Abstract
Estradiol (E) mediates increased synaptogenesis in the hippocampal CA1 stratum radiatum (sr) and enhances memory in young and some aged female rats, depending on dose and age. Young females rats express more estrogen receptor α (ERα) immunolabeling in CA1sr spine synapse complexes than aged rats and ERα regulation is E sensitive in young but not aged rats. The current study examined whether estrogen receptor β (ERβ) expression in spine synapse complexes may be altered by age or E treatment. Young (3–4 months) and aged (22–23 months) female rats were ovariectomized 7 days prior to implantation of silastic capsules containing either vehicle (cholesterol) or E (10% in cholesterol) for 2 days. ERβ immunoreactivity (ir) in CA1sr was quantitatively analyzed using post-embedding electron microscopy. ERβ-ir was more prominent postsynaptically than presynaptically and both age and E treatment affected its synaptic distribution. While age decreased the spine synaptic complex localization of ERβ-ir (i.e., within 60 nm of the pre- and post-synaptic membranes), E treatment increased synaptic ERβ in both young and aged rats. In addition, the E treatment, but not age, increased dendritic shaft labeling. This data demonstrates that like ERα the levels of ERβ-ir decrease in CA1 axospinous synapses with age, however, unlike ERα the levels of ERβ-ir increase in these synapses in both young and aged rats in response to E. This suggests that synaptic ERβ may be a more responsive target to E, particularly in aged females.
INTRODUCTION
In young rats, elevated levels of estrogens (either in the proestrus phase of the estrous cycle and with experimental replacement) increase axospinous synapse density on CA1 pyramidal cells (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1992, 1993). Parallel with changes in spine/synapse densities, estrogen treatment also has been shown to change levels of synaptic proteins in CA1, CA3 and the dentate gyrus (Brake et al., 2001; Waters et al., 2009) and activate second messenger pathways (Kelly and Ronnekleiv, 2009). In contrast, estrogen fails to increase CA1 synapse density in aged female rats (Adams et al., 2001c), which may be due in part to decreased synaptic ERα-immunoreactivity (ir) in CA1 synaptic profiles in aged rats as well as less down-regulation after estradiol (E) treatment (Adams et al., 2002). Interestingly, these axospinous synapses do display estrogen-induced alterations in synaptic glutamatergic N-methyl-D-aspartate (NMDA) receptors (Adams et al., 2001a; Adams et al., 2004b).
There are two known forms of classical estrogen receptors (ERs), ERα and ERβ, and they have a high and nearly equal affinity for estrogens (Levin, 2001). In the CNS, genomic actions of estrogen are mediated through nuclear receptors (McEwen Alves, 1999). Moreover, rapid, non-genomic actions of estrogen that may be mediated through extranuclear ERα and/or ERβ (Levin, 2001; McEwen et al., 2001). Electron microscopic studies have revealed that, like ERα (Milner et al., 2001; Towart et al., 2003), ERβ-ir is located at extranuclear sites: dendritic spines, axons, terminals, and glia (Milner et al., 2005). These findings strongly suggest that ERα and ERβ can mediate estrogen’s classical transcriptional/genomic actions as well as more rapid signaling in the rat hippocampal formation.
Although ERβ-ir has been analyzed in young and aged animals (Mehra et al., 2005; Milner et al., 2005), estrogen regulation of the synaptic distribution has not been studied. While the regulation of spine formation in CA1 pyramidal neurons by E may involve contributions from both ERβ and ERα, the role of each receptor plays during the decline in estrogen sensitivity with age is unclear. With increasing age, altered ER distribution would have important implications for estrogen signaling and hippocampal dependent function. Indeed, age-related alterations in ERβ distribution may lead to a decreased impact of E on aged synapses. To address this hypothesis, the subcellular distribution of ERβ-ir in the stratum radiatum (sr) of the CA1 region of the dorsal hippocampus was analyzed by quantitative electron microscopy in young and aged female rats that were ovariectomized and treated with either vehicle (Veh) or E.
RESULTS
Post-embedding immunocytochemistry was performed to quantitatively examine whether the levels or subcellular distribution of ERβ-ir in the CA1sr of the dorsal hippocampus are affected by age and/or E. Young and aged female rats were ovariectomized (OVX) for 7 days prior implanting silastic capsules containing either vehicle (Veh; cholesterol) or E (10% in cholesterol). Two days after the implant, the animals’ brains were perfusion-fixed with paraformaldehyde and glutaraldehyde and the dorsal hippocampus was embedded in Lowicryl embedding resin. Sections through CA1sr were cut on an ultratome, collected on grids and labeled with an antibody to ERβ. Immunoreactivity for ERβ was identified using gold-conjugated secondary antibodies. The sections then were examined under an electron microscope and the percentage of axodendritic synapses containing ERβ-ir and the number of ERβ immunogold particles in pre- and post-synaptic compartments quantified (see details in Experimental Procedures).
ERβ-ir is prominent in pre- and postsynaptic compartments in all groups
The subcellular distributions of ERβ-ir using post-embedding immunogold methods were consistent with previous studies using pre-embedding electron microscopic methods (Milner et al., 2005). Specifically, ERβ-ir was found in axon terminals, dendritic spines (Fig. 1) and shafts (Fig. 5A) in all groups. Within all groups, the distribution of ERβ-ir was more pronounced in the postsynaptic profile as compared to the presynaptic terminal and synaptic cleft. Moreover, the distribution of ERβ immunogold particles was non-uniform in both pre- and postsynaptic profiles. In tissue processed in primary antibody that had been preadsorbed with the antigenic peptide, in the absence of primary antibody or in the presence of non-gold conjugated IgG secondary antibody, 99% of the gold particles in both pre- and postsynaptic profiles were removed. Moreover, the gold particles were not limited to any particular cellular compartment.
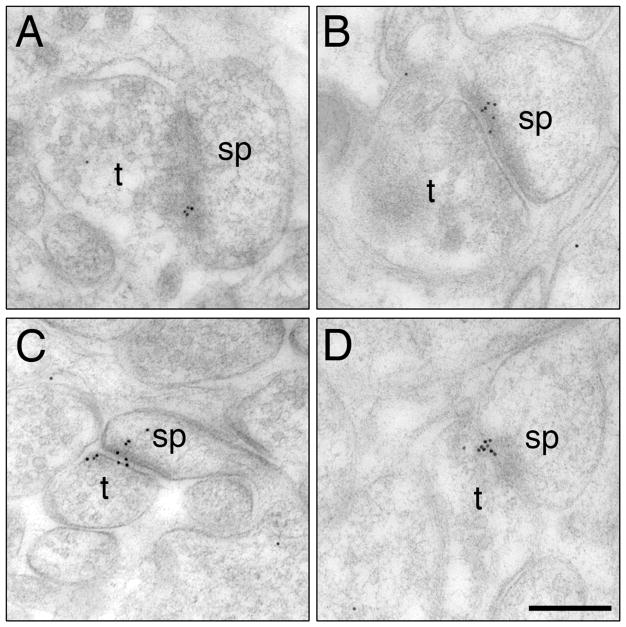
Fig. 1.
Representative electron micrographs show the distribution of ERβ immunogold particles (black puncta) in the CA1 stratum radiatum of young OVX + Veh (A), young OVX + E (B), aged OVX + Veh (C) and aged OVX + E (D) rats. t, terminal; sp, dendritic spine. Bar, 100 nm
Fig. 5.
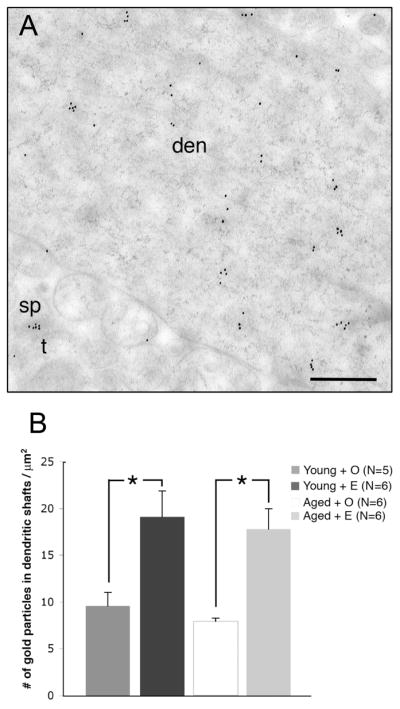
The overall numbers of ERβ immunogold particles in dendrites are not significantly different in aged rats compared to young rats. A. Clusters of ERβ immunogold particles are visible in dendrites in the CA1sr. B. ERβ immunogold particles are significantly increased following E-replacement in both young and aged rats. * p < 0.02
Both aging and estrogen, affect the percentage of ERβ-labeled dendritic spines
To determine whether the percent of axospinous synapses that contained ERβ changed with aging or E treatment, the percentage of ERβ labeled dendritic spines within a 3000 μm2 area in CA1sr was assessed. Dendritic spines that contained 2 or more immunogold particles were considered labeled.
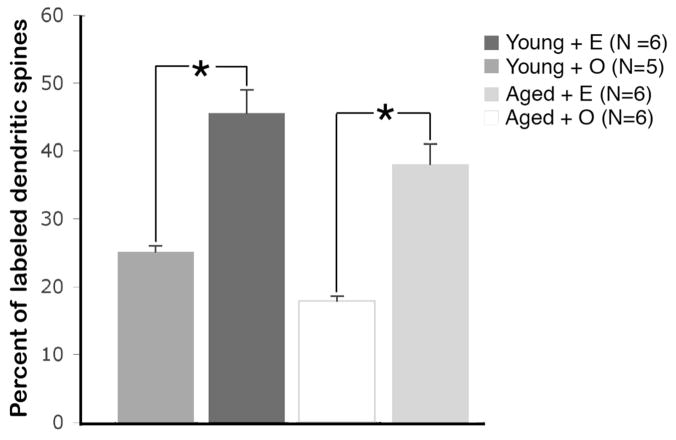
ERβ immunogold particles within axospinous synapses were significantly affected by age as well as E treatment (Fig. 2). Specifically, multiple comparison analysis followed by Tukey-Kramer post-hoc analysis revealed that the percentage of ERβ-immunoreactive synapses was significantly elevated in young compared to aged rats (p < 0.02). However, E significantly affected the percentage of ERβ immunoreactive axospinous synapses in both young and aged rats (Fig. 2). In particular, the young OVX + E group had 45% more ERβ immunoreactive synapses then young OVX + Veh group (p < 0.0001). Moreover, the aged OVX + E group demonstrated 53% more labeled synapses then aged OVX + Veh group (p < 0.0001).
Fig. 2.
The overall percentage of ERβ immunoreactive axospinous synapses labeled dendritic spines is not affected by age but is significantly increased following E-replacement in both young and aged OVX rats. N = number of animals. * p < 0.02
Aging decreases and estrogen treatment increases ERβ-ir within select compartments of spine synapse complexes
To determine if aging and/or E affected the subcellular distribution of ERβ-ir in the spine synapse complexes, the distribution of ERβ immunogold particles in dendritic spines and in terminals presynaptic to dendritic spines was quantitatively analyzed using the Synbin analysis (Adams et al., 2002; Adams et al., 2004b). The Synbin analysis divides the synapse into discrete areas for analysis of gold particle distribution (Fig. 3). In dendritic spines, postsynaptic ERβ immunogold particles were present in all areas including the core of the spine (Bin 4), near the postsynaptic density (PSD; Bins 1 and 2) and in the synaptic cleft (Bin 5). In terminals, ERβ immunogold particles were in the cytoplasmic compartment where they were often affiliated with vesicles (Bin 8) as well as near the synaptic junction (Bins 6 and 7). In all groups, almost no ERβ-ir was seen in the perisynaptic region (Bin 3) so this region was excluded from the analysis.
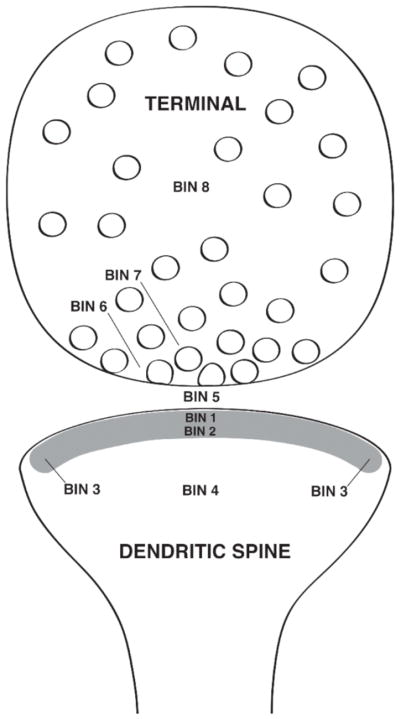
Fig. 3.
Schematic drawing showing a pre- and postsynaptic profile with the eight Bin divisions used in the EM analysis.
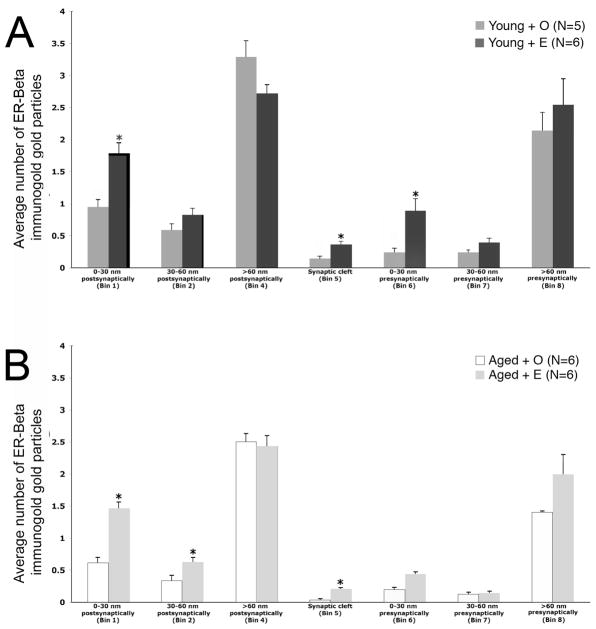
Multiple comparison analysis followed by Tukey-Kramer post-hoc analysis revealed that ERβ immunogold particles were significantly less in select cellular compartments in aged OVX animals compared to young OVX animals (Fig. 4). Specifically, aged OVX rats had significantly fewer ERβ immunogold particles within the 0–30 nm postsynaptic bin (Bin 1; p = 0.02), in the core of dendritic spines (Bin 4; p = 0.01) and in the cleft (Bin 5; p < 0.03). No significant differences were detected in the presynaptic bins (Bins 6–8; p > 0.05).
Fig. 4.
Both age and estrogen altered the subcellular distribution of ERβ immunogold particles in pre- and postsynaptic profiles in the CA1sr region. A. In young OVX rats, E administration significantly increased ERβ-ir presynaptically (Bin 6) and postsynaptically (Bin 1) and in the synaptic cleft (Bin 5). B. In aged OVX rats, E administration significantly increased ERβ-ir only in Bins 1 and 8. Moreover, E administration to aged OVX significantly decreased ERβ-ir in Bin 4. * p < 0.05
The post-hoc analysis also revealed that E administration altered the subcellular distribution of ERβ immunogold particles in pre- and postsynaptic profiles in young and aged animals. E increased ERβ immunogold particle counts in young animals within the 0–30 nm postsynaptic bin (Bin 1; p < 0.0001), cleft (Bin 5; p < 0.001) and 0–30 nm pre-synaptic bin (Bin 6; p < 0.0001). There was also a strong trend (p = 0.05) for E to increase ERβ-ir in the 30–60 nm region near the synaptic contact of the presynaptic terminal (Bin 7). A treatment effect for the aged animals was observed only in the postsynaptic Bins 1 (p < 0.0001) and 2 (p = 0.02) and in the synaptic cleft (Bin 5; p = 0.0006). Unlike young rats, E did not significantly increase ERβ–ir presynaptically (Bin 6; p > 0.05).
Estrogen, but not aging, affects ERβ-ir distribution in dendritic shafts
Like dendritic spines, ERβ-ir was detected in dendritic shafts in CA1sr in all groups. Thus, we determined if aging and/or E treatment affected the distribution of ERβ immunogold particles in dendritic shafts. Unlike dendritic spines, no significant (p > 0.05) overall effect of aging was detected in the density of ERβ immunogold particle in the dendritic shafts. However, like dendritic spines, E increased the density of ERβ immunogold particles in dendritic shafts in both young and aged animals (Fig. 5B) Multiple comparisons analysis with a Tukey-Kramer post hoc analysis revealed that OVX + E treated groups were significantly different from OVX + Veh groups (i.e. treatment effect) for young animals (p < 0.02) and for aged animals (p < 0.01). E increased the number of ERβ immunogold particles by approximately 50% in OVX + E groups compared to OVX + Veh groups in both young and aged animals.
Estrogen induces a clustering of ERβ immunogold particles in dendritic profiles
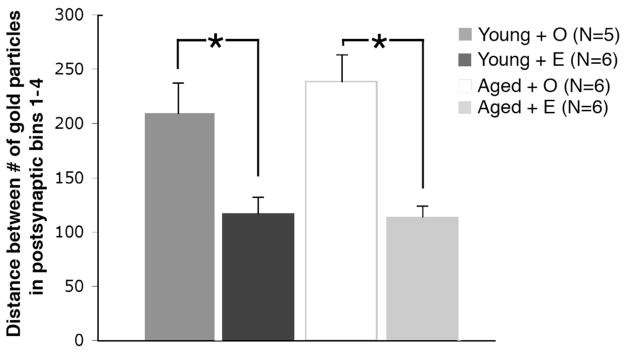
During the above analysis, we noticed that the ERβ immunogold particles in dendritic spines and dendritic shafts appeared to form clusters in the presence of E. Increased clustering would suggest a relationship between protein levels and their distribution within the cell. The clustering of ERβ gold particles in postsynaptic profiles was assessed by using average distance between gold particles analysis (Elste and Benson, 2006). This analysis revealed that the average distance between ERβ immunogold particles decreased 79% (p < 0.03) in young animals and 109% (p < 0.003) in aged animals following E treatment.
DISCUSSION
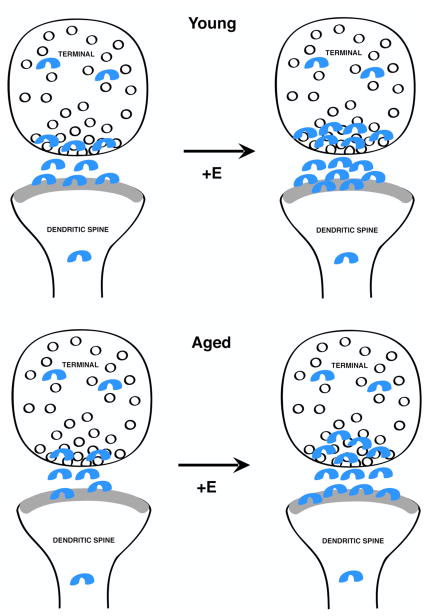
The present study demonstrates that like ERα the levels of ERβ-ir decrease in CA1 axospinous synapses with age, however, unlike ERα the levels of ERβ-ir increase in these synapses in both young and aged rats in response to E (Fig. 7). This suggests that synaptic ERβ may be a more responsive target to E, particularly in aged females.
Fig. 7.
Schematic diagram depicting the effects of E administration on the levels and subcellular distribution of ERβ immunogold particles in spine synapse complexes in young and aged rats. ERβ-ir was detected in pre- and postsynaptic compartments of asymmetric synapses in the CA1sr in young and aged females. Although synapses contained fewer ERβ immunogold particles in aged females compared to young females, in both groups E treatment increased synaptic ERβ-ir.
Dendritic ERβ is affected by estrogen in both young and aged rats
In agreement with our pre-embedding electron microscopic studies (Milner et al., 2005; Mitterling et al., 2010), ERβ-ir using post-embedding electron microscopic methods was detected in pre- and post-synaptic compartments in young and aged rat hippocampus. In CA1sr, synaptic ERβ-ir increased with E treatment in young and aged animals. Although the percentage of ERβ-labeled synapses was less in the aged animals compared to young animals, in both groups E treatment increased the number of ERβ immunogold particles. ERβ immunogold particles were seen in all of the sub-synaptic bins, they were highest in the postsynaptic compartment of the synapse. In dendritic shafts ERβ immunogold particle density was increased by E treatment in young and aged animals. Additionally, the average distance between the ERβ immunogold particles in dendrites decreased with E treatment resulting in clustering in both young and aged rats. These ERβ clusters may represent increased expression and/or movement to distal parts of the cell or even translocation of protein between subcellular compartments (Saneyorshi et al., 2010).
The detection of ERβ-ir in synaptic compartments may facilitate estrogen’s rapid effects on synaptic plasticity. The presence of ERs along with 125I-E binding in synapses (Milner et al., 2008) supports a role for E modulation of synaptic transmission and plasticity. Moreover, the detection of ERβ-ir on the plasma membrane of dendrites may be important in modulating estrogen-mediated plasticity associated with neuropeptides. For example, opioid peptide receptors are found on the plasma membranes of dendritic shafts and are modulated by estrogen levels (Torres-Reveron et al., 2009). Acute estrogen is reported to affect learning and memory particularly mnemonic processes and consolidation (Packard and Teather, 1997; Luine et al., 2003; Rhodes and Frye, 2006), facilitate long term potentiation (LTP) (Foy et al., 1999; Bi et al., 2000; Kramar et al., 2009), translocate glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Zadran et al., 2009), and polymerize actin (Kramar et al., 2009; Zadran et al., 2009). The contribution of ERβ to acute estrogen effects was demonstrated recently for LTP (Liu et al., 2008; Zadran et al., 2009) and actin polymerization (Zadran et al., 2009). Thus, extranuclear ERβ likely mediates estrogen’s rapid actions and its persistence during aging represents another opportunity to explore alterations in estrogen signaling with age.
Estrogens and aging affect ERβ-ir differently than ERα
Like ERα-ir (Adams et al., 2002), ERβ-ir were found in dendritic spine synaptic complexes. Although the SynBin analysis revealed that both ERα-ir and ERβ-ir had a similar subcellular distribution, responsiveness of individual bins to E and age differed between the two receptors. In particular, ERα-ir decreased only in the cytoplasmic portions of dendritic spines and terminals (e.g., bins 4 and 8) in young rats following E treatment. Additionally, ERα-ir did not change in aged rats following E treatment. In contrast, ERβ-ir increased in the regions of the dendritic spines and terminals that were closest to the synapse (i.e., bins 1, 5 and 6) in young rats treated with E. Moreover, E elevated ERβ-ir in synaptic regions the dendritic spine and synaptic cleft (i.e., bins 1, 2 and 5) in aged rats following E treatment. These data, support previous studies (Tetel and Pfaff, 2010) suggesting ERα and ERβ have functionally distinct roles in regulating synaptic function. For example, E may promote a translocation of ERβ-ir from cytoplasmic pools into the synapse, where ERβ may have an “active” role in synaptic transmission (Rizzoli and Betz, 2005). In contrast, E elevations ERα-ir in the presynaptic cytoplasmic pool, where synaptic vesicles reside, may promote neurotransmitter release (Becker 1990; Adams et al., 2002 ) and reuptake (O-Malley et al., 1987; Adams 2002).
In aged rats E enhancement of ERβ would increase the ratio of ERβ to ERα. This alteration in their ratio could contribute to the age-related loss of E induced synapse formation, since it has been suggested that ERβ may be responsible for inhibiting E-induced synapse formation (Szymczak et al., 2006). ERβ actions also have been associated with increased levels of the glutamatergic AMPA receptor subunit GluR2 (Waters et al., 2009), which may increase calcium impermeability and alter synaptic plasticity. The retention of E sensitive ERβ regulation with aging concurrent with the loss of ERα protein and regulation supports a greater role for ERβ affects without ERα opposition in the aging hippocampus.
ERβ and second messenger signaling pathways
Post-synaptic effects of E on ERs are mediated by second messenger signaling pathways. We have studied two, LIMK and Akt. Both phosphorylated LIMK and Akt are detected primarily in dendritic spines associated with the synapse and synaptic cleft (Yildirim et al., 2008; Yildirim et al., 2010). In young rats, both pLIMK and pAkt increase in response to E treatment. However, only pAkt increases in response to E treatment in aged rats. In mice, high estrogen levels increase pLIMK and pAkt in the hippocampus of young females (Spencer et al., 2008). Estrogen actions at a membrane estrogen receptor increases ERK phosphorylation and enhances memory in young mice (Fernandez et al., 2008). In middle-aged mice, estrogen mediated phosphorylation of ERK, PI3K and AKT enhances memory retention but this effect did not occur in aged mice (Fan et al., 2010). These findings suggest that the altered ERβ and ERα ratio contribute to signaling deficits that may affect memory and plasticity.
Implications for aging and hippocampal function
Loss of estrogen and processes affected by this loss are defined by a “window of opportunity” that closes with age and postmenopausal state. The persistence of E sensitive ERβ regulation in aged animals in both estrogen sensitivity and direction is the first demonstration of E sensitivity in acyclic animals. ERβ actions may offer an important route for maintaining steroid homeostasis and altering plasticity in the aged brain. Indeed, ERβ may be a major target for estrogen therapy in the aging hippocampus.
ERβ is a potential target and mechanism for understanding effects of estrogen in brain regions and circuits mediating cognitive processes such as memory (Shughrue et al., 1997; Rissman et al., 2002; Walf et al., 2009; Liu, et al., 2008). Estrogen influences hippocampal-dependent learning (Sandstrom and Williams, 2001; Korol and Kolo, 2002; Luine et al., 2003; Sandstrom and Williams, 2004) and is neuroprotective in brain injury, although it remains unclear which ER is mediating estrogen’s beneficial effects (Dubal et al., 2001; Miller et al., 2005). ERβ actions may be linked to Alzheimer’s Disease as ERβ disruption increased neurodegeneration and modulated beta-Amyloid metabolism (Zhang et al., 2004). With increasing time after estrogen loss, its beneficial effects cannot be simply restored with E replacement. In addition, the aged brain is fundamentally different from the young brain in its capacity for synaptic plasticity (Miranda et al., 1999; Adams et al 2001b), and hippocampal dependent functions (Burke and Barnes, 2010). Decreased levels of ERα and ERβ in the aged brain suggest that alterations in the receptor retinue along with a decline in circulating estrogen levels may be involved in loss of E actions.
The relative levels and location of ERα and ERβ in the brain, in particular in the hippocampal formation, provides a fruitful ground for understanding the effects of estrogens on neuronal plasticity. ERα and ERβ have been shown to work in a complex, both complementary and sometimes antagonistic, manner in a number of biological systems (Lindberg et al., 2003; Monroe et al., 2003). ER partial antagonists also reveal differences in how ERα and ERβ interact in the regulation of gene expression (Paech et al., 1997) and non-genomic signaling pathways (Razandi et al., 2004). Moreover, the balance of ERα and ERβ may determine the outcome of E action. For example, increased ERβ expression in hippocampal neurons has been related to decreased spine formation (Szymczak et al., 2006) and ERβ deletion in female mice results in spatial learning deficits (Rissman et al., 2002). ERα and ERβ are not only present in neuronal compartments at different levels, but they also change differentially as a result of aging and by E treatment. This implies a changing balance of ERα and ERβ effects in the aging compared to young brain and suggests that further research is needed to understand the interactions between steroids and their receptors particularly in the aging brain.
The onset of alterations in ERs that occur as a result of age and hormone availability are likely a primary characteristic of the “window of opportunity”. This “window” is a critical period that occurs during perimenopause and prior to postmenopause where hormone replacement could be initiated. ERβ targeted therapy for the central nervous systems may allow for a strategy that benefits the brain without activating untoward effects of estrogen in other organs.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley rats, including eleven young (3–4 month; 225 grams) and eleven aged (23–24 month; 350 grams), were obtained from Harlan (Indianapolis, IN) for use in post-embedding electron microscopic studies. These animals have been used in previous studies examining the interaction of estrogen replacement and aging on markers of synaptic plasticity (Adams et al., 2001a; Adams et al., 2001b; Adams et al., 2002; Adams et al., 2004b). Animals were housed in a temperature-controlled room (12 hour light/dark cycle; lights on at 0700) and had food and water available ad libitum. All experiments were conducted in accordance with Guidelines for the Care and Use of Experimental Animals, by using protocols approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine.
Ovariectomy and estrogen replacement
Seven days after bilateral OVX, a silastic capsule (capsule dimensions: inner diameter 1.96 mm; outer diameter 3.18 mm) filled with either 17β-Estradiol (E) (10% in cholesterol) or cholesterol (Veh) was implanted subcutaneously. Young animals received an implant that was 1 cm in length and aged animals received an implant that was 2 cm in length. Implant lengths was adjusted by age to account for differences in body weight, resulting in similar E levels in both groups of rats (Lauber et al., 1990; Funabashi et al., 1998). Prior to OVX, all young rats had normal estrous cycles. Aged rats have been previously shown to be acyclic (i.e., constant estrous or diestrus (Adams et al., 2001a). Effectiveness of the E replacement was determined by examination of the uterus from each animal at the time of sacrifice. Regardless of age, the uteri in vehicle treated animals were very small and atrophied, while uteri from E treated animals were hypertrophied. A similar E replacement paradigm after a longer-term ovariectomy in aged animals resulted in a similar uterine responses and circulating E levels were within a physiological range (Adams et al., 2002).
Tissue preparation
Transcardial perfusion under deep anesthesia (30% chloral hydrate, i.p.) was used to collect brains. Animals were perfused with 2% dextran in 0.1 M phosphate buffer (PB; pH 7.4, 50 ml/min) for 1 min, followed by 4% paraformaldehyde and 0.125% glutaraldehyde in PB for 10–15 min. Post-perfusion, the animals were inspected for the presence of the implant, to confirm complete removal of ovaries and to assess uterine condition. The brains were removed and postfixed in the same fixative overnight.
Post-embedding immunogold electron microscopic labeling
Tissue slices (1 mm) were prepared for low temperature embedding as described previously (van Lookeren Campagne et al., 1991; Adams et al., 2002; Janssen et al., 2005). Briefly, slices were cryoprotected in increasing concentrations of glycerol (10, 20, and 30%) in PB, then rapidly submerged in liquid propane cooled by liquid nitrogen (−190°C) in a Universal Cryofixation System KF80 (Reichert-Jung, Vienna). Next, samples were immersed in 1.5% uranyl acetate (for en bloc fixation) in anhydrous methanol (−90°C, 24 h) in a cryosubstitution Automatic Freeze-Substitution System unit (Leica, Vienna) and the temperature was increased in steps of 4°C/h from −90 to −45°C. After a final wash with anhydrous methanol, samples were infiltrated with Lowicryl HM20 resin (EMS, Washington, PA) at −45°C with an increasing ratio of resin to methanol for 1 h each, followed by pure Lowicryl overnight. UV light (360 nm) was used to induce polymerization at −45°C for 48 h and finally 24 h at room temperature.
From the dorsal hippocampus of each animal, two blocks (1 mm thick) were randomly selected and processed for post-embedding immunogold localization of ERβ. An area (150–200 μm from the cell bodies) in CA1sr was sectioned. Ultrathin sections (75 nm in thickness) were cut by diamond knife on a Reichert-Jung ultramicrotome and mounted on a nickel mesh grid. The mesh grids with ultrathin sections for the immunolabeling studies were treated with a saturated solution of NaOH in absolute ethanol, rinsed, and incubated at room temperature in 0.1% sodium borohydride and 50 mM glycine and Tris-buffered saline (TBS) containing 2% human serum albumin (HSA). The primary antibody (1:100; goat polyclonal anti-ER-β antibody; L-20; Santa Cruz Biotechnology, Santa Cruz, CA) was incubated overnight. The next day, grids were washed with TBS and incubated in secondary gold-tagged (10 nm) rabbit-anti-goat IgG antibody (diluted 1:75, EMS) in TBS containing 2% HSA and polyethyleneglycol 20,000 (5 mg/ml). Sections were washed and dried, counterstained with 1% uranyl acetate and Reynolds lead citrate. The sections were analyzed on a JEOL 1200EX electron microscope (JEOL, Tokyo, Japan). Images were captured using an Advantage charge-coupled device camera (Advanced Microscopy Techniques, Danvers, MA). For figures, images were prepared in Adobe Photoshop 9.0 to adjust brightness and contrast and sharpness. These changes did not alter the original content of the raw image.
To assess the degree of non-specific labeling by the anti-ERβ antibody, sections on grids were processed as described with primary antibody that had been preadsorbed with the immunogenizing peptide. For this, the primary antibody was diluted 1:100 and incubated with 10x the concentration of ERβ blocking peptide (sc-6822P, Santa Cruz Biotechnology), overnight at 4°C. The preadsorbed antibody was then spun at 4K for 5 minutes and the supernatant was then used as the primary antibody. Sections were also processed in the absence of primary antibody using the procedure described above. To assess the degree of nonspecific absorption of the protein-gold complex of the secondary antibody to the tissue, the secondary antibody was replaced with non-conjugated secondary antibody, at the same protein concentration.
ERβ antibody
The ERβ antibody used in this study was raised in goat against a peptide (aa 420–480) mapping near the C terminus of the human ERβ (L-20, sc-6822; Santa Cruz Biotechnology). This antibody reacts to human, rat and mouse ERβ (Santa Cruz spec. sheet). On Western blots, this antibody recognizes ERβ1 (~MW 59 kDa) and ERβ2 (~MW 53kD) and does not cross-react with ERα (Roger et al., 2005). Seminoma cells and platelets which are immunolabeled using the L-20 antibody also contain ERβ mRNA as determined with reverse transcriptase polymerase chain reaction (Roger et al., 2001; Alonso-Magdalena et al., 2008). Preadorption of the L-20 antibody with the immunizing peptide eliminates labeling in Western blots (Nealen et al., 2001; Royuela et al., 2001) as well as in human prostate and smooth muscle cells (Royuela et al., 2001; Speir et al., 2000). Additionally, omission of the L-20 antibody in pancreas, seminoma cells and smooth muscle cells processed for immunocytochemistry yields no labeling of these tissues (Alonso-Magdalena et al., 2008; Roger et al., 2005; Speir et al., 2000). The L-20 antibody has been used for light and electron microscopic examinations of ERβ labeling in the brain (Ishunina et al., 2000; Ishunina and Swaab, 2001; Milner et al., 2005). In the human supraoptic nucleus, the L-20 antibody yields an identical labeling pattern as that seen with the N-terminus antibody to ERβ (sc-6821, Santa Cruz Biotechnology) (Ishunina et al., 2000). In the rat hippocampus, L20 labeling exhibits similar labeling patterns at the ultrastructural level as three other ERβ antibodies (Merck 485; Zymed Z8P and Santa Cruz sc-6821) (Milner et al., 2005). In particular, all four antibodies label dendritic spines and terminals in a similar pattern when viewed under the electron microscope. In addition, the L-20 and Zymed Z8P antibodies labeled the same synaptic compartments in dual label colocalization studies (see supplemental data). In estrogen receptor beta knock out mice, no labeling is detected with the Z8P antibody (Snyder et al., 2010). Although the immunogenic peptide used to generate the L-20 antibody was selected to uniquely recognize ERβ, the possibility remains that it recognizes other proteins with similar structures, particularly other ERβ isoforms or estrogen binding proteins (Snyder et al., 2010).
Analysis of percentage of axospinous synapses labeled for ERβ
The analyses were performed by an experimenter “blind” to age and E treatment. In each block, a region of sr that was greater than 50 μm away from the pyramidal cell layer was randomly selected at low magnification (600x). The magnification was then increased to 7,500 and photographs were taken of the at least 150 synapses. An ERβ immunoreactive synapse was identified as containing two or more gold particles per pre- or postsynaptic profile (i.e., in the axon terminal, synaptic cleft, postsynaptic density, or spine head) (Adams et al., 2002; Adams et al., 2004a, 2004b).
Sub-Synaptic distribution of ERβ
The density and distribution of immunogold particles in the various synaptic compartments was analyzed using SYNBIN software (Adams et al., 2002; Adams et al., 2004b). This software employs the proximity to membranes principles established by Ruud and Blackstad (Ruud and Blackstad, 1999) where the position of each immunogold particle is determined in relation to pre- and postsynaptic membranes and structures. This allows each gold particle to be assigned to a bin, whose size and synaptic location was predetermined. Bin sizes are based on both the lateral resolution of the electron microscopy techniques and the optimal separation of synaptic and non-synaptic pools of receptors. This procedure generates a synaptic map of immunogold particles that accurately reflects both gold particle density and distribution in the synaptic complex. An average of 40 randomly chosen spines per animal were collected at a magnification of 40,000 and analyzed. The final analysis excluded any synapses that lacked clearly defined synaptic structures such as pre-and postsynaptic membranes, a synaptic cleft, and a postsynaptic density.
For this analysis bin width was set at 30 nm, which assures high resolution yet encompasses the theoretical limit of resolution of the immunogold particles (i.e., 25 nm). For each synapse, eight bins were established (Fig. 3). Postsynaptic bins defined included, [Bin 1] 0–30 nm from the inner leaflet of the postsynaptic membrane, [Bin 2] 30–60 nm from the postsynaptic membrane, [Bin 3] 15 nm lateral to both of the postsynaptic bins, [Bin 4] a cytoplasmic bin that included gold particles >60 nm from the postsynaptic membrane, and [Bin 5] comprised the synaptic cleft. Three presynaptic bins were established, [Bin 6] 0–30 nm from the inner border of the presynaptic membrane, [Bin 7] 30–60 nm from the inner border of the presynaptic membrane, and [Bin 8] >60 nm from the presynaptic membrane. With this layout, gold particles within the 0–30 nm of the synaptic membranes are unquestionably synaptic in location. All other postsynaptic bins then include the particles representing non-synaptic pools of ER-β-ir. Lateral bins established a “buffer zone” at the edges of the synapse (i.e., within 15 nm) to account for gold particles that might be labeling proteins associated with the postsynaptic density. When analyzed, gold particles in these lateral bins (Bin 3) were so insignificant that they were not included in this study.
Dendritic shaft analysis of ERβ
Density analysis of dendritic shafts was limited to primary dendrites or those with a minimum 2500 nm width. A minimum of 50 images was collected with an average of 10 dendrites per animal were analyzed. Dendritic images were captured at 30,000 magnification to give an area of 2 μm2 for each image, with a minimum total area of 100 μm2 analyzed per animal. Immunogold particles were mapped on dendrite tracings using Neurolucida 6.0 (MicroBrightField, Williston, VT) and the distances between gold particles were measured using NeuroExplorer 6.0 (MicroBrightField).
The average distance between markers analysis was performed for ERβ immunogold particles in dendrites. Using NeuroExplorer 6.0, the average distance between every pair of gold particles was computed. The average distance between markers analysis is useful in understanding the distribution of markers in a population or an area (Elste and Benson, 2006).
Statistical analysis
Statistical analyses were performed using StatView 5.0 (Abacus Concepts, Inc., Berkeley, CA). Group differences in percentage of labeled synapses and number of gold particles per synaptic compartment were evaluated between young ovariectomized Veh- and E-treated animals, as well as aged OVX Veh- and E-treated rats by two-way analysis of variance (ANOVA). Each bin was also compared with two-way ANOVA, and post-hoc comparisons were made by Tukey-Kramer adjustments. Significance was set at p < 0.05. All values are given as means ± SEM.
Supplementary Material
Fig. 6.
The average distance between ERβ immunogold particles in postsynaptic profiles (dendritic spines, Bins 1 – 4) significantly increased following E administration in both young and aged rats. * p < 0.02
Acknowledgments
NIH Grants: NIA PO1-AG16765 (JHM), NS07080 (BSM and TAM) and DA08259 (TAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Morrison JH, Gore AC. N-methyl-D-aspartate receptor mRNA levels change during reproductive senescence in the hippocampus of female rats. Exp Neurol. 2001a;170:171–179. doi: 10.1006/exnr.2001.7687. [DOI] [PubMed] [Google Scholar]
- Adams MM, Oung T, Morrison JH, Gore AC. Length of postovariectomy interval and age, but not estrogen replacement, regulate N-methyl-D-aspartate receptor mRNA levels in the hippocampus of female rats. Exp Neurol. 2001b;170:345–356. doi: 10.1006/exnr.2001.7716. [DOI] [PubMed] [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Diiferent modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001c;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Janssen WG, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004a;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Janssen WG, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004b;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elste AM, Benson DL. Structural basis for develomentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495:324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-mediated enhancement of object memroy consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kleopoulos SP, Kimura F, Mobbs CV. Changes in neurotensin mRNA expression by estrogen in the female rat preoptic are during aging: an in situ hybridization histochemistry study. Gen Comp Endocrinol. 1998;112:364–371. doi: 10.1006/gcen.1998.7139. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Increased expression of estrogen receptor alpha and beta in the nucleus basalis of Meynert in Alzheimer’s disease. Neurobiol Aging. 2001;22:417–426. doi: 10.1016/s0197-4580(00)00255-4. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Kruijver FP, Balesar R, Swaab DF. Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J Clin Endocrinol Metab. 2000;83:3283–3291. doi: 10.1210/jcem.85.9.6826. [DOI] [PubMed] [Google Scholar]
- Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, Morrison JH. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–44. doi: 10.1016/j.expneurol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitablity by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Mobbs CV, Howells RD, Pfaff DW. Estradiol induction of proenkephalin messenger RNA in the hypothalamus: dose-response and relation to reproductive behavior in the female rat. Brain Res Mol Brain Res. 1990;8:47–54. doi: 10.1016/0169-328x(90)90008-2. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quanitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endo. 2008;149:3306–3312. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J Neurosci. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, Riggs BL, Spelsberg TC. Mutual antagonism of estrogen receptors alpha and beta and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol. 2003;176:349–357. doi: 10.1677/joe.0.1760349. [DOI] [PubMed] [Google Scholar]
- Nealen ML, Vijayan KV, Bolton E, Bray PF. Human platelets contain a glygcosylated estrogen receptor beta. Circ Res. 2001;88:438–442. doi: 10.1161/01.res.88.4.438. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:389–394. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Roger C, Lambard S, Bouskine A, Mograbi B, Chevallier D, Nebout M, Pointis G, Carreau S, Fenichel P. Estrogen-induced growth inhibition of human seminoma cells expressing estrogen receptor beta and aromatase. J Mol Endocrinol. 2005;35:191–199. doi: 10.1677/jme.1.01704. [DOI] [PubMed] [Google Scholar]
- Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- Royuela M, de Miguel MP, Bethencourt FR, Sancherz-Chapado M, Fraile B, Arenas MI, Paniagua R. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Neuroendocrinol. 2001;168:447–454. doi: 10.1677/joe.0.1680447. [DOI] [PubMed] [Google Scholar]
- Ruud HK, Blackstad TW. PALIREL, a computer program for analyzing particle-to-membrane relations, with emphasis on electron micrographs of immunocytochemical preparations and gold labeled molecules. Comput Biomed Res. 1999;32:93–122. doi: 10.1006/cbmr.1999.1508. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Saneyorshi T, Fortin DA, Soderling TR. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr Opin Neurobiol. 2010;20:108–115. doi: 10.1016/j.conb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods. 2010 doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir E, Yu ZX, Takeda K, Ferrans VJ, Cannon ROr. Competition for p300 regulates transcription of estrogen receptors and nuclear factor-kappaB in human coronary smooth muscle cells. Circ Res. 2000;87:1006–1011. doi: 10.1161/01.res.87.11.1006. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, Merchenthaler I, Kaczmarek L. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Pfaff DW. Contributions of estrogen receptor-alpha and estrogen receptor-beta to the regulation of behavior. Biochem Biophys Acta. 2010 doi: 10.1016/j.bbagen.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome LF, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positionedfor direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Oestreicher AB, van der Krift TP, Gispen WH, Verkleij AJ. Freeze-substitution and Lowicryl HM20 embedding of fixed rat brain: suitability for immunogold ultrastructural localization of neural antigens. J Histochem Cytochem. 1991;39:1267–1279. doi: 10.1177/39.9.1833448. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling KL, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Lou WY, Akama KT, McEwen BS, Milner TA, Morrison JH. Effects of estrogen and aging on the synaptic distribution of phosphorylated Akt-immunoreactivity in the CA1 region of the female rat hippocampus. Brain Res. 2010 doi: 10.1016/j.brainres.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, Akama KT, McEwen BS, Milner TA, Morrison JH. Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in the CA1 region of the female rat hippocampus. Neuroscience. 2008;152:360–370. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Kim Y, Foy MR, Thompson R, Baudry M. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci U S A. 2009;106:21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QH, Huang YH, Hu YZ, Wei GZ, Han XF, Lu SY, Zhao YF. Disruption of estrogen receptor beta in mice brain results in pathological alterations resembling Alzheimer disease. Acta Pharmacol Sin. 2004;25:452–457. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.