Abstract
The totality of data indicate that the “window of opportunity” for reducing coronary heart disease (CHD) and overall mortality is initiation of hormone therapy (HT) within 6 years of menopause and/or before 60 years of age. Reduction of CHD risk and overall mortality with prolonged HT use in this subgroup of women is consistent across randomized controlled trials and observational studies. As such, HT use for 5 to 30 years in postmenopausal women who initiate HT in their 50s substantially increases quality-adjusted life-years (QALYs) by 1.5 QALYs and is highly cost-effective at $2,438 per QALY gained. Cumulated randomized controlled trial results indicate a consistency along with observational data that young postmenopausal women with menopausal symptoms who use HT for long periods of time have lower rates of CHD and overall mortality than comparable postmenopausal women who do not use HT.
Introduction
The consistency of data across approximately 40 observational studies that demonstrate a reduction in coronary heart disease (CHD) and overall mortality with exogenous postmenopausal hormone therapy (HT) with estrogen (Grodstein and Stampfer, 1995; Grodstein and Stampfer 1998; Prentice et al., 2006) and estrogen plus progestogen (Thompson et al., 1989; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein et al., 1996; Prentice et al., 2005) is unparalleled by any other potential primary prevention therapy for CHD in women. The consistency across observational studies led to formulation of the estrogen cardioprotective hypothesis that remained un-challenged until the 1990’s when a series of randomized controlled trials (RCTs) tested this hypothesis. Two well-established and consistent results from these 40 observational studies are supported by the cumulated RCTs: 1) the incidence of CHD and overall mortality is reduced in postmenopausal women who initiate HT in close proximity to menopause; and, 2) the beneficial effect of HT on CHD and overall mortality accumulates with duration of HT use.
Observational Studies and RCTs of HT Involved Different Populations of Women
Whereas observational studies have shown a 30–50% reduction in CHD and overall mortality in users versus nonusers of HT (Thompson et al., 1989; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998; Prentice et al., 2005; Prentice et al., 2006), RCTs have shown a null effect on these outcomes when analyzed over all randomized women without consideration of age (Hodis and Mack, 2008). However, as shown in Table 1, women selected for RCTs were a different population than the women included in observational studies. Because population-based observational studies reflect the pattern of HT use within the general population, women who used HT in observational studies were relatively young at the time of HT initiation (30–55 years old), recently postmenopausal (majority initiated HT at the time of menopause), were relatively lean (approximate body mass index of 25 kg/m2) and were predominantly symptomatic mainly with flushing and other menopausal symptoms since these symptoms were the primary reason for initiating HT. Many of the women in the observational studies who used HT did so for decades (10–40 years).
Table 1.
Comparison of Study Populations Included in Randomized Controlled Trials and Observational Studies of Postmenopausal Hormone Therapy
| Randomized Controlled Trials | Observational Studies | |
|---|---|---|
| Mean age or age range at enrollment (years) | >62 | 30–55 |
| Time since menopause (years) | >10 | <61 |
| Duration of therapy (years) | ≤8 | >10 |
| Menopausal symptoms (flushing) | excluded | predominant |
| Body mass index (mean) | ~29 kg/m2 | ~25 kg/m2 |
>80% of the women initiated hormone therapy within 2 years of menopause.
On the other hand, women selected for RCTs were much older with more than 90% of women older than 55 years of age and on average more than 10 years beyond menopause when randomized (range of trial averages, 13–23 years). Women with significant menopausal symptoms, predominantly flushing, were excluded from RCT participation. Mean duration of therapy (1–6.8 years) in RCTs was also considerably less than that of the HT users in observational studies. Additionally, women in RCTs were on average overweight (approximate body mass index of 29 kg/m2). It is clear that the characteristics of women selected for RCTs were markedly different from those of women studied from the general population in observational studies from which the estrogen cardioprotective hypothesis was generated. This accounts in part for the discordance of HT effects on CHD and overall mortality between observational studies and RCTs analyzed among all women regardless of age (Hodis and Mack, 2008).
Cardioprotective Effect of HT According to Age and Timing of Initiation
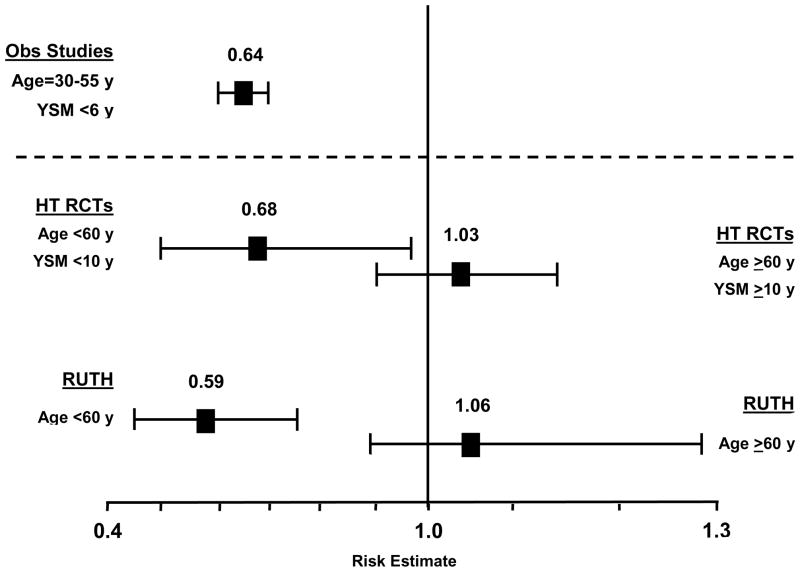
Although the effect of HT on CHD over all ages is null in RCTs, these trials also indicate that there are distinct populations of women in terms of response to HT. Specifically, the beneficial effects of HT on CHD and overall mortality occurs when HT is initiated in younger women in close proximity to menopause and a null effect when HT is initiated in older women remote from menopause (Hodis and Mack, 2008). The beneficial effect of HT on CHD risk according to timing of HT initiation has been demonstrated in a large meta-analysis of RCTs (Salpeter, et al., 2006). Using 23 RCTs (39,049 participants with 191,340 patient-years of follow-up) that reported at least 1 CHD event in postmenopausal women comparing HT with placebo of at least 6 months duration, the effect of HT on CHD events over all ages was null (Table 2). However, a statistically significant 32% reduction in CHD events was found for subjects younger than 60 years of age or within 10 years since menopause when randomized to HT relative to placebo (Table 2). The magnitude of the reduction in CHD events for the women younger than 60 years of age or within 10 years since menopause when randomized was similar to that of observational studies (Figure 1) (Thompson et al., 1989; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998; Prentice et al., 2005; Prentice et al., 2006).
Table 2.
Relative Risks for Coronary Heart Disease: Hormone Therapy (HT) and Raloxifene Compared to Placebo by Age and Years Since Menopause (YSM) at Randomization
| Study | HT Relative Risk | Number of Participants |
|---|---|---|
| HT Meta-analysis1 | OR (95% CI) | |
| All ages | 0.99 (0.88–1.11) | 39,049 |
| <60 years old or <10 YSM | 0.68 (0.48–0.96) | not given |
| ≥60 years old or ≥10 YSM | 1.03 (0.91–1.16) | not given |
| WHI2 (years since menopause) | HR (95% CI) | |
| CEE+MPA Trial | ||
| <10 | 0.88 (0.54–1.43) | 5,494 |
| 10–19 | 1.23 (0.85–1.77) | 6,041 |
| ≥20 | 1.66 (1.14–2.41) | 3,653 |
| P-value for trend | 0.05 | |
| CEE Trial | ||
| <10 | 0.48 (0.20–1.17) | 1,643 |
| 10–19 | 0.96 (0.64–1.44) | 2,936 |
| ≥20 | 1.12 (0.86–1.46) | 4,550 |
| P-value for trend | 0.15 | |
| Combined Trials | ||
| <10 | 0.76 (0.50–1.16) | 7,137 |
| 10–19 | 1.10 (0.84–1.45) | 8,977 |
| ≥20 | 1.28 (1.03–1.58) | 8,203 |
| P-value for trend | 0.02 | |
| RUTH3 (age, years) | ||
| <60 | 0.59 (0.41–0.83) | 1,670 |
| 60–69 | 1.06 (0.88–1.28) | 4,534 |
| ≥70 | 0.98 (0.82–1.17) | 3,897 |
| P-value for trend | 0.01 | |
OR = Odds ratio (95% Confidence Interval)
HR = Hazard ratio (95% Confidence Interval)
WHI = Women’s Health Initiative
CEE = conjugated equine estrogen
MPA = medroxyprogesterone acetate
RUTH = Raloxifene Use for the Heart
Figure 1.
Relative risks of coronary heart disease comparing meta-analysis of randomized controlled trials (RCTs) of hormone therapy (HT) (Salpeter et al., 2006) and the selective estrogen receptor modulator raloxifene from the Raloxifene Use for the Heart (RUTH) trial (Collins et al., 2009) to meta-analysis of observational (Obs) studies (Grodstein et al., 1995) in women who initiate hormone therapy before 60 years (y) of age and within 10 years since menopause (ysm). Even though the age of initiation and time since menopause were much earlier in observational studies relative to the randomized controlled trials, the reduction of coronary heart disease is strikingly similar across all studies.
On the other hand, the effect of HT relative to placebo on CHD incidence in women older than 60 years of age or more than 10 years since menopause when randomized was null and similar to that reported over all ages in RCTs (Table 2). The HT-associated relative risks for CHD events significantly differed between these 2 groups of women according to when HT was initiated, that is, initiation in women younger than 60 years of age or within 10 years since menopause versus initiation in women older than 60 years of age or more than 10 years since menopause (OR, 0.66; 95% CI, 0.46–0.95). This large meta-analysis of the cumulated RCTs of HT clearly demonstrates that there are 2 distinct populations of women who respond differently to HT according to when HT is initiated in relation to age and/or time since menopause.
The meta-analytical results are mirrored by the results from the Women’s Health Initiative (WHI), the largest RCT designed to evaluate HT on CHD (Rossouw et al., 2007). Relative to placebo, women who were randomized to the WHI-estrogen + progestin (EP) trial to daily conjugated equine estrogen + medroxyprogesterone acetate (CEE+MPA) within 10 years of menopause had a 12% reduction in CHD events whereas women randomized between 10–20 years after menopause had a 23% increased risk of CHD and women randomized more than 20 years after menopause had a 66% increased risk of CHD (Table 2). In the WHI-estrogen alone (E) trial, hysterectomized women randomized to daily conjugated equine estrogen (CEE) therapy within 10 years of menopause had a 52% reduction in CHD events, those randomized between 10–20 years after menopause had a 4% reduction in CHD events and those randomized more than 20 years after menopause had a 12% increased risk of CHD, all relative to placebo treated women (Table 2). Additionally, several categories of CHD events (nonfatal myocardial infarction, coronary death, confirmed angina and coronary artery revascularization) were significantly reduced 34–45% in the CEE-treated group relative to the placebo-treated group in women who were 50–59 years old when randomized but not in the women in the 60–69 or 70–79 year old age ranges (Hsia et al., 2006). With the WHI CEE+MPA and CEE trials combined, the trend in CHD reduction according to the time of initiation of HT is stronger than either trial alone, due to the greater sample size. Relative to placebo, women randomized to HT within 10 years of menopause had a 24% reduction in CHD events whereas women randomized between 10–20 years after menopause had a 10% increased risk of CHD and women randomized more than 20 years after menopause had a 28% increased risk of CHD (Table 2). Although only one-third of the women randomized to the WHI trials were younger than 60 years of age and less than 5% were within a few years of menopause, the subgroup of women randomized to these trials who are representative of the women in observational studies had reduced CHD with HT. On the other hand, older women (older than 60 years of age) who were randomized to HT many years beyond menopause (>10 years) who are not representative of the women in observational studies showed no reduction in CHD risk with HT, and even evidence of elevated risk when HT was initiated more than 20 years after menopause.
Reduction of Overall Mortality by HT According to Age of Initiation
The beneficial effect of HT on overall mortality according to age has also been demonstrated in a large meta-analysis of RCTs (Salpeter et al., 2004). The effect of HT on overall mortality according to age was examined using 30 RCTs (26,708 participants with 119,118 patient-years) that reported at least 1 death in postmenopausal women comparing HT with placebo of at least 6 months duration. In this study, the effect of HT on overall mortality was null over all ages (Table 3). However, when the data were examined by the age of subjects, a statistically significant reduction in overall mortality was found for subjects younger than 60 years of age (mean age 54 years) when randomized to HT relative to placebo (Table 3). The magnitude of the reduction in overall mortality of 39% for the women younger than 60 years of age was similar to that of observational studies (Thompson et al., 1989; Henderson et al., 1991; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998; Prentice et al., 2005; Prentice et al., 2006), notably comparable to that of the Nurses’ Health Study, the largest observational study (HR, 0.63; 95% CI, 0.56–0.70)(Grodstein et al., 2000). The age at initiation of HT among the women in the observational studies and the age of the younger women randomized to RCT’s examined in the meta-analysis is similar (Table 3). On the other hand, in this meta-analysis, the effect of HT on overall mortality in women who were older than 60 years of age (mean age 66 years) when randomized was similar to that reported over all ages in RCTs (Table 3).
Table 3.
Relative Risks for Overall Mortality: Hormone Therapy (HT) Compared to Placebo by Age at Randomization
| Study | HT Relative Risk | Number of Participants |
|---|---|---|
| HT Meta-analysis1 (age, years) | OR (95% CI) | |
| All ages | 0.98 (0.87–1.18) | 26,708 |
| <60 | 0.61 (0.39–0.95) | not given |
| >60 | 1.03 (0.90–1.18) | not given |
| WHI2 (age, years) | HR (95% CI) | |
| CEE+MPA Trial | ||
| 50–59 | 0.69 (0.44–1.07) | 5,494 |
| 60–69 | 1.09 (0.83–1.44) | 6,041 |
| 70–79 | 1.06 (0.80–1.41) | 3,653 |
| P-value for trend | 0.19 | |
| CEE Trial | ||
| 50–59 | 0.71 (0.46–1.11) | 1,643 |
| 60–69 | 1.02 (0.80–1.30) | 2,936 |
| 70–79 | 1.20 (0.93–1.55) | 4,550 |
| P-value for trend | 0.18 | |
| Combined Trials | ||
| 50–59 | 0.70 (0.51–0.96) | 7,137 |
| 60–69 | 1.05 (0.87–1.26) | 8,977 |
| 70–79 | 1.14 (0.94–1.37) | 8,203 |
| P-value for trend | 0.06 | |
OR = Odds ratio (95% Confidence Interval)
HR = Hazard ratio (95% Confidence Interval)
WHI = Women’s Health Initiative
CEE = conjugated equine estrogen
MPA = medroxyprogesterone acetate
Similar to the effect of HT on CHD, the meta-analytical results are mirrored by the results from the WHI trials, indicating that the effect of HT on overall mortality is related to the age at which HT is initiated (Rossouw et al., 2007). Relative to placebo, women who were 50–59 years old when randomized to CEE+MPA had a 31% reduction in overall mortality whereas women 60–69 years old when randomized had a 9% increased risk of overall mortality and women 70–79 years old when randomized had a 6% increased risk of overall mortality (Table 3). In the WHI-E trial, women who were randomized to CEE therapy between the ages of 50–59 years had a 29% reduction in overall mortality, those randomized between the ages of 60–69 years had a 2% increased risk of overall mortality and those randomized between the ages of 70–79 years had a 20% increased risk of overall mortality, all relative to placebo treated women (Table 3). With the CEE+MPA and CEE trials combined, overall mortality was significantly reduced 30% in women 50–59 years old when randomized, whereas women 60–69 years old when randomized had a 5% increased risk of overall mortality and women 70–79 years old when randomized had a 14% increased risk of overall mortality, all relative to placebo treated women (Table 3). The subgroup of younger women randomized to WHI is more representative of the women in observational studies, data from which also indicate that younger women who use HT relative to those who do not have a reduction in overall mortality (Thompson et al., 1989; Henderson et al., 1991; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998; Grodstein et al., 2000; Prentice et al., 2005; Prentice et al., 2006).
To address the risks and benefits of HT, a recent Bayesian meta-analysis was conducted using RCTs and observational studies to evaluate the effect of HT on overall mortality in young postmenopausal women who initiated HT in close proximity to menopause (Salpeter et al., 2009a). Results generated using a hierarchical random-effects Bayesian meta-analysis from 19 RCTs with 16,283 women (mean age 54.5 years) followed for 83,043 patient-years over 5.1 years (range, 1–6.8 years) showed an overall mortality reduction of 27% in those women randomized to HT relative to placebo (RR, 0.73; 95% credible interval (CrI), 0.52–0.96). The 95% CrI is a Bayesian analog of the 95% CI from traditional meta-analyses. Overall mortality was reduced 22% (RR, 0.78; 95% CrI, 0.69–0.90) in HT users relative to non-users from the pooled data of 8 prospective observational studies in which a total of 212,717 women were followed for 2,935,495 patient-years over a mean of 13.8 years (range, 6–22 years). When the RCT and prospective observational data were combined, the relative risk was 0.72 (95% CrI, 0.62–0.82). The results from this study indicate a convergence of evidence from a variety of sources that support a beneficial effect of HT on overall mortality in young postmenopausal women who initiate HT in close proximity to menopause. Further, the results from this meta-analysis indicate that RCTs and observational studies are similar, each with an overall mortality reduction of approximately 25%; results that are similar to the 30% reduction in overall mortality shown in postmenopausal women who were younger than 60 years of age when randomized to HT in the WHI trials (Table 3).
Other Estrogen Receptor-Binding Agents that Support the Timing Hypothesis
There are accumulating data that other classes of estrogen receptor binding agents exert the same beneficial effect on CHD in young postmenopausal women as HT, broadening support for the timing hypothesis. The most complete data derive from the Raloxifene Use for the Heart (RUTH) trial, a RCT of 10,101 postmenopausal women with a mean age of 67 years. In this trial, raloxifene, a selective estrogen receptor modulator, had no effect on the incidence of CHD over all ages after a median treatment period of 5.6 years. However, in the women who were younger than 60 years of age when randomized to raloxifene, the incidence of CHD was significantly reduced 41% relative to placebo (Table 2) (Collins et al., 2009). This finding with raloxifene is similar to that with CEE from the WHI-E trial in which CHD incidence was reduced 37% in women who were younger than 60 years of age when randomized to CEE relative to placebo (HR, 0.63; 95% CI, 0.36–1.09) (Rossouw et al., 2007). On the other hand, no difference in the incidence of CHD was found between treatment groups for the women who were 60–69 years old and 70 years old or older when randomized to raloxifene relative to placebo (Table 2). The HT-associated relative risks of CHD differed significantly by age (Table 2).
The totality of data from RCTs indicates that young postmenopausal women who initiate HT in close proximity to menopause have a reduced incidence of CHD and overall mortality. These results parallel the consistent reduction in CHD and overall mortality seen in observational studies where the majority of women initiated HT at the time of menopause and the remainder within 6 years of menopause (Figure 1) (Thompson et al., 1989; Henderson et al., 1991; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998; Grodstein et al., 2000; Prentice et al., 2005; Prentice et al., 2006).
An Atheroprotective Effect of HT Varies by Stage of Atherosclerosis
The effect of HT on atherosclerosis progression appears to be determined by the stage of atherosclerosis. This was demonstrated in the sister RCTs, the Women’s Estrogen-progestin Lipid Lowering Hormone Atherosclerosis Regression Trial (WELL-HART) (Hodis et al., 2003) and the Estrogen in the Prevention of Atherosclerosis Trial (EPAT) (Hodis et al., 2001). WELL-HART and EPAT were designed to determine the effects of HT on the progression of atherosclerosis in postmenopausal women with and without symptomatic pre-existing atherosclerotic vascular disease, respectively. The differing outcomes of no effect of HT on atherosclerosis progression in WELL-HART and a reduction in atherosclerosis progression in EPAT may be related to the timing of the intervention relative to the stage of atherosclerosis as reflected by the arterial imaging methods used in these 2 trials. Quantitative coronary angiography used in WELL-HART is a measure of symptomatic late stage atherosclerosis whereas common carotid artery intima-media thickness (CIMT) used in EPAT is a measure of asymptomatic early subclinical atherosclerosis (Blankenhorn and Hodis, 1994). CIMT was also used in a RCT to evaluate the effects of oral CEE and micronized 17B-estradiol with sequential progestogens on atherosclerosis in 121 perimenopausal women with a mean age of 47 years and without cardiovascular disease (de Kleijn et al., 1999). Although a high drop out rate of 48% prevented reliable conclusions from this RCT, women randomized to HT showed a reduction in the progression of CIMT compared with placebo after 2 years of treatment (de Kleijn et al., 1999). Even though the sample size was limited in this RCT, the magnitude of reduction in CIMT progression was similar to that of EPAT (Hodis et al., 2001).
Although a late component of atherosclerosis, it has been shown in the WHI-E trial that HT uniquely reduces coronary artery calcium in women who are younger than 60 years of age when randomized to CEE relative to placebo (Manson et al., 2007). Importantly, the reduction of coronary artery calcium with estrogen therapy was greatest in the women who were most compliant with CEE therapy and adhered to study treatment for at least 5 years. Although, unfortunately an older group of postmenopausal women in WHI-E were not included for comparison, these results are consistent with the large body of evidence that HT reduces atherosclerosis, CHD and overall mortality in women who initiate HT before 60 years of age. Additionally, these results are consistent with observational and case-control studies indicating that HT is significantly associated with a reduction in coronary artery plaque burden measured by coronary artery calcium (Akhrass et al., 2003; Barrett-Connor et al., 2005).
Human and animal studies indicate that an underlying healthy endothelium is required for HT to be atheroprotective (Hodis and Mack, 2007a). Since age and atherosclerosis are inextricably linked, further investigation will be required to determine which phenotype is most important in determining whether HT will be atheroprotective or ineffective. Age and/or time since menopause likely serve as chronological markers for vascular age (stage of atherosclerosis), which is the ultimate determinate as to whether HT will be cardioprotective (Hodis et al., 2003).
Duration of HT and Cardioprotection
Evidence for a long-term beneficial effect of HT on CHD is derived from both observational studies and RCTs including the Heart and Estrogen/progestin Replacement Study (HERS) and the WHI trials which have the longest randomized follow-up. There was a statistically significant trend toward a reduction in CHD in years 4 and 5 in the CEE+MPA treated group relative to the placebo group (p=0.009 for trend) in HERS despite no overall benefit in this population of women with pre-existing CHD (Hulley et al., 1998). In the WHI-EP trial, there was a statistically significant trend toward a reduction in CHD outcome in year 6 and beyond in the HT group relative to the placebo group (p=0.02 for trend) (Manson et al., 2003).
Further, the beneficial effect on CHD with longer duration of HT use is strongly supported by consistency between the WHI RCTs and the WHI observational study (Prentice et al., 2005; Prentice et al., 2006). Consistent with other observational studies (Thompson et al., 1989; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998), the WHI observational study showed a 50% decreased risk of CHD in estrogen + progestogen (E+P) users relative to nonusers and a 32% decreased risk of CHD in estrogen alone users relative to nonusers (Prentice et al., 2005; Prentice et al., 2006). In both the WHI-EP trial and the WHI observational study of E+P users (n=17,503 women), the relative risk for CHD decreased with increasing duration of HT use (Prentice et al., 2005). After 5 years of HT use, the risk for CHD in both the WHI-EP trial (HR, 0.66; 95% CI, 0.36–1.21) and the WHI observational study of E+P users (HR, 0.83; 95% CI, 0.67–1.01) was reduced relative to nonusers (Prentice et al., 2005).
Reduction of CHD risk with longer duration of HT use is also supported by the consistency between the WHI-E trial and the WHI observational study of estrogen use (Prentice et al., 2006). After 5 years of estrogen alone use, the risk for CHD in both the WHI-E trial (HR, 0.80; 95% CI, 0.57–1.12) and the WHI observational study of estrogen users (HR, 0.73; 95% CI, 0.61–0.84) was reduced relative to nonusers (Prentice et al., 2006). The WHI RCTs and observational studies of E+P and estrogen alone demonstrate a consistent reduction in the incidence of CHD with duration of HT use within the same study across 2 different HTs as well as consistency with other RCTs and observational studies. Although limited in duration relative to observational studies, RCTs clearly support the cardiovascular benefits of HT seen in the much longer-term observational studies.
Misperception of Long-Term HT and CHD
It has been suggested that prolonged HT in young postmenopausal women who initiate HT in close proximity to menopause will result in increased risk at older ages when CHD events become more frequent. However, considering the cumulated data, this assertion has no basis of support and, in fact is contradicted by the available data. HT has the longest observational follow-up than any other primary prevention therapy for CHD. There is absolutely no evidence from observational studies with decades of follow-up that HT causes an increased risk of CHD with long-term use where the majority of women across these studies initiated HT at the time of menopause. In fact, observational studies have demonstrated a long-term benefit of HT on CHD and overall mortality for up to 10–40 years of HT use (Thompson et al., 1989; Henderson et al., 1991; Falkeborn et al., 1992; Psaty et al., 1994; Grodstein and Stampfer, 1995; Grodstein et al., 1996; Grodstein and Stampfer 1998; Grodstein et al., 2000; Prentice et al., 2005; Prentice et al., 2006). In addition, HT has some of the longest RCT data, up to 8 years of randomized treatment. Over this period of time, there is no evidence that CHD risk increases with longer duration of HT when HT is initiated at any age (50–59 years, 60–69 years, 70–79 years) or at any time since menopause (<10 years, ≥10 years) (Hsia et al., 2006; Toh et al., 2010). In fact, as shown by the cumulative hazard in WHI-E, women between 50–59 years old when randomized to CEE therapy had a reduction in CHD events relative to placebo over the entire 8 years of HT (Hsia et al., 2006). The cumulative hazard in WHI-EP showed that women <10 years since menopause when randomized to CEE + MPA therapy had no increased risk within the first 2 years of therapy and the curves were essentially the same until at approximately 6 years when the curves crossed indicating a possible cardioprotective effect of CEE + MPA relative to placebo (Toh et al., 2010). In sum, there are no observational or RCT data available to suggest that prolonged HT use is associated with elevated CHD risk if use is continued into older ages; and, in fact, the cumulated data support increasing benefit when HT is initiated early and continued long-term.
Observational cohort and case-control studies indicate that longer duration of HT (9–10 years) relative to shorter-term use is associated with significantly less coronary artery plaque burden measured by coronary artery calcium (Akhrass et al., 2003; Barrett-Connor et al., 2005). Duration of HT is a significant predictor of lower coronary artery calcium after adjustment for age and cardiovascular disease risk factors, with maximum benefit of HT on coronary artery calcium observed after 23 years of use (Akhrass et al., 2003).
Reduction of CHD risk and atherosclerosis with prolonged HT use is consistent across the large RCTs, observational studies, case-control studies and arterial imaging studies (Henderson et al., 1991; Heckbert et al., 1997; Hulley et al., 1998; Akhrass et al., 2003; Chilvers et al., 2003; Ferrara et al., 2003; Manson et al., 2003; Barrett-Connor et al., 2005; Prentice et al., 2005; Prentice et al., 2006; Toh et al., 2010). Comparison of the results from RCTs, prospective observational studies and case-control studies indicates that confounding and selection biases do not explain the consistent evidence that HT is associated with a duration-dependent lowering of CHD risk. The RCTs along with observational studies suggest that this duration-dependent lowering of CHD risk may predominantly manifest in women initiating HT in close proximity to menopause (within 6 years) or before 60 years of age.
HT Cost Effectively Extends Life When Initiated at Younger Age
Based on the cumulated data, a recent cost-effectiveness analysis indicates that HT given to postmenopausal women in their 50s for 5–30 years results in a substantial increase in quality-adjusted life-years (QALYs) of 1.5 QALYs with a cost of $2,438 per QALY gained, compared with no therapy (Salpeter et al., 2009b). The data indicate that the net gains gradually increase over time with treatment durations of 5–30 years and that the results for younger women are robust to all sensitivity analyses with the treatment remaining highly cost effective (defined as < $10,000 per QALY gained). At $2,438 per QALY gained, the data indicate that HT is a highly cost-effective strategy for improving quality-adjusted life. The substantial increase in QALYs in the younger women is due to a net benefit in quality of life and reduced overall mortality compared with no therapy. On the other hand, for 65-year old postmenopausal women initiating HT there is a smaller net gain of 0.11 QALYs with a cost of $27,953 per QALY gained. The results for older postmenopausal women are sensitive to many of the assumptions and compared with no therapy there is a loss of QALYs in the first 9 years of HT before a gain is realized.
Clinical Perspective of HT Relative to Other Primary Preventive Therapies for Women
Although a detailed discussion of current primary prevention therapies for women is beyond the scope of this review, it is important to appreciate the effect of HT on the incidence of CHD and overall mortality in relation to these commonly employed therapies (Hodis and Mack, 2007b). Lipid-lowering therapy, predominantly with HMG-CoA reductase inhibitors (statins) is the mainstay for the primary prevention of CHD in women (Executive Summary, 2001; Mosca et al., 2004). The cumulated data however, do not provide convincing evidence for the significant reduction of CHD with lipid-lowering therapy relative to placebo when used for primary prevention of CHD in women and there is no evidence that such therapy reduces overall mortality (Table 4) (Walsh et al., 2004; Petretta et al., 2010). Data used to support lipid-lowering therapy for the primary prevention of CHD in women and upon which current recommendations are based are small compared with those of men (Walsh et al., 2004). Recommendations for lipid-lowering therapy in the primary prevention of CHD in women are predominantly extrapolated from data derived from men and from secondary prevention trials in women (Executive Summary, 2001; Mosca et al., 2004).
Table 4.
Comparison of Therapies in Women for the Primary Prevention of Coronary Heart Disease and Reduction of Overall Mortality
| Therapy (reference) | Coronary Heart Disease1 | Overall Mortality1 |
|---|---|---|
| Hormone2 (Salpeter et al., 2004; Salpeter et al., 2006) | 0.68 (0.48–0.96) | 0.61 (0.39–0.95) |
| Lipid-Lowering (Walsh and Pignone, 2004) | 0.89 (0.69–1.09) | 0.95 (0.62–1.46) |
| Statin (Petretta et al., 2010) | 0.95 (0.78–1.16) | 0.96 (0.81–1.13) |
| Aspirin-MI3 (Berger et al., 2006) | 1.01 (0.84–1.21) | 0.94 (0.74–1.19) |
| Aspirin-WHS4 (Ridker et al., 2005) | 0.91 (0.80–1.03) | 0.95 (0.85–1.06) |
Hazard ratio (95% Confidence Interval)
<60 years old and/or <10 years since menopause when randomized
Coronary heart disease outcome = myocardial infarction (MI)
Women’s Health Study; Coronary heart disease outcome = nonfatal myocardial infarction, nonfatal stroke or death from cardiovascular causes. MI outcome = 1.02 (0.84–1.25)
Additionally, prophylactic use of aspirin for the primary prevention of CHD in women is a common medical practice recommended by major health organizations (Mosca et al., 2004). However, the Women’s Health Study (WHS) of 39,876 healthy women randomized to aspirin 100 mg every other day or to placebo for 10 years showed a null effect of aspirin on the primary trial cardiovascular end point (Table 4) (Ridker et al., 2005). Overall mortality and cardiovascular death from any cause were also unaffected by aspirin (Ridker et al., 2005). Meta-analyses of the cumulated RCT data in women confirm these null results with aspirin therapy from the WHS (Table 4) (Berger et al., 2006). In a more recent study, the lack of efficacy of aspirin in the primary prevention of CHD was also demonstrated in women with diabetes mellitus (Ogawa et al., 2008).
Meta-analyses of the cumulated RCT data for the prevention of CHD indicate that there is a sex-specific efficacy for the major therapies used for the primary prevention of CHD. Both lipid-lowering and aspirin therapy have a null effect on the primary prevention of CHD in women and no effect on overall mortality (Table 4) (Walsh et al., 2004; Petretta et al., 2010; Ridker et al., 2005; Berger et al., 2006; Ogawa et al., 2008). In stark contrast to lipid-lowering and aspirin therapy for the primary prevention of CHD, the cumulated data across more than 2 dozen RCTs demonstrate a significant reduction in CHD and overall mortality in women who are younger than 60 years of age and within 6 years of menopause when initiating HT (Table 4) (Salpeter et al., 2004; Salpeter et al., 2006; Salpeter et al., 2009a; Salpeter et al., 2009b). Initiation of HT in close rather than remote proximity of menopause and continued for a prolonged duration appears to be key in the full expression of the cardioprotective and reduction of overall mortality effects of HT (Hodis et al., 2003; Hodis and Mack, 2007a; Hodis and Mack, 2007b; Hodis and Mack, 2008).
Test of the Estrogen Cardioprotective and Timing Hypotheses
Soon after publication of the discordant primary trial results from WHI-EP (July 2002) relative to the extensive observational literature and based on the results from HERS and the provocative disparate findings from WELL-HART and EPAT, the timing hypothesis was proposed and a new RCT was planned, designed and approved for submission to the NIH in October 2003. The Early vs. Late Intervention Trial with Estradiol (ELITE; clinicaltrials.gov NCT00114517) began enrollment in 2004 and randomized 643 healthy postmenopausal women in a 2×2, randomized, double-blind, placebo-controlled trial according to time since menopause. Healthy women without pre-existing clinical cardiovascular disease <6 years and >10 years since menopause were randomized to oral estradiol therapy and to placebo therapy (with vaginal progesterone gel or placebo) in each stratum. The primary trial end point is the progression of CIMT measured every 6 months. The secondary trial end point is the rate of cognitive decline. Based on the wealth of evidence that has accumulated over the ensuing years in support of the initial proposal to the NIH of the timing hypothesis, a 3-year extension of ELITE was funded. The 3 specific aims of the ELITE extension are to: 1) extend randomized treatment for an average of 6 years (range, 2 to 8.5 years); 2) add a secondary vascular end point using cardiac computed tomography to non-invasively measure coronary artery calcium and coronary artery lesions; and, 3) add a third cognitive measurement to extend the measurement of cognitive decline over an average of 5 years. Primary trial results from ELITE are expected in 2013.
Conclusion
The totality of data indicate that the effect of postmenopausal HT on CHD and overall mortality is modified by the timing of initiation (age and time since menopause) and the duration of therapy. The greatest benefit occurs in women who initiate HT below age 60 years or within 6 years of menopause. It is this latter group of women who are in the most need of symptomatic relief of menopausal symptoms such as flushing for which estrogen remains the most effective therapy (Nelson et al., 2006). RCTs are supported by approximately 40 observational studies that also indicate that initiation of HT early in the postmenopausal period and continued for a prolonged period of time results in a significant reduction of CHD and overall mortality. Comparison of the results from RCTs, observational studies and case-control studies indicates that selection bias does not explain the consistent evidence that HT is associated with a duration- and time-dependent lowering of CHD and overall mortality. Analyses of the subgroups of women within RCTs that resemble the women from observational studies indicate a consistency between the 2 types of study designs with similar benefit of HT on the reduction of CHD and overall mortality (Figure 1). The “window of opportunity” for maximal expression of the beneficial effects of HT on CHD and overall mortality appears to be initiation of HT within 6 years of menopause and/or before 60 years of age and continued for 6 years or more. Unlike lipid-lowering and aspirin therapy, HT reduces CHD and more importantly, overall mortality in this subgroup of women. Due to this reduced overall mortality, there is a substantial increase in QALYs in younger postmenopausal women who initiate HT in close proximity to menopause supporting HT as a highly-cost effective strategy for improving quality-adjusted life.
No other single preventive therapy offers systemic-wide effects for women and as such, the use of HT in the primary prevention of disease must be considered differently than medications currently used for prevention that are limited to a single organ system. Administration of exogenous estrogen during menopause should not be viewed as a therapy for any specific disease entity but as a replacement for a hormone that appears by the cumulated data to lessen the impact of aging on a multitude of organ systems such as the cardiovascular, skeletal and potentially the central nervous systems (Hodis and Mack, 2007a). Timing in the initiation of hormone replacement before tissue damage due to aging becomes too extensive appears to be the key for successful prevention and amelioration of any further damage (Hodis and Mack, 2007a).
In the final analysis, the discordance in the association of HT with CHD and overall mortality between RCTs and observational studies is a function of the differences in study design and the characteristics of the populations studied. As such, the cardioprotective hypothesis has yet to be appropriately tested in a population of women with the characteristics from which the hypothesis has been generated. ELITE is a RCT designed specifically for this purpose. As data from RCTs accumulate, it is important to realize how much the results look more and more like the more than 40 consistent observational studies that indicate that young postmenopausal women with menopausal symptoms who use HT for long periods of time have lower rates of CHD and overall mortality than comparable postmenopausal women who do not use HT.
Acknowledgments
Funding: Funded in part by the National Institute on Aging, National Institutes of Health R01AG-024154.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Howard N. Hodis, Email: athero@usc.edu, Harry J. Bauer and Dorothy Bauer Rawlins Professor of Cardiology, Professor of Medicine and Preventive Medicine, Professor of Molecular Pharmacology and Toxicology, Director, Atherosclerosis Research Unit, Keck School of Medicine, University of Southern California, 2250 Alcazar Street, CSC 132, Los Angeles, CA 90033, (323) 442-1478, (323) 442-2685 Fax
Wendy J. Mack, Email: wmack@usc.edu, Professor of Preventive Medicine, Atherosclerosis Research Unit, Keck School of Medicine, University of Southern California, 1540 Alcazar Street, CHP 234, Los Angeles, CA 90033, (323) 442-1820 323), 442-2993 Fax
References
- Akhrass F, Evans AT, Wang Y, Stuart R, Kannan CR, Fogelfeld L, Mazzoneet T. Hormone replacement therapy is associated with less coronary atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2003;88:5611–5614. doi: 10.1210/jc.2003-031008. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in asymptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40–48. doi: 10.1097/00042192-200512010-00009. [DOI] [PubMed] [Google Scholar]
- Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- Blankenhorn DH, Hodis HN. Duff Memorial Lecture: arterial imaging and atherosclerosis reversal. Arterioscl Thromb. 1994;14:177–192. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- Chilvers CED, Knibb RC, Armstrong SJ, Woods KL, Logan RFA. Postmenopausal hormone replacement therapy and risk of acute myocardial infarction: a case control study of women in the East Midlands, UK. Eur Heart J. 2003;24:2197–2205. doi: 10.1016/j.ehj.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, Effron MB, Dowsett SA, Barrett-Connor E, Wenger NK. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the raloxifene use for the heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119:922–930. doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleijn MJJ, Bots ML, Bak AAA, Westendorp ICD, Planellas J, Bennink HJC, Witteman JCM, Grobbee DE. Hormone replacement therapy in perimenopausal women and 2-year change of carotid intima-media thickness. Maturitas. 1999;32:195–204. doi: 10.1016/s0378-5122(99)00035-3. [DOI] [PubMed] [Google Scholar]
- Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Falkeborn M, Persson I, Adami HO. The risk of acute myocardial infarction after oestrogen and oestrogen-progestogen replacement. Br J Obstet Gynaecol. 1992;99:821–828. doi: 10.1111/j.1471-0528.1992.tb14414.x. [DOI] [PubMed] [Google Scholar]
- Ferrara A, Quesenberry CP, Karter AJ, Njoroge CW, Jacobson AS, Selby JV. Current use of unopposed estrogen and estrogen plus progestin and the risk of acute myocardial infarction among women with diabetes: the Northern California Kaiser Permanente diabetes registry, 1995–1998. Circulation. 2003;107:43–48. doi: 10.1161/01.cir.0000042701.17528.95. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiol Dis. 1995;38:199–210. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Stampfer M. Estrogen for women at varying risk of coronary disease. Maturitas. 1998;30:19–26. doi: 10.1016/s0378-5122(98)00055-3. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Heckbert SR, Weiss NS, Koepsell TD, Lemaitre RN, Smith NL, Siscovick DS, Lin D, Psaty BM. Duration of estrogen replacement therapy in relation to the risk of incident myocardial infarction in postmenopausal women. Arch Intern Med. 1997;157:1330–1336. [PubMed] [Google Scholar]
- Henderson BD, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–78. [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu CR, Liu CH, Azen SP. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, Faxon DP, Cashin-Hemphill L, Sanmarco ME, French WJ, Shook TL, Gaarder TD, Mehra AO, Rabbani R, Sevanian A, Shil AB, Torres M, Vogelbach KH, Selzer RH. Hormone therapy and the progression of coronary artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–545. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ. Randomized controlled trials and the effects of postmenopausal hormone therapy on cardiovascular disease: facts, hypotheses and clinical perspective. In: Lobo RA, editor. Treatment of the postmenopausal woman. 3. Philadelphia, PA: Elsevier; 2007a. pp. 529–564. [Google Scholar]
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007b;14:944–957. doi: 10.1097/gme.0b013e31802e8508. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ. Postmenopausal hormone therapy and cardiovascular disease in perspective. Clin Obstret Gyencol. 2008;51:564–580. doi: 10.1097/GRF.0b013e318181de86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternak RC, Pinn VW, Robertson RM, Schenck- Gustafsson K, Sila CA, Smith SC, Sopko G, Taylor AL, Walsh BW, Wenger NK, Williamset CL. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672–693. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhumoral therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- Petretta M, Costanzo P, Perrone-Filardi P, Chiariello M. Impact of gender in primary prevention of coronary heart disease with stain therapy: a meta-analysis. Int J Cardiol. 2010;138:25–31. doi: 10.1016/j.ijcard.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Langer R, Stefanick ML, Howard BV, Pettinger ML, Anderson GL, Barad D, Curb JD, Kotchen J, Kuller L, Limacher M, Wactawski-Wende J. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial. Am J Epidemiol. 2005;162:404–414. doi: 10.1093/aje/kwi223. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Langer R, Stefanick ML, Howard BV, Pettinger M, Anderson GL, Barad D, Curb JD, Kotchen J, Kuller L, Limacher M, Wactawski-Wende J. Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone therapy and cardiovascular disease. Am J Epidemiol. 2006;163:589–599. doi: 10.1093/aje/kwj079. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Heckbert SR, Atkins D, Lemaitre RN, Koepsell TD, Wahl PW, Siscovick DS, Wagneret EH. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med. 1994;154:1333–1339. [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JA, Manson JE, Wu LL, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2004;19:791–804. doi: 10.1111/j.1525-1497.2004.30281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JME, Greyber E, Salpeter EE. Coronary heart disease events associated with hormone therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2006;21:363–366. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009a;12:1016–1022. doi: 10.1016/j.amjmed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Buckley NS, Liu H, Salpeter EE. The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med. 2009b;122:42–52. doi: 10.1016/j.amjmed.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Meade TW, Greenberg G. The use of hormonal replacement therapy and the risk of stroke and myocardial infarction in women. J Epidemiol Community Health. 1989;43:173–178. doi: 10.1136/jech.43.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Hernandez-Diaz S, Logan R, Rossouw JE, Hernan MA. Coronary heart disease in postmenopausal recepients of estrogen plus progestin therapy: does the increased risk ever disappear? Ann Intern Med. 2010;152:211–217. doi: 10.1059/0003-4819-152-4-201002160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA. 2004;291:2243–2252. doi: 10.1001/jama.291.18.2243. [DOI] [PubMed] [Google Scholar]