Abstract
During visually-guided manual movements, gaze is usually fixated to a target until a pointing movement is completed to that target, showing gaze anchoring. We previously examined gaze anchoring during a two-segment eye-hand task under a low accuracy constraint. Eye movements were made to predetermined first and second targets, while hand movements were varied across two conditions: 1) stop at the first target and discontinue (HS1) and 2) stop at both the first and second targets (HS1S2). Young adults previously broke gaze anchoring at the first target only when the second pointing was excluded (HS1). However, older adults did not break gaze anchoring for either condition. The present study further investigated whether young and older adults break gaze anchoring through short-term practice under the same conditions. An HS1 practice proceeded to an HS1S2 practice. The results showed that the timing of terminating gaze anchoring relative to pointing completion oscillated considerably during the HS1 practice until it was stabilized. Conversely, that timing was stable during the HS1S2 practice. Nevertheless, the young adults benefited from the HS1 practice and broke gaze anchoring even when the second pointing was included in HS1S2. This indicates that gaze anchoring to pointing completion is not a prerequisite for the production of subsequent pointing. In contrast, older adults did not improve the timing of gaze anchoring termination for either practice condition, thereby failing to break gaze anchoring. Thus, aging compromises a predictive control of terminating gaze anchoring relative to pointing completion, which is difficult to overcome through short-term practice.
Keywords: Gaze anchoring, Saccade, Reaching, Limb motor control, Oculomotor control, Aging
INTRODUCTION
During manual movements, gaze is usually fixated to a reaching target until the reach is completed [1,7,9,10,12], showing gaze anchoring [9,10]. This gaze pattern allows for effective use of visual feedback to guide the hand to a target for precise reaching movements. We previously examined gaze anchoring in a two-segment aiming task where the eyes moved to both the first and second targets, while the hand moved to both targets or only to the first target. The extent of gaze anchoring was measured as the duration from hand movement offset at the first target to eye movement onset to the second target. We reported that the duration of gaze anchoring is modulated depending on at least two factors: (1) an accuracy constraint of pointing at the first target, and (2) whether or not to include a pointing movement to the second target [12]. Thus, the CNS takes into account the control demand of hand movement for the current and subsequent movement segments in modulating the extent of gaze anchoring.
Recent evidence suggests that the termination of gaze anchoring in relation to pointing completion is often controlled predictively [1,3,5,12]. For example, when an accuracy constraint of the first target was reduced in the above two-segment task and the second pointing was excluded, young adults broke gaze anchoring to the first target before pointing completion [12]. This suggests that the CNS predicts the pointing completion to a target prior to the actual completion, and terminates gaze anchoring based on that prediction. However, an inclusion of the second pointing resulted in the maintenance of gaze anchoring to the first target until shortly after pointing completion [12]. Thus, the production of the second pointing necessitates a longer gaze anchoring to accurately monitor the hand termination location on the first target. Consequently, the predictive termination of gaze anchoring is diminished. Furthermore, when we applied the same two-segment task to older adults, they did not break gaze anchoring regardless of whether or not the second pointing was included [13]. Hence, aging reduces the capacity of individuals to perform such predictive gaze anchoring termination.
These previous observations led to the following two questions which we investigated in this study: 1) whether young adults break gaze anchoring through short-term practice when a second pointing is included; and 2) whether older adults break gaze anchoring through practice under a more conducive condition, namely, the one without a second pointing. To our knowledge, no previous study examined the adaption of eye-hand coordination manifested in gaze anchoring through short-term practice or age-related deficits for that adaptation. Thus, we investigated these issues by using the above two-segment aiming task. Similar to our previous studies [12,13], pointing to the second target was eliminated in one condition (HS1), but included in another condition (HS1H2). The adaptation of gaze anchoring was investigated at the first target.
This study employed a fixed practice order: HS1 first, and then HS1H2. It is because young adults, not older adults, previously broke gaze anchoring when the second pointing was excluded (HS1) [13]. Thus, it is important to first establish whether the previous failure of older adults in breaking gaze anchoring under this condition is due to a fundamental deficit or the lack of practice. If older adults break gaze anchoring with HS1 practice, it would suggest that the difficulty older adults experience in breaking gaze anchoring is not a fundamental deficit from aging; hence it can be overcome with short-term practice. Furthermore, based on our previous study [12], young adults are expected to break gaze anchoring throughout the HS1 practice. Such practice would provide the best possibility, if any, to facilitate the breaking of gaze anchoring during the subsequent HS1S2 practice (where the second pointing is included). If young adults break gaze anchoring with HS1S2 practice, it would suggest that gaze fixation to pointing completion is not a prerequisite for the production of subsequent pointing.
MATERIAL AND METHODS
Participants
Ten young adults (range in age: 18–22 years [mean = 19.3 years]; 5 males and 5 females) and ten older adults (range in age: 66–82 years [mean = 73.2 years]; 5 males and 5 females) provided written informed consent and participated in the study. No participant had a history of diabetics, stroke, arthritis, or other neurological or movement impairments. The average total score of the Mini-Mental State Exam (MMSE, [4]) was 29.7±0.7 (mean±SD) and 29.7±0.7 for the young and older groups, respectively. All participants were right-handed and had normal or corrected-to-normal visual acuity.
Apparatus and Procedure
The current experimental setting was the same as the one used for a large first-target condition of our previous studies [12,13]. The participants were seated in front of a table, on which a starting position (SP, 5 mm in diameter), a first target (T1, 40 mm), and second target (T2, 5 mm) were horizontally aligned and displayed in black on a white background throughout each trial. T1 and T2 were located 20 cm and 30 cm left of SP, respectively. T1 was aligned along the participant’s midline. The viewing distance of the head relative to T1 was 60 cm, resulting in that visual angles from the SP to T1 and from T1 to T2 were 18.4° and 9.5°, respectively.
All participants held the stylus with their dominant hand as a pointing device, and performed two-segment eye-hand movements. More specifically, there were two hand movement conditions for short-term practice: 1) terminate the hand movement at T1, then discontinue (hand-stop1 condition [HS1]); and 2) terminate the hand movement at both T1 and T2 (hand-stop1-stop2 condition [HS1S2]). For both conditions, eye movements were always made to T1 and T2. In addition, a control condition (CTL) utilizing the same movement as HS1S2 was included to examine a baseline performance level. The HS1S2 movement was used as the control because this movement was most natural, and because both young and older adults previously did not break gaze anchoring under this movement [12,13].
At the beginning of each trial, the participants fixated their gaze on the SP and placed the tip of the stylus on the SP, and then the examiner said “ready.” After a random delay between one-two seconds, an auditory go-signal was delivered. In response to the go-signal, the participants initiated eye and hand movements from the SP to T1 (first segment), and then moved the eyes only or both the eyes and hand to T2 (second segment). All participants were instructed to slide the stylus tip on the table surface during pointing, and terminate movements at each target to make distinct two-segment movements. However, they were instructed to reach T2 as quickly and accurately as possible.
The experiment consisted of three successive conditions: a control condition (10 trials), the HS1 (50 trials), and the HS1S2 (50 trials). To keep the participants motivated, the fifty trials for each HS1 and HS1S2 were divided into 5 sets (10 trials/set). In between the sets, a brief break was given and the examiner reminded the participants to perform the task as quickly and as accurately as possible. A few familiarization trials were performed before data collection of each condition. A total of 110 trials were analyzed. For the recording of hand movements, an infrared emitting diode marker was attached to the stylus tip, and the marker position was recorded at 240 Hz (Optotrak system, Northern Digital). Horizontal eye movements were recorded at 240 Hz (ASL Eye-Trac 6000, Applied Science Laboratories).
Data Analysis
For hand movements, velocity was calculated as the first derivative of horizontal position data. Movement onset and offset was determined using a threshold criterion of 1.5 cm/s. To assess the general improvement of performance through practice, hand movement time to T1 was measured from the onset to the offset of the first segment. Inter-segment interval was also measured from the offset of the first segment to the onset of the second segment. For eye movements, velocity of saccades was calculated as the first derivative of horizontal position data from the left eye. Saccade onset to T2 was determined using a threshold criterion of 18.4°/s. To examine whether gaze anchoring was maintained or broken with practice, handoffset-to-eyeonset dwell time was measured from the offset of hand movement to T1 to the saccadic onset to T2. Further details of data processing were described previously [12].
For analysis, 50 trials within a practice condition were divided into ten blocks with five trials each. Data within a trial block were averaged for each participant, and this value was used to calculate the group’s mean for that block. Progressive changes in parameters across blocks were determined for each group using a linear regression analysis. To assess whether movements at the beginning or the end of each practice condition differed between experimental conditions, an average of the first ten trials or last ten trials was calculated in each practice condition for each participant. An average of ten trials of the control condition was calculated for each participant. Furthermore, the within-subject SD of handoffset-to-eyeonset dwell time was calculated for each participant for the last ten trials of HS1 and HS1S2 as well as the first ten trials of HS1S2. Based on these average or SD values, the differences between various phases (early or late) of practice conditions as well as the control condition were examined using a paired t-test for each group separately. Group difference was assessed using an independent t-test. The probability level for statistical significance was p<0.05.
RESULTS
Since adaptability of gaze anchoring through practice has never been examined before, normal practice effects are established first in young adults without any influence from aging effect (e.g., increased movement variability). Next, aging effect on adaptability of gaze anchoring is examined in the older group.
Hand movement time of the first segment
Young adults
Movement time (Fig. 1, black circles) was similar between the control condition (CTL) and that of the first ten trials of the HS1 condition (shown in the first two blocks of Fig. 1-HS1). Thereafter, it gradually decreased as more trials were performed for HS1. Accordingly, a negative trend across blocks was significant (correlation coefficient: r=0.95, p<0.001, slope=−8.0). When practice was switched from HS1 to HS1S2, the average value of the first-ten HS1S2 trials did not differ from that of the last-ten HS1 trials (p>0.05). The movement time continued to decrease across blocks of HS1S2 (Fig. 1, HS1S2), showing a significant negative trend (correlation coefficient: r=0.82, p<0.01, slope=−2.8). The average value of the last ten trials of HS1S2 was smaller than that of HS1 (t(9)=2.74, p<0.05).
Figure 1.
Changes in hand movement time of the first segment through practice. A mean value of ten trials from all participants is plotted for each group for the control condition (CTL). In the hand-stop1 condition (HS1) and the hand-stop1-stop2 condition (HS1S2), fifty trials for each condition were divided into ten blocks with five trials each. Mean values of all participants are plotted against blocks. Black and white circles refer to the young and old groups, respectively. The error bars represent standard errors
Older adults
Movement time of the older adults decreased with practice for both HS1 and HS1S2 (Fig. 1, white circles). Accordingly, a negative trend across blocks was significant (correlation coefficient: r=0.91, p<0.001, slope=−22.9 for HS1; r=0.93, p<0.001, slope=−9.8 for HS1S2). The movement time significantly increased for the first-ten HS1S2 trials compared to the last-ten HS1 trials (Fig. 1, t(9)=2.3, p<0.05). Thus, practice effect obtained from HS1 was not fully transferred to HS1S2. Movement time of the last ten trials did not differ between HS1 and HS1S2.
Group difference in the movement time was significant for the control condition (t(18)=4.0, p<0.01). However, it was minimized after the practice: only a trend of group difference was found for the last ten trials of HS1 (t(18)=1.7, 0.05<p<0.1) and that of HS1S2 (t(18)=1.9, 0.05<p<0.1). Overall, the hand movements became faster with practice in both groups for both practice conditions.
In addition, inter-segment interval of hand movements in the HS1S2 condition was measured to assess whether a pause made at the transition from the first to the second segment were different depending on aging and practice. The young adults maintained a similar inter-segment interval from the first ten trials (32±9 [mean±SE] ms) to the last ten trials (29±8 ms, p>0.05). The older adults tended to decrease the interval from the former (195±38 ms) to the latter (153±33 ms, t(9)=2.1, 0.05<p<0.1). The older adults produced significantly longer intervals compared to the young adults (first ten trials: t(18)=4.1, p<0.01; last ten trials: t(18)=3.6, p<0.01).
Gaze anchoring
Young adults
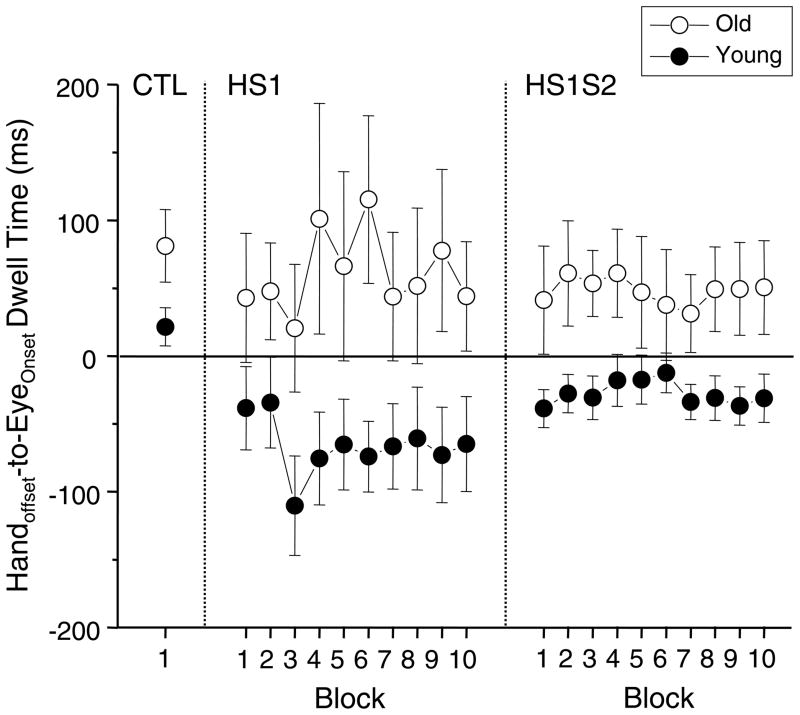
To illustrate changes of gaze anchoring through practice, the average handoffset-to-eyeonset dwell times across all participants are shown in Fig. 2 (black circles). The control condition (CTL) showed a positive value, indicating that gaze was fixated to T1 until shortly after pointing completion. Conversely, the average value for the first-ten HS1 trials was negative and tended to be smaller than that of CTL (t(9)=2.1, 0.05<p<0.1). The negative value indicates that the gaze fixation to T1 was terminated before pointing completion, thereby breaking gaze anchoring.
Figure 2.
Changes in handoffset-to-eyeonset dwell time through practice. The formats of the plots are the same as in Fig. 1
The dwell time oscillated substantially during the HS1 practice (Fig. 2, HS1). It decreased from the 2nd to the 3rd block, but increased again for the 4th block. Thereafter, it was stabilized. These changes seem to reflect the following: 1) the first practice set was used as a familiarization of eye-hand coordination under this task (1st and 2nd blocks); 2) thereafter, the timing of gaze anchoring termination was set to an earlier point than before for the next practice set in order to perform the task faster (3rd block); 3) the timing was evaluated as too early for this task, and it was compensated to a later timing (4th block); 4) the compensated timing was evaluated as preferable, and maintained thereafter. Due to this oscillation, a negative trend found in relation to trial blocks was non-significant (slope=−1.5). Nevertheless, the dwell times were negative for all trial blocks. The average value of the last-ten HS1 trials was −69 ms, which was significantly smaller than that (22 ms) of CTL (t(9)=2.41, p<0.05, see Fig. 2).
When practice was switched to HS1S2, the average dwell time of the first-ten HS1S2 trials was increased slightly, but non-significantly, from that of the last-ten HS1 trials (Fig. 2). However, that value of early HS1S2 trials was still negative, which contrasted to the positive value observed in CTL. Accordingly, the difference between HS1S2 (first ten trials) and CTL was significant (t(9)=4.4, p<0.01). During the HS1S2 practice, young adults maintained stable, but negative dwell times (Fig. 2, HS1S2). Thus, no significant trend of change across blocks was found (p>0.05). The average value of the last-ten HS1H2 trials (−34 ms) was significantly smaller than that of CTL (t(9)=3.46, p<0.01).
Additionally, within-subject variability of the dwell time was significantly reduced from the last-ten HS1 trials (59 ms on average across participants) to the first-ten HS1S2 trials (41 ms, t(9)=2.5, p<0.05). Across-subject variability was also smaller for HS1S2 compared with that of HS1, as can be seen in the error bars of Fig. 2. These results indicate that gaze anchoring was terminated in a narrower time window at around pointing completion in HS1S2 compared to that in HS1.
Older adults
The average dwell time was positive for the control condition, indicating that gaze was held to T1 until after pointing completion (Fig. 2-CTL, white circle). Contrary to the young adults, the older adults maintained positive average dwell times for all trial blocks of the HS1 practice. This parameter showed a large oscillatory change between an increase and a decrease during the 3rd to 6th blocks (Fig. 2, HS1). Consequently, a positive trend across blocks was non-significant (slope=1.5). The difference between CTL and the last ten trials of HS1 was non-significant (p>0.05), indicating that there was no improvement with practice.
When practice was switched to HS1S2, the dwell times for the first-ten HS1S2 trials were similar to those for the last-ten HS1 trials (Fig. 2-HS1S2, white circles). This parameter was stable throughout the practice blocks; hence, there was no significant trend across blocks. Furthermore, the average value of the last-ten HS1S2 trials was not significantly different from that of CTL (p>0.05). Thus, the older adults did not improve with the HS1S2 practice. Additionally, within-subject variability of the dwell time did not significantly differ between the last-ten HS1 trials (109 ms on average across participants) and the first-ten HS1S2 trials (73 ms) in the older adults (p>0.05), which stood in contrast to the young adults.
Regarding the group difference, the average dwell time of the older adults in the control condition tended to be greater than that of the young adults (t(18)=1.9, 0.05<p<0.1). However, after the practice, the group difference became more apparent. Accordingly, the average value of the last ten trials showed a significant group difference for both HS1 (t(18)=2.1, p<0.05) and HS1S2 (t(18)=2.3, p<0.05). Moreover, the older adults produced significantly greater within-subject variability in the dwell time compared to the young adults for the last ten trials of each practice condition (HS1: (t(18)=2.3, p<0.05; HS1S2: (t(18)=2.9, p<0.01).
DISCUSSION
Adaptation of gaze anchoring
Without practice, young adults maintained gaze anchoring until after pointing completion when the second pointing was included (control condition). Conversely, they broke gaze anchoring for the early HS1 trials where the second pointing was excluded. These findings are consistent with our previous study [12]. Thus, whether gaze anchoring is maintained or broken during pointing depends on the inclusion of a subsequent pointing movement. In other words, the oculomotor system takes into account the planning of a subsequent pointing before terminating gaze anchoring to a pointing target.
One purpose of this study was to examine whether young adults break gaze anchoring through practice when a subsequent pointing is included (i.e., HS1S2). Based on the prior HS1 practice, the young adults broke gaze anchoring from the early HS1S2 trials (Fig. 2). Thus, maintaining the gaze fixation until the pointing completion to T1 is not prerequisite for the planning of subsequent pointing. In both conditions, target size and location of T1 were the same. Hence, information obtained through the HS1 practice about the dynamics of the moving hand and its relationship with T1 must have contributed to the HS1S2 practice. As a result, the prediction of hand termination on T1 during pointing was improved, thereby facilitating the breaking of gaze anchoring.
It should be noted, however, that this study used a fixed condition order for practice (HS1 first, then HS1S2). Hence, it is not known whether practice specific to HS1 is prerequisite for breaking gaze anchoring in the HS1S2 condition. It is possible that general practice of HS1S2 per se is enough for that breaking. A future study that alters the practice order between HS1S2 and HS1 will be required to distinguish these two possibilities.
The handoffset-to-eyeonset dwell time was not modified during the HS1S2 practice. These results suggest that once the preferable timing of terminating gaze fixation is established based on a given task requirement and prior experience, further practice does not keep advancing that timing relative to pointing completion. This contention is supported by the results of HS1 practice in young adults. The dwell time was modified at the early HS1 blocks, but thereafter it was stabilized at about −70 ms on average (Fig. 2). Since there was no need to plan subsequent pointing under the HS1 condition, the main contribution of gaze anchoring during this condition is to guide the hand to T1 and assess pointing completion [1,3,5,12]. Hence, about 70 ms before the pointing completion seems to be a preferable timing under the current task for the oculomotor system to complete that assessment and terminate gaze anchoring.
It is noteworthy that across-subject and within-subject variability of the handoffset-to-eyeonset dwell time was substantially reduced for HS1S2 compared to HS1 in young adults. Furthermore, the average dwell times for HS1S2 were less negative compared to HS1 (Fig. 2), even though this difference was not statistically significant. These results suggest that planning of next pointing to T2 imposes a narrow range of timing for gaze anchoring termination which is close to the time of pointing completion. This is likely due to a need to register the initial hand location for the planning of a next pointing [2,14]. If such planning has to be taking place while the hand is still far away from T1, it would require the utilization of internal models capable of estimating the state of the neuro-muscular system involving the hand located at T1 ahead of time, and incorporate that information for planning. That would require more complex information processing than using information about the state where the hand is very near or on T1. Thus, the gazes likely have to be held until the hand is close to a pointing target while planning of the next pointing is taking place.
Aging and gaze anchoring
Without practice, the older adults did not break gaze anchoring for both control and HS1 (early trials) conditions, which coincides with our previous study [13]. Another purpose of this study was to examine whether older adults break gaze anchoring to T1 with practice when a second pointing is not included. The older adults failed to break gaze anchoring through practice when the second pointing was not included (HS1, Fig. 2), and also when it was included (HS1S2). Hence, the difficulty of older adults in breaking gaze anchoring is not due to the lack of practice, but due to an age-related deficit.
These results suggest that aging compromises the predictive assessment of pointing completion to terminate gaze anchoring. It should be noted, however, that when a hand termination at T1 was not required in our previous study (moving through T1 instead of stopping on it) [13], older adults were as capable as young adults in predicting the timing of the hand arrival to that target. However, they were less able to predict whether the hand would be stopped at a target, resulting in a delayed termination of gaze anchoring. The current study further demonstrated that such a deficit of older adults cannot be overcome with short-term practice. Furthermore, the same deficit likely attributed to a greater-than normal variability of handoffset-to-eyeonset dwell time found in the older adults. Possible underlying causes for this deficit are 1) that older adults have a problem to dissipate the energy of the moving hand to make a complete stop at the target, and 2) that they have difficulty to process various sensory signals to effectively determine the complete stop of hand movements. Indeed, an age-related proprioceptive degeneration [6,11] and position sense deterioration [8,14] were reported previously. These difficulties would necessitate utilizing more visual feedback to estimate the hand termination in older adults, thereby enhancing gaze anchoring.
Acknowledgments
This research was supported by a grant from National Institute on Aging AG31366. We thank Ms. Lydia Anderson for her help in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowman MC, Johansson RS, Flanagan JR. Eye-hand coordination in a sequential target contract task. Exp Brain Res. 2009;195:273–283. doi: 10.1007/s00221-009-1781-x. [DOI] [PubMed] [Google Scholar]
- 2.Desmurget M, Rossetti Y, Prablanc C, Stelmach GE, Jeannerod M. Representation of hand position prior to movement and motor variability. Can J Physiol Pharmacol. 1995;73:262–272. doi: 10.1139/y95-037. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424:769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- 4.Folstein M, Folstein S, McHugh P. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 5.Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci. 2001;21:6917–6932. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Exp Brain Res. 1996;108:129–139. doi: 10.1007/BF00242910. [DOI] [PubMed] [Google Scholar]
- 7.Hayhoe MM, Shrivastava A, Mruczek R, Pelz JB. Visual memory and motor planning in a natural task. J Vis. 2003;3:49–63. doi: 10.1167/3.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Madhavan S, Shields RK. Influence of age on dynamic position sense: evidence using a sequential movement task. Exp Brain Res. 2005;164:18–28. doi: 10.1007/s00221-004-2208-3. [DOI] [PubMed] [Google Scholar]
- 9.Neggers SFW, Bekkering H. Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol. 2000;83:639–651. doi: 10.1152/jn.2000.83.2.639. [DOI] [PubMed] [Google Scholar]
- 10.Neggers SFW, Bekkering H. Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol. 2001;86:961–970. doi: 10.1152/jn.2001.86.2.961. [DOI] [PubMed] [Google Scholar]
- 11.Petrella RJ, Lattanzio PJ, Nelson MG. Effect of age and activity on knee joint proprioception. Am J Phys Med Rehabil. 1997;76:235–241. doi: 10.1097/00002060-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Rand MK, Stelmach GE. Effects of hand termination and accuracy constraint on eye-hand coordination during sequential two-segment movements. Exp Brain Res. 2010a;207:197–211. doi: 10.1007/s00221-010-2456-3. [DOI] [PubMed] [Google Scholar]
- 13.Rand MK, Stelmach GE. Effects of hand termination and accuracy requirements on eye-hand coordination in older adults. Behav Brain Res. 2010b doi: 10.1016/j.bbr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero DH, Van Gemmert AWA, Adler CH, Bekkering H, Stelmach GE. Time delays prior to movement alter the drawing kinematics of elderly adults. Hum Mov Sci. 2003;22:207–220. doi: 10.1016/s0167-9457(02)00160-4. [DOI] [PubMed] [Google Scholar]