Abstract
Background
The fibroblast growth factor (FGF) system has been implicated in the pathophysiology of mood disorders in humans and in affective behavior in animal models. However, the studies have been either correlative or involved exogeneous administration of FGF2. None of them have directly linked endogenous FGF2 to changes in emotional responses. Therefore, we began a series of studies to knockdown FGF2 by RNA interference to examine the role of brain FGF2 in emotional responsiveness.
Methods
We assessed the efficacy of shRNA sequences targeted to FGF2 in COS7 cells transfected with a plasmid vector containing the full-length FGF2 sequence. We then sought to assess the effects of knocking down FGF2 gene expression in vivo on behavior. We microinjected a lentiviral vector containing either a short-hairpin RNA (shRNA) targeting FGF2 or a non-silencing sequence bilaterally into the dentate gyrus (DG) of the rat.
Results
In a reporter assay system, three different shRNA sequences resulted in significant FGF2 knockdown in vitro. Five weeks following a single microinjection of one of those sequences in vivo, we observed a significant decrease in FGF2 gene expression by mRNA in situ hybridization in the hippocampus. FGF2 knockdown increased the time spent in the closed arms of the elevated-plus maze (EPM), a test of anxiety behavior.
Conclusions
FGF2 knockdown in the hippocampus resulted in an anxiogenic effect. Together with our findings of an inverse correlation between anxiety and FGF2 expression levels, these results implicate FGF2 in the genesis and expression of anxiety disorders.
Keywords: fibroblast growth factor, dentate gyrus, elevated plus-maze, mRNA, lentiviral, gene
INTRODUCTION
The fibroblast growth factor (FGF) system modulates several important functions in the the central nervous system (CNS), including neurogenesis. The role of FGF2 in ischemia, as well as neurodegenerative disorders has been well studied (1–4). However, its role in mood disorders has only been investigated within the last decade. In post-mortem brains of individuals with major depressive disorder (MDD), FGF2 gene expression was downregulated in several cortical and limbic structures, including the hippocampus (5, 6).
Stress exposure or glucocorticoid administration increase FGF2 expression in the hippocampus in animal models (7–13). Specifically, hippocampal FGF2 gene expression was increased in response to anxiogenic stimuli, such as acute restraint (9). Moreover, acute corticosterone administration uregulated FGF2 in the hippocampus (11). The acute response of FGF2 upregulation in response to stress activation is thought to be neuroprotective. Under chronic conditions, such as repeated stress, adrenalectomy, and chronic administration of corticosterone, FGF2 gene expression was decreased (7–13). We previously have shown that repeated social defeat stress, an animal model of depression, decreased FGF2 gene expression in the hippocampus (14). We have also observed a positive correlation between the peak in gene expression of FGF2 in the hippocampus and the peak of corticosterone secretion (unpublished observations). However, glucocorticoid modulation or secretion has not yet been studied following FGF2 administration or in FGF2 knockout mice. Taken together, these finding lend support for chronic knockdown of FGF2 in the hippocampus resulting in an animal that may be more vulnerable to a stressor.
The hippocampus plays a variety of critical roles in processing emotionally salient information and controlling behavior (15–17). More recent evidence suggests that the hippocampus may play a functional role in modulating anxiety-like behavior. For example, hippocampal lesions and direct intra-hippocampal injections have been shown to alter anxiety-like behavior (15, 18–20). Furthermore, the role of the hippocampus in modulating individual differences in anxiety-like behavior has previously been documented (21). Here, animals that naturally differ in anxiety-like behavior exhibit differences in hippocampal glucocorticoid receptor (GR) expression. Specifically, high anxiety animals exhibit higher gene expression of GR compared to low anxiety animals. Moreover, the differences in hippocampal GR expression are likely responsible for the individual differences in anxiety-like behavior, as hippocampal microinjections with a GR antagonist disrupt the individual differences.
Further support for the role of FGF2 in mood disorders was found following pharmacological manipulations. For example, chronic antidepressants increased hippocampal FGF2 (22, 23). Studies from our laboratory have revealed antidepressant and anxiolytic effects of chronically administered FGF2 (24, 25). Interestingly, Perez et al. (18) reported an increase in the survival of adult-born neurons and glia in the dentate gyrus (DG) after chronic FGF2 administration. The effect on hippocampal neurogenesis was observed in animals that were genetically more prone to anxiety and exhibited an anxiolytic response to chronic FGF2 administration. FGF2 administration more than likely decreased the vulnerability to anxiety by increasing the survival of adult stem cells, particularly the generation of new astrocytes. This led us to the hypothesis that basal levels of FGF2 and the modulation of those levels, particularly in the hippocampus, play a role in vulnerability or resilience to anxiety.
The body of work described above, while highly suggestive, never directly established a causal relationship between endogenous levels of FGF2 in the hippocampus and anxiety behavior. To address this issue, it is critical to demonstrate two features of FGF2 in relation to affective behavior a) that basal levels of FGF2 mRNA in the hippocampus correlate with a behavioral index of emotionality and b) that direct manipulation of endogenous FGF2 expression has an impact on emotional responsiveness. In this study, we address the causal relationship between hippocampal FGF2 and anxiety behavior by correlating FGF2 expression with responsiveness in a test of anxiety, and then knocking down its expression in the hippocampus and ascertaining the results.
We relied on RNA interference (RNAi) as a method to selectively decrease FGF2 expression, as RNAi represents an efficient and specific tool to suppress gene expression in mammalian cells (26). In this study, we coupled RNAi to a viral vector, which would allow us to inject it once and allow recovery prior to any behavioral testing. RNA interference in vivo, using transgenic approaches or viral transfer, has recently proven to be useful in elucidating the neural function of a number of specific transcripts (27–32). In particular, when combined with lentiviral vectors, shRNAs have been shown to be stably expressed in the CNS in vivo for several months (27). Thus, in the present study, we describe the development of a targeted RNA interference system using a lentiviral vector expressing an shRNA sequence targeted to FGF2, and we test the hypothesis that its microinjection into the dentate gyrus increases anxiety-like behavior.
METHODS AND MATERIALS
shRNA and lentivirus construction
Target sites for rat FGF2 mRNA (accession number: NM_019305) were selected using RNAi design sites (Dharmacon and Invitrogen). Three short-hairpin RNAs (shRNA) targeting different regions of FGF2 mRNA and a scrambled non-silencing (NS) control shRNA that does not correspond to any mammalian mRNA were generated as nucleotide (nt) inverse repeats separated by a 9-nt loop sequence (UUCAAGAGA). The target sites were as follows: FGF2 shRNA-1 (784–804), FGF2 shRNA-2 (862–882), and FGF2 shRNA-3 (797–817), see Table 1. The shRNA sequences were inserted downstream of the U6 promoter in the lentiviral vector pLL3.7 which also expressed GFP, and the resultant knockdown efficacy was tested in vitro in COS7 cells. All shRNA constructs in the vector were verified before use by sequencing.
Table 1.
Non-silencing and FGF2 shRNA sequences used in the in vitro experiment.
| shRNA | Sequence |
|---|---|
| NS shRNA | UUCUCCGAACGUGUCACGUUU UUAAGAGGCUUGCACAGUGCA |
| FGF2 shRNA-1 | GAAGGAAGAUGGACGGCUGUU UUCUUCCUUCUACCUGCCGAC |
| FGF2 shRNA-2 | CUACAACACUUACCGGUCAUU UUGAUGUUGUGAAUGGCCAGU |
| FGF2 shRNA-3 | CGGCUGCUGGCUUCUAAGUGU UUGCCGACGACCGAAGAUUCA |
Based on its high efficacy and low toxicity in vitro, FGF2 shRNA-1 was chosen for use in vivo. Sequences with high toxicity (FGF2 shRNA-2 and FGF2 shRNA-3) typically exhibited a confluency of less than 40%, whereas confluency was 90–100% in non-toxic conditions. Lentiviral vectors containing either FGF2 shRNA-1 or NS shRNA were sent to the University of Michigan Vector Core and lentiviruses expressing FGF2 shRNA-1 and NS shRNA were generated, yielding LVshFGF2-1 and LVshNS, respectively (5×108 transducing units).
Cell culture studies
COS7 cells were grown in DMEM medium supplemented with 10% fetal bovine serum. Since COS7 cells do not express FGF2 constitutively, rat FGF2 cDNA was introduced into the cells by a plasmid vector. The psiCHECK2 vector (Promega) was chosen for this purpose because of its dual reporter specificity. Briefly, full length rat FGF2 cDNA (a generous gift from Dr. Peter Cattini) was subcloned downstream of the renilla luciferase (RL) reporter enabling expression of a fused mRNA, to which shRNAs bind and cleave resulting in the degradation of both products. The firefly luciferase (FL) reporter enabled normalization of RL expression. The ratio of RL/FL was used as a measure of knockdown efficiency.
One day after the cells were plated onto 6-well plates, the cells were co-transfected with the psiCHECK2 vector containing rat FGF2 cDNA and the pLL3.7 vector containing either the NS shRNA or one of the FGF2 shRNA sequences (n = 3 wells/group). The concentration of plasmid transfected was 1ng/μl. For a description of the lentivirus, readers are referred to Mahairaki et al. (30). T-2 transfection reagent was used to facilitate transfection (Dharmacon). Forty-eight hours after transfection, the cells were lysed with passive lysis buffer and RL and FL of the lysates were determined according to the manufacturer’s instructions (Promega).
Animals
Eleven male Sprague-Dawley rats from generation 5 of an in-house breeding colony weighing 385–450 g were used for the initial assessment of FGF2 gene expression and anxiety-like behavior, some of which had undergone 21 days of an enriched environment paradigm (for methods see reference 18). Twenty-three male Sprague-Dawley rats (Charles River Laboratories) weighing 350–400 g were used for the shRNA microinjection study. These animals were habituated to the housing conditions for one week before any manipulations. All animals were housed under a 12-hour light/dark cycle with food and water available ad libitum. The animals were singly housed for the first three days following surgery. For the remainder of the experiment, the animals were housed two per cage, and the weight of the animals was monitored carefully throughout the experiment. All of the procedures were performed in accordance with the National Institutes of Health Guidelines on Laboratory Animal Use and Care and in accordance with the guidelines set by the university committee on use and care of animals at the University of Michigan.
Surgeries
Under isoflurane anesthesia, 33-gauge microinjectors were lowered bilaterally into the DG (coordinates from bregma: AP −5.0, ML ± 3.5, DV −2.6). Microinjectors were connected to Hamilton syringes mounted on a syringe pump (Harvard Apparatus) by PE-50 tubing. Animals were microinjected with 1μl of either LVshFGF2-1 or LVshNS over 4 minutes at a rate of 0.25 μl/min. After 5 minutes the microinjectors were removed slowly over 4 minutes to allow for diffusion of the virus into the DG. The holes were filled with bone wax, and staples were used to close the wound. The staples were removed between days nine and thirteen for all of the animals. The animals received a pre-operative injection of the analgesic flunixamine (2mg/kg, s.c.) and a post-operative injection of 0.9% saline (1ml/kg, i.p.).
Elevated plus-maze (EPM)
Either one-week after habituation or four weeks following surgery, animals were tested for anxiety-like behavior on the EPM between 0800 and 1100. The EPM is a plus-shaped, black Plexiglas apparatus with four elevated arms. All arms are 70 cm high from the floor, 45 cm long, and 12 cm wide. Two opposing arms are surrounded by 45-cm-high walls (closed arms), whereas the other two arms are open (open arms). In the center of the arms is a 12 × 12 cm square with access to all four arms. The test was performed in a dimly lit room and behavior was monitored for 5 min using a computerized video tracking system (Ethovision, Noldus Information Technology). At the beginning of the test, each rat was placed in the center facing a closed arm. Total time spent in the center, open and closed arms was recorded for each animal and averaged for each group.
In situ hybridization
To determine the correlation between basal anxiety-like behavior and FGF2 gene expression, animals not injected with lentivirus were sacrificed immediately after testing in the elevated plus-maze. To determine the effect of FGF2 knockdown on gene expression, animals injected with the lentivirus were sacrificed one week after elevated plus-maze testing. The rationale for waiting a week in the injected animals to confirm FGF2 knockdown is because behavioral testing can have affect gene expression, and we did not want to introduce this confound. The brains were removed and snap frozen in isopentane (−80°C). Either 20 μm for the animals used in the correlation study, or ten-micrometer thick sections for animals injected with the lentivirus were taken from the hippocampus every 200 μm at −20°C. The difference in section thickness is because the correlation experiment was performed at a different point in time than the lentiviral experiment. Both section thicknesses yield quantifiable results, and the results were not compared between studies (24). The placement of the microinjectors was determined by cryostat sectioning through the infusion site. The method for in situ hybridization was previously described elsewhere (21). The FGF2, FGFR1 and β-actin probes were synthesized in our laboratory. The following sequences of rat FGF2 mRNA (accession number: NM_019305, bp 716–994), FGFR1 mRNA (accession number: NM_024146, bp 320–977), and βactin (accession number: NM_031144, bp 211–844) were used for generating the probes. Bactin and FGFR1 were quantified in sections adjacent to FGF2. Following the appropriate exposure times for FGF2 (17 days), FGFR1 (7 days), and β-actin (3 hours), the films were developed (Kodak D-19) and scanned. Radioactive signals were quantified using computer-assisted optical densitometry software (Scion Image). Integrated optical densities (IOD) were determined by outlining the region of interest for the dorsal hippocampus (500 μm before and after the injection site) and averaged for each group. This distance was selected because the spread of GFP corresponded to +/− 500 μm from the injection site. The rationale for the dorsal hippocampus is discussed in the discussion section. IOD measurements were corrected for background. Data were presented as mean ± S.E.M.
Immunofluorescent staining
Sections were fixed in 4% paraformaldehyde for 1 hour and then rinsed in 0.1M phosphate buffer (PB). Sections were blocked in blocking solution (0.3% Triton X-100, 1% normal goat serum, 1% bovine serum albumin in 0.1M PB) at room temperature (RT) for 1 hour and then incubated in a rabbit FGF2 antibody (Chemicon; 1:500) and a chicken GFP antibody (Abcam; 1:2000) at −4°C. Forty-eight hours later, tissue was rinsed and incubated in secondary antibodies (goat anti-rabbit AlexaFluor 546: 1:200; goat anti-chicken AlexaFluor 488: 1:200, for GFP and FGF2, respectively) for 2 hours at RT in the dark, rinsed and cover slipped with mounting media (Aquamount).
Statistical analyses
A Pearson’s correlation was performed to assess the relationship between FGF2 gene expression in the hippocampus and anxiety-like behavior, as assessed on the elevated plus-maze. The in vitro study was analyzed by a one-way analysis of variance (ANOVA) with Tukey’s posthoc comparisons. The elevated plus-maze and in situ hybridization studies for the lentiviral studies were analyzed by a Student’s t-test (SPSS 17.0, Chicago, Il.). All data are presented as mean +/− SEM.
RESULTS
Basal FGF2 gene expression and anxiety-like behavior
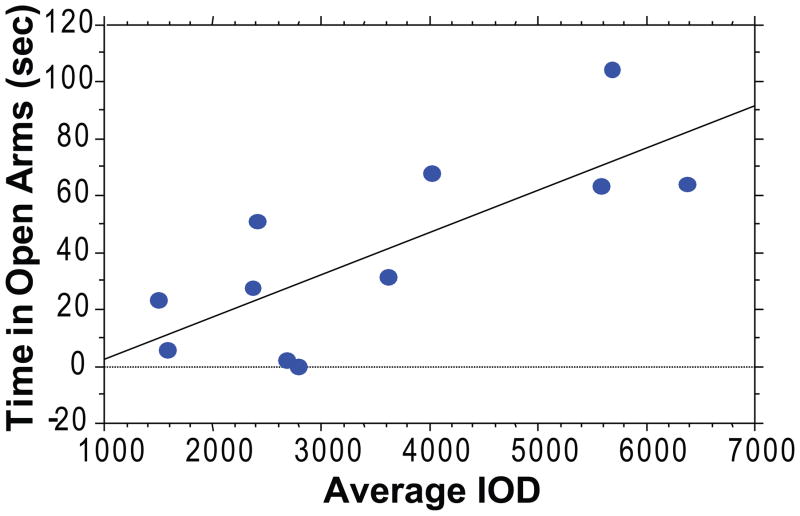
In order to investigate whether resting levels of FGF2 might modulate anxiety behavior, we correlated hippocampal expression of this growth factor with responsiveness in a test of spontaneous anxiety. Figure 1 is a scatterplot of the FGF2 average integrated optical density values for CA2 and the time spent in the open arms of the EPM for each animal. There was a positive correlation between hippocampal FGF2 gene expression and time spent in the open arms of the elevated plus-maze (r2 = 0.59, p < 0.004). This indicates that animals that are naturally less anxious exhibit higher expression of FGF2 endogenously. These results complement our previous findings in animals selectively bred to express differences in novelty-seeking and anxiety, in which we showed that the highly anxious animals have decreased expression of FGF2 in the dentate gyrus (18). Together, these findings point to the hippocampus as a key site in the modulation of anxiety behavior, and to FGF2 as a likely factor in this modulation. The remaining experiments were aimed at testing this idea more directly.
Figure 1. Relationship between FGF2 gene expression in the hippocampus and anxiety-like behavior on the elevated plus-maze.
There was a positive correlation between FGF2 gene expression in the hippocampus and time spent in the open arms of the elevated plus-maze. This indicates that lower levels of anxiety-like behavior are associated with higher FGF2 gene expression in CA2 (p < 0.003). Data are expressed as the mean integrated optical density value and the total time spent in the open arms of the elevated plus-maze for each animal (n = 11).
Screening of shRNA sequences in vitro targeted to FGF2
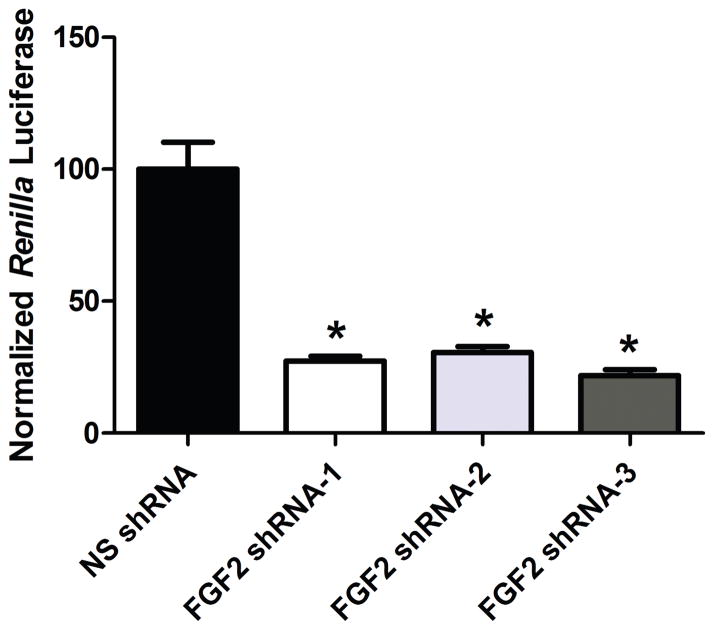
In order to find an efficient shRNA targeting FGF2 mRNA, the knockdown efficacy of three different FGF2 shRNAs was tested in COS7 cells. Cells were co-transfected with psiCHECK2 containing FGF2 cDNA and pLL3.7 expressing either the control NS shRNA or one of the three FGF2 shRNAs. The luminescence of the cell lysates was measured, and the ratio of RL/FL was calculated as a measure of FGF2 knockdown. The knockdown efficacies of the FGF2 shRNAs were normalized to the RL/FL ratio of the control group (pLL3.7-NS shRNA transfected cells). All three FGF2 shRNA sequences resulted in significantly reduced normalized Renilla luciferase levels and, hence, degradation of FGF2 given the fused construct (F(3,11) = 46.62, p < 0.001). Specifically, there was a 72.5% knockdown by FGF2 shRNA-1 (p < 0.001). There was a 69.5% knockdown by FGF2 shRNA-2 (p < 0.001), and a 78% knockdown by FGF2 shRNA-3 (p < 0.001), see Figure 2. Since we observed cellular toxicity with FGF2 shRNA-2 and FGF2 shRNA-3, we chose to proceed with FGF2 shRNA-1 for the subsequent in vivo study.
Figure 2. Knockdown efficacy of three different shRNA sequences targeted to FGF2 in COS7 cells.
The in vitro knockdown efficacies of FGF2 shRNA-1, FGF2 shRNA-2, FGF2 shRNA-3 were normalized to the RL/FL ratio of the control NS shRNA group (n = 3 wells/group). All three FGF2 shRNA sequences resulted in significant knockdown in vitro (p < 0.001). Data are expressed as mean +/− SEM.
Knockdown of FGF2 in rat hippocampus by LVshFGF2-1 microinjection
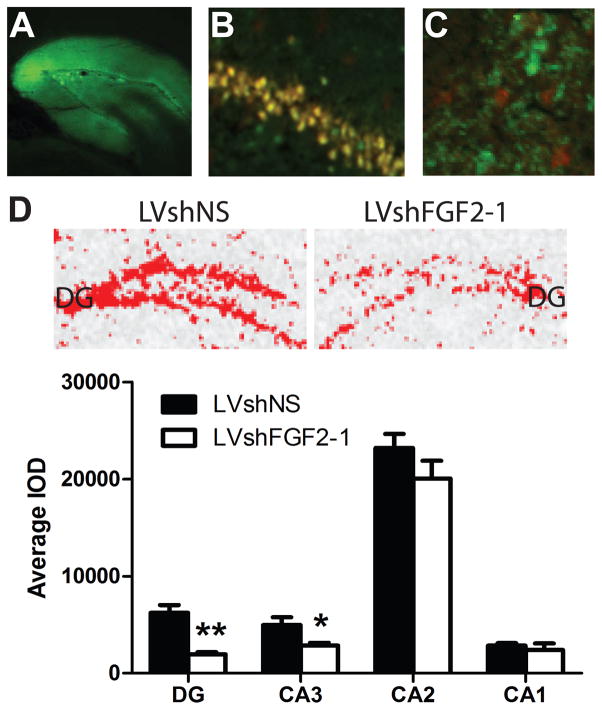
We then investigated whether FGF2 shRNA-1 would decrease FGF2 gene expression in vivo. For this purpose, we microinjected a lentivirus construct containing FGF2 shRNA-1 bilaterally into the DG (LVshFGF2-1). As a control we microinjected a lentivirus construct containing a non-silencing (NS) shRNA (LVshNS) bilaterally into the DG. The GFP signal was confined to the region containing the DG and the area between the granule cell layers (hilus and some of CA3), see Figure 3A. In the LVshNS group, GFP and FGF2 signals were found in the same cells in the LVshNS group, see Figure 3B. In the LVshFGF2-1 group, GFP and FGF2 signals were found in distinct cells, see Figure 3C. Since the FGF2 signal was not localized to cells labeled with GFP, we concluded that there was successful knockdown of FGF2 protein in the cells that were transduced with the lentivirus.
Figure 3. Knockdown efficacy of LVshFGF2-1 in the dentate gyrus.
(A) Confirmation of transduction of a lentivirus construct expressing GFP within and around the dentate gyrus by immunofluorescence (5X). (B) Representative photomicrograph illustrating FGF2 (shown in red) and GFP (shown in green) and their co-localization (shown in yellow) in CA3 of the hippocampus in the LVshNS condition (C) Representative photomicrograph illustrating that FGF2 (shown in red) was not expressed in the same cells that expressed GFP (shown in green) in CA3 of the hippocampus in the LVshFGF2-1 condition. (D) (Top panel) Representative FGF2 mRNA expression in the dentate gyrus of LVshNS animals (left panel) and LVshFGF2 animals (right panel). Red color denotes high level of expression. (Bottom panel) Average integrated optical density values for FGF2 gene expression by hippocampal subfield. The LVshFGF2-1 group showed less FGF2 expression in the DG and CA3 when compared to the control LVshNS group (**p < 0.0005, *p < 0.05). Data are expressed as mean +/− SEM (n = 7/group).
To determine whether the LVshFGF2-1 decreased FGF2 in the DG, FGF2 gene expression was determined by mRNA in situ hybridization in all hippocampal subfields. As shown in Figure 3D, FGF2 gene expression in the DG was significantly lower in the LVshFGF2-1 injected group than the LVshNS injected group (t12 = 4.82, p < 0.005). In the LVshFGF2-1 injected group the knockdown of FGF2 was also significantly lower in CA3 than the LVshNS injected group, a region adjacent to the injection site (t12 = 2.62, p < 0.05). However, FGF2 gene expression did not differ in either CA2 (t12 = 1.33, p = 0.21) or CA1 (t12 = 0.56, p = 0.59) between the two groups. Thus, FGF2 gene expression was decreased within the microinjection region and extended somewhat into CA3.
As a control, we also assessed one of the receptors for FGF2, FGFR1, by mRNA in situ hybridization. FGFR1 is also found in abundance in the DG and other regions of the hippocampus. As shown in Table 2, there were no differences in FGFR1 gene expression in the regions where FGF2 was altered. Specifically, FGFR1 was not different between LVshNS and LVshFGF2-1 in the DG (t12 = −0.22, p = 0.83), CA3 (t12 = −1.05, p = 0.31) or CA2 (t12 = −0.42, p = 0.68). Interestingly, FGFR1 gene expression was higher in LVshFGF2-1 than LVshNS in CA1 (t12 = −2.31, p < 0.05). This suggests that the manipulation was specific for FGF2 in the microinjection region. However, it also suggests that altering FGF2 expression long-term may result in compensatory alterations in FGFR1 in other subfields.
Table 2.
Average integrated optical density values for FGFR1 gene expression by hippocampal subfield.
| Group | DG | CA3 | CA2 | CA1 |
|---|---|---|---|---|
| LVshNS | 73985±13335.5 | 148281±12659.8 | 21257±2106.3 | 71859±7559.6 |
| LVshFGF2-1 | 77363±7022.4 | 163627±7365.1 | 22223±869.9 | 95601±6948.7* |
p < 0.05, n = 8/group
To assess structural integrity, we performed an additional control mRNA in situ hybridization experiment in the hippocampus using β-actin. There was no significant difference in β-actin gene expression between LVshNS and LVshFGF2-1 animals in either the dentate gyrus (t12 = −0.18, p = 0.86), CA3 (t12 = 1.28, p = 0.22), CA2 (t12 = .47, p = 0.65) or CA1 (t12 = 1.12, p = 0.29) subfield. Therefore, the structural integrity of the hippocampus was maintained following the knockdown of FGF2.
Effects of FGF2 knockdown on anxiety-like behavior
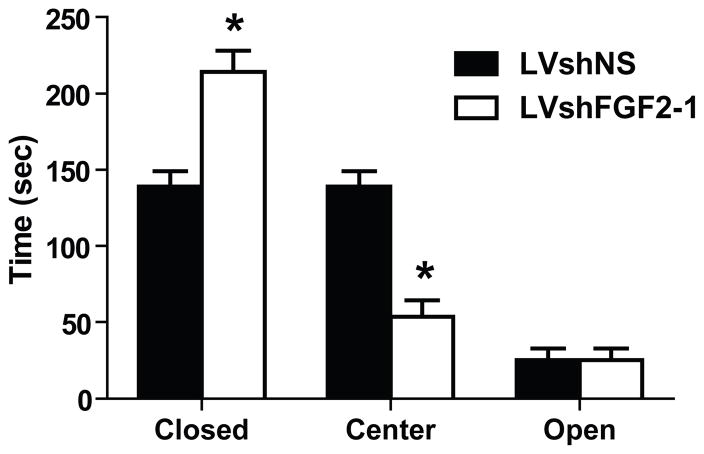
In order to examine the effects of FGF2 knockdown on anxiety-like behavior, animals were tested on the EPM. The LVshFGF2 group spent more time in the closed arms than the LVshNS group (t21 = −4.30, p < 0.001). Furthermore, the LVshFGF2 group spent less time in the center than the LVshNS group (t21 = 5.77, p < 0.001). However, there were no difference between the two groups in the open arms (t21 = 0.66, p = 0.52). Thus, as expected, the LVshFGF2-injected rats displayed higher anxiety-like behavior than the LVshNS-injected rats, see Figure 4.
Figure 4. Effect of FGF2 knockdown in the dentate gyrus on behavior.
Effect of FGF2 knockdown in the DG on anxiety-like behavior. LVshFGF2-1 microinjected group exhibited greater anxiety-like behavior on the EPM when compared with LVshNS animals (*p < 0.001). Data are expressed as mean +/− SEM (n = 11–12/group).
DISCUSSION
This study establishes a causal relationship between the expression of FGF2 in the hippocampus and the degree of spontaneous anxiety. We demonstrate that a) basal levels of FGF2 expression in the hippocampus negatively correlate with anxiety-like behavior- This is true both in the CA2 region, as shown in this current study in outbred animals, as well as in the dentate gyrus as we have previously shown in selectively bred animals; and b) knocking down the expression of FGF2 in the hippocampus by RNA interference results in increased anxiety behavior. This knockdown was effective, well-localized and offers convincing evidence of the role of endogenous hippocampal FGF2 in modulating affective behavior.
The careful validation of the lentiviral construct in vitro proved to be valuable in generating an effective tool for in vivo modulation of FGF2. We achieved highly efficient knockdown in the dentate gyrus where the shRNA targeting FGF2 mRNA was microinjected (69% decrease in endogenous mRNA levels) and in the adjacent CA3 region (43% decrease in expression level), whereas no knockdown effect was observed in other hippocampal subfields. Moreover, we did not observe any change in the expression of FGFR1 in DG or CA3 regions, confirming the specificity of the knockdown effect of the shRNA for FGF2.
Knockdown of FGF2 in the hippocampus resulted in an anxiogenic phenotype in rats, indicating a central role for FGF2 in mediating anxiety. These results are congruent with previous findings from our laboratory (18) that animals selectively bred for high anxiety/low novelty seeking, and who express lower FGF2 mRNA levels in the hippocampus, profit from chronic administration of FGF2 and reverse their highly anxious phenotype (24). It is worth recalling that beyond its role in modulating anxiety behavior, FGF2 is increased following chronic antidepressant treatments (22, 23). Moreover, we have shown that FGF2 has antidepressant-like effects when administered chronically to rodents (25). This may be of particular relevance in considering the translational implications of the findings on FGF2. The high comorbidity between anxiety disorders and depression, the longitidunal stability of comorbid anxiety and depression over 15 years (33) and findings demonstrating common genetic factors underlying both anxiety and depression (34) suggest that clincial anxiety and depression may be manifestations of the same underlying diathesis. Thus, our findings may also be interpreted as suggestive of a causative role of hippocampal FGF2 in vulnerability to depression, possibly via a greater vulnerability to anxiety. It was not possible to ascertain whether the decrease in FGF2 in post-mortem studies was a result of the disease process or causative (35). The body of evidence in animal models on the role of FGF2 both as a basal predisposing factor to anxiety behavior and as a transcript that responds readily to environmental stressors, strongly suggests that it might play a dual role in humans.
It would be interesting to consider whether other anxiety measures would also be altered following the knockdown of FGF2 in the hippocampus, such as social interaction. Similarly, it would be interesting to determine whether there are sex differences in anxiety-like behavior in response to the knockdown of FGF2. There are known sex differences in anxiety and depression, with women more susceptible to both disorders (36–38). In terms of sex differences in FGF2 in animal models, one study found that lesions of the medial forebrain bundled resulted in higher FGF2 immunoreactivity in the ventral tegmental area in males compared to females (39). It is plausible that there may be differences in anxiety-like behavior in male and female rats following FGF2 knockdown. However, we have not found sex differences in FGFR1 gene expression in the hippocampus under basal conditions (unpublished observations). Therefore, it is unlikely that knocking down FGF2 in the hippocampus would differentially affect anxiety-like behavior in males and females.
It should also be mentioned that there is regional dissociation of behavior in the hippocampus. The ventral hippocampus was found to be involved in the modulation of anxiety, while the dorsal hippocampus was found to be more involved in the modulation of learning (15) Although the ventral hippocampus has been traditionally thought of as the region that modulates anxiety, our lab has recently found alterations in FGF2 in the dorsal hippocampus that correspond to decreased anxiety-like behavior (24). Several reports have also found that direct microinjections into the dorsal hippocampus alters anxiety-like behavior, and this effect varies by neurotransmitter system (18–20). Taken together, these reports support the role of the dorsal hippocampus in anxiety.
While the FGF family plays an important role in neural development (40), less was known about the role in adulthood. The findings of the present study, coupled with the accumulating body of evidence from other recent work, underscore the central role of FGF2 during adulthood in the control of anxiety. Modulation of the FGF system during adulthood may be of relevance to the development of new strategies for the treatment of anxiety disorders. In conclusion, we successfully decreased FGF2 gene expression in the rat hippocampus by RNA interference. Knockdown of FGF2 in the DG and CA3 resulted in increased anxiety-like behavior. Thus, FGF2 may play a functional role in the development of anxiety disorders.
Acknowledgments
We would like to thank Sharon Burke, Jennifer Fitzpatrick, James Stewart and James Beals for their outstanding technical contributions. We would also like to thank Javier Perez for help with some of the experiments. This work was supported by the Pritzker Neuropsychiatric Disorders Research Consortium, NIH Conte Center Grant #L99MH60398, NIDA PPG #5P01DA021633-02 to HA & SJW, and ONR Grant #N00014-02-1-0879. This work was also supported by UL1RR024986 to CAT and by the Turkish Academy of Sciences as part of the fellowship program for integrated doctoral studies to EEK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ay H, Ay I, Koroshetz WJ, Finklestein SP. Potential usefulness of basic fibroblast growth factor as a treatment for stroke. Cerebrovasc Dis. 1999;9:131–135. doi: 10.1159/000015941. [DOI] [PubMed] [Google Scholar]
- 2.Date I, Yoshimoto Y, Imaoka T, Miyoshi Y, Gohda Y, Furuta T, et al. Enhanced recovery of the nigrostriatal dopaminergic system in MPTP-treated mice following intrastriatal injection of basic fibroblast growth factor in relation to aging. Brain Res. 1993;621:150–154. doi: 10.1016/0006-8993(93)90312-b. [DOI] [PubMed] [Google Scholar]
- 3.Otto D, Unsicker K. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci. 1990;10:1912–1921. doi: 10.1523/JNEUROSCI.10-06-01912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmer M, Cesnulevicius K, Winkler C, Kolb J, Lipokatic-Takacs E, Jungnickel J, et al. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci. 2007;27:459–471. doi: 10.1523/JNEUROSCI.4493-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akil H, Evans SJ, Turner CA, Perez J, Myers RM, Bunney WE, et al. The fibroblast growth factor family and mood disorders. Novartis Found Symp. 2008;289:94–96. doi: 10.1002/9780470751251.ch8. discussion 97–100, 193–105. [DOI] [PubMed] [Google Scholar]
- 6.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, et al. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank MG, Der-Avakian A, Bland ST, Watkins LR, Maier SF. Stress-induced glucocorticoids suppress the antisense molecular regulation of FGF-2 expression. Psychoneuroendocrinology. 2007;32:376–384. doi: 10.1016/j.psyneuen.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Fumagalli F, Bedogni F, Slotkin TA, Racagni G, Riva MA. Prenatal stress elicits regionally selective changes in basal FGF-2 gene expression in adulthood and alters the adult response to acute or chronic stress. Neurobiological Disorders. 2005;20:731–737. doi: 10.1016/j.nbd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Mocchetti I, Spiga G, Hayes VY, Isackson PJ, Colangelo A. Glucocorticoids differentially increase nerve growth factor and basic fibroblast growth factor expression in the rat brain. J Neurosci. 1996;16:2141–2148. doi: 10.1523/JNEUROSCI.16-06-02141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, et al. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- 12.Riva MA, Fumagalli F, Blom JM, Donati E, Racagni G. Adrenalectomy reduces FGF-1 and FGF-2 gene expression in specific rat brain regions and differently affects their induction by seizures. Brain Res Mol Brain Res. 1995;34:190–196. doi: 10.1016/0169-328x(95)00157-n. [DOI] [PubMed] [Google Scholar]
- 13.Riva MA, Fumagalli F, Racagni G. Opposite regulation of basic fibroblast growth factor and nerve growth factor gene expression in rat cortical astrocytes following dexamethasone treatment. J Neurochem. 1995;64:2526–2533. doi: 10.1046/j.1471-4159.1995.64062526.x. [DOI] [PubMed] [Google Scholar]
- 14.Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008;430:147–150. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 17.Roullet P, Lassalle JM. Genetic variation, hippocampal mossy fibres distribution, novelty reactions and spatial representation in mice. Behav Brain Res. 1990;41:61–70. doi: 10.1016/0166-4328(90)90054-i. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho MC, Masson S, Brandao ML, Silva MADS. Anxiolytic-like effects of substance P administration into the dorsal, but not ventral, hippocampus and its influence on serotonin. Peptides. 2008;29:1191–1200. doi: 10.1016/j.peptides.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- 20.Engin E, Treit D. The effects of intra-cerebral drug infusions on animals' unconditioned fear reactions: A systematic review. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1399–1419. doi: 10.1016/j.pnpbp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- 24.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21–nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 27.Delic S, Streif S, Deussing JM, Weber P, Ueffing M, Holter SM, et al. Genetic mouse models for behavioral analysis through transgenic RNAi technology. Genes Brain Behav. 2008;7:821–830. doi: 10.1111/j.1601-183X.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 28.Di Benedetto B, Wefers B, Wurst W, Kuhn R. Local knockdown of ERK2 in the adult mouse brain via adeno-associated virus-mediated RNA interference. Mol Biotechnol. 2009;41:263–269. doi: 10.1007/s12033-008-9125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garza JC, Kim CS, Liu J, Zhang W, Lu XY. Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. J Endocrinol. 2008;197:471–482. doi: 10.1677/JOE-08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahairaki V, Xu L, Farah MH, Hatfield G, Kizana E, Marban E, et al. Targeted knock-down of neuronal nitric oxide synthase expression in basal forebrain with RNA interference. J Neurosci Methods. 2009;179:292–299. doi: 10.1016/j.jneumeth.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santamaria J, Khalfallah O, Sauty C, Brunet I, Sibieude M, Mallet J, et al. Silencing of choline acetyltransferase expression by lentivirus-mediated RNA interference in cultured cells and in the adult rodent brain. J Neurosci Res. 2009;87:532–544. doi: 10.1002/jnr.21866. [DOI] [PubMed] [Google Scholar]
- 32.Wood L, Gray NW, Zhou Z, Greenberg ME, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J Neurosci. 2009;29:12440–12448. doi: 10.1523/JNEUROSCI.3321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merikangas KR, Zhang H, Avenevoli S, Acharyya S, Neuenschwander M, Angst J. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003;60:993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS. Major depression and generalised anxiety disorder. Same genes, (partly)different environments--revisited. Br J Psychiatry Suppl. 1996:68–75. [PubMed] [Google Scholar]
- 35.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young EA, Becker JB. Perspective: sex matters: gonadal steroids and the brain. Neuropsychopharmacology. 2009;34:537–538. doi: 10.1038/npp.2008.221. [DOI] [PubMed] [Google Scholar]
- 38.Young EA, Ribeiro SC, Ye W. Sex differences in ACTH pulsatility following metyrapone blockade in patients with major depression. Psychoneuroendocrinology. 2007;32:503–507. doi: 10.1016/j.psyneuen.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moroz IA, Rajabi H, Rodaros D, Stewart J. Effects of sex and hormonal status on astrocytic basic fibroblast growth factor-2 and tyrosine hydroxylase immunoreactivity after medial forebrain bundle 6-hydroxydopamine lesions of the midbrain dopamine neurons. Neuroscience. 2003;118:463–476. doi: 10.1016/s0306-4522(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 40.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]