Abstract
Introduction
Quantitative cartilage morphometry is a valuable tool to assess osteoarthritis (OA) progression. Current methodologies generally evaluate cartilage morphometry in a full or partial sub-region of the cartilage plates. This report describes the evaluation of a semi-automated cartilage segmentation software tool capable of quantifying cartilage loss in a local indexed region.
Methods
We examined the baseline and 24 month follow up MRI image sets of twenty-four subjects from the progression cohort of Osteoarthritis Initiative (OAI), using the Kellgren-Lawrence (KL) score of 3 at baseline as the inclusion criteria. A radiologist independently marked a single region of local thinning for each subject, and three additional readers, blinded to time point, segmented the cartilage using a semi-automated software method. Each baseline-24 month segmentation pair was then registered in 3D and the change in cartilage volume was measured.
Results
After 3D registration, the change in cartilage volume was calculated in specified regions centered at the marked point, and for the entire medial compartment of femur. The responsiveness was quantified using the SRM values and the percentage of subjects that showed a loss in cartilage volume. The most responsive measure of change was SRM = −1.21, and was found for a region of 10 mm from the indexed point.
Discussion
The results suggest that measurement of cartilage loss in a local region is superior to larger areas and to the total plate. There also may be an optimal region size (10 mm from an indexed point) in which to measure change. In principle, the method is substantially faster than segmenting entire plates or sub-regions.
Introduction
Osteoarthritis (OA) is the most common joint disorder and has a high social and economic cost. OA is one of the most frequent causes of pain, loss of function, and disability in adults and the impact on the healthcare system is increasing as the population ages1–2.
Evaluation of structural changes in joints, such as the knee, is important for tracking the progression and the effect of treatment on the course of OA3–6. Several radiological imaging techniques have been used to measure cartilage change. Magnetic resonance imaging (MRI) provides high-resolution visualization of the cartilage, and other soft tissue structures such as, ligaments, and meniscus and their pathological change, making it an ideal modality for OA assessment. It is also non-invasive and does not use ionizing radiation. Semi-quantitative scoring systems such as Whole-Organ Magnetic Resonance Imaging Score (WORMS), and the Boston Leeds Osteoarthritis Knee Score (BLOKS) are established measures for assessment of degenerative changes in joints with OA using MR images7–9. However, these systems are based fundamentally on a qualitative assessment of the knee joint and do not attempt to completely quantify the structural changes due to OA
Quantitative measurement of articular structures on MR images may reveal changes that are not observed with semi quantitative scoring. Several software methods have been described in the literature that provide tools to manually or semi-automatically segment (outline) the articular cartilage on MRI data sets3, 5, 10–33. Compared to more automated techniques, manual cartilage segmentation is time consuming and potentially less objective. However, the thin size of joint cartilage (often less than 1 mm.) combined with the low contrast between the cartilage and surrounding soft tissues, can make it difficult to create a fully automated segmentation software12–13. Semi automated techniques, in which an observer with anatomic knowledge is required to guide the software, are the most common approaches. Several different algorithms such as region growing21, shape modelling22, edge detection20, or active contour models33–34 are employed.
Many methods are laborious and the reader time can be a major component to the expense of studies that use these measurements. Large studies such as the OAI will require analysis methods that can be applied to the vast amount of MRI data sets in an efficient manner. After data from all time points are acquired, the OAI will have over 50,000 knee MRI image sets for each pulse sequence. A fast semi-automated software analysis tool would offer a great deal of promise for handling the task of processing these data. The cost of a clinical trial or other study can be greatly reduced using a method with lower reader time and improved responsiveness.
We previously published a validation study of a software tool that uses a hybrid approach. The tool had two components: core image-processing algorithms (low level software code) that provided automated image analysis, and a graphical user interface, which allowed the reader to view and correct the software output. An "active contour” edge detection algorithm was employed to automatically refine the segmented margins; this step applied an objective and consistent final refinement to the delineated edge and reduced the amount of variation due to the reader input. The software tool was also modified to permit the reading of paired data sets and demonstrated improved reproducibility, compared to a blinded reading, based on an analysis of duplicate acquisitions35.
Recently, OA research studies have begun to study changes in the cartilage thickness in different anatomic sub-regions of the knee joint, evaluating the effect of different risk factors on the rate of change detected in these areas25–26, 36. In principle, measuring the cartilage in sub-regions should be more responsive to change than in total plates and studies have demonstrated improved responsiveness of cartilage loss in the central femorotibial (FT) sub-region24, 36. Osteoarthritis can affect different sub-regions of the FT joint with variable intensity in each person. This can reduce the sensitivity of the measurements in detecting change in cartilage thickness in studies with a wide range of subjects. In order to overcome this issue Buck et al37 used the ordered values approach which only considered the magnitude of change separate from the location of the sub-region.
The goal of the current study was to validate a method that uses 3D image registration in a focussed region with more severe disease. We hypothesized that this approach would provide a substantial increase in responsiveness to cartilage change from OA progression. An additional goal of the research was to validate a technique that could represent the first step in developing a tool that can be used to provide measures of OA progression for large numbers of patients.
Materials and Methods
Twenty-four subjects were randomly selected from the OAI Progression Cohort Data Set 0.1.1 and Image Releases 0.B.1 and 1.B.1. Conditions for inclusion were a Kellgren-Lawrence (KL) grade of 3 at baseline and additional criteria described in a separate publication (43). Three readers (TI, RB, and AW) used the software method to segment the medial compartment femur for the baseline and 24 month visits. Our study used the sagittal 3D dual-echo steady-state (DESS) (sagittal, 0.456 mm × 0.365 mm, 0.7 mm slice thickness, TR 16.5 ms, TE 4.7 ms. Reformatted to 0.365 mm × 0.365 mm × 0.7 mm) pulse sequence and the segmentation was performed paired but with the readers blinded to time point.
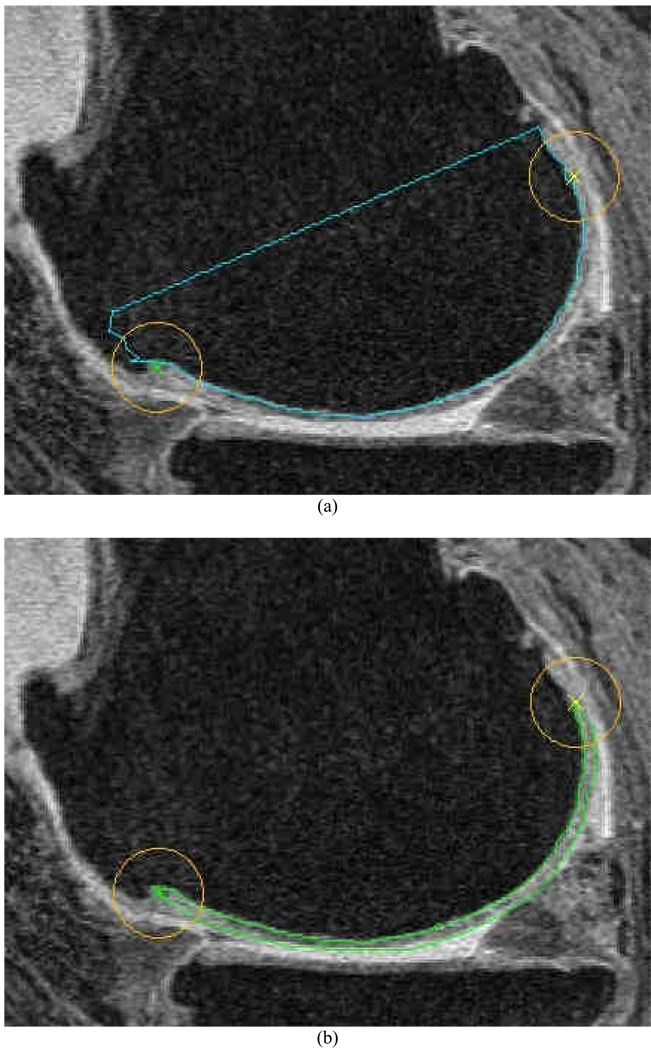
We used a previously documented semi-automated software method31 to perform the cartilage segmentation with several additional components described below. An initial segmentation of the bone-cartilage margins was first performed for each slice (Figure 1a) and was used to constrain the subsequent segmentation of the opposing cartilage-soft-tissue margin (Figure 1b). Once the bone-cartilage margin of the central slice had been segmented, the observer placed two points on the two "tips" of the cartilage on each slice. As shown in Figure 1, a 10 mm circular region around each of these points defines an “exclusion region” where the reader was instructed to ignore any inaccuracies in the segmentation. The use of the exclusion regions was employed to decrease the reader time by ignoring these locations where software failures are common and cartilage loss is less frequent. The reader then used the tool to segment the opposite free cartilage margin and the software initiated an automated active-contour algorithm to refine the segmentation (Figure 1b).
Figure 1.
(a) Example of segmentation of the bone-cartilage interface. The reader also places landmarks denoting the outer-most “tips” of the cartilage in the slice, which are used to constrain the segmentation. (b) shows an example of a segmented slice where the bone-cartilage margin is combined with the remainder of the cartilage border.
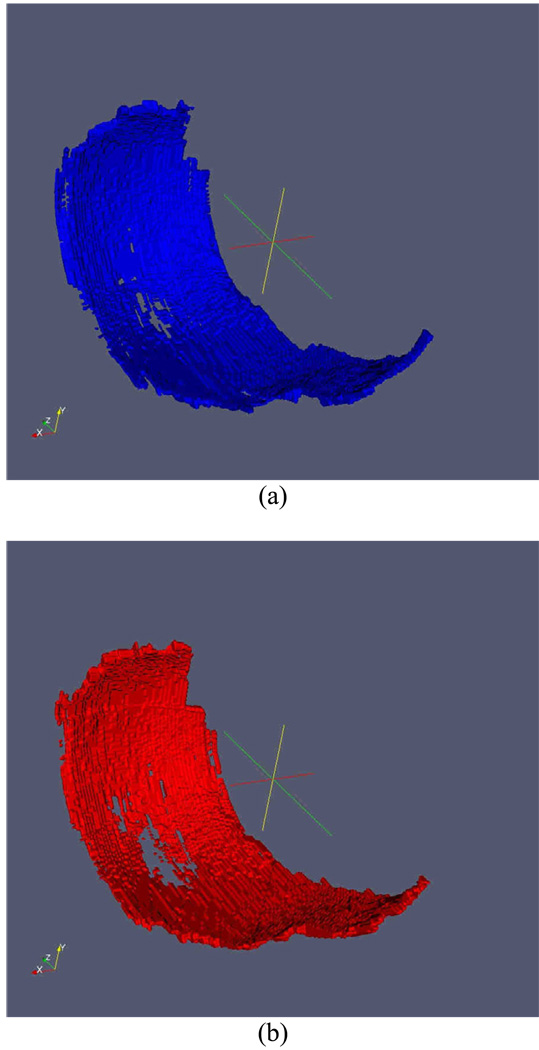
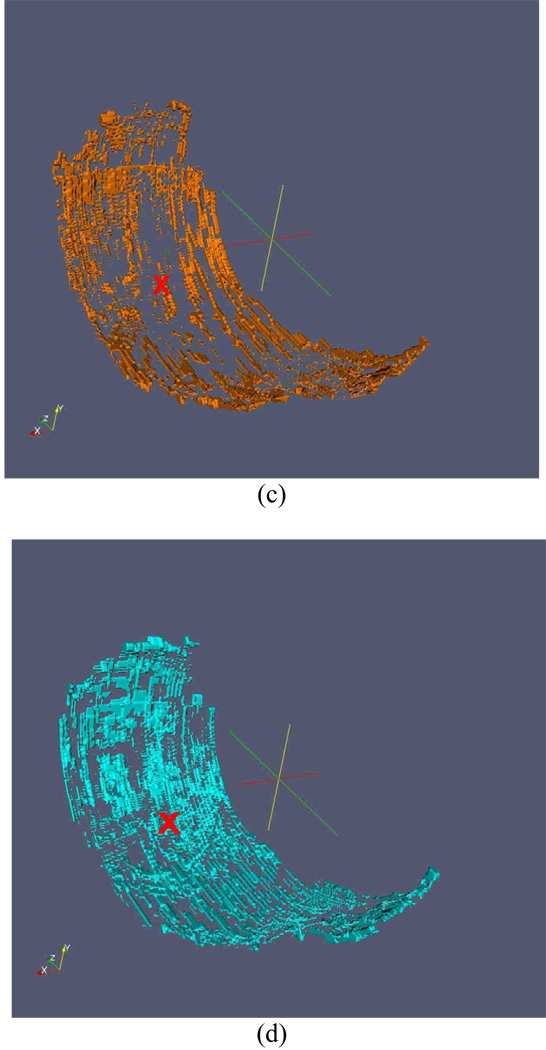
A radiologist (HY) independently marked the center of the largest cartilage lesion in the medial compartment for each subject on the 24-month visit MR image set. To avoid a bias towards a known area of thinning, this reader did not view the baseline data set and the images were evaluated using the eFilm (Merge Healthcare, Hartland, WI) software, and not the cartilage segmentation tool. This location was provided to the other readers on a randomly selected time point so as not to reveal the order of the scans. Once the segmentation was completed, the baseline (Figure 2a) and 24 month (Figure 2b) image sets were registered in three dimensions (3D) for each subject. Registration was performed on the 3D binary-segmented images by matching the voxels that were identified to be on the bone-cartilage interface according to a previously reported method38. After registration, 3D binary "gain" and "loss" images (Figure 2c and 2d) were produced consisting of voxel locations that contained cartilage on the baseline but not on the follow-up (loss) and those that did not contained cartilage on the baseline but did on the follow-up (gain).
Figure 2.
Example of 3D rendering of the baseline cartilage (a), follow-up cartilage (b), cartilage gained (c) and cartilage lost (d). (c) and (d) also show the indexed location as a red ‘×’.
The baseline to follow-up volume difference (ΔV) was calculated as the difference between loss and gain in a region centered on the location of thinning (red X on Figures 2c and 2d). ΔV was calculated for radii of 5.0 mm, 10.0 mm, 15.0 mm, 20.0 mm, 25.0 mm, 30.0 mm, 35.0 mm, and 40.0 mm, and for the total half plate. Responsiveness was measured using the average ΔV, the standard deviation of ΔV, the standardized response mean, (SRM) difference (Average of ΔV)/(Standard Deviation of ΔV), percent difference (ΔV)/(Average of V), and by calculating the percentage of subjects for which there was a net loss of cartilage.
Results
The results are given in Table 1. The SRM for the mean cartilage thickness was highest in magnitude for the 10 mm region (SRM=−1.21), while the lowest SRM was detected for total medial femoral condyle (SRM=−0.51). The smallest percent decrease was observed for the total medial femoral condyle (6.3%), while the largest was for the 5.0 mm region (22.6%). Based on the SRM and the percentage of knees with change (Columns 5 and 7), the results suggest an optimal region of 10 mm from the indexed point in which to measure the cartilage loss.
Table 1.
Responsiveness results. %Diff is defined by ratio of the Average Volume Change to the Average Volume. The 95% confidence intervals, based on 1000 bootstrap samples, are given for the SRM values.
| Region Diameter |
Average Volume (mm3) |
Average Volume Change (mm3) |
SD of Volume change (mm3) |
Standardized Response Means. 95% confidence intervals are given in parentheses. |
% Diff | Percentage of knees with a measured loss in cartilage volume |
|---|---|---|---|---|---|---|
| 5 mm | 118.3 | −26.7 | 34.7 | −0.77 (−1.24, −0.42) | −22.6 | 75% (18/24) |
| 10 mm | 527.1 | −106.8 | 88.6 | −1.21 (−1.84, −0.84) | −20.3 | 92% (22/24) |
| 15 mm | 1241.8 | −189.5 | 183.2 | −1.03 (−1.63, −0.65) | −15.3 | 92% (22/24) |
| 20 mm | 2015.3 | −231.6 | 306.8 | −0.76 (−1.35, −0.35) | −11.5 | 79% (19/24) |
| 25 mm | 2719.6 | −254.3 | 390.2 | −0.65 (−1.22, −0.27) | −9.4 | 79% (19/24) |
| 30 mm | 3280.2 | −272.3 | 481.6 | −0.57 (−1.16, −0.17) | −8.3 | 79% (19/24) |
| 35 mm | 3640.9 | −307.5 | 526.8 | −0.58 (−1.24, −0.15) | −8.4 | 83% (20/24) |
| 40 mm | 3981.7 | −325.0 | 566.1 | −0.57 (−1.26, −0.14) | −8.2 | 83% (20/24) |
| Half plate | 4874.9 | −307.6 | 600.9 | −0.51 (−1.22, −0.09) | −6.3 | 83% (20/24) |
Discussion
Results using our new technique demonstrate that measurement of cartilage volume loss in a local indexed region is more responsive than when assessed in larger areas and the total half plate. The data also suggest that there also may be an optimal region size of 10 mm in which to measure change. This is the first study to present the use of 3D registration for measuring cartilage change in located regions of the medial femoral condyle, and the effect of the size of the sampled region in the proximity of an indexed location.
A further advantage of the new method is the potential to dramatically reduce the reader time and cost necessary to perform the measurement. Segmentation of the bone-cartilage margin in the femur typically takes less than 10 minutes, and can be performed by a less skilled individual. Once this margin is delineated, the automated 3D registration step can take place without any additional reader time. For our current study, readers segmented the entire half plate as a comparison metric to understand the effect of sampling region size, and the reader time was similar to previously published results (40 minutes for the total femur) 31. For future studies or clinical trials it will be necessary to segment the free (non bone) cartilage margin only in the vicinity of the indexed location on each visit. With such an approach it is reasonable to suggest that a highly responsive cartilage volume measurement can be made in an average time of well under 15 minutes per knee for the trained reader. Based on this assumption, assessments for a two-visit study of 200 subjects could be made in less than three weeks of time by a single reader.
For this study, a single indexed location was identified for each medial compartment femur and we evaluated a set of individual fixed sampling regions that were applied equally to each knee. In practice it may be advantageous to determine custom sized sampling regions depending on the extent of the observed cartilage damage. We could also implement a method to measure multiple indexed locations for each cartilage plate. Such improvements may further increase the responsiveness of the technique.
Several other studies have investigated the change in cartilage morphometry in regions smaller than the total plate. These are generally done using standardized sub-regions that are defined using anatomical landmarks24–26, 36. Longitudinal studies have suggested that central FT sub-regions show more pronounced cartilage loss 24, 36. Hellio et al showed 6 months follow-up annualized cartilage loss in central medial FT sub-region was 3.7% (SRM −0.33) in KL grade 325. Wirth et al. showed that the rate of cartilage loss was greater in central sub-regions than the entire FT cartilage plates36. Pelletier et al. showed the cartilage loss with the rate of change of 12.4%, 12.0% and 4.4% and corresponding SRM values of 1.03, 1.04, and 0.56, in the anterior, central and posterior sub-regions of medial femoral condyle respectively24. Direct comparison with other studies is problematic as the patient and imaging characteristics are different, however our results compare quite favorably.
Our analysis is based on the assumption that cartilage should not gain in volume since there is not physiological basis for cartilage regeneration. Yet there is evidence for an increase in cartilage thickness in early stages (K/L grade of 2) of OA39. This cartilage thickening is due to the swelling or hypertrophy that can anticipate the cartilage atrophy. However this is a common issue with any studies that measure responsiveness, and the any volume gain can be considered part of the measurement noise, and a result of imperfections in the image acquisition and analysis methodologies.
Additional development of the core image-processing algorithm should enable further increase in the level of automation, thereby producing a quicker, more objective and robust tool, which is less influenced by reader bias. Furthermore, we plan to add features to the graphical user interface to permit segmentation of more than two visits of a longitudinal study, which will be beneficial to studies, such as the OAI, with many time points.
This study has limitations. The relatively small number of the subjects makes the results potentially influenced by outliers and it may be difficult to make definite conclusions without a higher-powered validation study. We observed the change in a population with the KLG grade of 3; studies with a more healthy population may demonstrate a reduced responsiveness. The methods will, most likely, perform differently for different levels and grades of initial cartilage thinning. The method was designed for focal cartilage thinning, which may not be common in cases with more generalized cartilage loss; for subjects with known generalized loss other methods may prove more responsive. However, since our test subjects were selected randomly, and not preferentially for knees with focal loss, this study provides a robust test of the methodology.
The goal of this study was to validate a method that has the potential to be used for studies with a large amount of MRI data. As it relies on a voxel by voxel baseline to follow-up comparison the method can not be used to measure cartilage thickness or surface area or to assess subjects cross-sectionally. The use of the “exclusion regions” improved the speed of analysis but also meant that cartilage thinning could not be measured at the outer edges of the femoral plate, however, these are regions where thinning is less likely. The method could include the full knee cartilage, if necessary, but with the reader time would be increased.
In conclusion, the results demonstrate that a more local measurement of cartilage change is superior to methods that use total plates or sub-regions. Furthermore we have also demonstrated that cartilage thinning can be observed using a method where only half, or potentially a much smaller fraction, of the femur cartilage is segmented. This technique has the potential to be a fast and highly effective tool to assess cartilage change for clinical studies of OA.
Acknowledgements
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), Grant R01AR056664 supported this work.
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation
Statistical support came from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Elders MJ. The increasing impact of arthritis on public health. J Rheumatol Suppl. 2000;60:6–8. [PubMed] [Google Scholar]
- 3.Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 4.Eckstein F, Westhoff J, Sittek H, Maag KP, Haubner M, Faber S, et al. In vivo reproducibility of three-dimensional cartilage volume and thickness measurements with MR imaging. AJR Am J Roentgenol. 1998;170:593–597. doi: 10.2214/ajr.170.3.9490936. [DOI] [PubMed] [Google Scholar]
- 5.Graichen H, von Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004;50:811–816. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 6.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 9.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)--inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102. doi: 10.1007/s00256-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 10.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein F, Buck RJ, Burstein D, Charles HC, Crim J, Hudelmaier M, et al. Precision of 3.0 Tesla quantitative magnetic resonance imaging of cartilage morphology in a multicentre clinical trial. Ann Rheum Dis. 2008;67:1683–1688. doi: 10.1136/ard.2007.076919. [DOI] [PubMed] [Google Scholar]
- 12.McWalter EJ, Wirth W, Siebert M, von Eisenhart-Rothe RM, Hudelmaier M, Wilson DR, et al. Use of novel interactive input devices for segmentation of articular cartilage from magnetic resonance images. Osteoarthritis Cartilage. 2005;13:48–53. doi: 10.1016/j.joca.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis Cartilage. 2003;11:725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Schnier M, Haubner M, Priebsch J, Glaser C, Englmeier KH, et al. Accuracy of cartilage volume and thickness measurements with magnetic resonance imaging. Clin Orthop Relat Res. 1998;352:137–148. [PubMed] [Google Scholar]
- 15.Piplani MA, Disler DG, McCauley TR, Holmes TJ, Cousins JP. Articular cartilage volume in the knee: semiautomated determination from three-dimensional reformations of MR images. Radiology. 1996;198:855–859. doi: 10.1148/radiology.198.3.8628883. [DOI] [PubMed] [Google Scholar]
- 16.Folkesson J, Dam E, Olsen OF, Pettersen P, Christiansen C. Automatic segmentation of the articular cartilage in knee MRI using a hierarchical multi-class classification scheme. Med Image Comput Comput Assist Interv. 2005;8:327–334. doi: 10.1007/11566465_41. [DOI] [PubMed] [Google Scholar]
- 17.Stammberger T, Eckstein F, Englmeier KH, Reiser M. Determination of 3D cartilage thickness data from MR imaging: computational method and reproducibility in the living. Magn Reson Med. 1999;41:529–536. doi: 10.1002/(sici)1522-2594(199903)41:3<529::aid-mrm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein F, Stammberger T, Priebsch J, Englmeier KH, Reiser M. Effect of gradient and section orientation on quantitative analysis of knee joint cartilage. J Magn Reson Imaging. 2000;11:469–470. doi: 10.1002/(sici)1522-2586(200004)11:4<469::aid-jmri16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis Cartilage. 1999;7:265–271. doi: 10.1053/joca.1998.0200. [DOI] [PubMed] [Google Scholar]
- 20.Kshirsagar AA, Watson PJ, Tyler JA, Hall LD. Measurement of localized cartilage volume and thickness of human knee joints by computer analysis of three-dimensional magnetic resonance images. Invest Radiol. 1998;33:289–299. doi: 10.1097/00004424-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Peterfy CG, van Dijke CF, Janzen DL, Glüer CC, Namba R, Majumdar S, et al. Quantification of articular cartilage in the knee with pulsed saturation transfer subtraction and fat-suppressed MR imaging: optimization and validation. Radiology. 1994;192:485–491. doi: 10.1148/radiology.192.2.8029420. [DOI] [PubMed] [Google Scholar]
- 22.Solloway S, Hutchinson CE, Waterton JC, Taylor CJ. The use of active shape models for making thickness measurements of articular cartilage from MR images. Magnetic resonance in medicin. 1997;37:943–952. doi: 10.1002/mrm.1910370620. [DOI] [PubMed] [Google Scholar]
- 23.Raynauld JP, Kauffmann C, Beaudoin G, Berthiaume MJ, de Guise JA, Bloch DA, et al. Reliability of a quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthritis Cartilage. 2003;11:351–360. doi: 10.1016/s1063-4584(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 24.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellio Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Subregional femorotibial cartilage morphology in women--comparison between healthy controls and participants with different grades of radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1177–1185. doi: 10.1016/j.joca.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 27.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68:349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum. 2004 50;:94–97. doi: 10.1002/art.11483. [DOI] [PubMed] [Google Scholar]
- 29.Bruyere O, Genant H, Kothari M, Zaim S, White D, Peterfy C, et al. Longitudinal study of magnetic resonance imaging and standard X-rays to assess disease progression in osteoarthritis. Osteoarthritis Cartilage. 2007;15:98–103. doi: 10.1016/j.joca.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14 Suppl A:A46–A75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Duryea J, Neumann G, Brem MH, Koh W, Noorbakhsh F, Jackson RD, et al. Novel fast semi-automated software to segment cartilage for knee MR acquisitions. Osteoarthritis Cartilage. 2007;15:487–492. doi: 10.1016/j.joca.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkesson J, Dam EB, Olsen OF, Pettersen PC, Christiansen C. Segmenting articular cartilage automatically using a voxel classification approach. IEEE Trans Med Imaging. 2007;26:106–115. doi: 10.1109/TMI.2006.886808. [DOI] [PubMed] [Google Scholar]
- 33.Kass M, Witkin A, Terzopoulos D. Snakes: Active contour models INTERNATIONAL JOURNAL OF COMPUTER VISION. 1988;1:321–331. [Google Scholar]
- 34.Lynch JA, Zaim S, Zhao J, Stork A, Peterfy CG, Genent HK. Cartilage segmentation of 3D MRI scans of the osteoarthritic knee combining user knowledge and active contours. SPIE proceedings series. 2000;3979:925–935. [Google Scholar]
- 35.Brem MH, Lang P, Neumann G, Schlechtweg PM, Schneider E, Jackson R, et al. Magnetic resonance image segmentation using semi-automated software for quantification of knee articular cartilage---initial evaluation of a technique for paired scans. Skeletal Radiol. 2009;38:505–511. doi: 10.1007/s00256-009-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Does the use of ordered values of subregional change in cartilage thickness improve the detection of disease progression in longitudinal studies of osteoarthritis? Arthritis Rheum. 2009;61:917–924. doi: 10.1002/art.24613. [DOI] [PubMed] [Google Scholar]
- 38.Duryea J, Magalnick M, Alli S, Yao L, Wilson M, Goldbach-Mansky R. Semiautomated three-dimensional segmentation software to quantify carpal bone volume changes on wrist CT scans for arthritis assessment. Med Phys. 2008;35:2321–2330. doi: 10.1118/1.2900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Summers GC, Merrill A, Sharif M, Adams MA. Swelling of articular cartilage depends on the integrity of adjacent cartilage and bone. Biorheology. 2008;45:365–374. [PubMed] [Google Scholar]