Abstract
Voltage-gated K+ (Kv) channels are key determinants of membrane excitability in the nervous and cardiovascular systems, functioning to control resting membrane potentials, shape action potential waveforms and influence the responses to neurotransmitters and neurohormones. Consistent with this functional diversity, multiple types of Kv currents, with distinct biophysical properties and cellular/subcellular distributions, have been identified. Rapidly activating and inactivating Kv currents, typically referred to as IA (A-type) in neurons, for example, regulate repetitive firing rates, action potential back-propagation (into dendrites) and modulate synaptic responses. Currents with similar properties, referred to as Ito,f (fast transient outward), expressed in cardiomyocytes, control the early phase of myocardial action potential repolarization. A number of studies have demonstrated critical roles for pore-forming (α) subunits of the Kv4 subfamily in the generation of native neuronal IA and cardiac Ito,f channels. Studies in heterologous cells have also suggested important roles for a number of Kv channel accessory and regulatory proteins in the generation of functional IA and Ito,f channels. Quantitative mass spectrometry-based proteomic analysis is increasingly recognized as a rapid and, importantly, unbiased, approach to identify the components of native macromolecular protein complexes. The recent application of proteomic approaches to identify the components of native neuronal (and cardiac) Kv4 channel complexes has revealed even greater complexity than anticipated. The continued emphasis on development of improved biochemical and analytical proteomics methods seems certain to accelerate progress and to provide important new insights into the molecular determinants of native ion channel protein complexes.
Keywords: Proteomics, Protein identification, Native ion channel protein complexes, Kv4 α subunits, Kv accessory subunits, Post-translational modifications, Quantitative mass spectrometry
1- Introduction
Voltage-gated K+ (Kv) channels contribute importantly to the regulation of neuronal excitability and, in most neurons, multiple types of Kv currents, with unique time- and voltage-dependent properties, are co-expressed [1–3]. This multiplicity has physiological significance in that Kv currents with distinct properties and subcellular distributions contribute differentially to the control of resting membrane potentials, input resistances, action potential waveforms, repetitive firing and synaptic transmission [2,3]. Insights into the molecular basis of functional Kv channel diversity were provided with the identification of a large number of Kv channel pore forming (α) subunits, and studies in heterologous cells have revealed that the various Kv α subunits give rise to Kv currents with distinct biophysical properties [3,4]. Rapidly activating and inactivating Kv4 α subunit-encoded currents, typically referred to as IA (A-type), for example, are broadly expressed in central and peripheral neurons [5,6], and IA channels function in the regulation of repetitive firing rates [7,8], back-propagation of action potentials into dendrites [9], neurotransmitter release [2,3] and in mediating the responses to synaptic inputs and synaptic plasticity [10,11]. In the heart, Kv4 α subunits also underlie the generation of fast transient Kv currents, Ito,f, which contribute importantly to determining the early phase of myocardial action potential repolarization and to regional heterogeneities in ventricular action potential waveforms [12]. Changes in the expression and/or functioning of IA or Ito,f channels have been implicated in inherited and acquired diseases of the nervous and cardiovascular systems [12,13].
The potential molecular and functional diversity of Kv channels is further expanded by the interaction of α subunits with accessory subunits and additional regulatory proteins, and accumulating evidence suggests that neuronal and cardiac Kv channels reflect the functioning of macromolecular protein complexes [1,3,5,6,12]. Numerous studies in heterologous cells have clearly demonstrated that Kv channel accessory/regulatory proteins can affect channel stability, subcellular distribution, cell surface expression and/or biophysical properties [3,5,6,12]. In addition, the expression and the properties of expressed Kv channels can be further regulated/modulated by post-translational modifications, such as phosphorylation [12,14], of channel pore-forming subunits, accessory subunits and/or channel regulatory proteins. Although considerable effort has been focused on identifying the physiological roles of Kv α subunits, very little is presently known about the functioning of accessory subunits and other regulatory proteins in the generation and regulation of native neuronal and/or cardiac Kv channels.
There is now consensus that native somatodendritic IA channels and cardiac Ito,f channels reflect the expression of pore-forming α subunits of the Kv4 subfamily, Kv4.2, Kv4.3 and/or Kv4.1 [7,8,15–20]. Although Kv4 α subunits expressed alone can assemble as homotetramers or as heterotetramers to generate functional channels in heterologous cells, a number of proteins have been shown to associate with Kv4 α subunits and to affect the properties of (heterologously expressed) Kv4 channels [5,6]. The Kv Channel Interacting Proteins, KChIPs, for example, were first identified in a yeast-two hybrid screen using the Kv4.2 N-terminus as the bait [21]. Members of the Neuronal Calcium Sensor superfamily, KChIPs are low molecular weight (20–30 kDa) cytosolic proteins, consisting of a variable N-terminal region and a highly conserved C-terminal core with four EF hand-like Ca2+-binding motifs [21]. In heterologous cells, KChIP co-expression increases the cell surface expression of Kv4 channels and modifies the time- and voltage-dependent properties of Kv4-encoded currents [21,22]. The interaction between the Kv4 and the KChIP proteins in native cells has been confirmed by co-immunoprecipitation from brain and heart [20,21,23,24]. A critical role for KChIP2 in the generation of cardiac Kv4 channels was also revealed with the demonstration that targeted disruption of the Kcnip2 (KChIP2) locus eliminates Ito,f in mouse ventricular myocytes [25].
Despite the implications of the many studies in heterologous cells, there has been very little emphasis on analyzing the molecular composition of Kv channels in situ and/or on defining the roles of accessory and regulatory proteins in the generation or functioning of native Kv channels. In addition, translating findings from heterologous systems to native cells has proven difficult. Several approaches, including mouse transgenesis or RNA interference targeting specific putative channel subunits, hold the promise of providing valuable insights into the functional relevance of findings obtained in heterologous cells. Recently, an alternative, unbiased approach, exploiting mass spectrometry (MS)-based proteomic analyses, has also emerged, and the potential power of these methodologies to identify the components of native channel complexes, as well as post-translational modifications of channel components, is already very clear [14,24,26–33]. The focus here is on neuronal (and cardiac) Kv4 channels and insights into the molecular compositions of Kv4 channel complexes that have been gleaned from applying MS-based proteomic approaches are discussed.
2- In-gel Proteomic Identification of Brain Kv4 Channel Complex Components
Although expression of Kv4 α subunits alone results in the generation of functional channels in heterologous cells and co-expression with KChIP accessory subunits recapitulates some of the properties of native Kv4-encoded currents, Kv4 inactivation kinetics are actually slowed in the presence of KChIP [21,22], leading to suggestions that additional accessory or regulatory proteins contribute to the generation of native IA and Ito,f channels. In quest of additional accessory subunits, Rudy and colleagues developed a proteomic strategy to purify and characterize native brain Kv4-encoded channel complexes [26]. In these analyses, an anti-Kv4.2 specific antibody was used to immunoprecipitate Kv4.2 channel complexes from (rat) brain lysates, and co-immunoprecipitated proteins were fractionated on one-dimensional polyacrylamide gels. Protein bands of interest, present in the Kv4.2 immunoprecipitates and absent in the control immunoprecipitates (conducted using crosslinked antibody-beads in the presence of an excess of immunogenic peptide, or beads crosslinked to preimmune sera), were selected and subjected to mass spectrometric (MS) identification.
Using this approach, diaminopeptidyl transferase-like protein 6, DPP6 (or DPPX), was identified as a protein co-immunoprecipitating with Kv4.2 from brain [26]. DPP6 is a member of the dipeptidyl-aminopeptidase (DPP) family of proteins which, in contrast to the homolog DPP4 (also called CD26), lacks enzymatic activity [34]. Subsequent studies in heterologous cells revealed that, like the KChIPs, DPP6 co-expression increases Kv4-encoded current densities and alters the time- and voltage-dependent properties of the currents [26]. In addition, when DPP6 is co-expressed with Kv4 α subunits in the presence of one of the KChIPs, the Kv currents generated more closely resemble neuronal IA, leading to suggestions that native IA channels reflect the multimeric assembly of Kv4 α subunits with KChIP and DPP6 accessory subunits [5]. Using the same in-gel proteomic approach, Rudy and colleagues subsequently went on to identify another member of the DPP-like family, named DPP10 (or DPPY), in rat brain Kv4.2 channel complexes, and demonstrated that this protein has regulatory effects similar to DPP6 on heterologously-expressed Kv4 channels [27,35,36].
Subsequent in-gel proteomic identification analyses have confirmed the presence of the KChIP (KChIP1, 2, 3 and 4), as well as of the DPP6 and DPP10 accessory subunits in Kv4.2 channel complexes immunoprecipitated from mouse brain [24]. Importantly, none of these proteins were identified in the (control) immunoprecipitates which, in these experiments, were derived from brains isolated from mice (Kv4.2−/−) harboring a targeted disruption of the Kcnd2 (Kv4.2) locus [17]. The KChIP and DPP families of proteins co-immunoprecipitated with Kv4.2 in a variety of different buffers and under a number of different detergent (for protein solubilization) stringency conditions [24]. Nevertheless, it is important to note that the yield of the Kv4.2 protein and the relative amounts of the DPPx and KChIPx proteins varied under different detergent conditions [24]. Stringent detergent conditions that provided high yields of Kv4.x proteins, for example, resulted in lower relative amounts of the DPPx and KChIPx proteins [24]. The specific experimental conditions will likely need to be varied and optimized for different ion channel proteins and ion channel macromolecular complexes.
Although the accumulated biochemical and mass spectrophotometric data clearly suggest that the DPP proteins are integral components of native neuronal Kv4 channel complexes, direct demonstration of the roles of these proteins in the generation and/or regulation of native Kv4 channels has been equivocal. Indeed, contrary to predictions based on the results of heterologous expression studies [5,26,36], it was recently reported that RNA interference targeting DPP6 in hippocampal pyramidal neurons affects the biophysical properties of IA without measurably affecting current densities [37]. More specifically, acute knockdown of DPP6 expression resulted in depolarizing shifts in the steady-state activation and inactivation curves for IA and the slope of the conductance-voltage plot for IA was reduced [37]. Taken together, these changes resulted in larger IA window currents. It has also been reported that DPP6 increases the unitary conductance of Kv4 channels in both heterologous cells and in cerebellar granule neurons [38], although it is not clear if a similar effect is evident in hippocampal pyramidal neurons [37]. The observed differences in the functional effects of DPP6 expression on heterologously expressed Kv4 currents and native neuronal IA emphasize the importance of developing in situ approaches to identify the molecular components of native Kv channel complexes and of completing experiments aimed at defining the physiological roles of identified accessory/regulatory proteins in the regulation of native Kv channel expression, localization and functioning.
3- In-solution Proteomic Identification of Brain Kv4 Channel Complex Components
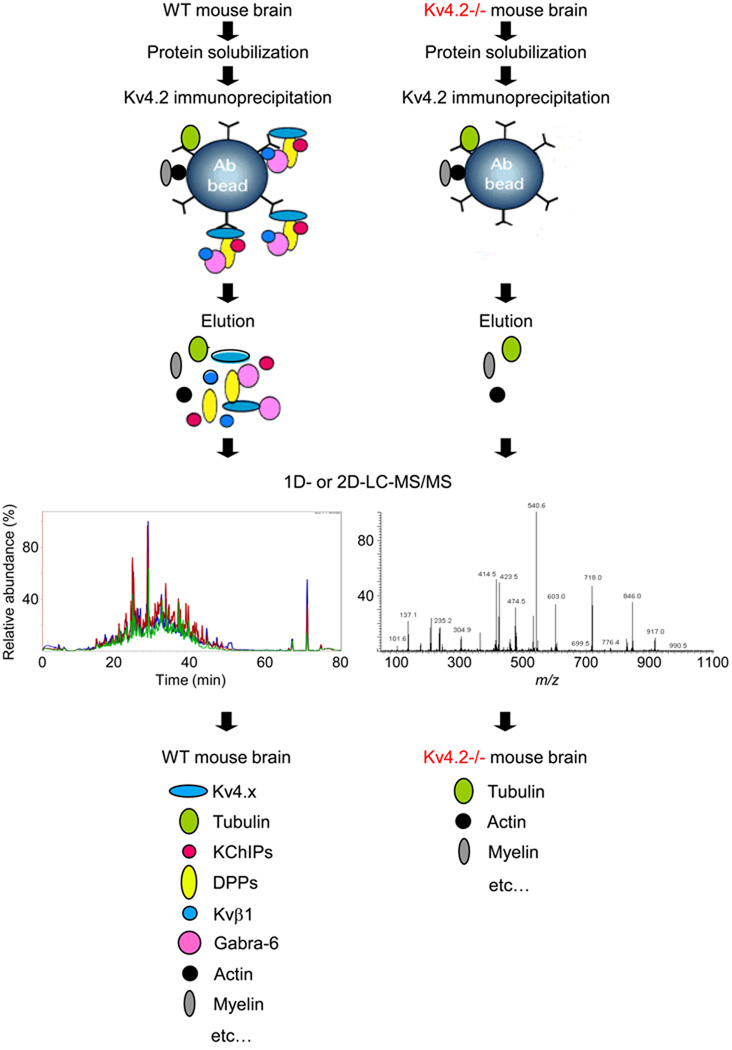
The in-gel proteomic approach described above offers several clear advantages over more classical methods, such as two-hybrid screening in bacteria or yeast or GST-pulldowns, for identifying interacting proteins. Nevertheless, this approach also has substantial limitations and, in particular, lacks sensitivity [24]. To enable the identification of greater numbers of interacting proteins, a more comprehensive, in-solution proteomic approach can also be undertaken. In these analyses, the entire immunoprecipitated sample is digested and sequenced by one- (1D) or two-(2D) dimensional liquid chromatography-tandem mass spectrometry (LC-MS/MS) [24]. The 2D-LC-MS/MS analysis is also called MudPIT, for Multidimensional Protein Identification Technology [39]. Although powerful and comprehensive, caution needs to be taken while using in-solution approaches as many proteins can non-specifically bind to the antibodies, beads and other reagents used in the immunoprecipitations, confounding efforts to identify specific channel interacting proteins. This problem has recently been minimized by the use of control samples from (knockout) mice with targeted genetic disruptions of genes encoding the specific α subunits being studied [24,32]; the approach is illustrated in Figure 1. Using this strategy, samples from wild-type (experimental) and knockout (control) mice can be processed in parallel using the same anti-channel α subunit specific-antibody. Direct comparison of the co-immunoprecipitated proteins identified in the two (experimental and control) samples can then be used to determine which proteins are present in both samples; these are typically very abundant proteins such as tubulin, actin, etc. (see Figure 1). Importantly, this comparison and subtraction of the proteins present in both the control and experimental samples also allows immediate identification of the proteins that are detected only in the experimental samples (e.g. specific interacting proteins). Using this approach for Kv4.2, several additional proteins (Table 1) were identified in the Kv4.2 immunoprecipitates from wild-type mouse brain [24]. These proteins were not detected in the control immunoprecipitates from Kv4.2−/− brains, consistent with the suggestion that these proteins correspond to accessory subunits and/or regulatory proteins interacting with native brain Kv4.2 α subunits.
Figure 1.
Proteomic strategy used to identify the components of native (mouse) brain Kv4.2 channel complexes. Once solubilized, proteins from wild-type (WT) and Kv4.2 targeted deletion (Kv4.2−/−) brains were immunoprecipitated using a polyclonal anti-Kv4.2 antibody [24]. After elution from the antibody-beads (Ab-beads), proteins were digested with trypsin, and the generated tryptic peptides were analyzed by one- (1D) or two- (2D) dimensional liquid chromatography-tandem mass spectrometry (LC-MS/MS). Comparison of the proteins identified in the immunoprecipitates from WT and Kv4.2−/− brains allowed the identification of Kv4.2 channel complex components.
Table 1.
Proteins identified in immunoprecipitated brain and heart Kv4.2 channel complexes.
| Brain Kv4.2-IP | PAF | Heart Kv4.2-IP | PAF |
|---|---|---|---|
| Kv4.2 | 2.1 | Kv4.2 | 0.7 |
| Kv4.1 | 0.8 | Kv4.3 | 0.1 |
| Kv4.3 | 1.4 | KChIP2 | 0.4 |
| KChIP2 | 1.7 | EHD4 | 0.3 |
| KChIP3 | 1.4 | TCP1 | 0.2 |
| KChIP4 | 5.6 | BICD2 | 0.1 |
| DPP6 | 5.3 | Actn2 | 0.1 |
| DPP10 | 2 | ||
| Kvβ1 | 0.7 | ||
| Gabra-6 | 1 | ||
| Gpr158 | 0.5 | ||
| Prkcβ1 | 0.5 |
The Protein Abundance Factor (PAF) for each protein is presented. None of the proteins listed were identified in the control immunoprecipitates (IP) from Kv4.2−/− mice. Abbreviations: Kv, Voltage-gated K+ channel; KChIP, K+ Channel Interacting Protein; DPP, Dipeptidyl-aminopeptidase; Gabra-6, α6 subunit of gamma-aminobutyric acid receptor; Gpr158, G protein-coupled receptor 158; Prkcβ1, β1 subunit of protein kinase C; EHD4, Esp15 homology domaincontaining protein; TCP1, t-complex protein 1; BICD2, Bicaudal D homolog 2; Actn2, α–actinin 2.
Among the proteins identified in these analyses [24] was the Kv channel accessory subunit, Kvβ1 (Table 1), which was originally identified as an accessory subunit of Kv1 α subunit-encoded channels [3,4]. The mass spectrometric identification of Kvβ1, however, is consistent with the results of previous studies suggesting physical and functional interactions between Kv4 and Kvβ subunits [40,41]. The α6 subunit (Gabra-6) of the gamma-aminobutyric acid (GABA-A) receptor as well as the G protein-coupled receptor 158 (Gpr158), which has been suggested to be a member of the glutamate G-protein coupled receptor subfamily [42], were also identified in brain Kv4.2 channel complexes (Table 1). Of note here, the interaction between Kv4.2 and Gabra-6 was subsequently validated by co-immunoprecipitation from mouse brain extracts under non-denaturing conditions and subsequent Western blot analyses (CM and JMN, unpublished data). These observations are particularly interesting in light of previous suggestions that Kv4.2 channels are localized at or near synapses and that these channels function in the regulation of synaptic responses and synaptic plasticity [10,11,43–45]. Another protein identified in these experiments [24], the β1 subunit (Prkcβ1) of Protein Kinase C (Table 1), is also potentially of interest, as this protein may play a role in the regulation of Kv4.2 channels by phosphorylation [46]. Interestingly, the diversity of proteins identified highlights the possibility that multiple Kv4 channel complexes, comprising distinct components, may exist in different neuronal subpopulations and/or subcellular compartments. Indeed, it was recently reported that KChIP3, but not the other KChIPs, regulates the voltage-dependence of inactivation of Kv4-encoded channels in cerebellar stellate cells in response to Ca2+ entry through voltage-gated Ca2+ (Cav) channels encoded by Cav3 α subunits [47].
The in-solution proteomic analyses also provided information about relative protein abundances in each sample, thereby making it possible to compare directly the relative proportions of the various interacting proteins in Kv4.2 channel complexes. These analyses of brain Kv4.2 immunoprecipitates clearly suggest that the KChIP and the DPP proteins are more abundant than any of the other interacting proteins identified [24], with protein abundance factors [48] in the range of 1.4 to 5.6 for the KChIP and the DPP proteins, as compared with values between 0.5 and 1 for Kvβ1, Gabra-6, Gpr158 and Prkcβ1 (Table 1). Although differences in the relative expression levels of these proteins in brain could explain the different abundances observed, it seems more likely that the various interacting proteins are not represented equally in brain Kv4.2 channel complexes. Contrary to the KChIP and the DPP proteins, one might suggest, for example, that the association of the other (Kvβ1, Gabra-6, Gpr158, Prkcβ1) proteins with Kv4.2 is not widespread in the central nervous system, but rather exists in specific neuronal subpopulations and/or subcellular compartments. Alternatively, the interactions of these proteins with Kv4 channels could be weak and occur transiently. Clearly, the physiological roles of these novel interacting proteins in the generation and regulation of native Kv4.2 channels warrants further investigation.
4- In-solution Proteomic Identification of Cardiac Kv4 Channel Complex Components
Although considerable evidence suggests that cardiac Kv4-encoded myocardial Ito,f channels likely also function in macromolecular complexes [12], the molecular components of native cardiac Ito,f channels remain to be identified. Indeed, although expression of DPP6 transcript was detected in human ventricles by quantitative RT-PCR, and was further verified at the protein level by Western blot analyses [49], it has not been demonstrated that DPP and Kv4 proteins are associated in situ and/or that these proteins can be co-immunoprecipitated from cardiac tissue. In addition, the functional roles of the DPP6 and DPP10 proteins in the generation of myocardial Ito,f channels have not been examined directly to date.
Using the in solution proteomic approach described above, experiments were also undertaken to characterize Kv4.2 channel protein complexes purified from adult mouse ventricles. Consistent with previous biochemical data [12,20], these analyses identified Kv4.2 and Kv4.3 α subunits, as well as the KChIP2 accessory protein, in cardiac Kv4.2 channel complexes (Table 1), whereas no DPP6/DPP10 proteins were detected. Several other novel proteins, however, were identified in Kv4.2 immunoprecipitates from wild-type mouse ventricles, and were absent in immunoprecipitates from Kv4.2−/− mouse ventricles (Table 1). One of these was the EH (Esp15 Homology) Domain-containing (EHD) protein 4 (EHD4) (Table 1). This finding is intriguing in light of recent findings suggesting a role for this family of EHD proteins in the targeting/trafficking network associated with ankyrin B in cardiomyocytes [50]. It is also interesting that several other proteins identified, including the t-complex protein 1 (TCP1) [51], Bicaudal D homolog 2 (BICD2) [52], and alpha-actinin 2 (Actn2) [53,54], are potentially relevant to protein chaperoning/folding, endosomal transport and cell surface protein targeting, respectively. More importantly, these preliminary proteomic data suggest possible roles for novel accessory/regulatory proteins in the generation of cardiac Ito,f channels. Clearly, further biochemical and functional studies will be necessary to decipher the roles of these newly identified interacting proteins in the regulation of native Ito,f channels.
5- Limitations of the Approach
A major limitation of using immunoprecipitation approaches in efforts focused on characterizing native channel complexes is associated with the lability of protein-protein interactions, or in other words, with the ability to maintain the integrity of native channel complexes during the numerous steps involved in the immunoaffinity purification. The impact of the detergent, solubilization and immunoprecipitation conditions on the efficiency of isolation of the protein targeted by the antibody, as well as of the other interacting proteins in the channel complex with the targeted protein, needs to be examined directly and to be optimized experimentally. Even then, it may be difficult (or impossible) to ensure that all of the complex components have been isolated and identified. For novel proteins, the impact of this problem is clear. As a consequence, it is very important in the development of these approaches to keep in mind that the absence of a protein does not necessarily mean that the protein is not a component of the in situ channel complex of interest. Although several previously identified Kv accessory subunits (KChIP and DPP), as well as novel interacting proteins were identified in brain Kv4.2 channel complexes using proteomic analyses [24], several other proteins that have been suggested to interact directly with Kv4 α subunits, such as filamin A [55] and syntaxin 1A [56] were not identified using MudPIT or in-gel analyses [24]. Similarly, although a recent, very elegant study by Anderson and colleagues identified an interaction between Cav3 and Kv4 α subunits in (rat) cerebellar stellate cells [47], no Cav3 α subunits were found to co-immunoprecipitate with Kv4 α subunits from whole mouse brain [24]. The absence of Cav3 α subunits in (whole) mouse brain Kv4.2 immunoprecipitates is somewhat surprising, and the reason(s) for this absence is not clear. It is certainly possible that Cav3-Kv4 interactions only occur in cerebellar stellate cells and, therefore, that these Ca2+ channel subunits are not abundant enough to be detected from immunoprecipitates from whole brain extracts. Alternatively, it is possible that the interactions between the Kv4 and Cav3 proteins are low affinity. Again, the importance of the conditions used for protein solubilization and immunoprecipitation cannot be over emphasized.
Another clear limitation of using methods based on immunoprecipitation of proteins is related to the fact that exogenous protein interactions, i.e., interactions that take place during the lysis and immunoaffinity purification steps cannot be excluded. This limitation emphasizes the necessity to validate the existence, as well as the functional roles, of newly identified interacting proteins in native cells using alternative experimental approaches.
The biggest limitation of using mass spectrometry to characterize immunoprecipitates has been the inability of instrumentation and software to acquire MS/MS spectra on a comprehensive set of peptide signals from the parent scans (MS1). Currently, LC-MS analysis is most often conducted in 'data-driven' mode whereby the most intense signals are used to 'trigger' MS/MS acquisition. With the limitations of instrument speed and peak widths in nano-liquid chromatography, only a fraction of the detected peptide signals produce MS/MS spectra, resulting in significant bias for sequencing peptides from the most abundant proteins. Importantly, tools are now available that allow sequencing of peptides based on differential abundances in control and experimental samples, rather than on absolute abundance in a sample.
6- Conclusions and Future Directions: Quantitative Proteomics of Channel Complexes
Quantitative mass spectrometric protein identification combined with affinity purification techniques has evolved as an important tool for the in-depth analyses of macromolecular protein complexes, as well as for analysis of post-translational modifications of the individual components of these complexes. As demonstrated with brain Kv4 channels, combining these approaches offers the advantages of allowing the characterization of native channel complexes and of being unbiased [24,26,27]. Nevertheless, the development and widespread application of these approaches has been hampered by experimental limitations inherent in both the biochemistry and the mass spectrometry [24]. Concerning the biochemistry in particular, three sinequanon conditions must be met: (i) the conservation of channel complex integrity; (ii) a mass spectrometric-detectable enrichment in channel proteins; and (iii) the use of an adequate control immunoprecipitation. Optimization of these three parameters has benefited considerably from the development and exploitation of technical strategies, including the development of specific and high affinity anti-channel subunit antibodies, as well as improvements in protein isolation and solubilization techniques. Additionally, as discussed above, the use of tissue from (knockout) animals lacking the gene of interest for the control immunoprecipitations has great advantages as the same antibody can be used in experimental and control immunoprecipitations. The use of this experimental strategy was successful in the analyses on neuronal Kv4.2 channel [24], as well as cardiac Kv4.2 channel (see above) interacting and/or regulatory proteins. Similar approaches have been exploited in studies on the two-pore domain K+ channel α subunit, TREK1, and have led to the identification of the microtubule-associated protein Mtap2, as well as with the A-Kinase Anchoring Protein, AKAP150, as TREK-1 interacting proteins [32,57,58].
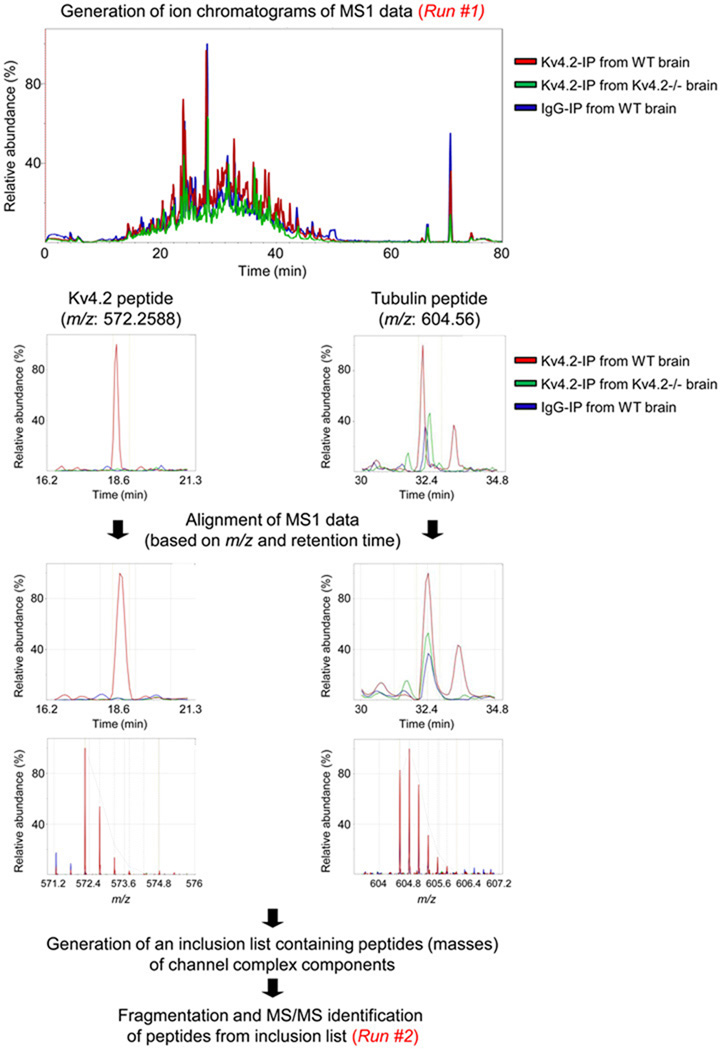
Enrichment in channel proteins is particularly important to avoid potential problems with the sensitivity limitations of mass spectrometry. Indeed, it is well-recognized that mass spectrometry of complex mixtures of peptides is limited by undersampling of signals for MS/MS analysis [59]. This phenomenon is intrinsically related to the technique itself, and reflects the fact that the analysis is typically intensity-driven. Only a small subset of the peptides generated and detected, the most abundant ones, are selected for fragmentation and sequencing. As a consequence, contaminating proteins, which can be abundant (and difficult to eliminate biochemically), are the first proteins to be sequenced after or in parallel with the channel proteins immunoprecipitated. Innovative advancements associated with the increased capability to acquire data with greater mass accuracy, combined with methods to quantify proteins from mass spectrometric data, are now making it possible to circumvent this issue. Using high resolution MS1 data for label-free quantification, followed by directed mass spectral acquisition data-driven (rather than intensity-driven) analyses [60–63], lower abundance peptides that are enriched in the experimental samples can be sequenced. The basic approach is illustrated in Figure 2.
Figure 2.
Label-free quantification using high mass resolution MS1 data followed by directed mass spectral acquisition. Experimental (Kv4.2-IP from WT brain) and control (Kv4.2-IP from Kv4.2−/− brain and IgG-IP from WT brain) immunoprecipitates (IP) are run (Run #1) in parallel to obtain MS1 data, and peptide ion chromatograms from the MS1 data are produced from the peptide signals obtained at high resolution. The selected ion chromatograms are then aligned and annotated for the three samples based on m/z ratios and retention times. Only the most abundant signals are annotated in the initial intensity-driven LC-MS analysis. Represented are peptides from the Kv4.2 and the tubulin proteins in the three MS1 runs before and after alignment. As is evident from the selected ion chromatograms, the Kv4.2 peptide is represented in the Kv4.2-IP from WT brain only, whereas the tubulin peptide is represented in the three IPs. The intensities of each of the peptides are compared for the different MS1 runs, and the relative quantitative information from the differential peptides is used to generate a list of masses (m/z values) and retention time pairs to constrain subsequent MS/MS acquisitions to only those signals that are enriched in the experimental samples.
Using this approach, quantitative information about differences in protein composition and abundance between control and experimental immunoprecipitations is obtained by comparing signal intensities of peptides extracted from the spectra. Contaminant proteins, which typically originate equally from control and experimental immunoprecipitates, are readily identified by abundance ratios of approximately one. In contrast, peptides from genuine components of native complexes are selectively enriched during the purification steps and exhibit abundance ratios significantly higher than one. The 'enriched' signals are then used to direct the mass spectrometer to acquire fragmentation spectra (MS/MS) on the differentially expressed peptides. In this strategy, MS/MS spectra are acquired from only peptides of interest, thereby enabling the identification of low-abundance, relevant peptides originating from genuine channel protein components against a high background of co-purified contaminants. Nevertheless, literature describing the use of this directed approach for the dissection of protein complexes is rather scarce [62]. Only recently have instrumentation and software tools become available for this complex proteomics workflow (Figure 2). The requirements for conducting differential peptide signal analysis, followed by directed mass spectral acquisition, are: i) sensitive (~ 10 fmol peptide detection limit), high resolution LC-MS; ii) software tools to detect peaks and align the thousands of peptide ion chromatograms from multiple control and experimental immunoprecipitates; and, iii) instrument software to perform directed mass spectral acquisition. Further developments in this area will almost certainly be a major advance in efforts to identify the components of native channel protein complexes, as well as of the post-translational modifications of the components of these complexes.
Acknowledgements
The authors acknowledge financial support provided to their laboratories by the National Institute of Health (R01-HL034161 and R21-NS065295), the National Center for Research Resources (NIH P41 RR000954 and UL1 RR024992), the NIH Neuroscience Blueprint Center Core (P30-NS057105), the Agence Nationale de la Recherche (ANR-08-GENO-006), and the Marie Curie 7th Framework Program of the European Commission (NavEx-256397). In addition, we would also like to thank Dr. Cheryl Lichti for assistance in the creation of the figures.
Abbreviations
- DPP
Dipeptidyl-aminopeptidase
- IA
A-type voltage-gated K+ current
- Ito,f
Fast transient outward voltage-gated K+ current
- KChIP
K+ Channel Interacting Protein
- Kv α subunit
Voltage-gated K+ channel pore-forming (α) subunit
- MS
Mass Spectrometry
- MS/MS
Tandem Mass Spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- 2.Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004;27:210–217. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett. 1999;452:31–35. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- 4.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 5.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;569:41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan W, Burkhalter A, Nerbonne JM. Functional role of the fast transient outward K+ current, IA, in pyramidal neurons in (rat) primary visual cortex. J Neurosci. 2005;25:9185–9194. doi: 10.1523/JNEUROSCI.2858-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Hoffman DA. Potassium channels: newly found players in synaptic plasticity. Neuroscientist. 2008;14:276–286. doi: 10.1177/1073858408315041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33:307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current Ito expression and regulation. J Mol Cell Cardiol. 2010;48:12–25. doi: 10.1016/j.yjmcc.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- 14.Park KS, Yang JW, Seikel E, Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology. 2008;23:49–57. doi: 10.1152/physiol.00031.2007. [DOI] [PubMed] [Google Scholar]
- 15.Barry DM, Xu H, Schuessler R, Nerbonne JM. Functional knockout of the transient outward current, long QT syndrome and cardiac remodeling in mice expressing a dominant negative Kv4 α subunit. Circ Res. 1998;83:560–567. doi: 10.1161/01.res.83.5.560. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, et al. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, et al. Targeted deletion of Kv4.2 eliminates Ito,f and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- 18.Nerbonne JM, Gerber BR, Norris A, Burkhalter A. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J Physiol. 2008;586:1565–1579. doi: 10.1113/jphysiol.2007.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris AJ, Nerbonne JM. Molecular dissection of IA in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci. 2010;30:5092–5101. doi: 10.1523/JNEUROSCI.5890-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, et al. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- 21.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 22.Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, et al. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–36454. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, et al. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marionneau C, LeDuc RD, Rohrs HW, Link AJ, Townsend RR, Nerbonne JM. Proteomic analyses of native brain Kv4.2 channel complexes. Channels. 2009;3:284–294. doi: 10.4161/chan.3.4.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 26.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 27.Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, et al. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005;280:18853–18861. doi: 10.1074/jbc.M410613200. [DOI] [PubMed] [Google Scholar]
- 28.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 29.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A. 2007;104:20055–20060. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte U, Thumfart JO, Klocker N, Sailer CA, Bildl W, Biniossek M, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron. 2006;49:697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, et al. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7:2188–2198. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandoz G, Lesage F. Protein complex analysis of native brain potassium channels by proteomics. Methods Mol Biol. 2008;491:113–123. doi: 10.1007/978-1-59745-526-8_9. [DOI] [PubMed] [Google Scholar]
- 33.Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain. J Proteome Res. 2010;9:1976–1984. doi: 10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorrell MD, Wang XM, Park J, Ajami K, Yu DM, Knott H, et al. Structure and function in dipeptidyl peptidase IV and related proteins. Adv Exp Med Biol. 2006;575:45–54. doi: 10.1007/0-387-32824-6_5. [DOI] [PubMed] [Google Scholar]
- 35.Jerng HH, Kunjilwar K, Pfaffinger PJ. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol. 2005;568:767–768. doi: 10.1113/jphysiol.2005.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–5623. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, et al. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaulin YA, De Santiago-Castillo JA, Rocha CA, Nadal MS, Rudy B, Covarrubias M. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J Neurosci. 2009;29:3242–3251. doi: 10.1523/JNEUROSCI.4767-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 40.Yang EK, Alvira MR, Levitan ES, Takimoto K. Kvbeta subunits increase expression of Kv4.3 channels by interacting with their C termini. J Biol Chem. 2001;276:4839–4844. doi: 10.1074/jbc.M004768200. [DOI] [PubMed] [Google Scholar]
- 41.Aimond F, Kwak SP, Rhodes KJ, Nerbonne JM. Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes. Circ Res. 2005;96:451–458. doi: 10.1161/01.RES.0000156890.25876.63. [DOI] [PubMed] [Google Scholar]
- 42.Bjarnadottir TK, Fredriksson R, Schioth HB. The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene. 2005;362:70–84. doi: 10.1016/j.gene.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Andrasfalvy BK, Makara JK, Johnston D, Magee JC. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. J Physiol. 2008;586:3881–3892. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. Differential expression of IA channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci. 2006;26:12274–12282. doi: 10.1523/JNEUROSCI.2599-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008;60:657–671. doi: 10.1016/j.neuron.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrader LA, Ren Y, Cheng F, Bui D, Sweatt JD, Anderson AE. Kv4.2 is a locus for PKC and ERK/MAPK cross-talk. Biochem J. 2009;417:705–715. doi: 10.1042/BJ20081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, et al. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. doi: 10.1038/nn.2493. [DOI] [PubMed] [Google Scholar]
- 48.Powell DW, Weaver CM, Jennings JL, McAfee KJ, He Y, Weil PA, et al. Cluster analysis of mass spectrometry data reveals a novel component of SAGA. Mol Cell Biol. 2004;24:7249–7259. doi: 10.1128/MCB.24.16.7249-7259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative beta-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol. 2005;565:751–756. doi: 10.1113/jphysiol.2005.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gudmundsson H, Hund TJ, Wright PJ, Kline CF, Snyder JS, Qian L, et al. EH Domain proteins regulate cardiac membrane protein targeting. Circ Res. 2010;107:84–95. doi: 10.1161/CIRCRESAHA.110.216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund PA. The roles of molecular chaperones in vivo. Essays Biochem. 1995;29:113–123. [PubMed] [Google Scholar]
- 52.Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw CI, Willemsen R, et al. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 2001;20:4041–4054. doi: 10.1093/emboj/20.15.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR, et al. Alpha-actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel) Proc Natl Acad Sci USA. 2009;106:18402–18407. doi: 10.1073/pnas.0908207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziane R, Huang H, Moghadaszadeh B, Beggs AH, Levesque G, Chahine M. Cell membrane expression of cardiac sodium channel Nav1.5 is modulated by alpha-actinin-2 interaction. Biochemistry. 2010;49:166–178. doi: 10.1021/bi901086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci. 2000;20:8736–8746. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamakawa T, Saith S, Li Y, Gao X, Gaisano HY, Tsushima RG. Interaction of syntaxin 1A with the N-terminus of Kv4.2 modulates channel surface expression and gating. Biochemistry. 2007;46:10942–10949. doi: 10.1021/bi7006806. [DOI] [PubMed] [Google Scholar]
- 57.Sandoz G, Tardy MP, Thummler S, Feliciangeli S, Lazdunski M, Lesage F. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandoz G, Thummler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, et al. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garza S, Moini M. Analysis of complex protein mixtures with improved sequence coverage using (CE-MS/MS)n. Anal Chem. 2006;78:7309–7316. doi: 10.1021/ac0612269. [DOI] [PubMed] [Google Scholar]
- 60.America AH, Cordewener JH. Comparative LC-MS: a landscape of peaks and valleys. Proteomics. 2008;8:731–749. doi: 10.1002/pmic.200700694. [DOI] [PubMed] [Google Scholar]
- 61.Neubert H, Bonnert TP, Rumpel K, Hunt BT, Henle ES, James IT. Label-free detection of differential protein expression by LC/MALDI mass spectrometry. J Proteome Res. 2008;7:2270–2279. doi: 10.1021/pr700705u. [DOI] [PubMed] [Google Scholar]
- 62.Nittis T, Guittat L, Leduc RD, Dao B, Duxin JP, Rohrs H, et al. Revealing novel telomere proteins using in vivo crosslinking, tandem affinity purification and label-free quantitative LC-FTICR-MS. Mol Cell Proteomics. 2010;9:1144–1156. doi: 10.1074/mcp.M900490-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oeljeklaus S, Meyer HE, Warscheid B. New dimensions in the study of protein complexes using quantitative mass spectrometry. FEBS Lett. 2009;583:1674–1683. doi: 10.1016/j.febslet.2009.04.018. [DOI] [PubMed] [Google Scholar]