Abstract
The neuroprotective effects of estrogen have been demonstrated consistently in cellular and animal studies but the evidence in women remains conflicted. We explored the window of opportunity hypothesis in relation to cognitive aging and dementia. In particular, we reviewed existing literature, reanalyzed some of our data, and combined results graphically. Current evidence suggests that estrogen may have beneficial, neutral, or detrimental effects on the brain depending on age at the time of treatment, type of menopause (natural versus medically or surgically induced), or stage of menopause. The comparison of women who underwent bilateral oophorectomy with referent women provided evidence for a sizeable neuroprotective effect of estrogen before age 50 years. Several case-control studies and cohort studies also showed neuroprotective effects in women who received estrogen treatment (ET) in the early postmenopausal stage (most commonly at ages 50–60 years). The majority of women in those observational studies had undergone natural menopause and were treated for the relief of menopausal symptoms. However, recent clinical trials by the Women’s Health Initiative showed that women who initiated ET alone or in combination with a progestin in the late postmenopausal stage (ages 65–79 years) experienced an increased risk of dementia and cognitive decline regardless of the type of menopause. The current conflicting data can be explained by the window of opportunity hypothesis suggesting that the neuroprotective effects of estrogen depend on age at the time of administration, type of menopause, and stage of menopause. Therefore, women who underwent bilateral oophorectomy before the onset of menopause or women who experienced premature or early natural menopause should be considered for hormonal treatment until approximately age 51 years.
Keywords: Oophorectomy, Menopause, Estrogen, Dementia, Cognitive impairment, Window of opportunity hypothesis
1. Introduction
Whether estrogen has beneficial neuroprotective effects on the brain of women remains controversial (Siegfried, 2007; Rocca et al., 2009; Henderson and Brinton, 2010; Hogervorst and Bandelow, 2010; Nejat and Chervenak, 2010; Rocca et al., 2010). The results of the Women’s Health Initiative (WHI) clinical trials have created a situation of uncertainty with experimental studies showing detrimental effects of estrogen on the heart and brain, and observational studies showing beneficial effects (Writing Group for the Women’s Health Initiative, 2002; Shumaker et al., 2003; Anderson et al., 2004; Shumaker et al., 2004; Manson et al., 2006).
The reaction of practicing clinicians and scientists to the results of the WHI trials has been mixed. Some have accepted the findings as final evidence and have discontinued the use of estrogen treatment in their practice or have opposed the funding of additional research on estrogen as a neuroprotectant. Other practicing clinicians and scientists have criticized the WHI findings and interpretations arguing that the effect of estrogen on the heart, brain, or other organs and tissues may vary by age at the time of administration, type of menopause, and stage of menopause. In addition, they suggested that the WHI data should be interpreted in light of the type (e.g. conjugated equine estrogens versus estradiol), regimen (e.g. oral versus transdermal), dosage (e.g. higher dose versus lower dose), and schedule of administration (e.g. continuous versus cyclic) of hormone therapy.
The idea that the beneficial or detrimental effects of estrogen treatment (ET) vary across women by age and stage of menopause has been called the “window of opportunity hypothesis” by some authors and the “timing hypothesis” by others (Manson et al., 2006; Mendelsohn and Karas, 2007; Siegfried, 2007; Brinton, 2008; Rocca et al., 2008; Rocca et al., 2009; Henderson and Brinton, 2010; Hogervorst and Bandelow, 2010; Rocca et al., 2010). We consider these two descriptions conceptually equivalent. From an epidemiologic perspective, the window of opportunity hypothesis can be described as an effect modification or an interaction (Szklo and Nieto, 2007; Porta and International Epidemiological Association, 2008).
In this review, we combine findings from both observational studies (case-control and cohort studies) and experimental studies (controlled clinical trials) to explore the window of opportunity hypothesis in relation to cognitive aging and dementia. However, this article does not review in depth the large body of experimental work on the effects of estrogen at the cellular level and in animal models.
2. Classification of women in relation to menopause
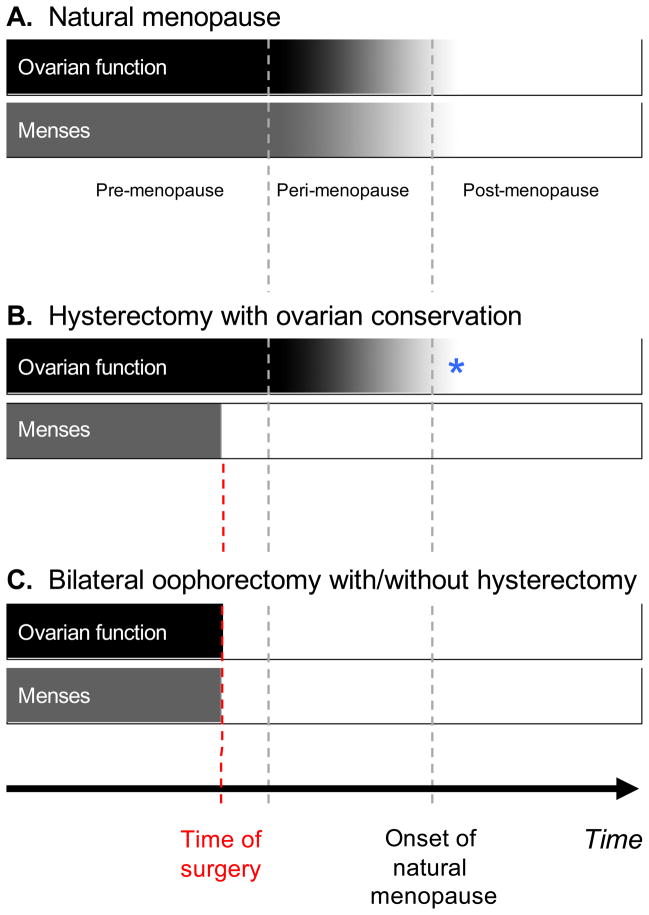
There is some confusion in the literature regarding age at the time of estrogen administration and type of menopause. To reduce this confusion, we suggest to first distinguish women who experienced natural menopause from those who underwent medically induced menopause primarily via surgery (Table 1, Fig. 1). Natural menopause is defined as cessation of menses for 12 continuous months or more in the absence of a medical or surgical cause (Utian, 2001), and the median age at natural menopause is approximately 51 years (Armstrong et al., 2004; Shuster et al., 2010). For naturally menopausal women, the cessation of menses is synchronic with the cessation of ovarian function because the ovaries control the cyclic changes in the endometrium (Fig. 1A). However, the variability of age at natural menopause is quite broad and some women experience premature (before age 40 years) or early (between ages 40 and 45 years) natural menopause (Shuster et al., 2010).
Table 1.
A classification of women in relation to type of menopause and age at menopause.
| Type of menopause | Cessation of menses | Cessation of ovarian function | Comments |
|---|---|---|---|
| Surgically induced menopause | |||
| Hysterectomy alonea | Abrupt at the time of surgery | Difficult to assess | Current belief is that hysterectomy does not influence ovarian functionb |
| Hysterectomy plus unilateral oophorectomya | Abrupt at the time of surgery | Difficult to assess | Current belief is that hysterectomy plus unilateral oophorectomy does not influence ovarian functionb |
| Bilateral oophorectomy with or without hysterectomy | Abrupt at the time of surgery | Abrupt at the time of surgery | Abrupt loss of ovarian function |
| Natural menopause | |||
| Premature menopause, age <40 years | Variable, sometimes with fluctuations | Variable, sometimes with fluctuations | Premature and variable ovarian insufficiencyc |
| Early menopause, age 40–45 years | Usually gradual and synchronized with cessation of ovarian function | Gradual | Early but usually gradual ovarian insufficiencyc |
| Normal menopause, age >45 years | Gradual and synchronized with cessation of ovarian function | Gradual | Expected natural ovarian insufficiency |
lt is ambiguous to refer to hysterectomy with one or both ovaries conserved as “surgical menopause”. Because there is cessation of menses, the women are in menopause. However, from an endocrinological perspective, these women will continue to have circulating hormones until the time of cessation of ovarian function.
lt has been suggested that women who undergo hysterectomy alone or hysterectomy plus removal of one ovary may actually experience an earlier cessation of endocrine function in the remaining ovary or ovaries (Farquhar et al., 2005; Rocca et al., 2007; Phung et al., 2010). Therefore, the current belief of no effect of hysterectomy on ovarian function may be incorrect.
Details about premature or early menopause were reported elsewhere (Shuster et al., 2010).
Fig. 1.
Graphic representation of ovarian function and menstrual activity in relation to menopause. Fig. 1A – For women who experience natural menopause, ovarian function, and menstrual activity decline gradually in a synchronized fashion (gradual shading of bars). Fig. 1B – Women who undergo hysterectomy with one or both ovaries conserved experience an abrupt cessation of menses at the time of the surgery (solid bar) but a gradual decline in ovarian function (gradual shading of bar). These women experience a lag time between hysterectomy and cessation of ovarian function that may last 10 or more years and may be difficult to measure correctly in the clinical setting. *In this figure, we are assuming that the removal of the uterus or of one ovary have no consequences on the remaining ovarian function (one or two conserved ovaries). In fact, removal of the uterus or of one ovary may lead to an earlier cessation of ovarian function by approximately 4 years (Farquhar et al., 2005; Rocca et al., 2007; Phung et al., 2010). Fig. 1C – Women who undergo bilateral oophorectomy with or without hysterectomy experience an abrupt cessation of both ovarian function and menstrual activity at the time of surgery (solid bars).
Women who undergo surgically induced menopause should be divided into those who had the uterus removed with one or both ovaries conserved and those who had bilateral oophorectomy with or without hysterectomy. For women who had the uterus or the uterus plus one ovary removed, there may be a long gap between cessation of menses and cessation of ovarian function. In addition, the time of cessation of ovarian function may be difficult to determine in the absence of menstruation (Fig. 1B). By contrast, the age at menopause due to bilateral oophorectomy is easily determined because cessation of menses and cessation of ovarian function coincide (Fig. 1C). Therefore, the term “surgical menopause” is ambiguous from an endocrinological perspective and should be avoided. In this review, we will not address the issue of the possible modifying effects of hysterectomy alone or of hysterectomy plus unilateral oophorectomy on the remaining ovarian function (one or two conserved ovaries) (Farquhar et al., 2005; Rocca et al., 2007; Phung et al., 2010).
3. Evidence of neuroprotective effects of estrogen in animal experiments
The mechanisms of action of estrogen on the brain are only briefly summarized here, and the readers are referred to some recent reviews and original reports of cellular or animal studies (Mendelsohn and Karas, 2005; Morrison et al., 2006; Umetani et al., 2007; Gibbs, 2010; Henderson and Brinton, 2010).
Most animal experiments on the neuroprotective effects of estrogen involved the removal of both ovaries and the subsequent administration of estrogen. Most of the studies utilized female rodents; however, some important studies of non-human primates are also available. These studies demonstrated several neuroprotective actions of estrogen: 1) Estrogen improves synapse formation on dendritic spines in hippocampi of ovariectomized rats (McEwen and Alves, 1999; Monk and Brodaty, 2000; Hao et al., 2003). 2) Estrogen improves cerebral blood flow and glucose metabolism, and it may act as an antioxidant (Gibbs and Aggarwal, 1998; McEwen and Alves, 1999; Monk and Brodaty, 2000). 3) Estrogen increases choline acetyltransferase activity in the basal forebrains and hippocampi of ovariectomized rats (Gibbs and Aggarwal, 1998; Markowska and Savonenko, 2002; Gibbs, 2010). 4) Estrogen reduces the deposition of β-amyloid in the brain, whereas progesterone has the opposite effect (Huang et al., 2004). 5) Estrogen prevents mitochondrial damage (Morrison et al., 2006; Brinton, 2008; Henderson and Brinton, 2010).
In addition, there is a growing body of literature suggesting that estrogen effects on the brain vary with age both in female rats and female monkeys (Hao et al., 2003). Additional experiments in ovariectomized monkeys have shown that the effects of estrogen on cognition are mediated by multiple brain regions including both the hippocampus and the prefrontal cortex (Rapp et al., 2003a; Tang et al., 2004). Thus, there is strong and consistent evidence of neuroprotective effects of estrogen in animal models (Siegfried, 2007; Gibbs, 2010; Henderson and Brinton, 2010). However, laboratory models and animal experiments are inexact representations of the processes underlying dementia in women (Henderson and Brinton, 2010).
4. Evidence of neuroprotective effects of estrogen in women before the age of natural menopause
Important observations on the effects of estrogen in younger women came from studies of women who underwent bilateral oophorectomy before reaching natural menopause. The hormonal changes occurring after bilateral oophorectomy in premenopausal women are different from those occurring during natural menopause or after bilateral oophorectomy in women who already experienced natural menopause (Table 1, Fig. 1). In particular, bilateral oophorectomy before menopause causes an abrupt decline of estrogen as well as progesterone and testosterone, and a disruption of the hypothalamic-pituitary-ovarian axis (Morrison et al., 2006). The disruption of the axis is associated with an abrupt increase in gonadotropins (luteinizing hormone and follicle stimulating hormone), which may have direct detrimental effects on the brain (Webber et al., 2005; Casadesus et al., 2006; Morrison et al., 2006; Casadesus et al., 2007; Rodrigues et al., 2008; Rocca et al., 2009).
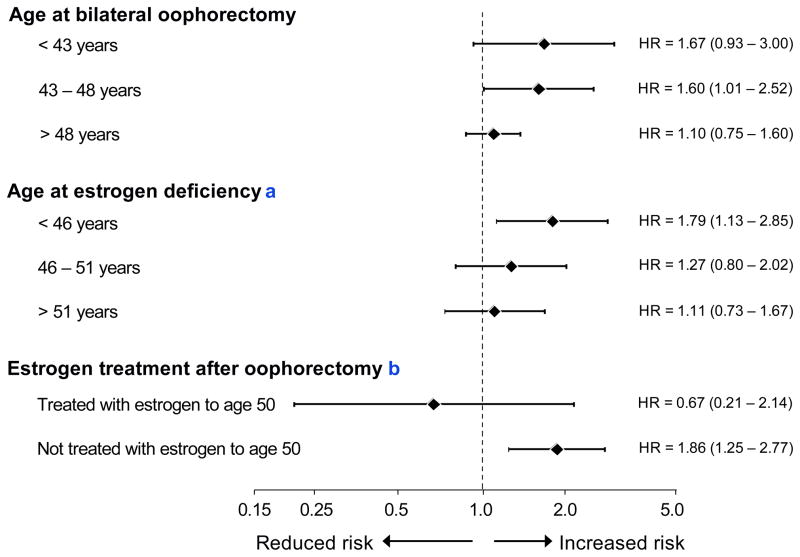
The available scientific evidence on cognitive sequelae of bilateral oophorectomy is limited (Rocca et al., 2007; Rocca et al., 2008; Shuster et al., 2008; Rocca et al., 2009; Phung et al., 2010; Rocca et al., 2010). However, the Mayo Clinic Cohort Study of Oophorectomy and Aging showed an almost doubled long-term risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause (Rocca et al., 2007). The study also showed a trend of increasing risk of cognitive impairment and dementia with younger age at the time of oophorectomy or at the time of estrogen deficiency. Time of estrogen deficiency was defined as the time of oophorectomy if the woman did not receive ET or the end of ET if the woman was treated (Fig. 2) (Rocca et al., 2007). A nationwide historical cohort study conducted in Denmark recently confirmed the Mayo Clinic cohort study findings (Phung et al., 2010).
Fig. 2.
Risk of cognitive impairment or dementia by age at bilateral oophorectomy and by age at estrogen deficiency in the Mayo Clinic Cohort Study of Oophorectomy and Aging (relative risk estimated by a hazard ratio and 95% confidence intervals, logarithmic scale). The hazard ratio increased linearly with decreasing age at oophorectomy (p for trend = 0.02) and with decreasing age at estrogen deficiency (p for trend = 0.02).
aAge at estrogen deficiency = age at oophorectomy plus total duration of subsequent estrogen treatment.
bBilateral oophorectomy in women younger than age 49 years.
It remains uncertain whether the detrimental effects of bilateral oophorectomy are mediated entirely by estrogen deficiency or also by other hormonal mechanisms (Morrison et al., 2006; Rocca et al., 2009). However, women who underwent bilateral oophorectomy before age 49 years but received ET through age 50 years or longer did not experience an increased risk of cognitive impairment or dementia (Fig. 2) (Rocca et al., 2007). These analyses suggest that estrogen deprivation plays a key role in the detrimental effect of oophorectomy. We emphasize that only 60% of the women in the Mayo Clinic study were prescribed ET after bilateral oophorectomy, and only 20% were treated with estrogen through age 50 years (Rocca et al., 2007).
The only experimental evidence specific for the use of ET after bilateral oophorectomy comes from the clinical trials of Sherwin and colleagues who studied small groups of women with short-term treatment and minimal follow-up (2–3 months). These short-term trials consistently suggested a neuroprotective effect of estrogen (Sherwin, 1988; Sherwin and Phillips, 1990; Phillips and Sherwin, 1992). Unfortunately, none of the ongoing clinical trials of ET after menopause are focusing on women who underwent bilateral oophorectomy or who experienced premature or early natural menopause (Lethaby et al., 2008).
5. Difference between confounding and interaction
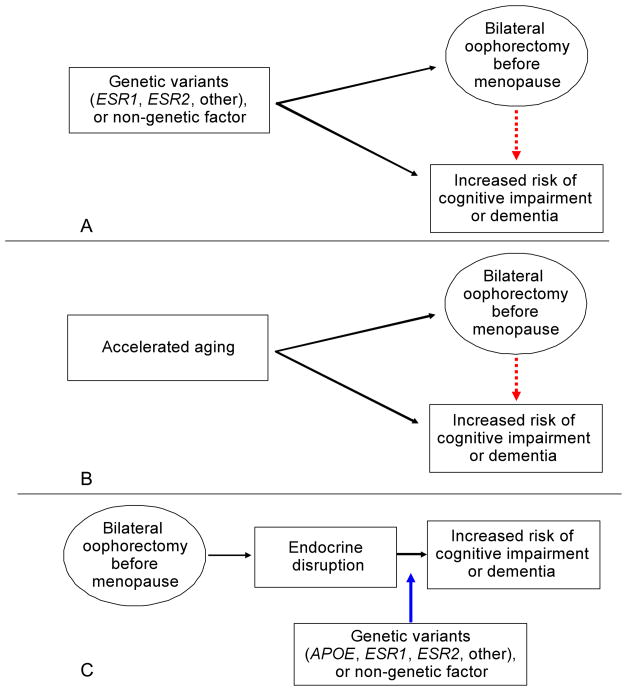
There is some disagreement on the interpretation of the findings from the Mayo Clinic cohort study (Hogervorst and Bandelow, 2007; Rocca et al., 2009). Bilateral oophorectomy may be a true risk factor for the subsequent increased risk of cognitive impairment or dementia or the association may be spurious and caused by confounding. For example, there may be confounding by genetic factors (e.g. genetic variants), confounding by non-genetic factors, and confounding by accelerated aging (Fig. 3).
Fig. 3.
Three possible explanations for the association of bilateral oophorectomy with increased risk of cognitive impairment or dementia. Fig. 3A – Possible confounding by genetic variants and possible confounding by non-genetic factors (red arrow). Fig. 3B – Confounding by accelerated aging (red arrow). Fig. 3C – Chain of causality in which genetic variants or non-genetic factors are effect modifiers (or interaction variables; blue arrow) rather than confounders.
Genetic variants in the estrogen synthesis or responsiveness pathway may predispose women to develop uterine abnormalities such as fibroids or adenomyosis leading to menorrhagia or metrorrhagia that, in turn, may prompt the removal of the uterus. In approximately 50% of the cases, women and their physicians elect to perform a prophylactic bilateral oophorectomy in conjunction with the hysterectomy (American College of Obstetricians and Gynecologists, 2008). Similarly, genetic variants that predispose women to ovarian cysts or endometriosis may prompt a bilateral oophorectomy to treat the lesions, to prevent recurrences, or to prevent subsequent ovarian cancer. If the same genetic variants that increase the risk of benign uterine or ovarian lesions also increase the risk of neurological diseases through unknown hormonal or non-hormonal mechanisms, bilateral oophorectomy has no causal role but is merely a marker of the underlying genetic predisposition (Fig. 3A) (Rocca et al., 2009).
In support of this hypothesis, there is evidence that genetic factors predispose women to hysterectomy. In a twin study, heritability was 63% for age at natural menopause, 59% for hysterectomy prior to natural menopause, 69% for fibroids, and 55% for menorrhagia (Snieder et al., 1998). Another study showed that women with a variant in the estrogen receptor 1 gene (ESR1; SNP rs2234693) are more likely to undergo surgical menopause (Weel et al., 1999). By contrast, the evidence for an association between genetic variants and ovarian cysts or endometriosis that might prompt bilateral oophorectomy remains limited (Cramer et al., 2000; Di and Guo, 2007; Montgomery et al., 2008; Rocca et al., 2009).
For the genetic variants to act as a confounder, they must be associated not only with oophorectomy but also with cognitive impairment or dementia (Szklo and Nieto, 2007; Porta and International Epidemiological Association, 2008). However, the evidence linking certain variants of the genes in the estrogen synthesis and responsiveness pathway with the risk of cognitive impairment or dementia remains limited (Porrello et al., 2006; Hogervorst and Bandelow, 2007; Luckhaus and Sand, 2007).
Alternatively, confounding could be caused by another non-genetic risk factor. We can postulate that some early life events, such as the use or non-use of oral contraceptives, or the number and outcome of pregnancies, might predispose women to uterine or ovarian diseases leading to bilateral oophorectomy, and independently might predispose women to cognitive impairment or dementia (Fig. 3A). In this situation, bilateral oophorectomy has no causal role. However, we are not aware of any evidence in support of this hypothesis.
Some authors have suggested that premature or early natural menopause is the result of an accelerated aging process determined by genetic or non-genetic causes and involving all tissues and organs throughout the body, including the ovaries (Snowdon et al., 1989). This hypothesis of accelerated aging has been proposed to interpret the association between premature or early natural menopause and increased mortality and morbidity (Shuster et al., 2010); however, it can be extended to bilateral oophorectomy if the surgical indication for the oophorectomy is itself a manifestation of accelerated aging (Fig. 3B). Under this hypothesis, the hormonal changes following the removal of the ovaries have no causal role in the development of cognitive impairment or dementia. However, the evidence in support of this hypothesis is limited (Shuster et al., 2010).
If the association of bilateral oophorectomy with cognitive decline or dementia is due to confounding, changes in surgical practice favoring ovarian conservation in young women would not affect the risk of subsequent neurological diseases. Strong evidence against a confounding effect is the observation that women who underwent bilateral oophorectomy before age 49 years but received ET through age 50 years or longer did not experience an increased risk of cognitive impairment or dementia in the Mayo Clinic cohort study (Rocca et al., 2007). Against a confounding effect is also the similar risk of cognitive impairment or dementia among women who underwent bilateral oophorectomy for benign conditions or for prophylaxis of ovarian cancer. These findings suggest that the risk was independent of the indication for the oophorectomy (Rocca et al., 2009). Although the possibility that confounding is the explanation of the observed associations cannot be completely ruled out at this time, the evidence for a confounding mechanism is limited.
We suggest that genetic variants and non-genetic factors are involved in the association of bilateral oophorectomy with cognitive decline or dementia as effect modifiers (or interaction variables) rather than as confounders (Fig. 3C) (Szklo and Nieto, 2007; Porta and International Epidemiological Association, 2008). Thus, bilateral oophorectomy is the key initial step in a chain of causality leading to accelerated brain aging, and variants of the APOE gene, the ESR1 or ESR2 genes, or other genes, and smoking, alcohol consumption, obesity, diabetes mellitus, or other non-genetic factors may reduce or accelerate the causal process (Rocca et al., 2009). Under this causal model, ovarian conservation at the time of hysterectomy would reduce the risk of cognitive impairment or dementia in the general population (Rocca et al., 2009), even though the consequences of bilateral oophorectomy may vary in severity among women (Parker et al., 2009).
Consideration of inter-individual variations in the response to estrogen deprivation is important but should not distract attention from the primary public health objective of reducing the risk of cognitive decline. We argue that changing surgical practices regarding prophylactic bilateral oophorectomy is the most important step toward prevention (Parker et al., 2009). A similar argument has been used to explain that inter-individual variations are not the most important variables to explain the rapid increase in obesity experienced in the USA in the last 20 years and that interventions at the individual level may be insufficient to stop the obesity epidemic (Schwartz and Carpenter, 1999; Sommer, 2009).
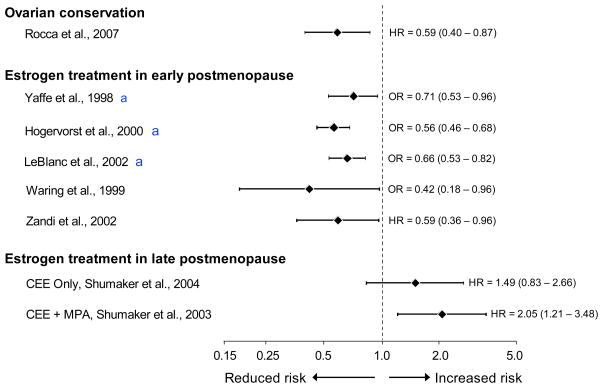
6. Evidence of neuroprotective effects of estrogen in women in the early postmenopausal stage
Case-control and cohort studies have consistently shown a beneficial effect of estrogen on cognition when ET was started in early postmenopause (most commonly at ages 50–60 years, Fig. 4) (Yaffe et al., 1998; Waring et al., 1999; Hogervorst et al., 2000; LeBlanc et al., 2001; Zandi et al., 2002). However, some studies did not confirm the beneficial effect (Roberts et al., 2006; Petitti et al., 2008). The majority of women in these observational studies had undergone natural menopause and were treated for the relief of menopausal symptoms. The observational studies showing beneficial effects of estrogen have been criticized because these findings could be the result of confounding. It has been argued that other factors such as higher socioeconomic status, higher education, or better general health may be the real causes of neuroprotection and that ET is only a surrogate marker of such confounders. By contrast, we suggest that the observational findings are not due to confounding and that the contradiction between observational studies and experimental studies (WHI clinical trials) is due to timing differences. Again, there may be some confusion in the literature between confounding variables and interaction variables (Szklo and Nieto, 2007; Porta and International Epidemiological Association, 2008).
Fig. 4.
The effect of estrogen on the risk of cognitive decline or dementia varies with age at the time of treatment, type of menopause, and stage of menopause (relative risk estimated by an odds ratio or a hazard ratio and 95% confidence intervals, logarithmic scale). Women with ovarian conservation have a reduced long-term risk of cognitive decline or dementia compared to women who underwent bilateral oophorectomy before menopause (most commonly before age 50 years). Treatment with estrogen in the early postmenopausal stage (most commonly at ages 50–60 years) is associated with a reduced long-term risk of cognitive decline or dementia. However, initiation of estrogen treatment in the late postmenopausal stage (ages 65–79 years) is associated with an increased risk of cognitive impairment or dementia. CEE = conjugated equine estrogen; HR = hazard ratio; MPA = medroxyprogesterone acetate; OR = odds ratio; WHI = Women’s Health Initiative Study (Yaffe et al., 1998; Waring et al., 1999; Hogervorst et al., 2000; LeBlanc et al., 2001; Zandi et al., 2002; Shumaker et al., 2003; Shumaker et al., 2004; Rocca et al., 2007) [Modified from W.A. Rocca et al., Neurodegenerative Dis 2010;7:163–166]. aThese three publications were meta-analyses.
A historical illustration of the tension between evidence from observational studies and from clinical trials is provided by the debate over the association of cigarette smoking with lung cancer in the 1950s. Two prominent statisticians, Ronald Fisher and Joseph Berkson, criticized the belief that smoking causes lung cancer because of the lack of experimental evidence (from controlled clinical trials) and because of the possibility of confounding (Parascandola, 2004). The theory that smoking is a major risk factor for lung cancer was ultimately accepted without any clinical trial evidence, prompted major public health interventions, and led to a sizeable decline in the risk of lung cancer over half a century (Parascandola, 2004; Sommer, 2009).
7. Evidence of deleterious effects of estrogen initiated in the late postmenopausal stage
The WHI clinical trials showed an increased risk of dementia or mild cognitive impairment (MCI) among women who initiated treatment with estrogen alone or in combination with progestin at ages 65–79 years (Fig. 4) (Rapp et al., 2003b; Shumaker et al., 2003; Espeland et al., 2004; Shumaker et al., 2004). However, these trials focused on the effects of ET initiated many years after the onset of natural or surgical menopause, and the discrepancy between the WHI results and observational data may be explained by the timing of initiation of estrogen (Manson et al., 2006; Siegfried, 2007; Brinton, 2008; Rocca et al., 2008; Henderson and Brinton, 2010; Rocca et al., 2010). Indeed, the effects of estrogen on the brain are probably beneficial when initiated early, but when vascular or degenerative lesions have occurred, estrogen cannot reverse the lesions or halt progression (Rocca et al., 2009). By contrast, the detrimental effects of estrogen, primarily the pro-thrombotic effects, may become predominant in older women and may cause an increased risk of cognitive impairment or dementia (Mendelsohn and Karas, 1999; Shumaker et al., 2003; Shumaker et al., 2004; Mendelsohn and Karas, 2005; Mendelsohn and Karas, 2007).
The WHI trials considered only women of ages 65–79 years at randomization because of statistical rather than biological considerations. The trials were designed to have sufficient statistical power to find a difference in dementia risk between women randomized to hormonal treatment and women randomized to placebo. Because the risk of dementia is relatively low before age 65 years, the investigators of the WHI decided to only enroll older women in the memory subproject (WHIMS). However, the idea of randomizing women at ages 65–79 years to hormonal treatment de novo had no biological support. Thus, the findings of the WHI trials for dementia are correct statistically but have no relevance to the treatment of women before age 65 years.
8. History of the window of opportunity hypothesis
Table 2 provides a brief history of the window of opportunity hypothesis (or timing hypothesis) from a broad perspective including several outcome diseases and overall mortality. We organized the events and the publications in chronological order and grouped them into four periods: 1) a phase of great optimism and expectation for estrogen treatment that was abruptly terminated by the premature interruption of the WHI trials; 2) a phase of introduction and support of the window of opportunity hypothesis; 3) a phase of opposition to and criticism of the hypothesis; and 4) the current phase of uncertainty about and new support of the hypothesis.
Table 2.
A brief history of the window of opportunity hypothesis for estrogen treatment.
| Year | Event | Reference |
|---|---|---|
| Phase of great expectation for estrogen treatment. | --- | |
| 1993–1998 | Enrollment in WHI CEE Trial: conjugated equine estrogen (CEE; 0.625 mg/day) - Randomized 10,739 women (with hysterectomy) - ages 50–79 years. | --- |
| 1993–1998 | Enrollment in WHI CEE/MPA Trial: CEE + medroxyprogesterone acetate (MPA; 2.5 mg/day) -Randomized 16,608 women - ages 50–79 years | --- |
| 1993–1998 | Enrollment in WHI Observational Study: recruited 93,676 women - ages 50–79 years. | --- |
| 2002 | WHI CEE/MPA Trial stopped at 5.6 years of follow-up: increased risk for invasive breast cancer, coronary heart disease (CHD), stroke, pulmonary embolism (reduced risk of colorectal cancer and hip fracture). Increased global index. | (Writing Group for the Women's Health Initiative, 2002) |
| 2004 | WHI CEE Trial stopped at 7.1 years of follow-up: increased risk of stroke (reduced risk of hip fracture; suggestive reduced risk of invasive breast cancer). Similar global index. | (Anderson et al., 2004) |
| Introduction and support of the window of opportunity hypothesis. | (Harman et al., 2005; Grodstein et al., 2006; Hsia et al., 2006) | |
| 2005 | KEEPS Trial: Kronos Early Estrogen Prevention Study (KEEPS) begins. 720 women aged 42–58 years treated with CEE (0.45 mg/day) or transdermal 17β estradiol (50 μg/day) + micronized progesterone (200 mg/day × 12 days/month). | (Harman et al., 2005; Manson et al., 2006) |
| 2007 | Analyses of WHI CEE and CEE/MPA Trial data: suggestive reduced risk of CHD and total mortality for treatment started within 10 years of menopause. No trend for stroke. | (Rossouw et al., 2007) |
| 2007 | Ancillary sub-study of WHI CEE Trial (WHI Coronary Artery Calcium Study [WHI-CACS]): calcified-plaque burden lower if estrogen given at ages 50–59 years. | (Manson et al., 2007) |
| Opposition to and criticism of the window of opportunity hypothesis. | --- | |
| 2009 | WHI CEE and CEE/MPA + observational study data: increased risk of invasive breast cancer and total invasive cancer for both CEE and CEE/MPA initiated early. For CEE/MPA, also increased risk of venous thromboembolism, stroke, and global index (reduced risk of hip fractures). For CEE, also increased risk of venous thromboembolism and stroke (reduced risk of hip fractures). | (Prentice et al., 2009) |
| May 2009 | Editorial against the window of opportunity hypothesis. | (Banks and Canfell, 2009) |
| 2010 | Analyses from the WHI suggest that CEE/MPA does not reduce the risk of coronary heart disease in women who initiated therapy close to menopause. | (Tohetal., 2010) |
| Phase of uncertainty about and new support of the window of opportunity hypothesis. | --- | |
| 2009 | Meta-analysis indicating a 25% reduced mortality in younger women taking hormone therapy compared with no treatment. | (Salpeter et al., 2009) |
| 2010 | Ancillary sub-study of WHI CEE Trial (WHI Coronary Artery Calcium Study [WHI-CACS]): calcified-plaque burden lower if estrogen given more immediately after onset of menopause and for a shorter duration in women with vasomotor symptoms. | (Allison et al., 2010) |
| 2010 | Observational study in UK general practices: transdermal estrogen at low dose does not increase the risk of stroke in postmenopausal women. | (Renoux et al., 2010) |
| 2010 | Reviews of animal data for the window of opportunity hypothesis of brain aging. | (Gibbs, 2010; Henderson and Brinton, 2010) |
| 2010 | Reviews of clinical data for the window of opportunity hypothesis of brain aging. | (Henderson and Brinton, 2010; Rocca et al., 2010) |
The WHI trials started with great confidence that they would demonstrate the benefit of estrogen alone and combined with progestin. However, the trial with estrogen plus progestin was stopped in 2002 because of the following risk-benefit balance: increased risk of invasive breast cancer, coronary artery disease, stroke, pulmonary embolism, and increased global index (a composite outcome) versus reduced risk of colorectal cancer and hip fractures (Writing Group for the Women’s Health Initiative, 2002). The trial with estrogen alone was stopped 2 years later in 2004 for the following risk-benefit balance: increased risk of stroke versus reduced risk of hip fractures and suggestive reduced risk of invasive breast cancer. The global index was similar (Anderson et al., 2004). It is interesting to note that the risk-benefit profiles were quite different for estrogen plus progestin versus estrogen alone.
In reaction to the widespread controversy and discontent following the publication of the WHI trials, some of the WHI investigators, along with investigators outside of the WHI, proposed the window of opportunity hypothesis to explain the paradoxical findings. The hypothesis prompted a re-examination of the WHI data and the publication of results from ancillary studies suggesting that estrogen in younger women is safe and beneficial (Manson et al., 2007; Rossouw et al., 2007). In addition, the hypothesis prompted the design of the Kronos Early Estrogen Prevention Study (KEEPS trial, NCT00154180) and the Early Versus Late Intervention Trial with Estradiol Study (ELITE trial, NCT00114517) (Harman et al., 2005; Grodstein et al., 2006; Hsia et al., 2006). By the end of 2007, there was growing evidence in favor of a more positive interpretation of estrogen treatment at least for cardiovascular disease. However, the literature remained quite cautious about the overall risk-benefit balance (Manson and Bassuk, 2007; Mendelsohn and Karas, 2007).
Starting in 2009, there has been a new phase of opposition to and criticism of the window of opportunity hypothesis. Another subgroup of the WHI investigators attacked the window of opportunity hypothesis using complicated reanalyses combining data from the estrogen plus progestin trial, the estrogen alone trial, and the observational study. The study also combined estrogen treatment that was reported historically by the women at the time of recruitment and estrogen treatment that was administered as part of the trials. These reanalyses showed that ET alone or in combination with progestin is not beneficial even when started early after menopause (Prentice et al., 2009). The arguments against the window of opportunity hypothesis are also reflected in the editorial that accompanied the paper (Banks and Canfell, 2009). Another reanalysis of WHI data published in 2010 confirmed that estrogen plus progestin does not reduce the risk of coronary heart disease in women who initiated therapy close to menopause (Toh et al., 2010). We argue that the WHI trials were not designed to address the window of opportunity hypothesis and, therefore, cannot be used to address the hypothesis. We hope that the results of the KEEPS and ELITE trials will bring new clarifying data (Harman et al., 2005; Grodstein et al., 2006; Hsia et al., 2006).
We are now left with an open controversy and with uncertainty about the final interpretation of the WHI data. Several recent publications bring new evidence in favor of the window of opportunity hypothesis. Salpeter and colleagues argued in a meta-analysis that younger women treated with hormone therapy have a 25% reduced mortality compared with no treatment (Salpeter et al., 2009). One additional report from the WHI ancillary study of coronary artery calcium showed that women with vasomotor symptoms who were given estrogen more immediately after the onset of menopause and for a shorter duration had a reduced calcified-plaque burden (Allison et al., 2010). Finally, a new large case-control study based on UK general practices showed that estrogen alone or combined with a progestogen is not a risk factor for stroke if the estrogen is used at low doses and administered transdermally (Renoux et al., 2010).
The window of opportunity hypothesis for brain aging was reviewed in 2010 from the animal data and laboratory perspective (Gibbs, 2010; Henderson and Brinton, 2010) and from the clinical perspective (Henderson and Brinton, 2010; Rocca et al., 2010). Fig. 4 provides a graphic combination of all of the evidence available in support of the hypothesis. The findings for ovarian conservation were derived from the original analyses in the Mayo Clinic study by inverting the definition of exposure from oophorectomy to ovarian conservation (Rocca et al., 2007; Rocca et al., 2010)
9. Conclusions
A combination of current scientific evidence from animal studies and from both observational studies and clinical trials suggests that estrogen is neuroprotective; however, the neuroprotective effects are dependent on age at the time of initiation, type of menopause, and stage of menopause. The apparent contradiction of results from observational studies versus results from clinical trials may be explained by the window of opportunity hypothesis (or timing hypothesis, Fig. 4).
The results from the WHI clinical trials have been inappropriately extrapolated from women in the late postmenopausal stage to younger women in the early postmenopausal stage, and even further to women who underwent bilateral oophorectomy before the onset of natural menopause or experienced premature or early natural menopause (Table 1). Thus, after publication of the WHI results, many women discontinued ET or avoided starting ET at all ages, including before age 51 years (Buist et al., 2004; Haas et al., 2004; Hersh et al., 2004). In agreement with the 2010 guidelines of the European Menopause and Andropause Society (EMAS), we suggest that women who undergo bilateral oophorectomy before the onset of natural menopause or experience premature or early natural menopause should be considered for hormonal treatment until the average age of natural menopause, and that the results from the WHI trials should not be applied to them (Shuster et al., 2010; Vujovic et al., 2010).
Acknowledgments
This research was supported by grants from the NINDS NS033978 and NIA AG034676.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison MA, Manson JE, Aragaki A, Langer RD, Rossouw J, Curb D, Martin LW, Phillips L, Stefanick ML, Cochrane BB, Sarto G, Barnhart J, O’Sullivan MJ, Johnson KC, Gass M, Trevisan M, Woods NF. Vasomotor symptoms and coronary artery calcium in postmenopausal women. Menopause. 2010;17 doi: 10.1097/gme.0b013e3181e664dc. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 89 Elective and risk-reducing salpingo-oophorectomy. Obstet Gynecol. 2008;111:231–241. doi: 10.1097/01.AOG.0000291580.39618.cb. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- Banks E, Canfell K. Invited Commentary: Hormone therapy risks and benefits--The Women’s Health Initiative findings and the postmenopausal estrogen timing hypothesis. Am J Epidemiol. 2009;170:24–28. doi: 10.1093/aje/kwp113. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist DS, Newton KM, Miglioretti DL, Beverly K, Connelly MT, Andrade S, Hartsfield CL, Wei F, Chan KA, Kessler L. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-[beta] deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Petterson KS, Barbieri RL, Huhtaniemi IT. Reproductive hormones, cancers, and conditions in relation to a common genetic variant of luteinizing hormone. Hum Reprod. 2000;15:2103–2107. doi: 10.1093/humrep/15.10.2103. [DOI] [PubMed] [Google Scholar]
- Di W, Guo SW. The search for genetic variants predisposing women to endometriosis. Curr Opin Obstet Gynecol. 2007;19:395–401. doi: 10.1097/GCO.0b013e328235a5b4. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956–962. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm Behav. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health. 2006;15:35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta–analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Should surgical menopausal women be treated with estrogens to decrease the risk of dementia? Neurology. 2007;69:1070–1071. doi: 10.1212/01.wnl.0000279584.03800.3d. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66:56–71. doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- Huang J, Guan H, Booze RM, Eckman CB, Hersh LB. Estrogen regulates neprilysin activity in rat brain. Neurosci Lett. 2004;367:85–87. doi: 10.1016/j.neulet.2004.05.085. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta–analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2008:CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaus C, Sand PG. Estrogen Receptor 1 gene (ESR1) variants in Alzheimer’s disease. Results of a meta-analysis. Aging Clin Exp Res. 2007;19:165–168. doi: 10.1007/BF03324684. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Harman SM, Brinton EA, Cedars MI, Lobo R, Merriam GR, Miller VM, Naftolin F, Santoro N. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause. 2006;13:139–147. doi: 10.1097/01.gme.0000177906.94515.ff. [DOI] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS. Invited Commentary: Hormone therapy and risk of coronary heart disease--why renew the focus on the early years of menopause? Am J Epidemiol. 2007;166:511–517. doi: 10.1093/aje/kwm213. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- Monk D, Brodaty H. Use of estrogens for the prevention and treatment of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2000;11:1–10. doi: 10.1159/000017206. [DOI] [PubMed] [Google Scholar]
- Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, Kennedy SH, Zondervan KT. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14:447–457. doi: 10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejat EJ, Chervenak JL. The continuum of ovarian aging and clinicopathologies associated with the menopausal transition. Maturitas. 2010;66:187–190. doi: 10.1016/j.maturitas.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Parascandola M. Two approaches to etiology: the debate over smoking and lung cancer in the 1950s. Endeavour. 2004;28:81–86. doi: 10.1016/j.endeavour.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Parker WH, Jacoby V, Shoupe D, Rocca W. Effect of bilateral oophorectomy on women’s long-term health. Womens Health (Lond Engl) 2009;5:565–576. doi: 10.2217/whe.09.42. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Crooks VC, Chiu V, Buckwalter JG, Chui HC. Incidence of dementia in long-term hormone users. Am J Epidemiol. 2008;167:692–700. doi: 10.1093/aje/kwm362. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord. 2010;30:43–50. doi: 10.1159/000314681. [DOI] [PubMed] [Google Scholar]
- Porrello E, Monti MC, Sinforiani E, Cairati M, Guaita A, Montomoli C, Govoni S, Racchi M. Estrogen receptor alpha and APOEepsilon4 polymorphisms interact to increase risk for sporadic AD in Italian females. Eur J Neurol. 2006;13:639–644. doi: 10.1111/j.1468-1331.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- Porta MS. A Dictionary of Epidemiology. Oxford University Press; Oxford; New York: International Epidemiological Association, 2008. [Google Scholar]
- Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord. 2006;20:141–146. doi: 10.1097/00002093-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis. 2008;5:257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., 3rd Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7:163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Verdile G, Foster JK, Hogervorst E, Joesbury K, Dhaliwal S, Corder EH, Laws SM, Hone E, Prince R, Devine A, Mehta P, Beilby J, Atwood CS, Martins RN. Gonadotropins and cognition in older women. J Alzheimers Dis. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009;122:1016–1022.e1011. doi: 10.1016/j.amjmed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Carpenter KM. The right answer for the wrong question: consequences of type III error for public health research. Am J Public Health. 1999;89:1175–1180. doi: 10.2105/ajph.89.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Phillips SJ. Estrogen and cognitive functioning in surgically menopausal women. Ann N Y Acad Sci. 1990;592:474–475. [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried T. Neuroscience: it’s all in the timing. Nature. 2007;445:359–361. doi: 10.1038/445359a. [DOI] [PubMed] [Google Scholar]
- Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, Iso H, Jacobs DR, Jr, Phillips RL. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79:709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A. Getting What We Deserve: Health and Medical Care in America. Johns Hopkins University Press; Baltimore: 2009. [Google Scholar]
- Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Jones and Bartlett Publishers; Sudbury, MA: 2007. [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Toh S, Hernandez-Diaz S, Logan R, Rossouw JE, Hernan MA. Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: does the increased risk ever disappear? A randomized trial. Ann Intern Med. 2010;152:211–217. doi: 10.1059/0003-4819-152-4-201002160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- Utian WH. Semantics, menopause-related terminology, and the STRAW reproductive aging staging system. Menopause. 2001;8:398–401. doi: 10.1097/00042192-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Vujovic S, Brincat M, Erel T, Gambacciani M, Lambrinoudaki I, Moen MH, Schenck-Gustafsson K, Tremollieres F, Rozenberg S, Rees M. EMAS position statement: Managing women with premature ovarian failure. Maturitas. 2010;67:91–93. doi: 10.1016/j.maturitas.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Waring SC, Rocca WA, Petersen RC, O’Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52:965–970. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- Webber KM, Casadesus G, Marlatt MW, Perry G, Hamlin CR, Atwood CS, Bowen RL, Smith MA. Estrogen bows to a new master: the role of gonadotropins in Alzheimer pathogenesis. Ann N Y Acad Sci. 2005;1052:201–209. doi: 10.1196/annals.1347.020. [DOI] [PubMed] [Google Scholar]
- Weel AE, Uitterlinden AG, Westendorp IC, Burger H, Schuit SC, Hofman A, Helmerhorst TJ, van Leeuwen JP, Pols HA. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J Clin Endocrinol Metab. 1999;84:3146–3150. doi: 10.1210/jcem.84.9.5981. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative, I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321 – 333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]