrated improvement in other clinical parameters such as response and progression free survival [1]. Another study targeting protein kinase C-α (PKCα) demonstrated the feasibility of adding the ASO LY900003 to chemotherapy in patients with non-small cell lung cancer, although clinical improvement was not seen [2]. However, evidence of single agent activity against ovarian cancer and non-Hodgkin’s lymphoma was seen in other trials [3, 4].
Ribonucleotide reductase (RR) is of interest as a target for ASO. RR is a highly regulated enzyme critical for the growth of mammalian cells [5]. It is responsible for the de novo conversion of ribonucleoside diphosphates to deoxy-ribonucleoside diphosphates, which are essential for DNA synthesis and repair [6]. The enzyme, consisting of two subunits, M1 and M2, is elevated in S-phase, is rate limiting for the synthesis of DNA, and plays an important role in the regulation of cell proliferation [7, 8]. Alterations in the levels of RR can have significant effects on the biological properties of cells such as tumor promotion and progression. Changes in enzyme activity and gene expression of the M1 and/or M2 subunit are observed after exposure of tumor cells to transforming growth factor β1, 12-0-tetra-decanoylphorbol-13-acetate, and during repair of chemotherapy DNA damage [9–13]. In vitro studies have also shown that aggressive tumor proliferation results in increase RR expression [14, 15]. The human RRM2 subunit can also act as a tumor promoter, cooperating with a variety of oncogenes to enhance cellular transformation and malignant potential [16, 17].
Preclinical data suggest that an antisense approach to inhibiting RR may be clinically eVective. Inducible RRM1 and M2 antisense constructs were designed and transfected into human KB cells with a 50% growth inhibition noted with the M2 antisense cDNA, but not the M1 construct [18]. GTI-2040 is a 20-mer ASO that is complementary to a coding region in the mRNA of M2. Preclinical studies demonstrate that GTI-2040 decreases mRNA and protein levels of M2 in a sequence-specific and dose-dependent manner. In vivo studies have shown that GTI-2040 significantly inhibits growth of a range of tumors including colon adenocarcinoma, pancreatic adenocarcinomas, liver tumors, lung tumors, breast tumors, renal tumors, ovarian tumors, melanoma, glioblastoma-astrocytoma, prostatic tumors, and cervical tumors [19]. A phase I clinical study supported a recommended phase II dose of 185 mg/m2 as a 21-day infusion every 28 days [20]. Reversible hepatotoxicity was seen at 222 mg/m2 with other patients noting fatigue and anorexia. Preclinical and clinical studies have shown improved activity when RR inhibitors are combined with chemotherapy, which provides the basis for the current phase I trial of GTI-2040 in combination with oxaliplatin and capecitabine [21].
Patients and methods
Patient eligibility
Patients at least 18 years of age with advanced incurable solid tumors and a Karnofsky performance status of ≥60% were eligible. One prior chemotherapy regimen for advanced disease or relapse within 12 months of adjuvant chemotherapy was required. Patients could not have received prior oxaliplatin, but prior fluoropyrimidines, including capecitabine, were allowed. Patients with baseline neuropathy >grade 1, abnormal bilirubin, or alkaline phosphatase, ALT, or AST ≥2.5 times upper limit of normal were ineligible. In addition, patients with baseline pulmonary frbrosis, known brain metastases, active or chronic hepatitis B or C, HIV on antiviral therapy, or patients on full anticoagulation were ineligible. A creatinine clearance ≥60 ml/min, an absolute neutrophil count ≥1,500/μl, and a platelet count ≥100,000/μl were required. Patients were required to be of previous therapy for >21 days, and for >6 weeks for mitomycin-C or nitrosoureas. Pregnant or nursing women were excluded and eVective contraception was required of all patients with reproductive potential. All patients signed an IRB-approved consent prior to participation.
Treatment plan
Treatment cycles were 21 days. In each cycle, GTI-2040 was given as a continuous intravenous infusion over 14 days, oxaliplatin as a 2-h intravenous infusion on day 1, and capecitabine orally twice a day for 14 days. In cycle 1 only, oxaliplatin and capecitabine were started on day 2 to allow the effect of GTI-2040 on ribonucleotide reductase mRNA levels to be measured with and without concurrent oxaliplatin and capecitabine treatment.
Study design
Patients were enrolled in cohorts of 3, beginning at dose level 1 (Table 1). Adverse events (AEs) were graded using the NCI common toxicity criteria (CTCAE) version 3.0. Grade 3 non-hematologic AEs not resolving to≤grade 2 within 96 h, grade 4 non-hematologic AEs, and grade 3 or 4 ANC or platelet counts that did not resolve to≤grade 2 within 7 days were defined as dose-limiting toxicities (DLTs). Toxicities were evaluated in all patients receiving any amount of GTI-2040. However, to be considered in the dose escalation scheme, patients must have completed one full cycle of treatment (21 days) or have experienced DLT reasonably associated with the treatment regimen during the first cycle. Patients not meeting these criteria were replaced. National Cancer Institute attributions of possibly, probably, and definitely related were considered reasonably associated with treatment. Dose escalations in cohorts followed a standard 3 + 3 design. If none of the three patients experienced a DLT, the next three patients were to be enrolled at the next higher dose level. If exactly one of the first three patients treated at a given dose level experienced a DLT, three additional patients were to be enrolled at the same dose level. As soon as two or more patients experienced a first-cycle DLT at any given dose level, accrual to that dose level and higher dose levels ceased and patients were accrued to the next lower dose level. The maximally tolerated dose was defined as the highest dose level tested at which no more than one patient of six patients experienced a DLT during the first cycle of treatment.
Table 1.
Dose escalation scheme
| Dose level | GTI-2040 (mg/kg per day) | Capecitabine (mg/m2 per BID) | Oxaliplatin (mg/m2) |
|---|---|---|---|
| −1 | 3 | 600 | 100 |
| 1 | 3 | 600 | 130 |
| 2a |
Due to dose-limiting toxicities at dose level 1, planned increases in GTI-2040 and capecitabine were not tested
Pharmacokinetic studies
Peripheral blood samples were drawn prior to the start of treatment and then on days 7 and 14 (immediately prior to the end of infusion) for determination of plasma GTI-2040. GTI-2040 levels in plasma were measured using HPLC–tandem mass spectrometry (LC–MS/MS). Average steady-state plasma (Cpss) concentrations were determined by averaging the plasma levels measured on days 7 and 14. Steady-state clearance (CLss) of GTI-2040 was calculated according to the formula; CLss = Dose rate (mg/h)/Cpss(mg/L), and AUC was calculated according to the formula; AUC0–∞ = total dose/CLss, where total dose was the single daily dose times 14.
Following addition of a 23-mer phosphoramidate oligonucleotide (internal standard, I.S.), GTI-2040 was extracted from plasma by phenol/chloroform/isoamyl alcohol extraction. LC–MS/MS analysis was performed using an Agilent Technologies LC 1100 series system (Palo Alto, CA, USA) interfaced with a Micromass Quattro Ultima Triple Quadrupole Mass Spectrometer (Micromass, Inc., Beverly, MA, USA). LC separation was achieved using a Clarity 3μ Oligo-RP 50 × 2.0 mm analytical column (Phenomenex, Torrance, CA, USA) preceded by a Phenomenex Clarity SecurityGuard cartridge (Torrance, CA, USA). Column temperature was maintained at 50°C and the flow rate was 0.2 ml/min. Solvent A consisted of 100 mM 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP; Sigma-Aldrich, St Louis, MO) and 8.6 mM triethylamine (TEA; Sigma-Aldrich, St Louis, MO) buffer, pH 8.35. Solvent B was a 1:1 mixture of methanol and 200 mM HFIP, 17.2 mM TEA buffer, pH 8.35. The isocratic mobile phase conditions were 60% solvent A and 40% solvent B. The flow rate was 0.2 ml/min and the total run time was 20 min. The electrospray ionization source of the mass spectrometer was operated in negative ion mode with a cone gas flow of 160 L/h and a desolvation gas flow of 620 L/h. The capillary voltage was 4 kV, and the cone and collision cell voltages were optimized to 100 V and 42 eV for GTI-2040 and 88 V and 40 eV for the internal standard (I.S.), respectively. The source temperature was 125°C and the desolvation temperature was 350°C. MassLynx version 3.5 software was used for data acquisition and processing.
Negative electrospray ionization of GTI-2040 and I.S. produced abundant molecular ions at m/z 2,124.1 and 1,893.5, representing the triply-charged parent ions, respectively. The fragmentation of the molecular ions was obtained by collision induced dissociation. The precursor → product ion combinations at m/z 2,124.1 → 2,073.4 for GTI-2040 and 1,893.5 → 1,842.8 for I.S. were subsequently used in multiple reaction monitoring (MRM) mode for quantitation. MS/MS experimental conditions, such as collision energy and collision cell pressure, were optimized using continuous flow injection of standard solutions. Under optimized assay conditions, the retention times for GTI-2040 and I.S. were 12.8 and 12.6 min, respectively. The final standard curve ranged from 1–16 μg/ml, and the inter- and intra-day precision and accuracy of the assay over the range of the standard curve were determined to be within 10% of the expected concentrations. Ten different sources of blank plasma were tested and all were found to be free of interfering peaks.
Pharmacodynamic studies
Expression of RR M1 and M2 mRNAs were measured in peripheral blood mononuclear cells (PBMC) as indicators of the biologic activity of GTI-2040 as described previously [22]. Blood was collected before the start of treatment, 4 and 24 h after the start of the GTI-2040 infusion (prior to capecitabine and oxaliplatin), 4 and 24 h after the first dose of capecitabine and oxaliplatin (28 and 72 h from the start of GTI-2040), at the end of the 14-day infusion of GTI-2040 infusion, and 24 h after the end of the infusion.
Results
Fourteen patients were enrolled between May 2004 and January 2006. Demographic data are presented in Table 2. All 14 patients had at least one prior chemotherapy treatment for advanced disease. Two patients were replaced in the dose escalation scheme because they stopped treatment early for reasons aside from a DLT, one patient due to problems with a biliary stent and the other due to individual preference.
Table 2.
Patient characteristics
| Total number | 14a |
| Median age (years) | 61 (35–70) |
| Gender | 5 Males, 9 females |
| Race/ethnicity | 12 Caucasian, 2 Asian |
| Karnofsky performance status | |
| 90% | 5 |
| 80% | 6 |
| 70% | 1 |
| 60% | 2 |
| Prior treatment | 2.5 (1–6) |
| Primary sites | |
| Lung cancer (non-small cell) | |
| Colorectal | 2 |
| Esophagus | 1 |
| Breast | 1 |
| Gastric | 1 |
| Salivary gland | 1 |
| Cervix | 1 |
| Unknown | 1 |
| Metastatic sites | 3 (1–8) |
Two patients were not evaluable for toxicity
Toxicity
Eight patients were enrolled on the first level. As noted, two patients did not complete the first cycle of treatment and were replaced. Of the six patients eligible for dose escalation decisions, two experienced a DLT. One patient was a 48-year-old male with metastatic esophageal cancer who presented on the tenth day of treatment with melena, a platelet count of 6/μl, and an ANC of 0.9/μl. His clinical condition deteriorated with the development of ascites and he expired 8 days later of progressive disease. The second patient was a 44-year-old female with a salivary gland tumor with lung and mediastinal metastases who had received four prior chemotherapy treatments as well as neck and chest radiation. During the first course, the patient experienced grade 3 thrombocytopenia lasting >7 days. No other significant hematologic or non-hematologic toxicity was noted. Based on this, further patients were treated at dose level −1.
Six patients completed at least one cycle of treatment at level −1 without a DLT, establishing this as the MTD. Toxicity was mild at this level with no hematologic or non-hematologic toxicity greater than grade 2.
Cumulative toxicity was manageable in the four patients that received more than two treatment cycles. Two patients required a dose reduction after two cycles due to grade 3–4 thrombocytopenia. No further dose reductions occurred after cycle 2. One patient had grade 3 peripheral neuropathy on cycle 4 of treatment. This patient had previously received treatment with paclitaxel as well as bortezomib.
Clinical response
At dose level 1, a 48-year-old female patient with non-small cell lung cancer had a partial response. The patient had had four prior chemotherapy regimens, including a carboplatin-containing regimen. She had also progressed on gefitinib, and had received cranial radiation for brain metastases. The patient received a total of five cycles of treatment before progression. At the same dose level, a patient with colorectal cancer had stable disease for five treatment cycles. At level −1, two patients, one with breast cancer and one with lung cancer, had stable disease for five and six cycles, respectively. Progressive disease was the best response in the remaining eight patients evaluable for response.
Pharmacokinetics
GTI-2040 pharmacokinetics were evaluated in 12 subjects, and the data are summarized by dose level in Table 3. Mean GTI-2040 steady-state plasma concentrations in patients on dose levels −1 and 1 were 1.9 ± 0.8 mg/L and 1.0 ± 0.05 mg/L, respectively (P = 0.06). Mean clearances on the two different dose levels were 5.1 ± 1.8 L/h and 10.4 ± 3.2 L/h, respectively (P = 0.008). As a result of the lower clearances in the patients on dose level −1, the mean AUC was higher in these patients than in those on dose level 1 (643 ± 274 vs. 347 ± 176 mg h/L; P = 0.06). There was wide inter-patient variability in GTI-2040 pharmacokinetics overall, with a three- to four-fold range in steady-state plasma concentrations, clearances, and AUC’s within the different dose levels. Moreover, there were no pharmacokinetic differences in the patients with stable or partially responsive disease compared to the others.
Table 3.
Pharmacokinetic summary
| PK parameter | Dose level −1 (n = 6) | Dose level 1 (n = 6) | P value* |
|---|---|---|---|
| Cpss (mg/L) | |||
| Mean | 1.9 | 1.0 | 0.06 |
| SD | 0.8 | 0.5 | |
| Median | 1.8 | 0.9 | |
| Range | 1.1–3.4 | 0.6–2.0 | |
| CLsys(L/h) | |||
| Mean | 5.1 | 10.4 | 0.008 |
| SD | 1.8 | 3.2 | |
| Median | 5.2 | 11.6 | |
| Range | 3.4–6.4 | 4.0–12.4 | |
| AUC (mg/L h) | |||
| Mean | 643 | 347 | 0.06 |
| SD | 274 | 176 | |
| Median | 595 | 307 | |
| Range | 383–1,148 | 189–681 | |
Two-tailed t test
Pharmacodynamic studies
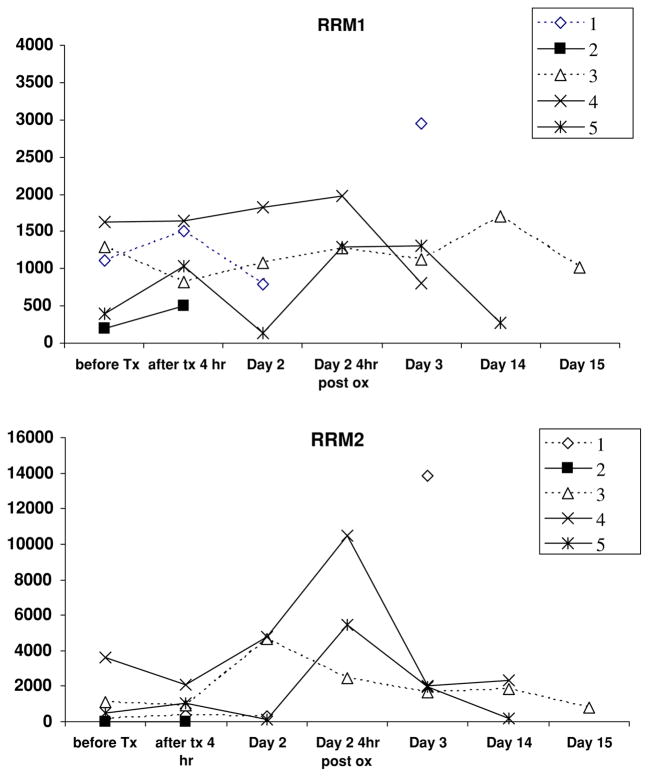
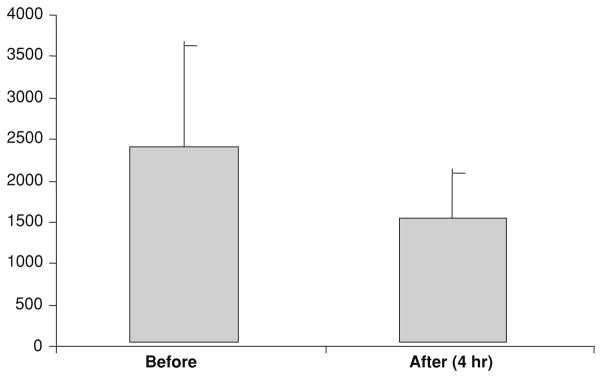
PBMC were assayed for RRM1 and M2 expression in samples from five patients with adequate specimens. There was little consistent change in RRM2 or M1 expression during the GTI-2040 infusion (Fig. 1). However, the mean RRM2 expression was slightly lower after 4 h of GTI-2040 alone (Fig. 2). In Fig. 1, patients 2, 4, and 5 had stable disease clinically with data not available in the patient with the PR. The RRM2 level is the lowest in patients 2 and 5 at baseline, which may suggest that clinical outcome may be related to RRM2 levels. However, there is no significant statistical correlation between RRM2 expression and outcome.
Fig. 1.
Expression of ribonucleotide reductase RR-M1 and -M2 in PMBCs in patients treated with infusional GTI-2040 combined with oxaliplatin and gemcitabine. All subjects received single-agent GTI-2040 at a dose of 3 mg/kg per day for the first 24 h, but patients 1, 2 were subsequently treated on dose level 1 and patients 3–5 were on dose level −1
Fig. 2.
Expression of RR-M2 in PMBCs before and after 4 h of GTI-2040
Discussion
This study demonstrated that GTI-2040 at a dose of 3 mg/kg per day as a 14-day continuous infusion could be combined with oral capecitabine at 600 mg/m2 twice daily for 14 days and a 2-h infusion of oxaliplatin at 100 mg/m2 once in each 21-day cycle.
The primary toxicity at the initial level (oxaliplatin at 130 mg/m2) was hematologic, leading to a reduction in the dose of oxaliplatin to 100 mg/m2 for the next cohort of patients, which proved to be the MTD. At the MTD level, stable disease was noted in two patients.
In this study, toxicity was seen at relatively low levels of oxaliplatin and capecitabine. In other studies, tolerated doses of oxaliplatin have generally been between 120 and 130 mg/m2 with capecitabine doses between 1,250 and 850 mg/m2 twice a day; the degree of pretreatment affected the tolerated dose [23–25]. The primary adverse events associated with single-agent GTI-2040 were hepatic toxicity and diarrhea; other toxicities included fatigue, anorexia and grade 1–2 anemia, thrombocytopenia and neutropenia [20]. GTI-2040 at a dose of 185 mg/m2 as a continuous infusion over 21 days in combination with capectabine 880 mg/m2 twice daily for 21 days every 28 days was well tolerated, albeit with a low objective response rate, in renal cancer patients previously treated with chemotherapy [26]. Toxicities included gastrointestinal and hematologic events. When GTI-2040 was combined with docetaxel in patients with non-small cell lung cancer, the MTD was 5 mg/kg per day of GTI 2040 for 14 days with 75 mg/m2 of docetaxel once every 3 weeks [27]. These studies represent the ability to combine GTI-2040 with relatively standard doses of chemotherapeutic agents. It is likely that the relatively low doses at the MTD in this study are a result of using a 3-drug combination in a heavily pre-treated patient population. Despite the relatively low doses, there was evidence for clinical activity including a partial response in a heavily pre-treated patient with non-small cell lung cancer suggesting that there may be a therapeutic interaction among the agents. The study design, however, precludes conclusively determining a contribution of GTI-2040.
Cumulative side effects were relatively low, with hematologic toxicity being the primary reason for dose adjustments. One patient had grade 3 peripheral neuropathy after five cycles of treatment and a total oxaliplatin dose of 500 mg/m2 even though chronic oxaliplatin neuropathy is most often seen with cumulative doses above 700–800 mg/m2 [28]. Other patients on this study receiving similar amounts of oxaliplatin had no significant neuropathy.
It is possible that complement activation may have played a role in the DLTs noted [29–31]. Complement activation has been associated with ASOs, especially when given by rapid infusions. Transient neutropenia and hypotension has been noted with associated changes in plasma concentrations of Bb, C3a, and C5a in female cynomolgus monkeys [31]. In the first patient with neutropenia, the patient presented at day 10 at about the time a chemotherapy related nadir would be expected. In the second case, grade 3 thrombocytopenia was noted also in a time frame most compatible with a chemotherapy related effect. In addition, the DLTs were noted at GTI-2040 level of 3 mg/kg per day, a dose below that which was well tolerated in other studies.
The plasma pharmacokinetics of GTI-2040 administered as a single agent have been reported previously [20]. Prior investigations have demonstrated that the plasma half-life of GTI-2040 is quite short (<1 h), leading to rapid disappearance of the drug following discontinuation of the infusion. Steady-state plasma levels are achieved quickly within the first 24 h after initiation of the infusion, and achievable drug concentrations are above those required to down-regulate RR in vivo [19]. Moreover, significant inter-patient variability has been seen in GTI-2040 pharmacoki-netics, leading to three- to four-fold differences in steady-state concentrations in patients receiving identical doses.
While the pharmacokinetic results from the current study are consistent with the previous reports with respect to the steady-state plasma concentrations, high inter-patient variability, and short half-life, there was a potential affect of oxaliplatin dose on GTI-2040 pharmacokinetics. Patients treated at dose level −1, in which the oxaliplatin dose was reduced from 130 to 100 mg/m2, had a significantly lower GTI-2040 plasma clearance. The lower clearance translated into a higher GTI-2040 AUC, although this did not reach statistical significance. This may be due to the inter-patient variability and relatively small patient numbers as has been seen in other studies [20]. Another possible explanation for this observation could be that oxaliplatin is reacting with the antisense oligonucleotide, much like it does with DNA and RNA. Therefore, higher oxaliplatin plasma levels could be leading to a higher degree of GTI-2040 platination and could be an issue for future study.
The small sample size in the correlative studies precludes definitive conclusions. There was intra-patient variation in the level of RRM2 expression in PBMCs despite a continuous infusion of GTI-2040. GTI-2040 did not profoundly depress the levels of RR M2 mRNA at the dose rate tested (3 mg/kg per day).
There were no significant differences noted in pharmacokinetics or in PBMC RR-M2 when patients with stable disease or a partial response were compared to non-responders.
In addition, no difference in PBMC RR expression levels was noted. In patients with refractory AML, patients less than age 60 treated with GTI-2040 and AraC had a decrease of RR protein over 120 h that correlated at 24 h with a complete response [32]. Non-responders had a corresponding increase in levels despite treatment in this time period. The lack of correlation in our study may be due to the small sample size or due to the use of PBMCs as a surrogate marker.
In summary, combination therapy with GTI 2040, capecitabine, and oxaliplatin was feasible. Although doses were relatively low, there was evidence of clinical activity with the combined regimen with two patients achieving stable disease at the MTD.
Acknowledgments
This study was supported by U01 CA062505 and P30 CA033572 from the NCI.
Contributor Information
Stephen I. Shibata, Email: sshibata@coh.org, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
James H. Doroshow, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA
Paul Frankel, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA.
Timothy W. Synold, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
Yun Yen, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA.
David R. Gandara, University of California at Davis Cancer Center, Sacramento, CA, USA
Heinz-Josef Lenz, University of Southern California Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Warren A. Chow, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
Lucille A. Leong, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
Dean Lim, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA.
Kim A. Margolin, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
Robert J. Morgan, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
George Somlo, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA.
Edward M. Newman, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Road, Duarte, CA 91010, USA
References
- 1.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 2.Villalona-Calero MA, Ritch P, Figueroa JA, Otterson GA, Belt R, Dow E, et al. A phase I/II study of LY900003, an antisense inhibitor of protein kinase C-alpha, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2004;10(18 Pt 1):6086–6093. doi: 10.1158/1078-0432.CCR-04-0779. [DOI] [PubMed] [Google Scholar]
- 3.Advani R, Peethambaram P, Lum BL, Fisher GA, Hartmann L, Long HJ, et al. A phase II trial of aprinocarsen, an antisense oligonucleotide inhibitor of protein kinase C alpha, administered as a 21-day infusion to patients with advanced ovarian carcinoma. Cancer. 2004;100(2):321–326. doi: 10.1002/cncr.11909. [DOI] [PubMed] [Google Scholar]
- 4.Rao S, Watkins D, Cunningham D, Dunlop D, Johnson P, Selby P, et al. Phase II study of ISIS 3521, an antisense oligodeoxy-nucleotide to protein kinase C alpha, in patients with previously treated low-grade non-Hodgkin’s lymphoma. Ann Oncol. 2004;15(9):1413–1418. doi: 10.1093/annonc/mdh359. [DOI] [PubMed] [Google Scholar]
- 5.Cory JG, Sato A. Regulation of ribonucleotide reductase activity in mammalian cells. Mol Cell Biochem. 1983;53–54(1–2):257–266. doi: 10.1007/BF00225258. [DOI] [PubMed] [Google Scholar]
- 6.Thelander L, Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thelander M, Graslund A, Thelander L. Subunit M2 of mammalian ribonucleotide reductase. Characterization of a homogeneous protein isolated from M2-overproducing mouse cells. J Biol Chem. 1985;260(5):2737–2741. [PubMed] [Google Scholar]
- 8.Yen Y, Grill SP, Dutschman GE, Chang CN, Zhou BS, Cheng YC. Characterization of a hydroxyurea-resistant human KB cell line with supersensitivity to 6-thioguanine. Cancer Res. 1994;54(14):3686–3691. [PubMed] [Google Scholar]
- 9.Hurta RA, Samuel SK, Greenberg AH, Wright JA. Early induction of ribonucleotide reductase gene expression by transforming growth factor beta 1 in malignant H-ras transformed cell lines. J Biol Chem. 1991;266(35):24097–24100. [PubMed] [Google Scholar]
- 10.Hurta RA, Wright JA. Alterations in the activity and regulation of mammalian ribonucleotide reductase by chlorambucil, a DNA damaging agent. J Biol Chem. 1992;267(10):7066–7071. [PubMed] [Google Scholar]
- 11.McClarty GA, Chan AK, Wright JA. Hydroxyurea-induced conversion of mammalian ribonucleotide reductase to a form hypersensitive to bleomycin. Cancer Res. 1986;46(9):4516–4521. [PubMed] [Google Scholar]
- 12.Choy BK, McClarty GA, Wright JA. Transient elevation of ribonucleotide reductase activity, M2 mRNA and M2 protein in BALB/c 3T3 fibroblasts in the presence of 12-0-tetradeca-noylphorbol-13-acetate. Biochem Biophys Res Commun. 1989;162(3):1417–1424. doi: 10.1016/0006-291x(89)90832-2. [DOI] [PubMed] [Google Scholar]
- 13.Mann GJ, Musgrove EA, Fox RM, Thelander L. Ribonucle-otide reductase M1 subunit in cellular proliferation, quiescence, and differentiation. Cancer Res. 1988;48(18):5151–5156. [PubMed] [Google Scholar]
- 14.Srinivasan PR, Tonin PN, Wensing EJ, Lewis WH. The gene for ornithine decarboxylase is co-amplified in hydroxyurea-resistant hamster cells. J Biol Chem. 1987;262(26):12871–12878. [PubMed] [Google Scholar]
- 15.Hurta RA, Wright JA. Mammalian drug resistant mutants with multiple gene amplifications: genes encoding the M1 component of ribonucleotide reductase, the M2 component of ribonucle-otide reductase, ornithine decarboxylase, p5-8, the H-subunit of ferritin and the L-subunit of ferritin. Biochim Biophys Acta. 1990;1087(2):165–172. doi: 10.1016/0167-4781(90)90201-c. [DOI] [PubMed] [Google Scholar]
- 16.Fan H, Villegas C, Huang A, Wright JA. The mammalian ribonucleotide reductase R2 component cooperates with a variety of oncogenes in mechanisms of cellular transformation. Cancer Res. 1998;58(8):1650–1653. [PubMed] [Google Scholar]
- 17.Huang A, Fan H, Taylor WR, Wright JA. Ribonucleotide reductase R2 gene expression and changes in drug sensitivity and genome stability. Cancer Res. 1997;57(21):4876–4881. [PubMed] [Google Scholar]
- 18.Chen S, Zhou B, He F, Yen Y. Inhibition of human cancer cell growth by inducible expression of human ribonucleotide reductase antisense cDNA. Antisense Nucleic Acid Drug Dev. 2000;10(2):111–116. doi: 10.1089/oli.1.2000.10.111. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Vassilakos A, Feng N, Lam V, Xie H, Wang M, et al. GTI-2040, an antisense agent targeting the small subunit component (R2) of human ribonucleotide reductase, shows potent antitumor activity against a variety of tumors. Cancer Res. 2003;63(11):2802–2811. [PubMed] [Google Scholar]
- 20.Desai AA, Schilsky RL, Young A, Janisch L, Stadler WM, Vogelzang NJ, et al. A phase I study of antisense oligonu-cleotide GTI-2040 given by continuous intravenous infusion in patients with advanced solid tumors. Ann Oncol. 2005;16(6):958–965. doi: 10.1093/annonc/mdi178. [DOI] [PubMed] [Google Scholar]
- 21.Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets. 2006;6(5):409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- 22.Juhasz A, Vassilakos A, Chew HK, Gandara D, Yen Y. Analysis of ribonucleotide reductase M2 mRNA levels in patient samples after GTI-2040 antisense drug treatment. Oncol Rep. 2006;15(5):1299–1304. [PubMed] [Google Scholar]
- 23.Borner MM, Dietrich D, Stupp R, Morant R, Honegger H, Wernli M, et al. Phase II study of capecitabine and oxaliplatin in first- and second-line treatment of advanced or metastatic colorec-tal cancer. J Clin Oncol. 2002;20(7):1759–1766. doi: 10.1200/JCO.2002.07.087. [DOI] [PubMed] [Google Scholar]
- 24.az-Rubio E, Evans TR, Tabemero J, Cassidy J, Sastre J, Eatock M, et al. Capecitabine (Xeloda) in combination with oxalipla-tin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol. 2002;13(4):558–565. doi: 10.1093/annonc/mdf065. [DOI] [PubMed] [Google Scholar]
- 25.Zeuli M, Costanzo ED, Sdrobolini A, Gasperoni S, Paoloni FP, Carpi A, et al. Capecitabine and oxaliplatin in advanced colorectal cancer: a dose-finding study. Ann Oncol. 2001;12(12):1737–1741. doi: 10.1023/a:1013562914125. [DOI] [PubMed] [Google Scholar]
- 26.Stadler WM, Desai AA, Quinn DI, Bukowski R, Poiesz B, Kardi-nal CG, et al. A phase I/II study of GTI-2040 and capecita-bine in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2008;61(4):689–694. doi: 10.1007/s00280-007-0524-6. [DOI] [PubMed] [Google Scholar]
- 27.Leighl NB, Laurie SA, Knox JJ, Ellis PM, Shepherd FA, Burkes RL, et al. A phase I/II study of GTI-2040 plus docetaxel in 2nd-line treatment in non-small cell lung cancer (NSCLC) an other solid tumors. J Clin Oncol. 2005;23 doi: 10.1097/JTO.0b013e3181a949b2. (ASCO annual meeting proceedings) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semin Oncol. 2002;29(5 Suppl 15):11–20. doi: 10.1053/sonc.2002.35524. [DOI] [PubMed] [Google Scholar]
- 29.Henry SP, Giclas PC, Leeds J, Pangburn M, Auletta C, Levin AA, et al. Activation of the alternative pathway of complement by a phosphorothioate oligonucleotide: potential mechanism of action. J Pharmacol Exp Ther. 1997;281(2):810–816. [PubMed] [Google Scholar]
- 30.Henry SP, Beattie G, Yeh G, Chappel A, Giclas P, Mortari A, et al. Complement activation is responsible for acute toxicities in rhesus monkeys treated with a phosphorothioate oligodeoxynu-cleotide. Int Immunopharmacol. 2002;2(12):1657–1666. doi: 10.1016/s1567-5769(02)00142-x. [DOI] [PubMed] [Google Scholar]
- 31.Galbraith WM, Hobson WC, Giclas PC, Schechter PJ, Agrawal S. Complement activation and hemodynamic changes following intravenous administration of phosphorothioate oligonucleo-tides in the monkey. Antisense Res Dev. 1994;4(3):201–206. doi: 10.1089/ard.1994.4.201. [DOI] [PubMed] [Google Scholar]
- 32.Klisovic RB, Blum W, Wei X, Liu S, Liu Z, Xie Z, Vukosavljevic T, Kefauver C, Huynh L, Pang J, Zwiebel JA, Devine S, Byrd JC, Grever MR, Chan K, Marcucci G. A phase I study of GTI-2040, an antisense to ribonucleotide reductase, in combination with high-dose AraC in acute myeloid leukemia. Clin Cancer Res. 2008;14(12):3889–3895. doi: 10.1158/1078-0432.CCR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]