Abstract
Purpose
The IGF1/IGF-1R signaling pathway has emerged as a potential determinant of radiation resistance in human cancer cell lines. Therefore we investigated the potency of monoclonal anti-IGF-1R antibody, A12, to enhance radiation response in upper respiratory tract cancers.
Methods and Materials
Cell lines were assessed for IGF-1R expression and IGF1-dependent response to A12 or radiation using viability and clonogenic cancer cell survival assays. In vivo response of tumor xenografts to 10 or 20 Gy and A12 (0.25–2 mg × 3) was assessed using growth delay assays. Combined treatment effects were also analyzed by immunohistochemical assays for tumor cell proliferation, apoptosis, necrosis and VEGF expression at days 1 and 6 after start of treatment.
Results
A12 enhanced the radiosensitivity of HN5 and FaDu head and neck carcinomas in vitro (p<0.05) and amplified the radioresponse of FaDu xenografts in a dose-dependent manner with enhancement factors ranging from 1.2 to 1.8 (p<0.01). Immunohistochemical analysis of FaDu xenografts demonstrated that A12 inhibited tumor cell proliferation (P<0.05) and VEGF expression. When A12 was combined with radiation, this resulted in apoptosis induction that persisted till 6 days from the start of treatment and in increased necrosis at day 1 (p<0.01, respectively). Combined treatment with A12 and radiation resulted in additive or sub-additive growth delay in H460 or A549 xenografts, respectively.
Conclusions
The results of this study strengthen the evidence for investigating how anti-IGF-1R strategies can be integrated into radiation and radiation-cetuximab regimen in the treatment of cancer of the upper aero-digestive tract cancers.
Keywords: IGF-1R, A12, radiation, head and neck cancer, lung cancer
INTRODUCTION
An emerging target in molecular cancer therapy is the insulin-like growth factor receptor 1 (IGF-1R) (1–5). The IGF-1R is a hetero-tetrameric protein with 2 identical α-subunits containing an IGF binding site and two transmembrane β-subunits that possess intrinsic tyrosine kinase activity. Binding of IGF results in conformational changes of the IGF-1R and autophosphorylation of tyrosine residues (6). Major downstream signalling pathways of IGF-1R are Ras-Raf-mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI3K)/Akt signaling cascades (7–9). IGF-1R signaling can stimulate a wide variety of responses in cells, including proliferation, differentiation, adhesion and motility, angiogenesis and survival (6). Experiments using a constitutively active IGF-1R construct demonstrated that altered IGF-1R signaling promoted tumorigenesis in the mouse mammary gland (10). Further, expression of a dominant-negative IGF-1R blocked transformation of murine fibroblasts by Ras (11). The fundamental role of IGF-1R as a cell survival factor has been shown in a variety of cell types and IGF-1R expression is inversely related to susceptibility to apoptosis (12–13). Clinical studies demonstrated that circulating IGF1 levels are associated with increased risk for development of colorectal, prostate, breast and lung cancer (3). High expression of IGFs and IGF-1R has been associated with tumor metastasis in preclinical studies (14–16). However, in MCF-7 breast cancer cells, lower expression of IGF-1R was associated with metastasis (17).
There is increasing evidence suggesting that IGF-1R mediates treatment resistance to cytotoxic agents (8, 18–22) and ionizing radiation (23–27). For example, it was demonstrated that downregulation of IGF-1R by antisense RNA impaired activation of ATM kinase and enhanced radiosensitivity in mouse melanoma cells (25). Mechanistically, IGF-1R inhibition was found to inhibit repair of radiation-induced DNA damage manifested by prolonged expression of phosphorylated histone H2AX (23) and through interference with Ku-DNA binding and Ku86 expression (24). An additional mechanism of radiosensitization demonstrated in vitro in non-small cell lung cancer cells included radiation-induced activation of the IGF-1R as a cell-protective stress response because its impairment enhanced lung cancer cell radiosensitivity (24).
Several preclinical studies were conducted using monoclonal anti-IGF-1R antibody A12 (Imclone Systems Incorporated, NY) (20, 28). A12 demonstrated activity against a wide range of human tumor types in vitro as well as in xenograft and orthotopic tumor models. The effects of A12 were initially evaluated in a series of studies involving human MCF7 breast, BxPC-3 pancreas and Colo205 colon carcinomas (28). In these tumors, A12 demonstrated significant inhibition of growth based on antiproliferative and proapoptotic effects (28). A12 also demonstrated potency to enhance the effects of cytotoxic agents. In myeloma models, A12 enhanced the effects of melphalan or bortezomib thereby prolonging survival (21). In an androgen-independent prostate cancer model, the combination of A12 and docetaxel resulted in greater anticancer activity than docetaxel alone (22). A recent study by Allen and colleagues showed that A12 enhances the effect of radiation in different lung cancer cell lines in vitro (23). In H460 lung cancer xenografts, the combination of 1.5 Gy given once a week with twice-weekly A12 (1 mg per mouse) for a total period of 4 weeks was shown to significantly inhibit tumor growth (23).
Based on preclinical results an extensive clinical research program has been initiated testing A12 (cixutumumab) alone or in combination with other agents in various cancers, including non-small cell lung cancer (NSCLC) and head and neck carcinoma (HNC). So far, 29 clinical trials were registered on the NIH website (clinicaltrials.gov). However, none of these trials tested A12 in combination with radiation.
The present study was undertaken to first investigate whether A12 potentiates the response of a human HNC and NSCLC models to radiation and to quantify the magnitude of enhancement achievable and its dependence on IGF-1R expression level.
MATERIALS AND METHODS
Cell culture
Human HNC cell line HN-5 (kindly provided by Dr. Zhen Fan, University of Texas M. D. Anderson Cancer Center, Houston, TX) and NSCLC cell lines H460 and A549 (ATCC; Manassas, Va) were maintained in DMEM/F-12 medium supplemented with 10% fetal calf serum and 10,000 U/ml of penicillin-streptomycin. A human HNC, FaDu (ATCC; Manassas, Va.) was maintained in MEM medium supplemented with 10% fetal calf serum, 10,000 U/ml penicillin-streptomycin and 1% non-essential amino acids.
A12 monoclonal antibody
The fully human IgG1 monoclonal antibody IMC-A12 (A12) was provided by ImClone Systems Incorporated (NY). This antibody was engineered to selectively bind to the IGF-1R and was developed by screening a human Fab phage display library to specifically yield a high-affinity monoclonal antibody (4.11 × 10−11 mol/L; IC50, 0.6-1 nmol/L). A12 readily cross-reacts with the mouse IGF-1R (20).
Clonogenic cell survival determination
Between 50 and 400 cells were plated in 6 cm dishes in triplicate. The next day, the cells were exposed to A12 (100 nM), and 5 h later they were irradiated with graded doses (2 or 4 Gy) of γ-rays using a 137Cs source (3.7 Gy/min). The cells were left in the incubator with A12 in the medium. The medium was changed 67h after radiation (total 72 h of A12 treatment) and the cells were incubated in drug free medium. Cells were stained after 14 days with 0.5% crystal violet in absolute ethanol, and colonies with more than 50 cells were counted under a dissection microscope. For treatment with IGF1 with or without A12, FaDu cells were plated in 10% serum containing culture media and after 24 hours media was changed to 0.5% serum media. The next day (16 h later) cells were exposed to A12, IGF1 (50 ng/ml), or A12 + IGF1 followed by radiation at five hours after A12 treatment. When A12 and IGF1 were combined, A12 was added 4 hours before IGF1, because such time period resulted in inhibition of IGF-1R activity including receptor downregulation (28). Radiation survival curves were plotted after normalizing for the cytotoxicity induced by A12 alone. Clonogenic survival curves were constructed by fitting the survival levels using least squares regression by the linear quadratic model (29). Clonogenic assays were plated in triplicate and were repeated as independent experiments at least twice.
Immuno-precipitation and Western blot analysis
In vitro cell cultures were exposed to IGF1, A12 or both, similar to regimens summarized above, and cell lysates were subjected to immuno-precipitation using anti-IGF-1R antibody, after which Western analysis was performed as described (30) to detect phosphorylated form of IGF-1R (p-IGF-1R). Anti-IGF-1R and p-IGF-1R antibodies were purchased from Cell Signaling (Danvers, MA).
Tumors and mice
Nude (nu/nu) mice were used for xenograft experiments, female mice for FaDu and male mice for H460 and A549. Animals were housed 5 per cage in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the U.S. Department of Agriculture and Department of Health and Human Services. Tumor cell suspensions were prepared from cells grown as monolayer in vitro. For generation of intramuscular (i.m.) tumors FaDu, H460 and A549 tumor cells were inoculated at concentrations of 1 × 106 (H460 and FaDu) or 5 × 106 (A549) into the hind legs of mice. The studies were initiated when the tumors had grown to 7–8 mm in diameter. Groups consisted of 8–10 mice each. All animals were examined twice to thrice weekly after commencement of experiments for measurement of tumor diameter. For growth delay assays mice were sacrificed when tumors had grown to 14–15 mm in diameter.
In vivo tumor irradiation
Irradiation was performed using a 137Caesium unit (dose rate 3.7 Gy/min). Mice were immobilized in a jig, and tumors were centered in a 3-cm-diameter circular field.
Immunohistochemistry
Three tumors for each condition were excised at three days after the second dose of A12. After fixation in 10% neutral-buffered formalin, 4-μm tissue sections were cut and mounted on silane-coated slides. The sections were then deparaffinized in xylene and rehydrated through decreasing ethanol concentrations to water. After deparaffinization and rehydration as above, the sections were washed twice for 5 min in PBS. For proliferation marker Ki-67 staining, tissue sections were incubated with purified mouse anti-human Ki-67 antibody (clone B56, BD Pharmingen, San Diego, CA) at a dilution of 1:30. The Ki-67 labeling index (LI) was defined as the percentage of positive nuclei out of at least 103 cells counted per tumor. For detection of VEGF expression a rabbit anti-human anti-VEGF antibody (A-20, Santa Cruz Biotechnologies, Santa Cruz, CA) was used at 1:100 dilution. VEGF and Ki-67 were detected with secondary donkey anti rabbit -FITC (Jackson ImmunoResearch, West Grove, PA), goat anti mouse – FITC (Sigma, St. Louis, MO) 1:100 respectively, followed by counterstaining with propidium iodide. IGF-1 expression was investigated by using a rabbit polyclonoal antibody (H-70, Santa Cruz Biotechnologies, Santa Cruz, CA) at 1:50 dilution and detection was performed by Rabbit Elite Kit (Vector Laboratories, Burlingame, CA). Apoptosis was investigated by the terminal nucleotidyl transferase–mediated nick end labeling (TUNEL) method according to the protocol of the manufacturer (Apop TAG Kit Intergen, Burlington, MA).
Statistical analysis
For statistical comparisons unpaired t-test with two tailed p-values was performed using graph pad software.
RESULTS
Sensitivity of head and neck cancer cell lines to A12 and radiation
The level of IGF-1R expression in HNC cell lines was measured by immunoblotting of total protein derived from HN5 and FaDu cells. Fig. 1A shows that IGF-1R was expressed at higher levels in HN5 than in FaDu cells. Subsequently, we determined the effect of the drug alone on the cell lines using the MTT assay. The cells were treated with increasing concentrations of A12 (1 to 500 nM) for 3 days. Fig. 1B shows that A12 had moderate effects in reducing the number of viable cells and that the effects were correlated with the level of IGF-1R expression.
Fig. 1.
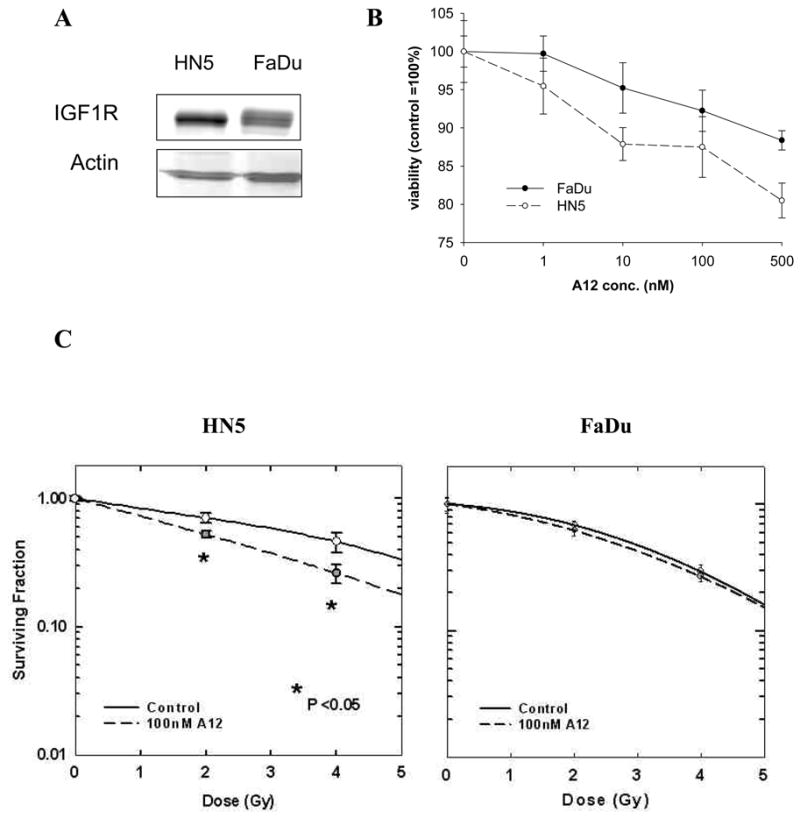
Sensitivity of head and neck squamous cell carcinoma (HNSCC) cell lines HN5 and FaDu to treatment with A12 in combination with radiation. (A) Western blot analysis of IGF-1R expression. (B) The cells were incubated with increasing concentrations of A12 (1–100 nM) and viability of treated cells relative to untreated cells at 72 hours was assessed using MTT colorimetric assay. Statistical significance of A12 effect was reached for HN5 at 10 nM and for FaDu at 100 nM dose levels (p<0.05). (C) Effect of A12 on radiosensitivity of HNSCC cells in culture. Cells were plated in defined cell numbers for 24 hours, then treated with A12 (100 nM) for five hours before radiation. The numbers of colonies were counted 14 days later and the survival curves were constructed with normalized values for the cytotoxicity induced by A12 alone. The average results of three independent experiments (HN5, *p<0.05, EF=1.74 at 0.5 survival fraction) or representative single experiments in triplicate (FaDu) are shown. Bars represent ± SE.
The effect of A12 on cellular radiosensitivity was assessed by the clonogenic cell survival assay. At 24 hours after plating, cells were exposed to 100 nM A12 for five hours before radiation with 2 or 4 Gy. After 14 days the colonies were stained and counted, and the survival curves were constructed. Fig. 1C shows radiation enhancement by A12 in HN5 (p<0.05; EF=1.74 at 0.5 survival fraction), whereas the effect was only additive in FaDu cells grown in 10% serum conditions.
Effect of ligand-receptor interaction on radiation response (IGF1-induced radiation resistance is abrogated by A12 in FaDu)
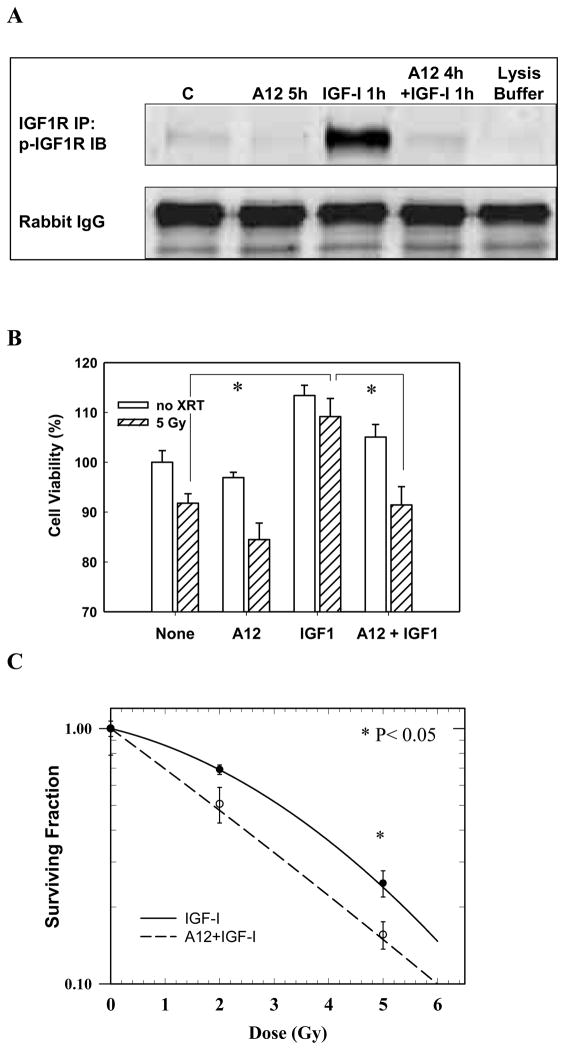
This component of the study addressed whether the lack of radiation enhancement in FaDu by A12 was dependent on the presence of IGF1, a ligand that is often upregulated in tumors such as FaDu (31). FaDu cells were plated in 10% medium and after 24 hours of serum deprivation (0.5% FCS) cells were exposed to A12, IGF1, or A12 + IGF1 with or without radiation given at five hours after A12 treatment. The effects of IGF1 and A12 on receptor expression were assessed with Western Blot. Since FaDu cells in vitro express low baseline p-IGF-1R, immuno-precipitation of IGF-1R protein was performed first and then analyzed for expression of p-IGF-1R by Western blots. As shown in Fig. 2A, exposure of cells to IGF1 induced phosphorylation of IGF-1R, which was completely abolished by A12. Fig. 2B shows that incubation of FaDu cells with IGF1 (50 ng/ml) increased cell viability and radiation resistance and A12 counteracted the effects of IGF1 (p<0.05, respectively). Next we performed a clonogenic cell survival assay in low serum (0.5% FCS) + IGF1 conditions (50 ng/ml). For this experiment FaDu cells were plated in normal growth medium, serum-deprived after 24 hours (0.5% FCS) and treated with A12 (5 hours pretreatment) and radiation (2 or 5 Gy) in the presence of IGF1 (50 ng/ml). At 67 hours after radiation the medium was changed to normal growth medium. As shown in Fig. 2C, A12 enhanced the dose-dependent reduction of clonogenic survival by radiation in FaDu cells in IGF1 rich conditions (p<0.05, EF=1.40 at 0.5 survival fraction).
Fig. 2.
Effects of IGF1 on cell viability and radiation response of FaDu tumor cells in vitro. The cells were plated in 10% serum and allowed to attach overnight, then they were incubated in serum deprived (0.5%) medium for 72 hours. At 24 hours after start of serum deprivation the cells were irradiated. A12 was added four hours before IGF1 and five hours before radiation. (A) Immuno-precipitation and Western blot analysis of FaDu lysates after exposure to IGF-1, A12 or both. IGF-1R antibody was used to immuno-precipitate IGF-1R protein and p-IGF-1R antibody was used in immuno-blot analysis. (B) Cell viability was assessed at 48 hours after radiation (and 72 hours after serum deprivation) using the MTT colorimetric assay. *p<0.05. (C) Radiation cell survival curves with or without A12. A12 significantly enhanced radiation response of FaDu to 5 Gy (p<0.05, EF=1.40 at 0.5 survival fraction). In A and B the results of two independent experiments (in triplicate) are shown. Bars represent ± SE.
A12 enhanced the response of FaDu xenografts to radiation
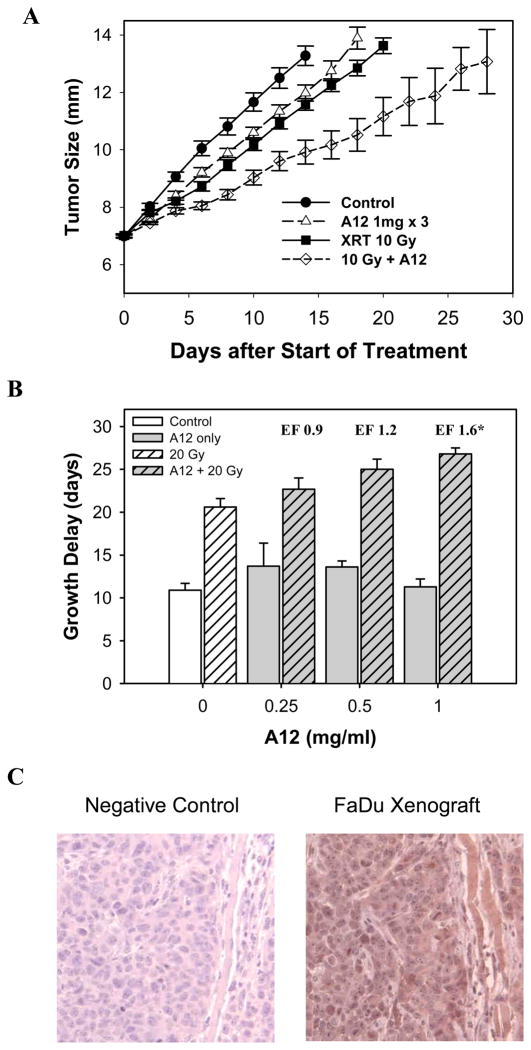
To recapitulate in vitro findings, tumor xenografts established in nude mice were treated with increasing doses of A12 (between 0.25 and 2 mg) and single dose radiation (10 or 20 Gy) when reaching 7–8 mm in diameter. When given together, A12 was injected 6 hours before radiation. As shown in Table 1 and Fig. 3, A12 at doses from 0.25 to 2 mg (data of 2 mg not shown in Fig. 3) induced negligible tumor growth delay without a clear dose-dependency. However, combining A12 with radiation resulted in dose-dependent and significant enhancement at the 1 to 2 mg dose level (p<0.01). The enhancement factors ranged from 1.2 to 1.8 (Table 1). Immunohistochemical analysis showed that FaDu xenograft expressed high levels of IGF-1 in vivo (Fig. 3C, right panel).
Table 1.
Effect of A12 dose on radioresponse of FaDu xenograft tumors assayed by tumor growth delay.
| Schedule* | Time (days) for tumor to grow from 7–8 mm to 12 mm | Growth Delay | Enhancement Factor§ | p-values‡ (95% CI) | |
|---|---|---|---|---|---|
| AGD† | NGD†† | ||||
| Control | 10.9 ± 0.8 | - | - | - | - |
| 20 Gy × 1 | 20.6 ± 1.0 | 9.7 | - | - | - |
| A12 0.25 mg × 3 | 13.7 ± 2.7 | 2.8 | - | - | - |
| 20 Gy + 0.25 mg A 12 | 22.7 ± 1.3 | 11.8 | 9.0 | No Enh. | - |
| A12 0.5 mg × 3 | 13.6 ± 0.7 | 2.7 | - | - | - |
| 20 Gy + 0.5 mg A12 | 25.0 ± 1.2 | 14.1 | 11.4 | 1.2 | 0.45 (−2.2–4.6)|| |
| A12 1 mg × 3 | 11.3 ± 0.9 | 0.4 | - | - | - |
| 20 Gy + 1.0 mg A12 | 26.8 ± 0.7 | 15.5 | 15.1 | 1.6 | <0.01 (2.7–8.0) |
| Control | 10.9 ± 0.8 | - | - | - | - |
| 10 Gy | 15.5 ± 0.6 | 4.6 | - | - | - |
| A12 1 mg × 3 | 14.2 ± 0.7 | 3.3 | - | - | - |
| 10 Gy + 1 mg A12 | 21.3 ± 1.4 | 10.4 | 7.1 | 1.5 | 0.10 (−0.6–5.4) |
| A12 2 mg × 3 | 13.8 ± 1.2 | 2.9 | - | - | - |
| 10 Gy + 2 mg A12 | 22.1±1.3 | 11.2 | 8.3 | 1.8 | 0.02 (0.7–6.7) |
Nude mice (10 per group) bearing FaDu tumors in the hind legs were used. A12 was administered in three injections given three days apart. Treatment was initiated when tumors were 7–8 mm in diameter. Local tumor radiation was given as a single dose of 10 or 20 Gy. On the day of radiation, A12 was given at 6 h before radiation in the combined treatment groups.
Absolute tumor growth delay (AGD) caused by A12, radiation, or their combination is defined as the time in days required for tumors to grow from 7–8 mm to 12 mm minus the time in days in untreated tumors.
Normalized tumor growth delay (NGD) is defined as the time in days for tumors to reach 12 mm in the mice treated with the combination of A12 and radiation minus the time in days to reach 12 mm in mice treated with A12 alone.
Enhancement factors were obtained by dividing the NGD in mice treated with A12 plus radiation by the AGD in mice treated with radiation alone.
Statistical analysis was performed by comparing the means of the NGD of A12 plus radiation with the AGD of radiation alone using unpaired t-test with two-tailed p-values. 95% confidence intervals of the difference between NGDs.
Two mice bearing tumors that never reached 12mm after combined treatment had to be excluded from analysis.
Fig. 3.
Effect of A12 and radiation on the growth of FaDu xenograft tumors. Nude mice bearing 7 mm tumors in hind legs were treated with A12 (three injections of 0.25 to 1 mg per mouse given three days apart) and/or local tumor radiation (10 or 20 Gy). When both treatments were combined A12 was given at 6 hours before radiation. (A) Representative growth delay assay with 1 mg A12 x3. In the group treated with 10 Gy + A12, two out of nine tumors regressed completely at 24 and 28 days, respectively. (B) The graph shows normalized growth delays (time to grow from 8 to 12 mm in the mice treated with the combination of A12 and radiation minus the time in days to reach 12 mm in mice treated with A12 alone) of 20 Gy single dose in combination with increasing doses of A12. *p<0.01. (C) Immunohistochemical analysis of IGF-1 expression in FaDu xenografts (right panel).
In vivo effect of A12 on tumor cell proliferation, apoptosis and angiogenesis
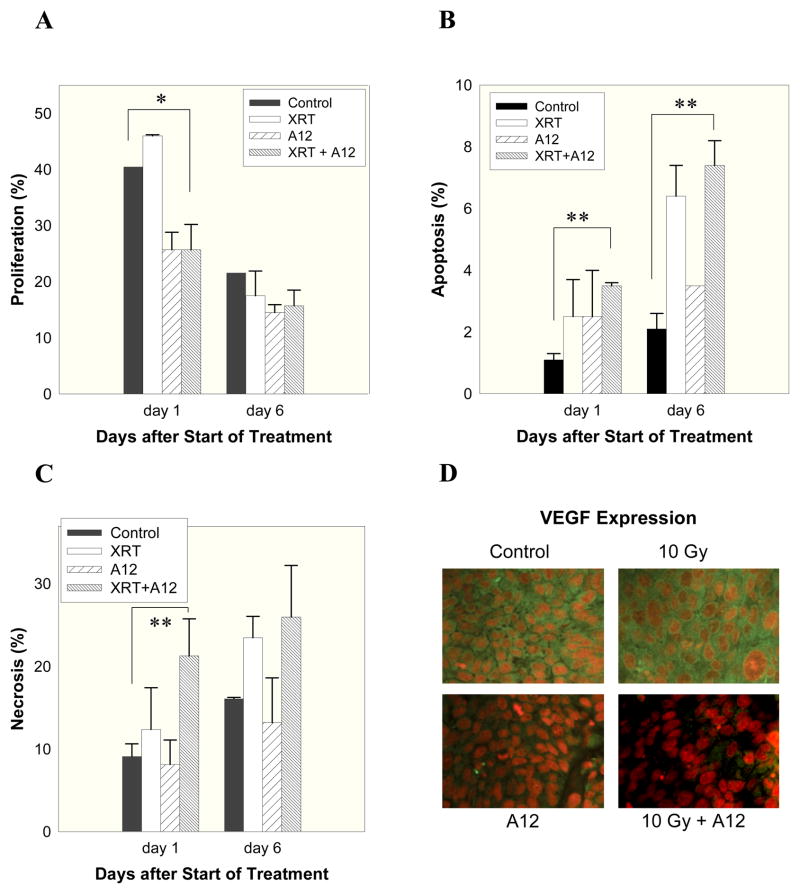
Immunohistochemical analysis was subsequently performed to assess the effect of A12 and/or radiation at cellular level. Tumors were excised, three per treatment group and time point, at days 1 and 6 after start of A12 treatment (1 mg × 3), radiation (10 Gy), or their combination. Tumor cell proliferation was assessed by Ki-67 staining, apoptosis by terminal nucleotidyl transferase–mediated nick end labeling (TUNEL), and necrosis by quantitative pixel analysis of H&E stained sections. Fig. 4 shows that at 24 hours after start of combined treatment with A12 and radiation tumors exhibited a significant reduction in proliferation (p<0.05) and an increase in the proportion of apoptotic cells and necrotic area relative to controls (p<0.01, Fig. 4A, B and C) and that the significant increase in apoptosis persisted until day 6 (p<0.01, Fig. 4B).
Fig. 4.
Immunohistochemical analyses of FaDu tumors. Mice were treated with A12 (1 mg × 3), radiation (10 Gy), or their combination at a tumor diameter of 7 mm. When both treatments were combined A12 was given 6 hours before radiation. Cell proliferation (Ki-67), apoptosis (TUNEL), and necrosis (area by pixel analysis on H&E stained tumor sections) were assessed at 1 and 6 days (panels A–C) after start of treatment. At least three tumors per treatment group and time point were analyzed and at least 103 tumor cells were counted. Panel D shows pictures (x 100) of VEGF expression (yellow) and nuclear staining (red) at 24 hours after radiation (i.e., 30 hours after A12 treatment). Bars represent mean values ± standard errors (SE). *p<0.05, **p<0.01.
It has been demonstrated that IGF-1R signaling is involved in regulation of VEGF expression in many tumor models in vitro (32–34). Therefore, we performed VEGF staining of tumor sections, which showed (Fig. 4D) that FaDu tumors express significant levels of VEGF and treatment with A12, particularly in combination with radiation, potently inhibited VEGF expression at 30 hours (corresponding to 24 hours after radiation). This effect could still be observed at day 6 (data not shown).
Effect of A12 and radiation on lung cancer xenografts
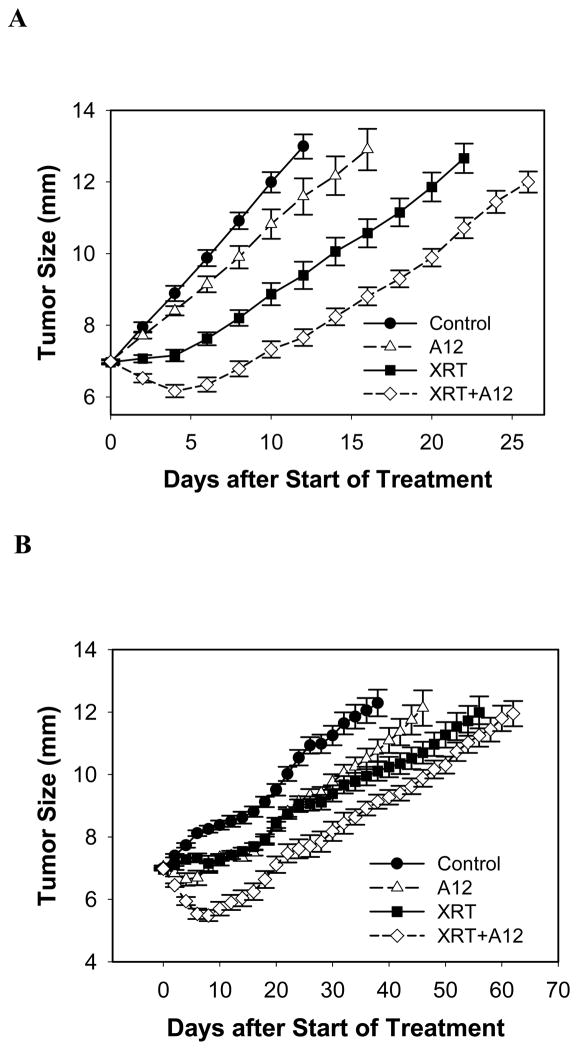
It has been recently demonstrated that A12 (1 mg) given twice weekly in combination with a weekly 1.5 Gy regimen for 4 weeks induces significant reduction of tumor growth in H460 lung carcinoma xenografts. To investigate if A12 would enhance tumor radiation response in human lung cancer xenografts with different growth kinetics, we performed tumor growth delay studies on two human lung cancer lines grown as xenografts in mice. Human H460 and A549 non-small cell lung cancer (NSCLC) xenografts were treated at 7 mm in diameter with A12 (1mg × 3), 10 Gy or combined. In H460 tumors (Fig. 5A), A12 by itself induced a minor delay in tumor growth, which was less than the effect of 10 Gy single dose radiation. Combining A12 with RT increased tumor growth delay but the effect was not supra-additive (EF 1.2, p=0.2). In A549 tumors (Fig. 5B), A12 and RT given alone delayed tumor growth considerably. Adding A12 to RT, however, only induced a transient enhancement of tumor regression for up to about 15 days, after which the effect was minimal (EF 1.1, p = 0.7) or even less than additive after 45 days.
Fig. 5.
Effect of A12 on the response of H460 and A549 lung cancer xenografts to radiation. Nude mice bearing 7 mm tumors in the hind legs were treated with A12 (1 mg per mouse × 3 given three days apart) and/or local tumor radiation (10 Gy). When both treatments were combined A12 was given 6 hours before radiation. Combined treatment with RT and A12 resulted in at least additive growth delay (time from 7 to 12 mm) in H460 and, after initial transient tumor regression, in additive to sub-additive growth delay in A549 xenografts at 60 days after start of treatment (Enhancement factors: 1.2 and 1.1; p-values: 0.17 and 0.7; respectively).
DISCUSSION
The combination of inhibitors of IGF1/IGF-1R signaling pathway with ionizing radiation has been considered a promising novel treatment approach (23–27). The concept emerged from preclinical studies using IGF-1R and ATM gene knock-out systems demonstrated IGF-1R-dependent radiation sensitivity and capacity of AT and mouse melanoma cells to repair radiation-induced DNA damage (25–26). Several in vitro studies demonstrated that IGF-1R antagonists induced tumor cell apoptosis (5, 12, 35–37) and cell cycle arrest (36, 38).
One recent study showed that stimulation of U87 glioblastoma cells with IGF1 induced radiation resistance (24). However, only one study so far investigated the effect of IGF-1R inhibition and radiation in an in vivo xenograft model (23). This study showed, using H460 human NSCLC xenograft, that treatment with 1 mg of A12 twice weekly in combination with weekly doses of 1.5 Gy induced significant inhibition of tumor growth. Given the potential for clinical application, we investigated the effects of IGF-1R inhibition by A12 in combination with radiation in HNC and NSCLC models with different IGF-1R expression and growth kinetics. Data obtained from in vitro work on HNC cell lines suggest that radiation sensitization by A12 correlated not only with the expression levels of IGF-1R but also with the presence of its ligand IGF1 in the culture media. Western blot analysis showed that IGF1 induced activation of IGF-1R that was blocked by A12 corroborating the clonogenic cell survival data. These findings point to the importance of accounting for tumor microenvironment when conducting in vitro studies.
In FaDu xenografts, we showed that A12 significantly enhanced tumor response to RT. Morphologically, A12 alone significantly suppressed proliferation and inhibited VEGF expression whereas RT alone induced apoptosis and terminal differentiation (data not shown). The combination of A12 with RT resulted in the occurrence of patchy tumor necrosis that did not exhibit TUNEL staining. This observation in combination with the finding of reduced VEGF expression by immunohistochemistry suggests that, in addition to its direct effect on cellular radiation sensitivity, A12 exerts an indirect antiangiogenic activity, particularly when combined with RT. Potent suppression of VEGF by inhibition of IGF1/IGF-1R signaling has been demonstrated in a panel of colorectal carcinoma cell lines (14, 32, 34) and L3.6pl pancreatic cancer (33). In addition, one of our previous experiments testing selective antibodies against VEGFR2 demonstrated that FaDu xenografts are highly sensitive to inhibition of VEGF/VEGFR2 in combination with RT.
In human lung cancer models H460 and A549, A12 and RT resulted in additive (H460) or near additive growth delay (A549). Of note is that in A549, combined treatment resulted in substantial early tumor regression but tumors began to regrow around day 10, which was 4 days after the last A12 administration. These data suggest that a prolonged administration of A12 might be necessary in some tumors. Comprehension of molecular mechanisms governing tumor response to inhibitors of epidermal growth factor receptor (EGFR) has been improving steadily over the last few years. Similar lines of investigation are needed for elucidating which tumors mostly depend on IGF-1R signaling and how to best target this pathway for enhancing tumor response will contribute to rational therapy selection.
One of the current treatment options in patients with locally advanced HNSCC is the combination of a monoclonal antibody against EGFR, cetuximab, and radiotherapy (39). Interestingly, several recent studies demonstrated that IGF1/IGF-1R signaling confers resistance to treatment with EGFR inhibitors (7, 40–42). One study, for example, showed that stimulation of head and neck cancer cells with either IGF or EGF resulted in IGF-1R and EGFR receptor heterodimerization, but only IGF caused activating phosphorylation of both receptors (43). This observation suggests that upregulation of IGF-1R could be an important escape mechanism circumventing the effects of EGFR inhibition. In further support of this notion is the finding that combined treatment with cetuximab and A12 was more effective than either treatment alone at reducing cell proliferation and migration in TU159 orthotopic tongue cancer xenografts (43). Thus, the integration of inhibitors of IGF-1R and EGFR with radiation is promising and requires well-organized preclinical and clinical investigation.
Acknowledgments
Financial Support: P01 CA06294 (K.K. Ang, L. Milas); UICC American Cancer Society International Fellowship (O. Riesterer), University of Zurich, Switzerland, supplemented by the Stiftung für medizinische Forschung und Entwicklung, and the Emil Boral-Stiftung (O. Riesterer), and the Gilbert H. Fletcher Memorial Distinguished Chair (K.K. Ang).
Footnotes
Conflicts of interest: K. K. Ang and L. Milas have had Sponsored Agreement with and have served on Advisory Board of Imclone Systems Incorporated, New York, N.Y.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller BS, Yee D. Type I insulin-like growth factor receptor as a therapeutic target in cancer. Cancer Res. 2005;65:10123–10127. doi: 10.1158/0008-5472.CAN-05-2752. [DOI] [PubMed] [Google Scholar]
- 3.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 4.Tao Y, Pinzi V, Bourhis J, et al. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 5.Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog. 1997;8:71–92. doi: 10.1615/critrevoncog.v8.i1.40. [DOI] [PubMed] [Google Scholar]
- 6.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 8.Rochester MA, Riedemann J, Hellawell GO, et al. Silencing of the IGF1R gene enhances sensitivity to DNA-damaging agents in both PTEN wild-type and mutant human prostate cancer. Cancer Gene Ther. 2005;12:90–100. doi: 10.1038/sj.cgt.7700775. [DOI] [PubMed] [Google Scholar]
- 9.Yeh AH, Bohula EA, Macaulay VM. Human melanoma cells expressing V600E B-RAF are susceptible to IGF1R targeting by small interfering RNAs. Oncogene. 2006;25:6574–6581. doi: 10.1038/sj.onc.1209674. [DOI] [PubMed] [Google Scholar]
- 10.Carboni JM, Lee AV, Hadsell DL, et al. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 2005;65:3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- 11.Sell C, Dumenil G, Deveaud C, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peruzzi F, Prisco M, Dews M, et al. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol. 1999;19:7203–7215. doi: 10.1128/mcb.19.10.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 14.Reinmuth N, Liu W, Fan F, et al. Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clin Cancer Res. 2002;8:3259–3269. [PubMed] [Google Scholar]
- 15.Zeng H, Datta K, Neid M, et al. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochem Biophys Res Commun. 2003;302:46–55. doi: 10.1016/s0006-291x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Lin M, van Golen KL, et al. Multiple signaling pathways are activated during insulin-like growth factor-I (IGF-I) stimulated breast cancer cell migration. Breast Cancer Res Treat. 2005;93:159–168. doi: 10.1007/s10549-005-4626-8. [DOI] [PubMed] [Google Scholar]
- 17.Pennisi PA, Barr V, Nunez NP, et al. Reduced expression of insulin-like growth factor I receptors in MCF-7 breast cancer cells leads to a more metastatic phenotype. Cancer Res. 2002;62:6529–6537. [PubMed] [Google Scholar]
- 18.Gooch JL, Van Den Berg CL, Yee D. Insulin-like growth factor (IGF)-I rescues breast cancer cells from chemotherapy-induced cell death--proliferative and anti-apoptotic effects. Breast Cancer Res Treat. 1999;56:1–10. doi: 10.1023/a:1006208721167. [DOI] [PubMed] [Google Scholar]
- 19.Perer ES, Madan AK, Shurin A, et al. Insulin-like growth factor I receptor antagonism augments response to chemoradiation therapy in colon cancer cells. J Surg Res. 2000;94:1–5. doi: 10.1006/jsre.2000.5923. [DOI] [PubMed] [Google Scholar]
- 20.Rowinsky EK, Youssoufian H, Tonra JR, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 21.Wu KD, Zhou L, Burtrum D, et al. Antibody targeting of the insulin-like growth factor I receptor enhances the anti-tumor response of multiple myeloma to chemotherapy through inhibition of tumor proliferation and angiogenesis. Cancer Immunol Immunother. 2007;56:343–357. doi: 10.1007/s00262-006-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JD, Haugk K, Coleman I, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–6160. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 23.Allen GW, Saba C, Armstrong EA, et al. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67:1155–1162. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- 24.Cosaceanu D, Budiu RA, Carapancea M, et al. Ionizing radiation activates IGF-1R triggering a cytoprotective signaling by interfering with Ku-DNA binding and by modulating Ku86 expression via a p38 kinase-dependent mechanism. Oncogene. 2007;26:2423–2434. doi: 10.1038/sj.onc.1210037. [DOI] [PubMed] [Google Scholar]
- 25.Macaulay VM, Salisbury AJ, Bohula EA, et al. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene. 2001;20:4029–4040. doi: 10.1038/sj.onc.1204565. [DOI] [PubMed] [Google Scholar]
- 26.Peretz S, Jensen R, Baserga R, et al. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tezuka M, Watanabe H, Nakamura S, et al. Antiapoptotic activity is dispensable for insulin-like growth factor I receptor-mediated clonogenic radioresistance after gamma-irradiation. Clin Cancer Res. 2001;7:3206–3214. [PubMed] [Google Scholar]
- 28.Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 29.Fertil B, Malaise EP. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981;7:621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- 30.Raju U, Nakata E, Mason KA, et al. Flavopiridol, a cyclin-dependent kinase inhibitor, enhances radiosensitivity of ovarian carcinoma cells. Cancer Res. 2003;63:3263–3267. [PubMed] [Google Scholar]
- 31.Kim WY, Jin Q, Oh SH, et al. Elevated epithelial insulin-like growth factor expression is a risk factor for lung cancer development. Cancer Res. 2009;69:7439–7448. doi: 10.1158/0008-5472.CAN-08-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akagi Y, Liu W, Zebrowski B, et al. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998;58:4008–4014. [PubMed] [Google Scholar]
- 33.Stoeltzing O, Liu W, Reinmuth N, et al. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003;163:1001–1011. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren RS, Yuan H, Matli MR, et al. Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J Biol Chem. 1996;271:29483–29488. doi: 10.1074/jbc.271.46.29483. [DOI] [PubMed] [Google Scholar]
- 35.Gilmore AP, Valentijn AJ, Wang P, et al. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem. 2002;277:27643–27650. doi: 10.1074/jbc.M108863200. [DOI] [PubMed] [Google Scholar]
- 36.Liu B, Fang M, Lu Y, et al. Fibroblast growth factor and insulin-like growth factor differentially modulate the apoptosis and G1 arrest induced by anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 2001;20:1913–1922. doi: 10.1038/sj.onc.1204277. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Sun Y. Insulin-like growth factor receptor-1 as an anti-cancer target: blocking transformation and inducing apoptosis. Curr Cancer Drug Targets. 2002;2:191–207. doi: 10.2174/1568009023333863. [DOI] [PubMed] [Google Scholar]
- 38.Sachdev D, Li SL, Hartell JS, et al. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–635. [PubMed] [Google Scholar]
- 39.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 40.Jones HE, Goddard L, Gee JM, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 42.Morgillo F, Woo JK, Kim ES, et al. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 43.Barnes CJ, Ohshiro K, Rayala SK, et al. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13:4291–4299. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]