Abstract
The developmental mechanisms regulating cell differentiation and patterning during the secondary growth of woody tissues are poorly understood. Class III HD ZIP transcription factors are evolutionarily ancient and play fundamental roles in various aspects of plant development. Here we investigate the role of a Class III HD ZIP transcription factor, POPCORONA, during secondary growth of woody stems. Transgenic Populus (poplar) trees expressing either a miRNA-resistant POPCORONA or a synthetic miRNA targeting POPCORONA were used to infer function of POPCORONA during secondary growth. Whole plant, histological, and gene expression changes were compared for transgenic and wild-type control plants. Synthetic miRNA knock down of POPCORONA results in abnormal lignification in cells of the pith, while overexpression of a miRNA-resistant POPCORONA results in delayed lignification of xylem and phloem fibers during secondary growth. POPCORONA misexpression also results in coordinated changes in expression of genes within a previously described transcriptional network regulating cell differentiation and cell wall biosynthesis, and hormone-related genes associated with fiber differentiation. POPCORONA illustrates another function of Class III HD ZIPs: regulating cell differentiation during secondary growth.
Introduction
Secondary vascular development involves the coordination of several developmental processes, including the patterning of secondary vascular tissues and differentiation of complex cell types [1]. In the model forest tree genus Populus, the cambium is typically believed to contain a single layer of initials, from which xylem mother cells are derived towards the inside (towards the pith) of the stem and phloem mother cells towards the outside (towards the epidermis) [2]. Xylem and phloem mother cells divide one or more times before differentiating into cell types within the secondary xylem (wood) or secondary phloem (inner bark), respectively. Conspicuous, lignified phloem fibers differentiate in the periphery of the phloem. In addition to these vertically oriented tissue systems, the cambium contains ray initials, which ultimately produce rays that transverse secondary xylem and phloem and transport water and solutes radially in the stem. The resulting woody stem is thus the result of coordination between radial patterning processes that produce tissues in appropriate positions, and differentiation of secondary vascular cell types within those tissues.
Although still poorly defined, insights into mechanisms regulating cell differentiation during secondary growth are emerging, and have been assisted by the observation that at least some of the key regulatory genes that regulate shoot apical meristems and primary vascular development are also expressed during secondary growth [3], [4], [5]. For example, Class I KNOX genes are well characterized for their roles in regulating stems cells and cell differentiation in shoot apical meristems, but they also play important roles in negatively regulating the differentiation of cambial and cambial daughter cells [6], [7]. In contrast, NAC-domain containing transcription factors have been identified that promote differentiation of vessel elements including VASCULAR-RELATED NAC-DOMAIN (VND), NAC SECONDARY CELL WALL THICKENING (NST), and SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN (SND) proteins, likely through direct regulation of MYB-class transcription factors that in turn regulate expression of cell differentiation and cell wall-related genes {reviewed in [8]}. For example, overexpression of VND6 or VND7 results in ectopic differentiation of vessel elements in Arabidopsis thaliana, which is accompanied by overexpression of MYB genes (including MYB46, MYB63, MYB83, MYB85, and MYB103) and downstream genes encoding enzymes involved in cell wall biosynthesis and vessel differentiation (including CESA4/IRX5, CESA7/IRX3, CESA8/IRX1, IRX8, IRX10, CCoAOMT7, IRX12/LAC4, and XCP1) [9]. However, this putative transcriptional network has not been evaluated during secondary growth.
While putative genes and mechanisms regulating cell differentiation are becoming better described, less is known about regulation of tissue patterning during secondary growth. In Arabidopsis thaliana, the Class III HD ZIPs comprise a small family of five genes, PHABULOSA (PHB), PHAVOLUTA (PHV), REVOLUTA (REV), ATHB8, and CORONA/ATHB15 (CNA). Combinations of loss of function mutants, gain of function mutants, and cross-complementation studies indicate overlapping yet distinct roles for A. thaliana Class III HD ZIPs [10], [11], [12], [13]. Functional differences among Class III HD ZIPs are likely attributable both to differences in expression patterns, as well as differences in protein function [10]. PHB, PHV, and REV form a subclade, and have been implicated in acting antagonistically with KANADI transcription factors to regulate polarity and patterning of lateral organs and vascular bundles [12], [13], [14], [15], [16], [17]. CORONA/ATHB15/INCURVATA4 (CNA) and ATHB8 form the second subclade of A. thaliana Class III HD ZIPs. ATHB8 is expressed in procambial cells in embryos and developing organs, and ATHB8 expression is induced by exogenous auxin [18]. Overexpression of ATHB8 is associated with precocious differentiation of xylem and lignification of cell types that are normally not lignified [19]. All known land plant Class III HD ZIPs contain a binding site for negative regulation posttranscriptionally by miRNA165/166 [20]. Mutations that abolish the miRNA binding sequence without changing amino acid sequence result in dominant phenotypes for Class III HD ZIPs [12], [13], [21], [22], [23], [24].
CNA is expressed in provascular cells of leaves and roots [25], in the shoot meristem, in floral meristems, and in ovules [11]. A Zinnia elegans ortholog of CNA, ZeHB-13, is expressed in leaf provascular cells and developing xylem parenchyma of leaf vascular bundles, but is not expressed in mature leaves [25]. In A. thaliana, overexpression of a miRNA-resistant ATHB15 results in moderate dwarfing, upcurling of leaves, and drastic reduction in xylem and lignified interfascicular tissues [21]. Similar phenotypes are seen in mutants carrying semidominant alleles of CNA with a single point mutation abolishing normal miRNA regulation [26], [27], [28]. Antisense ATHB15 transgenics are severely dwarfed, and display expansion of xylem and interfascicular tissues, and lignification into the pith of stems [21]. In contrast, EMS-induced cna mutants show slightly increased shoot apical meristem size [11] but no other dramatic phenotypes [11], [27], and RNAi suppression of CNA transcripts was reported not to result in obvious phenotypic changes [27]. Interestingly, cna is a dramatic enhancer of meristem size in clavata1/2/3 mutants, indicating that CNA may function with CLV genes to promote organ formation [11]. However, neither the genes directly regulated by CNA nor the biological functions influenced by CNA have been identified, and CNA function during secondary growth in plants has not been examined.
Class III HD ZIP transcription factors are evolutionarily ancient and found in all major lineages of land plants [17], [20], [29]. Importantly, Class III HD ZIPs predate evolution of vascular tissues and adaxial polarity of lateral organs [29], suggesting that many of the functions assigned to Class III HD ZIPs in angiosperms are derived. Ancestral functions of Class III HD ZIPs could include regulation of apical or meristematic growth [20], [29], and auxin transport [30]. While Class III HD ZIPs roles during secondary growth have not been characterized, they are known to be expressed during secondary growth from microarray analysis of wood forming tissues in Populus [5], [31], [32]. Class III HD ZIPs are thus candidates for regulating fundamental developmental processes acquired during the evolution of secondary growth.
We report here the cloning and characterization of a Populus CNA ortholog, POPCORONA (PCN). We defined roles for PCN during secondary growth by examining PCN expression and mutant phenotypes in transgenic Populus expressing either an artificial miRNA targeting PCN transcripts or overexpressing a miRNA-resistant form of PCN. Together, our results suggest that PCN regulates development of secondary vascular tissues, potentially through regulation of transcriptional modules associated with cell differentiation and/or hormone-mediated processes.
Results
POPCORONA encodes a Class III HD ZIP transcription factor
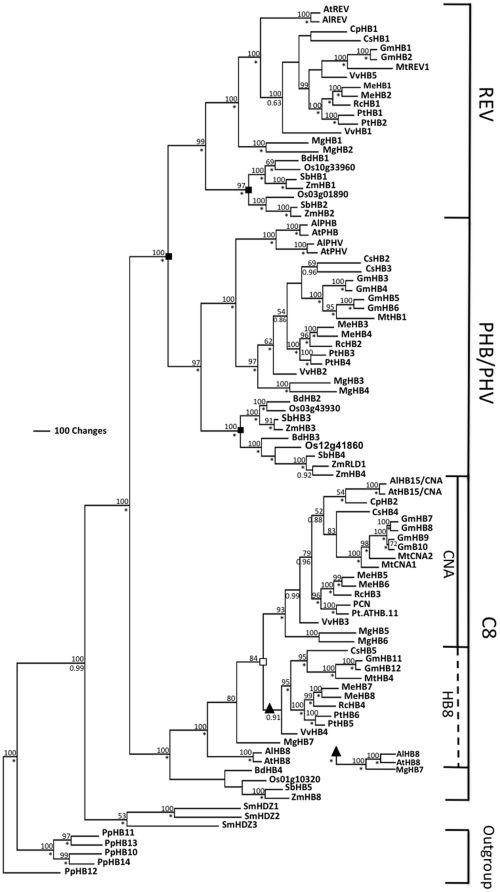
The POPCORONA (PCN) gene (also called Pt-ATHB.12; Joint Genome Institute Populus v.1.1 gene model fgenesh4_pm.C_LG_I000560; Phytozome Populus v2.0 gene model POPTR_0001s18930: GenBank XM_002299699) was amplified from cDNA of the sequenced Populus trichocarpa individual [33] based on similarity to the Arabidopsis thaliana ATHB15/CORONA (Materials and Methods). To determine the relationship of PCN to other Class III HD ZIPs, a phylogenetic analysis of Class III HD ZIP sequences from whole-genome sequencing projects was undertaken (Materials and Methods). Maximum parsimony analysis from the nucleotide sequence alignment of the coding sequences of Class III HD ZIPs found in 17 plant species and deduced from amino acid sequence alignments yielded a single tree, with clade support from Bayesian and bootstrap analysis (Fig. 1). Sequences from Physcomitrella patens, a moss, were used as outgroups to root the tree. Sequences from the lycophyte representative, Selanginella moellendorffii, formed a clade (53% bootstrap, 1.00 Bayesian) sister to sequences from the angiosperm taxa, which formed a strongly supported (100% bootstrap, 1.00 Bayesian) clade. These results all reflect previously reported Class III HD ZIP gene family relationships [17], [20]. Among the angiosperm sequences, three strongly supported clades were formed, REV, PHB/PHV, and C8, which is in agreement with previous reports [17]. REV and PHB/PHV clades are sister to each other forming a larger clade that is sister to C8. Relationships within each of the three clades are generally consistent with currently accepted ideas about angiosperm phylogeny [34]. Our analysis includes four representative species from the grass lineage; O. sativus, Z. mays, B. distachyon, and S. bicolor, allowing further details in the divergence of homologues within this monocot lineage. Sequences from these four species form monophyletic clades separate from the eudicot species. Within these clades two duplication events appear to have occurred after the monocot-eudicot split. This duplication likely represents the grass whole genome duplication event [35], [36].
Figure 1. Phylogenetic relationships among Class III HD ZIP gene family in land plants determined using maximum parsimony analysis.
Bootstrap support values above 50% are presented above branches and Bayesian support values above 0.50 are presented below branches, where * indicates maximum 1.00 support. Black squares indicate major duplication events, while the empty square represents evidence of a duplication event without bootstrap support. Major clades are presented by longitudinal lines to the right of the tree, where solid lines represent fully supported monophyletic clades (PHB, C8, and CNA) and dashed lines indicate clade supported by Bayesian, but not bootstrap support. Black triangles represent where the AtHB8, AlHB8, MgHB7 clade is supported according to Bayesian analysis. Species abbreviations: At, Arabidopsis thaliana; Bd, Brachypodium distachyon; Al, Arabidopsis lyrata; Cp, Carica papaya; Cs, Cucumis sativus; Gm, Glycine max; Me, Manihot esculenta; Mt, Medicago truncatula; Mg, Mimulus guttatus; Os, Oryzas sativa; Pt, Populus trichocarpa; Pp, Physcomitrella patens; Rc, Ricinus communis; Sm, Selanginella moellendorffii, Sb, Sorghum bicolor; Vv, Vitis vinifera; Zm, Zea mays.
The major duplication events previously reported are mostly in agreement with our phylogenetic tree, with a few exceptions. Our phylogeny does not include representatives of species within the ferns, basal angiosperms, or gymnosperms, and therefore our analysis cannot dispute or support duplication events that were suggested previously to have occurred in these lineages. In our parsimony tree (Fig. 1), bootstrap supports AtHB8 and AlHB8 (100% bootstrap), and MgHB7 (80% bootstrap) as sister to the rest of the CNA/HB8 eudicot clade, while Bayesian analysis supports them within the HB8 clade (1.00 Bayesian). Supporting AtHB8, AlHB8, and MgHB7 within the HB8 clade is evidence for a duplication event resulting in the HB8 and CNA clades, as previously reported (Prigge and Clark, 2006). Therefore, our parsimony and Bayesian analysis are not in agreement with their placement, and only partially support previous suggestions of a duplication event in C8 clade. This inconsistency may be an artifact of limited and uneven sampling, as discussed above.
Within Populus, each Class III HD ZIP is represented by two paralogs (Fig. 1), reflecting the genome duplication event whose signature was found in analysis of the P. trichocarpa genome [33]. Independent duplication events are present in the PHV/PHB clade in the A. thaliana and Populus lineages (Fig. 1). Two paralogs of A. thaliana ATHB15/CNA are present, including PCN and Pt-ATHB.11 (Phytozome Populus v2.0 gene model POPTR_0003s04860; Joint Genome Institute Populus v1.1 gene model estExt_fgenesh4_pg.C_LG_III0436).
PCN is expressed during secondary growth
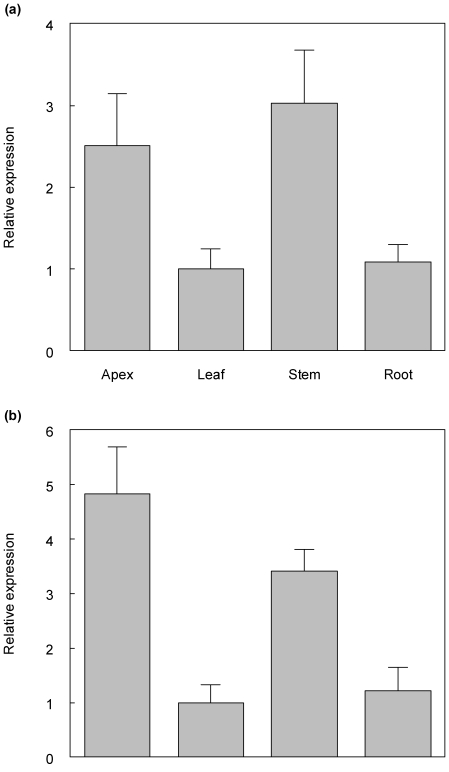
PCN is expressed in shoot apices, leaves, stems, and roots of young Populus plants. Transcript levels were determined for organs of tissue culture-grown Populus alba x tremula using quantitative real time PCR (Materials and Methods). Primers amplifying PCN transcripts revealed highest expression in stems and apices, with lower but significant expression in leaves and roots (Fig. 2a). Primers amplifying a PCN paralog, Pt-ATHB.11, showed similar expression patterns for this gene, although expression was slightly higher in apices relative to stem tissues (Fig. 2b).
Figure 2. Expression of PCN (Fig. 2a) and PCN paralog Pt-ATHB.11 (Fig. 2b) in organs, as assayed by Quantitative Real Time PCR.
Relative expression of PCN and paralog Pt-ATHB.11 in apices, leaves, roots, and stem was determined using Quantitative Real Time PCR (QRT-PCR) of two month old tissue culture grown Populus tremula x alba. PCN and paralog Pt-ATHB.11 are expressed in all tissues assayed, and are highly expressed in shoot apexes and stem tissue with active cambium. Stem tissue samples were confirmed to have a vascular cambium by phloroglucinol staining of secondary xylem. Relative expression (Mean ± SE) was calculated from triplicate QRT-PCR reactions of independent RNA samples prepared from different trees.
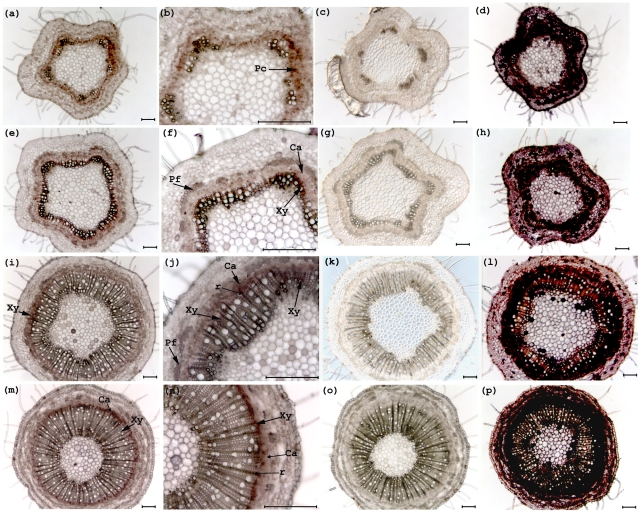
PCN is expressed broadly in the cambial zone and xylem of Populus shoots. Whole mount in situ hybridization was used to visualize PCN expression in tangential sections from different developmental stages of Populus stems (Materials and Methods). During primary growth and the transition to secondary growth, PCN is expressed broadly in the cambial zone and in developing xylem (Fig. 3a,b). Later in development, phloem fiber differentiation becomes evident (Fig. 3e,f) and weak signal is seen outside of the cambial zone at this stage in the developing phloem, including the phloem fibers (Fig. 3e,f). Moving further down the stem into developmentally older tissues, PCN expression is maintained within developing xylem, and is most pronounced in rays (Fig. 3 i,j). At the base of the stem, strongest expression is found in the cambial zone, with reduced expression in the secondary xylem (Fig. 3m,n). However, expression is still pronounced within the rays traversing the secondary xylem (Fig. 3n). In comparison to sense-probe negative controls (Fig. 3c,g,k,o), the in situ staining of experimental sections with the PCN antisense probe is specific and has relatively low background. However, based on comparison of PCN antisense and sense negative control sections we cannot exclude the possibility of low PCN expression, cross hybridization to related genes, or diffusion of the stain in epidermis, pith cells, or other tissues/cell types. Differential staining caused by differences in cytoplasmic density of cell types is revealed by an antisense probe for a presumably ubiquitously expressed gene encoding a 50S ribosomal protein (Fig. 3d,h,l,p).
Figure 3. Expression of PCN during Populus stem development revealed by whole mount in situ hybridization.
Antisense PCN (first and second columns), sense negative control (third column), and positive control (fourth column) probes were hybridized to stem sections from two month old tissue culture grown trees. (a) Section from first elongating internode hybridized with antisense PCN probe. PCN is expressed broadly during primary growth, with strongest expression associated with procambium. (b) Higher magnification of first elongating internode hybridized with antisense PCN probe. (c) Section from first elongating internode hybridized with sense PCN probe (negative control), showing minimal background hybridization. (d) Section from first elongating internode hybridized with antisense pop50S probe (positive control). (e) Section from the fourth internode, hybridized with antisense PCN probe. PCN is expressed broadly in the cambial zone, and strongly in differentiating xylem. (f) Higher magnification of (e). (g) Section from the fourth internode hybridized with negative control sense PCN probe. (h) Section from fourth internode hybridized with positive control antisense pop50S probe. (i) Section from seventh internode hybridized with antisense PCN probe. PCN expression is mostly associated with differentiating xylem cells and lightly in cambial zone. (j) Higher magnification of (i). (k) Section from seventh internode hybridized with sense PCN probe (negative control). (l) Section from seventh internode hybridized with positive control antisense pop50S probe. (m) Section from the base internode hybridized with antisense PCN probe. PCN expression is largely limited to the differentiating xylem cells and cambial zone. (n) Higher magnification of (m). (o) Section from the base internode hybridized with sense PCN probe (negative control). (p) Section from the base internode hybridized with positive control antisense pop50S probe. Cambial zone (Ca), Phloem fiber (Pf), Procambium (Pc), Ray (r), Xylem (Xy), Bar = 100 µm.
Misregulation of PCN results in whole plant phenotypes
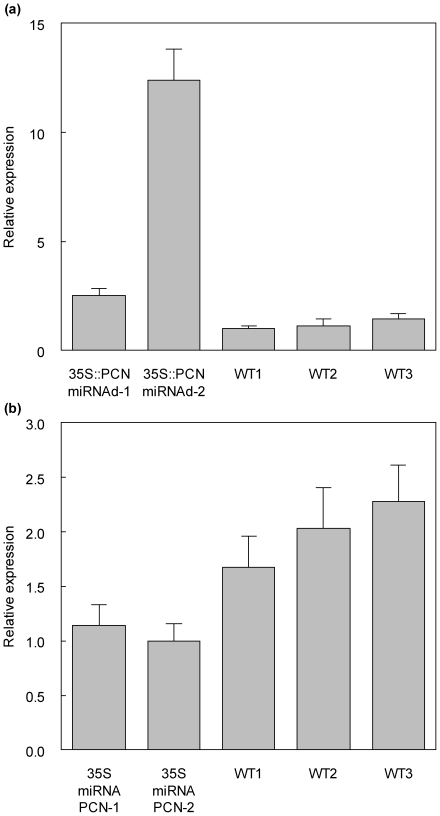
To examine the function of PCN during plant development, the Populus clone INRA 717-IB4 (Populus alba x P. tremula) was transformed with recombinant DNA constructs to either up or down-regulate PCN transcript levels. To upregulate PCN transcript levels, a PCN cDNA was modified to change the miRNA165/166 recognition sequence without changing the amino acid sequence when translated into protein, thus making the transcript transparent to negative regulation by miRNA165/166 (Materials and Methods). This cDNA was cloned behind a 35S cauliflower mosaic virus promoter in a T-DNA vector to create construct 35S::PCN-miRNAd, and transformed into Populus using an Agrobacterium-based method (Materials and Methods). To down-regulate PCN transcripts levels, an artificial miRNA [37], [38] was designed to target both PCN and its paralog, Pt-ATHB.11, and cloned behind the 35S promoter in a T-DNA vector to create construct 35S::miRNA-PCN, which was then transformed into Populus (Materials and Methods). Two plants independently transformed with 35S::PCN-miRNAd, and two plants independently transformed with 35S::miRNA-PCN were recovered and used in the analyses here. Quantitative Reverse Transcriptase Polymerase Chain Reaction (Q-RT-PCR) analysis of the transformants and wild-type controls showed that for plants transformed with 35S::PCN-miRNAd, one line (35S::PCN-miRNAd-4-1) had approximately two-fold increase of PCN transcript abundance relative to controls, while line 35S::PCN-miRNAd-4-3 had over twelve-fold increase in PCN transcript abundance (Fig. 4a). Only modest downregulation of PCN transcript abundance was found for artificial miRNA lines (up to approximately two-fold reduction in line 35S::miRNA-PCN-3-4), indicating the artificial miRNA was only partially effective in reducing PCN transcripts (Fig. 4b).
Figure 4. PCN expression levels in PCN 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN knockdown transgenic plants relative to wild-type controls.
PCN expression levels were detected by Quantitative Real Time PCR (Materials and Methods). Relative expression levels (mean ± SE) were calculated from triplicate qRT-PCR reactions of independent RNA samples for each transgenic and the wild-type prepared from different batches of two month-old plants. T test (P<0.05) comparison showed significant differences of expression in all transgenics compared to the wild-types. (a) Comparison PCN transcripts in wild-type and 35S::PCN-miRNAd gain of function plants. (b) Comparison PCN transcripts in wild-type and 35S::miRNA-PCN plants.
Compared with matched wild-type controls (Fig. 5a), 35S::PCN-miRNAd-4-3 plants have shorter internodes, darker green leaves, and large stipules (Fig. 5b). The mutants did not present any obvious polarity defects, barren axils, or root phenotypes. Plants transformed with 35S::miRNA-PCN did not show any consistent whole plant phenotypes in culture, perhaps reflecting that the modest decrease in transcript abundance for PCN and its paralog in these plants, or that loss of PCN function may not result in a strong phenotype.
Figure 5. Phenotypes of PCN 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN knockdown plants compared to wild-type controls.
(a) Wild-type plants (2 months old). (b) PCN 35S::PCN-miRNAd gain of function (2 months old) plants have changes to plant architecture, shorter plants length, darker green color in leaf. (c) 35S::miRNA-PCN knockdown plants (2 month old) have no strong differences from the wild-type. Bar = 2.5cm.
Misregulation of PCN alters secondary growth
Wild-type Populus make a gradual transition from primary to secondary growth. Under our culture conditions, wild-type Populus clone INRA 717-IB4 (Populus alba x P. tremula) is already transitioning to secondary growth by the fourth elongating internode from the apex, as seen in stem cross sections stained with toluidine blue (Fig. 6). By the seventh internode, secondary xylem characterized by cell files derived from the cambial initials is apparent (Fig. 6a). Nascent phloem fibers are apparent by the seventh node but are not yet highly lignified. At the base of the stem, cell files of lignified secondary xylem can be seen emanating from the cambial zone, and fully differentiated and lignified phloem fibers are seen at the periphery of the phloem (Fig. 6c). At lower magnification (Fig. 6d), the radial organization of the stem is seen, with successive layers of pith, secondary xylem, cambial zone, secondary phloem and phloem fibers, cortex, and epidermis.
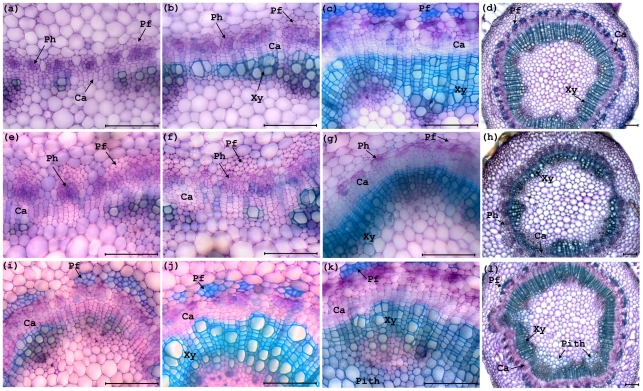
Figure 6. Transverse sections of stems from two month old wild-type and 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN knockdown Populus.
(a) Section from fourth internode of wild-type Populus stem during primary growth. (b) Section from seventh internode of wild-type Populus stem during transition to secondary growth, showing secondary xylem tissue formation. (c) Section from bottom internode of wild-type Populus stem showing secondary phloem fibers and secondary xylem tissue. (d) Lower magnification view of bottom internode of wild-type stem. (e) Section from fourth internode of 35S::PCN-miRNAd gain of function Populus stem during primary growth, showing increased cambium cell layers. (f) Section from seventh internode of 35S::PCN-miRNAd gain of function Populus stem during transition to secondary growth, showing delayed secondary xylem formation. (g) Section from bottom internode of 35S::PCN-miRNAd gain of function Populus stem showing no lignified phloem fibers formation and decreased xylem tissue. (h) Lower magnification of section from bottom internode of 35S::PCN-miRNAd gain of function Populus stem showing no lignified phloem fibers formation and decreased xylem tissue. (i) Section from fourth internode of 35S::miRNA-PCN knockdown Populus stem showing early formed lignified phloem fibers and xylem cells by comparing with the wild-type. (j) Section from seventh internode of 35S::miRNA-PCN knockdown Populus stem showing increased secondary phloem fibers and xylem tissue formation by comparing with the wild-type. (k) Section from bottom internode of 35S::miRNA-PCN knockdown Populus stem showing ectopic lignifications in pith cells. (l) Lower magnification of section from bottom internode of 35S::miRNA-PCN knockdown Populus stem showing ectopic lignifications in pith cells. Cambial zone (Ca), Phloem (Ph), Phloem fiber (Pf), Xylem (Xy), Bar = 100 µm.
In 35S::PCN-miRNAd-4-3 plants, cell files within the cambial zone are more readily apparent than in wild-type control plants at the fourth internode (Fig. 6e). The premature appearance of differentiating phloem fibers also distinguishes these stems from the wild-type, although the fibers are not lignified (Fig. 6e). Internode seven of 35S::PCN-miRNAd-4-3 plants is characterized by a distinct cambial zone, modest secondary xylem, and unlignified cells presumed to be differentiating phloem fibers (Fig. 6f). A section from the base of the stem shows relatively well-developed secondary xylem, but is largely lacking the lignified phloem fibers that conspicuously occur in the periphery of the phloem of wild-type plants. Thus, although phloem fiber differentiation appears to initiate prematurely in 35S::PCN-miRNAd plants, they do not complete normal differentiation and lignification.
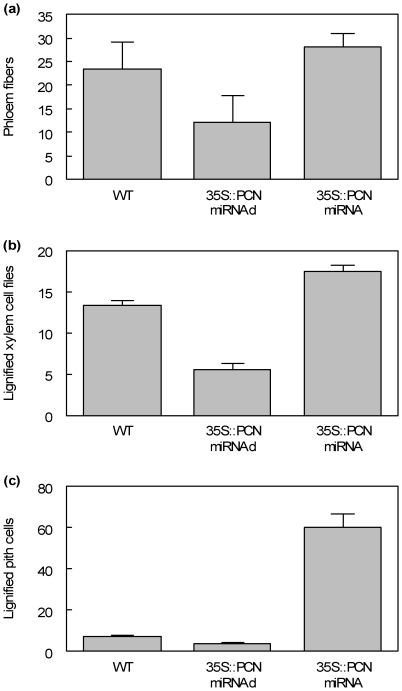
As mentioned, 35S::miRNA-PCN plants had only a modest reduction in PCN transcript abundance (Fig. 4), and no whole plant phenotype was apparent (Fig. 5). However, cross sections revealed subtle defects in stem development. At the fourth internode from the apex, lignified phloem fibers are already apparent in 35S::miRNA-PCN plants, while they are lacking at this stage of development in the wild-type (Fig. 6I). Also at this position, cambium activity is evident as cell files within the cambial zone, and modest amounts of secondary xylem have formed. By internode seven, 35S::miRNA-PCN plants have more abundant secondary and noticeably more highly lignified phloem fibers than the wild-type (Fig. 6j). Sections through the bottom of the stem are similar to the wild-type, except that there is abnormal lignification of some cells within the pith (Fig. 6 k,l). These abnormally lignified cells are primarily found adjacent to the position of primary vascular bundles (Fig. 6k,l). In summary, misexpression or down-regulation of PCN results in differences in the number of phloem fibers, number of xylem cell layers, and number of lignified pith cells, as quantified in Fig. 7.
Figure 7. Quantification of phenotypes in bottom internode of 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN knockdown transgenics.
(a) Comparison of number of phloem fibers in the bottom internodes of wild-type, 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN. (b) Comparison of number of lignified xylem cell layers in the bottom internodes of wild-type, 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN. (c) Comparison of number of lignified pith cells in the bottom internodes of wild-type, 35S::PCN-miRNAd gain of function and 35S::miRNA-PCN. Relative expression levels (mean ± SE) were calculated from three cross-sections of the bottom internodes of three independent wild type plants, three miRNAd gain of function transgenics, three 35S::PCN-miRNAd transgenics prepared from different batches of two month-old plants.
Misregulation of PCN changes expression of genes associated with gene regulation, hormones and cell differentiation
To begin to understand the biological processes and genes regulated directly or indirectly by PCN during secondary growth, global transcript abundance was assayed using microarrays for stems from wild-type control and 35S::PCN-miRNAd-4-3 plants (Materials and Methods). RNA was isolated from stems of four plants each of 35S::PCN-miRNAd-4-3 and matched wild-type controls (Materials and Methods). The RNA quality and quantity was assayed with Agilent Bioanalyzer before being labeled and hybridized to Affymetrix Populus whole transcriptomes gene chips (Materials and Methods). The resulting data were normalized and analyzed using dChip software (http://biosun1.harvard.edu/complab/dchip/) to identify genes with statistically differential expression between the wild-type and 35S::PCN-miRNAd stems (Materials and Methods). Data are available as accession GSE19467 through NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/).
Of the approximately 45,000 genes in the Populus genome [33], 237 genes showed statistically significant two-fold or greater differential transcript abundance between the wild-type and 35S::PCN-miRNAd stems (see Supplemental Table S3 for analysis of all probes with statistically different transcript levels). Of these genes, those belonging to three biological categories (transcription, hormone-related, and cell wall-related) are discussed here in more detail with the idea that they are potentially associated with the function of PCN and the phenotype of PCN transgenics. It should be noted that presumably only a subset of these genes may be direct targets of PCN.
Sixteen genes encoding transcription factors from ten different families are misregulated in 35S::PCN-miRNAd stems (Table 1). Interestingly, this group includes Populus orthologs of A. thaliana transcription factors that have been implicated in a transcriptional network controlling vascular cell differentiation and lignification. SECONDARY CELL WALL-ASSOCIATED NAC DOMAIN (SND) and VASCULAR RELATED NAC DOMAIN (VND) proteins have been shown to positively regulate expression of specific MYB transcription factors [9], [39], [40], [41]. In 35S::PCN-miRNAd stems, transcript levels of Populus orthologs of NAC7/VND4 (gw1.III.864.1) and SND2 (eugene3.01240095) are upregulated, as are orthologs of previously proposed VND/SND targets MYB83 (gw1.IX.3293.1), MYB85 (gw1.IX.3293.1), MYB52 (eugene3.00120054) [9], [39]. Additionally, a Populus ortholog (fgenesh4_pm.C_scaffold_66000095) of XYLEM ENDOPEPDIDASE 1 (XCP1) as well as categories (cellulose synthesis-related, lignin-related) and specific orthologs of cell wall-related genes (including IRREGULAR XYLEM3 and IRX8 orthologs) that are targets of these MYB transcription factors [39] are misregulated in 35S::PCN-miRNAd stems (Table 2).
Table 1. Transcription factors mis-regulated in PCN transgenics.
| JGI Gene Accessiona | Fold Changeb | Arabidopsisc | Definition Lined |
| grail3.0041013401 | 4.25 | AT1G71692 | AGAMOUS-like 12 (AGL12) |
| gw1.IX.3293.1 | 4.16 | AT3G08500 | MYB domain protein 83 (MYB83) |
| gw1.III.861.1 | 4.01 | AT4G22680 | MYB domain protein 85 (MYB85) |
| eugene3.00700057 | 3.62 | AT2G30580 | putative C3HC4 zinc finger protein |
| gw1.III.864.1 | 3.47 | AT1G12260 | NAM protein, NAC7/VND4 |
| eugene3.00120054 | 3.36 | AT1G17950 | MYB domain protein 52 (MYB52) |
| *grail3.0001068602 | 3.24 | AT1G10200 | transcription factor LIM |
| estExt_fgenesh4_pg.C_570199 | 2.99 | AT4G36740 | A. thaliana homeobox protein 40 (ATHB40) |
| gw1.V.991.1 | 2.96 | AT3G22830 | heat shock transcription factor A6B (HSFA6B) |
| eugene3.01310019 | 2.7 | AT1G09540 | MYB domain protein 61 (MYB61) |
| eugene3.01240095 | 2.65 | AT4G28500 | NAM protein, SND2 |
| gw1.II.2517.1 | −2.83 | AT3G60530 | GATA transcription factor 4 (GATA4) |
| gw1.XI.848.1 | −3.26 | AT1G01030 | DNA-binding protein |
| fgenesh4_pg.C_LG_XIV000644 | −4.28 | AT1G67030 | zinc finger protein (ZFP6) |
| *gw1.I.8677.1 | −4.96 | AT4G23750 | AP2 domain transcription factor, CRF2/TMO3 |
| estExt_Genewise1_v1.C_LG_XIV3374 | −7.1 | AT4G22070 | WRKY DNA-binding protein 31 (WRKY31) |
Accession number assigned by the Joint Genome Institute (http://genome.jgi-psf.org).
Fold Change is expressed as the ratio of gene expression in PCN gain of function transgenenics to wild type control.
Accession number of the best Arabidopsis BLAST return using the JGI gene model as query.
Definition line is from the Arabidopsis accession at TAIR (http://www.arabidopsis.org).
* Transcript level differences confirmed by qRT-PCR.
Table 2. Genes up or down-regulated in PCN transgenics involved in hormone related processes.
| JGI Gene Accessiona | Fold Changeb | Arabidopsisc | Definition Lined |
| *fgenesh4_pm.C_LG_II000702 | 4.67 | AT4G25420 | gibberellin 20-oxidase |
| *grail3.0050017401 | 3.78 | AT3G62100 | auxin-responsive protein |
| *estExt_fgenesh4_pg.C_LG_XI0670 | 3.16 | AT1G78440 | gibberellin 2-oxidase |
| *grail3.0061005101 | 3.08 | AT3G62100 | auxin-responsive protein |
| gw1.VI.2253.1 | −2.41 | AT5G21482 | putative cytokinin oxidase |
| estExt_Genewise1_v1.C_LG_X3745 | −3.12 | AT3G16770 | ethylene-response element binding protein |
JGI gene accession refers to the accession number assigned by the Joint Genome Institute (http://genome.jgi-psf.org).
Fold Change is expressed as the ratio of gene expression in PCN gain of function transgenenics to wild type control.
Arabidopsis refers to the accession number of the best Arabidopsis BLAST return using the JGI gene model as query.
Definition line is from the Arabidopsis accession at TAIR (http://www.arabidopsis.org).
* Transcript level differences confirmed by qRT-PCR.
Nineteen cell wall-related genes have altered transcript levels in 35S::PCN-miRNAd (Table 2). Four of the eight cell wall-related genes with increased transcript levels are involved in cellulose biosynthesis, including the previously mentioned orthologs of IRX3 and IRX8 as well as orthologs of CESA1/RSW1 (grail3.0052005901) and CSLA9/RAT4 (estExt_fgenesh4_pm.C_LG_VIII0087). The remaining eleven wall-related genes have lower transcript levels in 35S::PCN-miRNAd and participate in functions including lignification (putative laccase, cinnamoyl CoA reductase, chalcone synthase), and pectin biosynthesis and modification (putative pectin methylesterases and pectinesterase).
Six hormone-related genes are misregulated in 35S::PCN-miRNAd (Table 3). Two putative auxin-responsive genes (grail3.0050017401, grail3.0061005101) show elevated transcript levels in 35S::PCN-miRNAd. While two gibberellin-related genes have higher transcript levels in 35S::PCN-miRNAd, one (GA 20-oxidase, fgenesh4_pm.C_LG_II000702) is putatively involved in GA biosynthesis [42] while the other (GA 2-oxidase, estExt_fgenesh4_pg.C_LG_XI0670) is putatively involved in GA catabolism [43]. Transcript abundance for a gene encoding a putative cytokinin oxidase (gw1.VI.2253.1) involved in inactivation of cytokinin [44] and a gene encoding a cytokinin response factor (Table 1, gw1.I.8677.1) are lower in 35S::PCN-miRNAd. Auxin, cytokinin, ethylene, and gibberellins have been implicated in various studies as regulators of vascular development and secondary growth, although their precise roles remain uncertain [1].
Table 3. Genes up or down-regulated in PCN transgenics involved in cell wall synthesis related processes.
| JGI Gene Accessiona | Fold Changeb | Arabidopsisc | Definition Lined |
| *estExt_Genewise1_v1.C_LG_VI2188 | 3.76 | AT5G17420 | cellulose synthase, CESA7/IRREGULAR XYLEM 3 (IRX3) |
| estExt_fgenesh4_pm.C_LG_VIII0087 | 3.43 | AT5G03760 | cellulose synthase like, CSLA9/RAT4 |
| grail3.0052005901 | 3.35 | AT4G32410 | cellulose synthase, CESA1/RADIALLY SWOLLEN1 (RSW1) |
| eugene3.00111083 | 3.10 | AT5G54690 | galacturonosyltransferase, IRX8 |
| gw1.XV.2531.1 | 2.89 | AT5G61750 | germin-like protein-like |
| fgenesh4_pg.C_LG_I003312 | 2.82 | AT1G23460 | polygalacturonase |
| gw1.VII.534.1 | 2.72 | AT4G28380 | extensin-like protein |
| gw1.VI.781.1 | 2.44 | AT4G30420 | nodulin-like protein; MtN21 gene product |
| gw1.XIII.2619.1 | −2.52 | AT2G23910 | cinnamoyl CoA reductase-like |
| gw1.131.165.1 | −2.55 | AT1G56710 | polygalacturonase |
| grail3.0048017501 | −2.61 | AT1G03870 | fasciclin-like arabinogalactan 9 (FLA9) |
| gw1.856.4.1 | −2.83 | AT3G22142 | proline-rich cell wall protein |
| estExt_Genewise1_v1.C_LG_VII1401 | −2.97 | AT5G09760 | pectin methylesterase-like protein |
| gw1.VIII.2100.1 | −3.37 | AT5G05390 | LACCASE 12 (LAC12) |
| eugene3.00140920 | −3.55 | AT5G13930 | chalcone synthase, TRANSPARENT TESTA 4 |
| estExt_fgenesh4_pm.C_1480004 | −3.78 | AT2G44480 | glycosyl hydrolase family 1 |
| fgenesh4_pg.C_LG_V000014 | −3.95 | AT5G09760 | pectin methylesterase-like protein |
| gw1.X.3259.1 | −4.32 | AT3G10720 | pectinesterase |
| gw1.III.2711.1 | −6.6 | AT1G62500 | putative proline-rich cell wall protein |
Accession number assigned to the assayed gene model by the Joint Genome Institute (http://genome.jgi-psf.org).
Fold Change is expressed as the ratio of gene expression in PCN gain of function transgenenics to wild type control.
Accession number of the best Arabidopsis BLAST return using the JGI gene model as query.
Definition line is from the Arabidopsis accession at TAIR (http://www.arabidopsis.org).
* Transcript level differences confirmed by qRT-PCR.
Discussion
Class III HD ZIP transcription factors have been implicated in regulating diverse developmental processes, but their function during secondary vascular development has not been addressed. In addition, the genes and cellular processes regulated by Class III HD ZIPs are poorly understood. We examined here the expression and function of a Populus Class III HD ZIP, POPCORONA (PCN), during secondary growth and correlated PCN expression with changes in anatomy and gene expression that are consistent with a role in influencing cell differentiation.
Class III HD ZIPs are best characterized in Arabidopsis thaliana, where they have been shown to be involved in meristem initiation and function, polarity of lateral organs, and vascular development. Interestingly, among the Class III HD ZIPs in A. thaliana, only REVOLUTA and CORONA (CNA) present loss of function phenotypes [10], [11], [45], [46], [47], [48]. The cna loss of function phenotype is subtle, and largely limited to a slightly increased meristem size [11]. In A. thaliana, CNA is among the earliest expressed markers of vascular development [11], [25], and is expressed in procambial cells in leaves, shoot apical meristems, floral meristems, stamens, and carpels [11], [25]. A zinnia ortholog of CNA, ZeHB-13, is also expressed in procambial cells of developing leaves, but is not expressed at detectable levels in mature leaves [25]. In addition, ZeHB-13 is expressed in tracheary elements differentiating in vitro, but is not directly induced by cytokinin or auxin in that system [25]. We found that PCN is not restricted to provascular cells or primary xylem in Populus stems during primary growth. In Populus, PCN is also expressed during secondary growth, with strongest expression in phloem fibers, the cambial zone, developing xylem, and potentially pith (Figure 3). Interestingly, PCN expression is maintained in older secondary xylem, and appears to be associated with rays (Figure 3). The observation that PCN expression is maintained in secondary vascular tissues but not primary vasculature of leaves could reflect differences among species, but more likely reflects differences among these tissue types. Specifically, cambial and ray tissues are not present in primary vasculature of leaves.
To examine the function of PCN, we created transgenics expressing either an artificial miRNA targeting PCN transcripts (35S::miRNA-PCN) or a PCN cDNA with the endogenous miRNA recognition site mutated (35S::PCN-miRNAd). 35S::miRNA-PCN plants showed a subtle phenotype, which includes precocious differentiation of phloem fibers, and abnormal lignification of pith cells in proximity to primary vascular bundles (Figure 6). This subtle phenotype likely reflects the observation that the artificial miRNA was only partially effective in reducing PCN transcript levels, but could also reflect functional redundancy with other family members as has been described for A. thaliana Class III HD ZIPs [10]. While the A. thaliana cna loss of function phenotype is subtle [11], A. thaliana plants expressing an antisense CNA transgene present a strong phenotype that includes dwarfing, expansion of xylem and interfascicular tissues, and lignification into the pith (Kim et al., 2005). Possible explanations for the discrepancy between these phenotypes include that cna alleles characterized may not be complete loss of CNA function, or that the sense transgene affected expression of multiple Class III HD ZIP family members.
Populus overexpressing a miRNA-resistant (non-cleavable) PCN (35S::PCN-miRNAd) had stronger stem phenotypic changes, with delayed differentiation of secondary xylem and severe reduction of phloem fiber differentiation (Figure 6). Cells consistent with nascent phloem fibers form in appropriate positions in 35S::PCN-miRNAd plants, but fail to complete differentiation or fully lignify. Phloem fibers could be considered abaxial in their position within the stem, as their position is analogous to and continuous with the abaxial phloem of primary vascular tissues in leaves. Although one possible interpretation of this phenotype is that overexpression of PCN promoted adaxialization of the stem affecting phloem fiber development, we do not favor this interpretation, in part because phloem fibers initiate in an appropriate position, and simply fail to properly complete differentiation. Additional evidence supporting primary affects of PCN on cell differentiation vs. patterning was provided by gene expression profiling of PCN mutant stems. Genes associated with the biosynthesis, response, or catabolism of auxin, gibberellin, and cytokinin were misregulated in 35S::PCN-miRNAd stems (Table 3). In addition, an AP2-like transcription factor orthologous to Cytokinin Response Factor 2 (CRF2) is downregulated in 35S::PCN-miRNAd stems. CRF2 is upregulated by cytokinin, and acts with other CRFs to regulate a large fraction of genes involved in cytokinin response [49]. Interestingly, these same hormones have all been implicated in fiber differentiation, with auxin and gibberellin flowing basipitally through the stem from leaves [50], [51], and cytokinin from roots [52]. Indeed, mutations in the related A. thaliana Class III HD ZIP REVOLUTA/INTERFASCICULAR FIBERLESS 1 can result in defects in interfascicular fiber differentiation [47], which are associated with polar auxin transport defects in stems [48]. These results are also consistent with the implication of Class III HD ZIP genes in affecting expression of PIN1, an auxin efflux transporter, during embryogenesis in A. thaliana [30]. Thus, we favor the interpretation that PCN phenotypes do not reflect patterning or polarity defects, but more likely hormone-related defects and changes in genes involved in cell differentiation and cell wall biosynthesis, as discussed below.
In addition to hormone-related genes discussed above, other transcription factors as well as cell wall biosynthetic genes are misexpressed in 35S::PCN-miRNAd stems (Table 1, 2). Interestingly, these genes include homologs of SECONDARY CELL WALL-ASSOCIATED NAC DOMAIN (SND), VASCULAR RELATED NAC DOMAIN (VND), and MYBs that have been implicated in a hierarchical pathway regulating genes involved with tracheary element development and cell wall synthesis and lignification [9], [39], [40], [41]. Cell differentiation and cell wall-related genes associated with these transcriptional regulators also show misregulation in 35S::PCN-miRNAd (see Results). The changes in cell wall–related genes, including downregulation of lignin-related genes in 35S::PCN-miRNAd stems are consistent with anatomical phenotypes, which include inhibition of phloem fiber lignification in 35S::PCN-miRNAd and abnormal lignification of cells in the pith of 35S::miRNA-PCN. Importantly, it should be noted that the two SND/VND genes misexpressed in 35S::PCN-miRNAd are orthologous to SND2 and NAC7/VND4, not the better characterized SND1 and VND6&7 [8], and the expression relationships of MYBs to putative targets is altered. Thus, in addition to differences expected between species and tissue types, the relationships among the transcriptional networks underlying secondary growth in Populus and primary growth in A. thaliana will require further study to comprehensively compare.
Given that Class III HD ZIPs are evolutionarily ancient and predate evolution of vascular tissues [29], the function of PCN during secondary growth must be derived. PCN and its orthologs in A. thaliana and Zinnia [11], [17], [25] are not uniquely expressed during secondary vascular development, and the proteins’ biochemical functions are presumably the same in the different tissues where it is expressed. However, it is possible that target genes of PCN vary between tissues, because of differences in available partners for dimerization, dominant negative LITTLE-ZIPPERs [53], [54], different co-factors, or differences in regulation by miRNA165/166 in different tissues. Although beyond the scope of the work presented here, it will ultimately be very informative to examine changes in gene expression for PCN or related mutants in the various tissues in which these genes are normally expressed. Unfortunately there are no published reports of gene expression profiling for A. thaliana CNA mutants to date. An example of a potentially important difference for PCN/CNA function among tissues is illustrated by the observation that cna mutations dramatically enhance CLAVATA (CLV) mutant phenotypes in A. thaliana, and result in misexpression of CLV3 and WUSCHEL [11]. Interestingly, microarray profiling of gene expression in Populus stems undergoing secondary growth revealed that Populus orthologs of CLV3 and WUS are not expressed during secondary growth [5]. Although PCN may interact with other family members related to WUS and CLV3 in the cambium, this nonetheless indicates significant differences for PCN function during primary and secondary growth.
Materials and Methods
Phylogenetic Analysis of Class III HD Zips
Gene sequences from the Class III HD Zips gene family were recovered from species that have complete genome sequence using the BLAST function in phytozome.net (Table S1). Genome databases searched included Arabidopsis lyrata, Brachypodium distachyon, Cucumis sativus, Glycine max, Manihot esculenta, Mimulus guttatus, Ricinus communis, Selanginella moellendorffii, Zea mays Arabidopsis thaliana, Carica papaya, Clamydomonas reinhardtii, Medicago truncatula, Oryzas sativa, Populus trichocarpa, Physcomitrella patens, Sorghum bicolor, and Vitis vinifera. 94 unique sequences were found from the 18 complete species genomes. The sequences were aligned with known Class III HD Zips from previous work for identification (Prigge & Clark, 2006; Floyd et al., 2006). For Z. mays three additional sequences were found and in M. truncatula one additional sequence was identified. POPCORONA (PCN) is variously known as Pt-ATHB.12; Joint Genome Institute Populus v.1.1 gene model fgenesh4_pm.C_LG_I000560: Phytozome Populus v2.0 gene model POPTR_0001s18930.
Nucleotide sequences were translated into amino acid sequences using DAMBE [55]. Amino acid alignment was achieved using ClustalX (version 1.8) [56] with all default settings. The alignment was modified manually using MacClade 3.08 [57] and exported in NEXUS format for further analysis with the final alignment containing 3261 sites. A nucleotide alignment was created based on the amino acid alignment using DAMBE [55].
Maximum parsimony analysis of the nucleotide alignment was performed using PAUP* version 4.0b10 for Macintosh [58], with heuristic searches using the TBR branch-swapping algorithm and 1000 random taxon addition replicates; the maxtrees setting was allowed to increase automatically as necessary. Gaps within the alignment were treated as missing data. Relative support for clades was assessed using 1000 bootstrap replicates with 10 random taxon addition replicates per bootstrap replicate and maxtrees capped at 100. Bayesian analyses, using the GTRIG model of sequence evolution as selected by MrAIC [59], were implemented in MrBayes 3.1.1 [60]. Double analyses were run with four chains for 1,000,000 generations, sampling every 100 generations. Trees from the first 250,000 generations (2,500 trees) from each run were discarded as burn-in, following the authors’ recommendations and consistent with the observation that the likelihood scores from both runs had stabilized. The sampled trees from both analyses were pooled and majority-rule consensus trees were constructed from the resulting 15,000 trees in order to estimate Bayesian clade credibility values.
Plant Cultivation and Transformation
Hybrid aspen clone INRA 717-IB4 (Populus alba × P. tremula) was used for all experiments. Plants were propagated and transformed using previously published methods [61]. Two independently transformed lines were used for PCN gain of function analysis (35S::PCN-miRNAd 2-1 and 35S::PCN-miRNAd 2-3). Two independently transformed lines were used for PCN knock down analysis (35S::miRNA-PCN 6-1 and 35S::miRNA-PCN 6-4). All experiments were repeated at least twice using each of the above transformed lines and matched wild-type controls with similar results, unless otherwise stated.
Whole Mount in situ Hybridization
Whole mount in situ hybridization was performed as previously described [7]. A 220-bp fragment from the 5′ end of the PCN coding region and a 292-bp fragment from the 5′ end of the Pop50S coding region were selected to design primers to generate the template of probes using the gene-specific primers:. PtCN-F:5′CTTCTGGTTGTTGCGTTATAC-3′; PtCN-R:5′ CTCGGGCCATTTTGAGTATTT-3′. Pop50S-F:5′CCTAGTGTTCCTGTAACTCGCATTGG-3′; Pop50S-R:5′CTCCCACCACCATGTTGTCCGTAAGTG-3′. T7 promoter sequence 5′-TAATACGACTCACTATAGGG was added to the 5′ end of the PCN-R primer and Pop50S-R primer sequence to generate templates of antisense probes for PCN and pop50S. T7 promoter sequence was added to the 5′ end of PCN-F primer sequence and Pop50S-F primer sequence to generate templates of sense probes for PCN and pop50S.
Recombinant DNA Constructs
The overexpression of Class III HD Zip genes is not expected to yield strong phenotypes, as these genes contain a microRNA binding site which negatively regulates transcript levels. To prevent this mechanism masking the effects of overexpression of the gene of interest, the microRNA binding site of PCN was changed to abolish miRNA recognition while leaving the protein sequence unchanged, as previously described [13]. The coding sequence of PCN was amplified with PCR primers which replaced base pairs in the microRNA binding site PCN_F 5′-CTGGAATGAAGCCTGGaCCaGATTCCAG-3′ and PCN_R 5′-GCAACGATTCCACTGGAATCtGGtCCA-3′ and sub-cloned into vector ART7 to make the entry clone ART7-PCN. The insert was recombined into ART27 to generate 35S::PCN-miRNAd.
The 35S::miRNA-PCN construct was assembled to drive expression of a synthetic miRNA by the 35S promoter. A 21 nucleotide sequence (5′ TTGCGTTATACTTCTGTTTTA 3′) specific to PCN and its paralog, Pt-ATHB.11 (Phytozome Populus v2.0 gene model POPTR_0003s04860; Joint Genome Institute Populus v1.1 gene model estExt_fgenesh4_pg.C_LG_III0436) was targeted based on published targeting parameters [62], [63], [64] and uniqueness to PCN and its paralog, Pt-ATHB.11. The exact complementary sequence (miRNA) is 5′ TAAAACAGAAGTATAACGCAA. Mismatches were introduced at positions four, nine, and ten, and in positions 20 and 21 in the complementary strand, to produce the following miRNA and miRNA* sequences.
miRNA: 5′ TAAAACAGAAGTATAACGCAA 3′
miRNA*: 5′ ACGCGTTATACAACTGTATTA 3′
A DNA was synthesized with these sequences replacing the normal miRNA and miRNA* sequences within MIR164b [62] to produce the following sequence containing Xho and Xba restriction sites at the 5’ and 3’ ends, respectively. The sequence CACC was added at 5’ end for directional cloning:
Xho-Xba MIR-PCN
5’CACCCTCGAGGAGAATGATGAAGGTGTGTGATGAGCAAGATAAAACAGA
AGTATAACGCAATTACTAGCTCATATATACACTCTCACCACAAATGCGTGTAT
ATATGCGGAATTTTGTGATATAGATGTGTGTGTGTGTTGAGTGTGATGATATG
GATGAGTTAGTTCACGCGTTATACAACTGTATTATCATGACCACTCCACCTTG
GTGACGATGACGACGAGGGTTCAAGTGTTACGCACGTGGGAATATACTTATA
TCGATAAACACACACGTGCGTCTAGA3’
Microarray Analysis and Q-RT-PCR
For each genotype, three bulks of three plants each were combined for RNA isolation from shoot apices, leaves, stem, and root tissue of two-month-old tissue culture trees for microarray analysis and Q-RT-PCR. Affymetrix GeneChip® Poplar Genome Array oligonucleotide microarrays were used for all microarray hybridizations. For microarray hybridizations, total RNA was isolated from entire, defoliated stems of two months old tissue culture grown plants. Three independent biological replicate RNAs were isolated for each of three overexpression and miRNA lines, and four independent biological replicates of matched wild-type controls. Total RNA quantity and quality was determined using an Agilent Bioanalyzer (Agilent Technologies, USA). Biotin labeling of target RNAs was performed with one-cycle target kit (One-Cycle Target Labeling and Control Reagents, Affymetrix, P/N 900493), and hybridized according to the manufacturer’s protocol.
Analysis of microarray data was performed with dChip (http://biosun1.harvard.edu/complab/dchip/). Data were normalized across arrays using median probe intensity of the baseline array. Preliminary analysis established filtering and statistical cutoff thresholds, using as criteria the false discovery rate, the identification of biologically meaningful genes, and the inclusion of genes confirmed as being misexpressed by Q-RT-PCR as primary criteria. In the final analysis, model-based expression data were filtered by removing genes whose representative probes did not exceed > = 40% (presence call %) on a given array and > = 50% among arrays. Filtered genes were then compared based on fold expression difference and t-test (p-value of 0.05). False discovery rate was determined by 50 permutations to be 2.4% in the final analysis. MIAME-compliant information about samples, array platform, microarray data and further details of the samples are available through NCBI GEO (GSE19467). Output from microarray analysis is shown at the probe-level in Table S2 (and includes probes designed against Populus ESTs), and for P. trichocarpa gene models in Table S3.
Gene expression differences estimated by microarray analysis were confirmed using qRT-PCR for the indicated genes in Tables 1, 2 and 3. Gene-specific PCR primers were designed to target genes showing differential expression in the microarray comparison of 35S::PCN-miRNAd, 35S::miRNA-PCN and wild-type trees. Primers with Tm of >59°C were designed to produce a product 200-300bp. A tubulin-encoding gene (JGI accession estExt_fgenesh4_pm.C_LG_III0736) was used as reference gene for Q-RT-PCR. Q-RT-PCR was performed with an MJ Mini Opticon (BioRad) following the manufacturer’s protocols.
Supporting Information
Gene sequences from the Class III HD Zips gene family recovered from species that have complete genome sequence using the BLAST function in phytozome.net.
(TXT)
Output from microarray analysis at the probe-level, including probes designed against Populus spp. ESTs.
(XLS)
Output from microarray analysis at the level of P. trichocarpa gene models, excluding probes designed against Populus spp. ESTs.
(XLS)
Acknowledgments
We thank Gayle Dupper for creating and maintaining all transgenic lines, Maichi Phan for assisting with laboratory work and plant photography, and Dan Potter for guidance in phylogenetic analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by DOE grant DE-A102-05ER64115 to AG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Groover A, Nieminen K, Helariutta Y, Mansfield SD. Wood formation in Populus. 2010. in, Genetics and Genomics of Populus Jansson, Bhalerao, and Groover (eds) Springer.
- 2.Larson PR. The Vascular Cambium. 1994. Springer-Verlag.
- 3.Groover AT. What genes make a tree a tree? Trends in Plant Science. 2005;10:210–214. doi: 10.1016/j.tplants.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Spicer R, Groover A. The evolution of development of the vascular cambium and secondary growth. New Phytol. 2010;186:577–592. doi: 10.1111/j.1469-8137.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- 5.Schrader J, Nilsson J, Mellerowicz E, Berglund A, Nilsson P, et al. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell. 2004;16:2278–2292. doi: 10.1105/tpc.104.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du J, Mansfield SD, Groover AT. The Populus homeobox gene ARBORKNOX2 regulates cell differentiation during secondary growth. The Plant Journal. 2009;60:1000–1014. doi: 10.1111/j.1365-313X.2009.04017.x. [DOI] [PubMed] [Google Scholar]
- 7.Groover A, Mansfield S, DiFazio S, Dupper G, Fontana J, et al. The Populus homeobox gene ARBORKNOX1 reveals overlapping mechanisms regulating the shoot apical meristem and the vascular cambium. Plant Molecular Biology. 2006;61:917–932. doi: 10.1007/s11103-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 8.Zhong R, Ye Z-H. Transriptional regulation of lignin biosynthesis. Plant Signalling and Behavior. 2009;4:1028–1034. doi: 10.4161/psb.4.11.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Goue N, Igarashi H, Ohtani M, Nakano Y, et al. VASCULAR-RELATED NAC-DOMAIN6 (VND6) and VND7 Effectively Induce Transdifferentiation into Xylem Vessel Elements under Control of an Induction System. Plant Physiol. 2010. 110.154013. [DOI] [PMC free article] [PubMed]
- 10.Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, et al. Class III Homeodomain-Leucine Zipper Gene Family Members Have Overlapping, Antagonistic, and Distinct Roles in Arabidopsis Development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green KA, Prigge MJ, Katzman RB, Clark SE. CORONA, a Member of the Class III Homeodomain Leucine Zipper Gene Family in Arabidopsis, Regulates Stem Cell Specification and Organogenesis. Plant Cell. 2005;17:691–704. doi: 10.1105/tpc.104.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, et al. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- 13.Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, et al. Radial Patterning of Arabidopsis Shoots by Class III HD-ZIP and KANADI Genes. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 14.McConnell JR, Barton MK. Leaf polarity and meristem formation in Arabidopsis. Development. 1998;125:2935–2942. doi: 10.1242/dev.125.15.2935. [DOI] [PubMed] [Google Scholar]
- 15.Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Current Biology. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- 16.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- 17.Prigge MJ, Clark SE. Evolution of the class IIIHD-Zip gene family in land plants. Evolution & Development. 2006;8:350–361. doi: 10.1111/j.1525-142X.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 18.Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, et al. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;121:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- 19.Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, et al. The Arabidopsis ATHB-8 HD-Zip Protein Acts as a Differentiation-Promoting Transcription Factor of the Vascular Meristems. Plant Physiol. 2001;126:643–655. doi: 10.1104/pp.126.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floyd SK, Zalewski CS, Bowman JL. Evolution of class III homeodomain-leucine zipper genes in streptophytes. Genetics. 2006;173:373–388. doi: 10.1534/genetics.105.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Jung J-H, Reyes JL, Kim Y-S, Kim S-Y, et al. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. The Plant Journal. 2005;42:84–94. doi: 10.1111/j.1365-313X.2005.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, et al. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5[prime] region. EMBO J. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes & Development. 2002;16:1616–1626. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams L, Grigg SP, Xie M, Christensen S, Fletcher JC. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development. 2005;132:3657–3668. doi: 10.1242/dev.01942. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi-Ito K, Fukuda H. HD-Zip III Homeobox Genes that Include a Novel Member, ZeHB-13 (Zinnia)/ATHB-15 (Arabidopsis), are Involved in Procambium and Xylem Cell Differentiation. Plant Cell Physiol. 2003;44:1350–1358. doi: 10.1093/pcp/pcg164. [DOI] [PubMed] [Google Scholar]
- 26.Ochando I, González-Reig S, Ripoll J-J, Vera A, Martínez-Laborda A. Alteration of the shoot radial pattern in Arabidopsis thaliana by a gain-of-function allele of the class III HD-Zip gene INCURVATA4. International Journal of Developmental Biology. 2008;52:953–961. doi: 10.1387/ijdb.072306io. [DOI] [PubMed] [Google Scholar]
- 27.Ochando I, Jover-Gil S, Ripoll JJ, Candela H, Vera A, et al. Mutations in the MicroRNA Complementarity Site of the INCURVATA4 Gene Perturb Meristem Function and Adaxialize Lateral Organs in Arabidopsis. Plant Physiol. 2006;141:607–619. doi: 10.1104/pp.106.077149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano-Cartagena J, Candela H, Robles P, Ponce MR, Perez-Perez JM, et al. Genetic Analysis of incurvata Mutants Reveals Three Independent Genetic Operations at Work in Arabidopsis Leaf Morphogenesis. Genetics. 2000;156:1363–1377. doi: 10.1093/genetics/156.3.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Floyd SK, Bowman JL. The ancestral developmental tool kit of land plants. International Journal of Plant Sciences. 2007;168:1–35. [Google Scholar]
- 30.Izhaki A, Bowman JL. KANADI and Class III HD-Zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell. 2007;19:495–508. doi: 10.1105/tpc.106.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko JH, Prassinos C, Han KH. Developmental and seasonal expression of PtaHB1, a Populus gene encoding a class III HD-Zip protein, is closely associated with secondary growth and inversely correlated with the level of microRNA (miR166). New Phytol. 2006;169:469–478. doi: 10.1111/j.1469-8137.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 32.Hertzberg M, Aspeborg H, Schrader J, Andersson A, Erlandsson R, et al. A transcriptional roadmap to wood formation. Proc Natl Acad Sci USA. 2001;98:14732–14737. doi: 10.1073/pnas.261293398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, et al. The Genome of Black Cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 34.AngiospermPhylogenyGroup. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- 35.Thiel T, Graner A, Waugh R, Grosse I, Close T, et al. Evidence and evolutionary analysis of ancient whole-genome duplication in barley predating the divergence from rice. Bmc Evolutionary Biology. 2009;9:209. doi: 10.1186/1471-2148-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salse J, Bolot S, Throude M, Jouffe V, Piegu B, et al. Identification and Characterization of Shared Duplications between Rice and Wheat Provide New Insight into Grass Genome Evolution. Plant Cell. 2008;20:11–24. doi: 10.1105/tpc.107.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly Specific Gene Silencing by Artificial MicroRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, et al. Endogenous and Synthetic MicroRNAs Stimulate Simultaneous, Efficient, and Localized Regulation of Multiple Targets in Diverse Species. Plant Cell. 2006;18:1134–1151. doi: 10.1105/tpc.105.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy RL, Zhong R, Ye Z-H. MYB83 Is a Direct Target of SND1 and Acts Redundantly with MYB46 in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell Physiol. 2009;50:1950–1964. doi: 10.1093/pcp/pcp139. [DOI] [PubMed] [Google Scholar]
- 40.Zhong R, Demura T, Ye Z-H. SND1, a NAC Domain Transcription Factor, Is a Key Regulator of Secondary Wall Synthesis in Fibers of Arabidopsis. Plant Cell. 2006;18:3158–3170. doi: 10.1105/tpc.106.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masatoshi Yamaguchi MK, Fukuda Hiroo, Demura Taku. VASCULAR-RELATED NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. The Plant Journal. 2008;55:652–664. doi: 10.1111/j.1365-313X.2008.03533.x. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, et al. Gibberellin Biosynthesis and Response during Arabidopsis Seed Germination. Plant Cell. 2003;15:1591–1604. doi: 10.1105/tpc.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, et al. Genetic Analysis Reveals That C19-GA 2-Oxidation Is a Major Gibberellin Inactivation Pathway in Arabidopsis. Plant Cell. 2008;20:2420–2436. doi: 10.1105/tpc.108.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakakibara H. CYTOKININS: Activity, Biosynthesis, and Translocation. Annual Review of Plant Biology. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- 45.Prigge MJ, Clark SE. Evolution of the class III HD-Zip gene family in land plants. Evolution & Development. 2006;8:350–361. doi: 10.1111/j.1525-142X.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 46.Talbert PB, Adler HT, Parks DW, Comai L. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development. 1995;121:2723–2735. doi: 10.1242/dev.121.9.2723. [DOI] [PubMed] [Google Scholar]
- 47.Zhong R, Ye Z-H. IFL1, a Gene Regulating Interfascicular Fiber Differentiation in Arabidopsis, Encodes a Homeodomain-Leucine Zipper Protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong R, Ye Z-H. Alteration of Auxin Polar Transport in the Arabidopsis ifl1 Mutants. Plant Physiol. 2001;126:549–563. doi: 10.1104/pp.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, et al. A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proceedings of the National Academy of Sciences. 2006;103:11081–11085. doi: 10.1073/pnas.0602038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aloni R. Role of auxin and gibberellin in differentiation of primary phloem fibers. Plant Physiol. 1979;63:609–614. doi: 10.1104/pp.63.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aloni R. Role of Auxin and Gibberellin in Differentiation of Primary Phloem Fibers. Plant Physiology. 1979;63:609–614. doi: 10.1104/pp.63.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aloni R. Role of Cytokinin in Differentiation of Secondary Xylem Fibers. Plant Physiol. 1982;70:1631–1633. doi: 10.1104/pp.70.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y-S, Kim S-G, Lee M, Lee I, Park H-Y, et al. HD-ZIP III activity is modulated by competitive inhibitors via a feedback loop in Arabidopsis shoot apical meristem development. Plant Cell. 2008;20:920–933. doi: 10.1105/tpc.107.057448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenkel S, Emery J, Hou B-H, Evans MMS, Barton MK. A Feedback Regulatory Module Formed by LITTLE ZIPPER and HD-ZIPIII Genes. Plant Cell. 2007;19:3379–3390. doi: 10.1105/tpc.107.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia X, Xie Z. DAMBE: Software Package for Data Analysis in Molecular Biology and Evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- 56.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 57.Maddison WP, Maddison DR. Sunderland, MA: Sinauer; 1999. MacClade version 3.08. [Google Scholar]
- 58.Swofford DL. Sinauer Associates; 2002. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods). [Google Scholar]
- 59.Nylander JAA. MrAIC. pl. 2004. Evolutionary Biology Centre, Uppsala University.
- 60.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 61.Han KH, Meilan R, Ma C, Strauss SH. An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids (genus Populus). Plant cell reports. 2000;19:315–320. doi: 10.1007/s002990050019. [DOI] [PubMed] [Google Scholar]
- 62.Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, et al. Specific effects of microRNAs on the plant transcriptome. Dev Cell. 2005;8:517–527. doi: 10.1016/j.devcel.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, et al. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5' region. EMBO J. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene sequences from the Class III HD Zips gene family recovered from species that have complete genome sequence using the BLAST function in phytozome.net.
(TXT)
Output from microarray analysis at the probe-level, including probes designed against Populus spp. ESTs.
(XLS)
Output from microarray analysis at the level of P. trichocarpa gene models, excluding probes designed against Populus spp. ESTs.
(XLS)