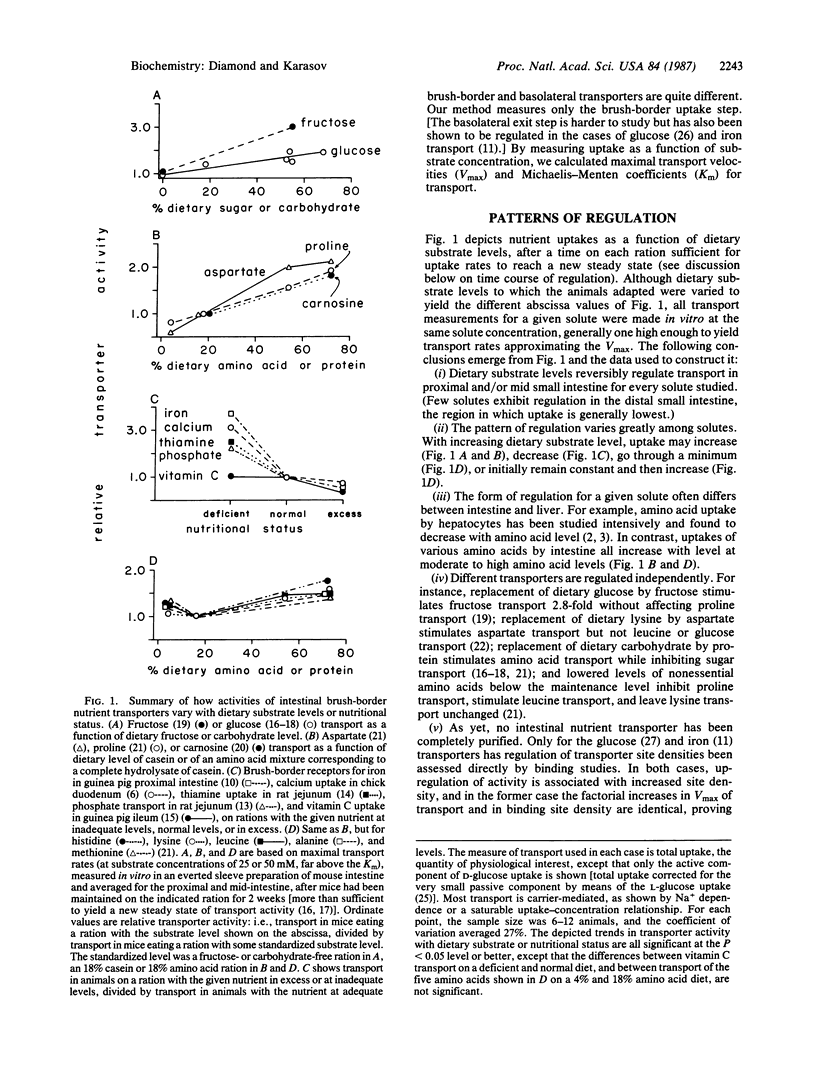
Abstract
Because most eukaryotic somatic cells are bathed in a constant internal milieu, most of their proteins are constitutive, unlike the adaptive enzymes of bacteria. However, intestinal mucosal cells, like bacteria, face a varying milieu. Hence, we tested for adaptive regulation of intestinal nutrient transporters, sought its functional significance, and compared it with regulation of bacterial proteins. All 12 transporters studied proved to be regulated by dietary substrate levels. Regulation in the intestine is slower than in bacteria and shows lower peak-to-basal activity levels. Regulatory patterns vary greatly among transporters: two sugars and two nonessential amino acids monotonically up-regulate their transporters, two vitamins and three minerals monotonically down-regulate their transporters, and two transporters of essential amino acids respond nonmonotonically to levels of their substrates. These varied patterns arise from trade-offs among four factors: transporter costs, calories yielded by metabolizable substrates, fixed daily requirements of essential nutrients, and toxicity of certain nutrients in large amounts. Based on these trade-offs, we predict the form of regulatory pattern for intestinal transporters not yet studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. H. Calcium bioavailability and absorption: a review. Am J Clin Nutr. 1982 Apr;35(4):783–808. doi: 10.1093/ajcn/35.4.783. [DOI] [PubMed] [Google Scholar]
- Charlton R. W., Bothwell T. H. Iron absorption. Annu Rev Med. 1983;34:55–68. doi: 10.1146/annurev.me.34.020183.000415. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H., Cary C., Enders D., Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984 Apr;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Why do disused proteins become genetically lost or repressed? Nature. 1986 Jun 5;321(6070):565–566. doi: 10.1038/321565a0. [DOI] [PubMed] [Google Scholar]
- Favus M. J. Factors that influence absorption and secretion of calcium in the small intestine and colon. Am J Physiol. 1985 Feb;248(2 Pt 1):G147–G157. doi: 10.1152/ajpgi.1985.248.2.G147. [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. M. Use of phlorizin binding to demonstrate induction of intestinal glucose transporters. J Membr Biol. 1986;94(1):77–82. doi: 10.1007/BF01901015. [DOI] [PubMed] [Google Scholar]
- Harper A. E., Benevenga N. J., Wohlhueter R. M. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev. 1970 Jul;50(3):428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Diamond J. M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am J Physiol. 1983 Oct;245(4):G443–G462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Pond R. S., 3rd, Solberg D. H., Diamond J. M. Regulation of proline and glucose transport in mouse intestine by dietary substrate levels. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7674–7677. doi: 10.1073/pnas.80.24.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov W. H., Solberg D. H., Chang S. D., Hughes M., Stein E. D., Diamond J. M. Is intestinal transport of sugars and amino acids subject to critical-period programming? Am J Physiol. 1985 Dec;249(6 Pt 1):G770–G785. doi: 10.1152/ajpgi.1985.249.6.G770. [DOI] [PubMed] [Google Scholar]
- Karasov W., Solberg D., Carter S., Hughes M., Phan D., Zollman F., Diamond J. Uptake pathways for amino acids in mouse intestine. Am J Physiol. 1986 Oct;251(4 Pt 1):G501–G508. doi: 10.1152/ajpgi.1986.251.4.G501. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Han H. P., Barber E. F., Chiles T. C. Adaptive regulation of neutral amino acid transport System A in rat H4 hepatoma cells. J Cell Physiol. 1985 Feb;122(2):290–298. doi: 10.1002/jcp.1041220219. [DOI] [PubMed] [Google Scholar]
- Kimber C. L., Mukherjee T., Deller D. J. In vitro iron attachment to the intestinal brush border. Effect of iron stores and other environmental factors. Am J Dig Dis. 1973 Sep;18(9):781–791. doi: 10.1007/BF01070848. [DOI] [PubMed] [Google Scholar]
- Lee D. B., Walling M. W., Brautbar N. Intestinal phosphate absorption: influence of vitamin D and non-vitamin D factors. Am J Physiol. 1986 Mar;250(3 Pt 1):G369–G373. doi: 10.1152/ajpgi.1986.250.3.G369. [DOI] [PubMed] [Google Scholar]
- Linder M. C., Munro H. N. The mechanism of iron absorption and its regulation. Fed Proc. 1977 Jun;36(7):2017–2023. [PubMed] [Google Scholar]
- Maenz D. D., Cheeseman C. I. Effect of hyperglycemia on D-glucose transport across the brush-border and basolateral membrane of rat small intestine. Biochim Biophys Acta. 1986 Aug 21;860(2):277–285. doi: 10.1016/0005-2736(86)90524-9. [DOI] [PubMed] [Google Scholar]
- Morrissey R. L., Wasserman R. H. Calcium absorption and calcium-binding protein in chicks on differing calcium and phosphorus intakes. Am J Physiol. 1971 May;220(5):1509–1515. doi: 10.1152/ajplegacy.1971.220.5.1509. [DOI] [PubMed] [Google Scholar]
- Patrini C., Cusaro G., Ferrari G., Rindi G. Thiamin transport by rat small intestine "in vitro": influence of endogenous thiamin content of jejunal tissue. Acta Vitaminol Enzymol. 1981;3(1):17–26. [PubMed] [Google Scholar]
- Rose R. C., Nahrwold D. L. Intestinal ascorbic acid transport following diets of high or low ascorbic acid content. Int J Vitam Nutr Res. 1978;48(4):382–386. [PubMed] [Google Scholar]
- Rose R. C. Water-soluble vitamin absorption in intestine. Annu Rev Physiol. 1980;42:157–171. doi: 10.1146/annurev.ph.42.030180.001105. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Swaminathan R., Sommerville B. A., Care A. D. The effect of dietary calcium on the activity of 25-hydroxycholecalciferol-1-hydroxylase and Ca absorption in vitamin D-replete chicks. Br J Nutr. 1977 Jul;38(1):47–54. doi: 10.1079/bjn19770060. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Brindak M. E., Meyer S. A., Fullmer C. S. Evidence for multiple effects of vitamin D3 on calcium absorption: response of rachitic chicks, with or without partial vitamin D3 repletion, to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7939–7943. doi: 10.1073/pnas.79.24.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffram S., Scharrer E. Effect of feeding a high protein diet on amino acid uptake into rat intestinal brush border membrane vesicles. Pflugers Arch. 1984 Jan;400(1):34–39. doi: 10.1007/BF00670533. [DOI] [PubMed] [Google Scholar]



