Abstract
Background
S-phase kinase protein 2 (Skp2), an F-box protein, targets cell cycle regulators via ubiquitin-mediated degradation. Skp2 is frequently overexpressed in a variety of cancers and associated with patient survival. In melanoma, however, the prognostic significance of subcellular Skp2 expression remains controversial.
Methods
To investigate the role of Skp2 in melanoma development, we constructed tissue microarrays and examined Skp2 expression in melanocytic lesions at different stages, including 30 normal nevi, 61 dysplastic nevi, 290 primary melanomas and 146 metastatic melanomas. The TMA was assessed for cytoplasmic and nuclear Skp2 expression by immunohistochemistry. The Kaplan-Meier method was used to evaluate the patient survival. The univariate and multivariate Cox regression models were performed to estimate the harzard ratios (HR) at five-year follow-up.
Results
Cytoplasmic but not nuclear Skp2 expression was gradually increased from normal nevi, dysplastic nevi, primary melanomas to metastatic melanomas. Cytoplasmic Skp2 expression correlated with AJCC stages (I vs II–IV, P<0.001), tumor thickness (≤2.00 vs >2.00 mm, P<0.001) and ulceration (P = 0.005). Increased cytoplasmic Skp2 expression was associated with a poor five-year disease-specific survival of patients with primary melanoma (P = 0.018) but not metastatic melanoma (P>0.05).
Conclusion
This study demonstrates that cytoplasmic Skp2 plays an important role in melanoma pathogenesis and its expression correlates with patient survival. Our data indicate that cytoplasmic Skp2 may serve as a potential biomarker for melanoma progression and a therapeutic target for this disease.
Introduction
Cutaneous melanoma is an aggressive cancer type originating from melanocytes in the human skin [1], [2]. Although early diagnosed melanoma is curable with surgical excision, up to 20% of patients will develop metastatic tumors due to its high capability of invasion and rapid metastasis to other organs [3], [4]. Despite many advances in cancer treatment over the last several decades, the prognosis for patients with advanced melanoma remains poor. The 5-year survival rate for patients with distant metastases is less than 10% [5].
The main hallmark of cancer is uncontrolled cellular proliferation with alterations in the expression or activity of proteins that are intimately involved in cell cycle regulation, differentiation and apoptosis [6]. These processes are regulated by transcription, translation, post-translational modifications and degradation of key regulatory proteins, and as such, the ubiquitin–proteasome system has a crucial role in maintaining and regulating cellular homeostasis [7]. In the ubiquitin–proteasome degradation pathway, the ubiquitin is transferred and covalently attached to substrates by the sequential action of three enzymes, namely E1 (ubiquitin-activating enzyme), one of many E2s (ubiquitin-conjugating enzymes) and one of many E3s (ubiquitin ligases) [8]. The E3 ligases are mainly classified into two classes on the basis of structural similarities: the RING-finger proteins and the HECT-domain proteins [7]. Most of RING-finger type ubiquitin ligases contain a cullin (Cul) protein subunit, which were also named as cullin RING ubiquitin ligases (CRLs) [9]. The SCF (Skp1-Cul1-F-box protein) ubiquitin ligases are the best characterized mammalian CRLs, and the F-box protein provides the substrate targeting specificity of the complex [10]. Till now, sixty-nine human F-box proteins that have been identified, and among them, S-phase kinase-associated protein 2 (Skp2) has emerged as a key regulator in different cellular processes [7], [11]. Skp2 is an oncogenic protein that targets tumor suppressor proteins for degradation, including cyclin-dependent kinase (CDK) inhibitors p21Cip1, p27Kip1 and p57Kip2 [7], [11]. Increased levels of Skp2 and reduced levels of p27 are observed in many types of cancer, and these levels are used as independent prognostic markers in several cases [12].
In melanoma, Li et al. have shown that gain of Skp2 protein expression are implicated in melanoma progression and Skp2 cytoplasmic expression predicted worse 10-year overall survival in primary melanoma, suggesting that cytoplasmic expression of Skp2 defines a subset of aggressive melanomas [13]. The study by Woenckhaus et al. suggests that Skp2 could contribute to melanoma progression and vertical growth phase (VGP) melanomas show significant higher nuclear Skp2 expression when compared with the radial growth phase (RGP) [14]. However, they found that the nuclear but not the cytoplasmic Skp2 expression correlated with a reduced survival time in melanoma [14]. These studies indicate that Skp2 expression plays a key role in melanoma development, but the correlation between the sub-cellular Skp2 expression and melanoma patient survival remains contradictory which may be due to small sample size in their studies. To further examine the role of Skp2 subcellular expression in melanoma development and its prognostic significance, we evaluated both nuclear and cytoplasmic staining in 30 normal nevi, 61 dysplastic nevi, 290 primary melanomas and 146 metastatic melanomas.
Materials and Methods
Ethics statement
The use of human skin tissues and the waiver of patient consent in this study were approved by the Clinical Research Ethics Board of the University of British Columbia. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Patient specimens and tissue microarray construction
TMA construction and immunohistochemistry of TMA were described elsewhere [15], [16]. Briefly, formalin-fixed, paraffin-embedded tissues from 49 normal nevi, 100 dysplastic nevi, 403 primary melanomas, and 161 metastatic melanomas were used for our present study. All specimens were obtained from the 1990 to 2009 archives of the Department of Pathology, Vancouver General Hospital. The most representative tumor area was carefully selected and marked on the hematoxylin and eosin-stained slide. The TMAs were assembled using a tissue-array instrument (Beecher Instruments, Silver Spring, MD). Duplicate 0.6-mm-thick tissue cores were taken from each biopsy specimen. Multiple 4-µm sections were cut with a Leica microtome (Leica Microsystems Inc, Bannockburn, IL) and transferred to adhesive-coated slides. One section from each TMA was routinely stained with hematoxylin and eosin. The remaining sections were stored at room temperature for immunohistochemical staining.
Immunohistochemistry of TMA
TMA slides were dewaxed at 55°C for 30 min followed by three 5-min washes with xylene. The rehydration of tissues was performed by 5-min washes in 100, 95, and 80% ethanol and distilled water. Antigen retrieval was performed by heating the samples at 95°C for 30 min in 10 mM sodium citrate (pH 6.0). Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 30 min. After 30 min blocking with the universal blocking serum (Dako Diagnostics, Carpinteria, CA), the sections were incubated with monoclonal mouse anti-Skp2 antibody (1∶100 dilution; clone A-2; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The sections were then incubated for 30 min each with a biotin-labeled secondary antibody and then streptavidin-peroxidase (Dako Diagnostics). The samples were developed using 3,3′-diaminobenzidine substrate (Vector Laboratories, Burlington, Ontario, Canada) and counterstained with hematoxylin. Dehydration was then performed following a standard procedure and the slides were sealed with coverslips. Negative controls were performed by omitting the Skp2 antibody during the primary antibody incubation.
Evaluation of immunostaining
Positive Skp2 immunostaining is defined as either cytoplasmic or nuclear staining and graded according to both intensity and percentage of cells with positive staining. The evaluation of Skp2 staining was blindly and independently examined by two observers, including one dermatopathologist. In few cases with discrepancy, between the two observers, the immunostained slides were reviewed in a double viewing microscope so that the discrepancy was settled. Skp2 staining intensity was scored as 0, 1+, 2+, and 3+. The percentage of Skp2-positive cells was also scored into 4 categories: 1 (0–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). In the cases with a discrepancy between duplicated cores, the average score from the two tissue cores was taken as the final score. The level of Skp2 staining was evaluated by immunoreactive score (IRS) [17], which is calculated by multiplying the scores of staining intensity and the percentage of positive cells. Based on the IRS, Skp2 staining pattern was defined as low staining (0–6) and high staining (8–12).
Statistical analysis
Differences in demographic and clinical characteristics and expression levels of Skp2 were evaluated by χ2 tests between patient subgroups. Survival time was calculated from the date of melanoma diagnosis to the date of death or last follow-up. The Kaplan-Meier method and log-rank test were used to evaluate the effects of Skp2 expression on the overall and disease-specific survival of patients. Univariate or multivariate Cox proportional hazards regression models were preformed to estimate the crude hazard ratios (HRs) or adjusted HRs and their 95% confidential intervals (CIs). SPSS version 11.5 (SPSS Inc, Chicago, IL) software was used for all analyses.
Results
Increased cytoplasmic Skp2 expression in melanoma
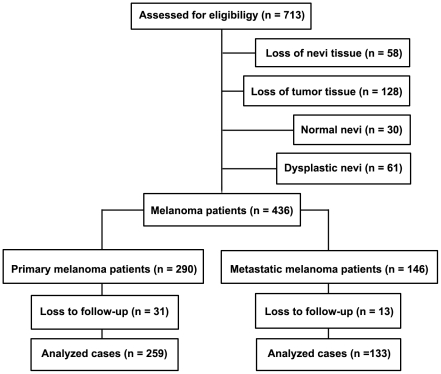
We investigated Skp2 expression in human melanocytic biopsies using TMA and immunohistochemistry. A total of 713 patients were enrolled. Due to loss of biopsy cores or insufficient tumor cells present in the cores, 30 normal nevi, 61 dysplastic nevi, 290 primary melanomas and 146 metastatic melanomas could be evaluated for Skp2 staining and included in the present study (Figure 1). For the 436 melanoma cases, there were 258 men and 178 women, with age ranging from 7 to 95 years (median, 60 years). 167 tumors were at AJCC stage I, 123 at stage II, 59 at stage III, and 87 at stage IV. Among the 290 primary melanoma cases, 176 tumors were ≤2.00 mm, and 114 tumors were >2.00 mm. Ulceration was observed in 53 cases. Seventy-one melanomas were located in sun-exposed sites (head and neck), and 219 were located in sun-protected sites (other locations) (Table 1).
Figure 1. CONSORT diagram for patient inclusion and exclusion.
Table 1. Cytoplasmic Skp2 staining and clinicopathological characteristics of melanomas.
| Variables | Cytoplasmic Skp2 Staining | |||
| Low staining | High staining | Total | P † | |
| All melanoma (n = 436) | ||||
| Age (years) | ||||
| ≤60 | 126 (56.3%) | 98 (43.7%) | 224 (51.4%) | 0.133 |
| >60 | 104 (49.1%) | 108 (50.9%) | 212 (48.6%) | |
| Sex | ||||
| Male | 135 (52.3%) | 123 (47.7%) | 258 (52.8%) | 0.830 |
| Female | 95 (53.4%) | 83 (46.6%) | 178 (47.2%) | |
| AJCC stage | ||||
| I | 112 (67.1%) | 55 (32.9%) | 167 (38.3%) | <0.001‡ |
| II | 54 (43.9%) | 69 (56.1%) | 123 (28.2%) | 0.958§ |
| III | 25 (42.4%) | 34 (57.6%) | 59 (13.5%) | |
| IV | 39 (44.8%) | 48 (55.2%) | 87 (20.0%) | |
| Primary melanoma (n = 290) | ||||
| Age (years) | ||||
| ≤60 | 84 (60.4%) | 55 (39.6%) | 139 (47.9%) | 0.292 |
| >60 | 82 (54.3%) | 69 (45.7%) | 151 (52.1%) | |
| Sex | ||||
| Male | 89 (56.3%) | 69 (43.7%) | 158 (54.5%) | 0.731 |
| Female | 77 (58.3%) | 55 (41.7%) | 132 (45.5%) | |
| Tumor thickness (mm) | ||||
| ≤2.00 | 118 (67.0%) | 58 (33.0%) | 176 (60.7%) | <0.001 |
| >2.00 | 48 (42.1%) | 66 (57.9%) | 114 (39.3%) | |
| Ulceration | ||||
| Absent | 141 (59.5%) | 96 (40.5%) | 237 (81.7%) | 0.005 |
| Present | 25 (47.2%) | 28 (52.8%) | 53 (18.3%) | |
| Site* | ||||
| Sun-protected | 124 (56.6%) | 95 (43.4%) | 219 (75.5%) | 0.707 |
| Sun-exposed | 42 (59.2%) | 29 (40.8%) | 71 (24.5%) | |
| Metastatic melanoma (n = 146) | ||||
| Age (years) | ||||
| ≤60 | 42 (49.4%) | 43 (50.6%) | 85 (58.2%) | 0.109 |
| >60 | 22 (36.1%) | 39 (63.9%) | 61 (41.8%) | |
| Sex | ||||
| Male | 46 (46.0%) | 54 (54.0%) | 100 (68.5%) | 0.437 |
| Female | 18 (39.1%) | 28 (60.9%) | 46 (31.5%) | |
*Sun-protected sites: trunk, arm, leg and feet; Sun-exposed sites: head and neck.
χ2 test.
Comparison between all AJCC stages.
Comparison between AJCC stage II, III and IV.
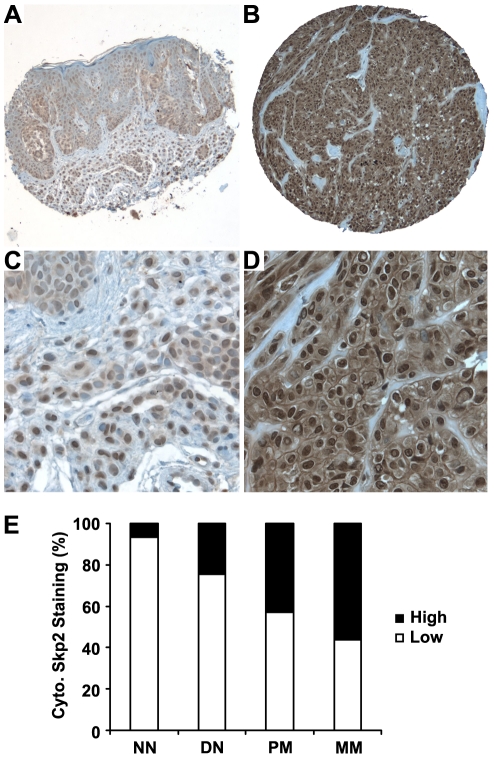
As shown in Figure 2 and Supporting Information Figure S1, various levels of cytoplasmic and nuclear Skp2 staining were observed in nevi and melanoma biopsies. The Skp2 staining was found consistent when two different antibodies were used in a small TMA including 20 cases of nevi and 20 case of melanomas (Supporting Information Figure S2). There are differences in the pattern of cytoplasmic Skp2 expression in melanocytic lesions at different stage, with increased levels of expression from normal nevi to dysplastic nevi and melanoma. Significant differences for Skp2 staining were observed between normal and dysplastic nevi (P = 0.039, χ2 test), dysplastic nevi and primary melanoma (P = 0.008, χ2 test), and primary and metastatic melanoma (P = 0.008, χ2 test) (Figure 2E). However, there was no significant difference for nuclear Skp2 staining among different stages of melanocytic lesions (P>0.05, χ2 test) (Figure S1).
Figure 2. Cytoplasmic Skp2 expression is increased in human melanomas.
Representative images of cytoplasmic Skp2 immunohistochemical staining in human melanocytic lesions. (A and C) Low cytoplasmic Skp2 staining, (B and D) High cytoplasmic Skp2 staining. (E) Cytoplasmic Skp2 expression is increased from normal nevi to dysplastic nevi and melanoma. Significant differences for Skp2 staining are observed between normal and dysplastic nevi (P = 0.039, χ2 test), dysplastic nevi and primary melanoma (P = 0.008, χ2 test), and primary and metastatic melanoma (P = 0.008, χ2 test). NN, normal nevi; DN: dysplastic nevi; PM: primary melanoma; MM: metastatic melanoma. Magnification: ×100 for A and B; ×400 for C and D.
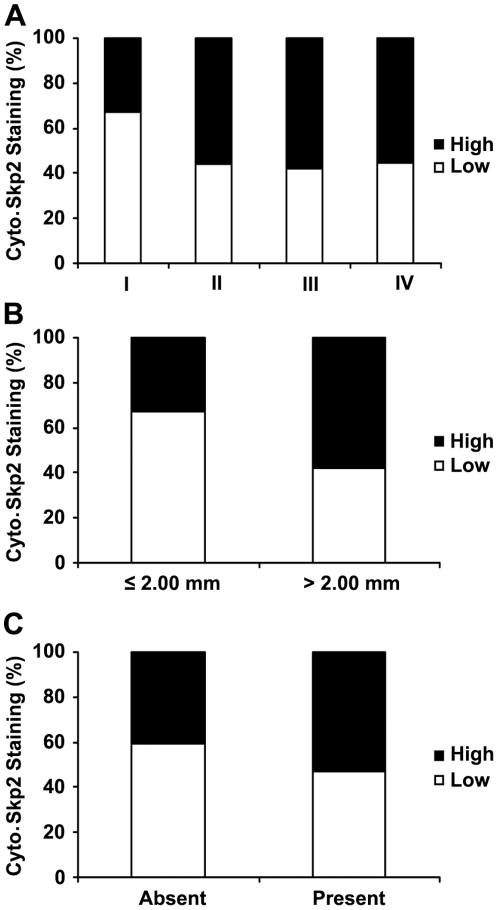
Cytoplasmic Skp2 expression correlates with AJCC stages, tumor thickness and ulceration
Since cytoplasmic Skp2 expression was correlated with melanoma progression, we next examined the correlation between cytoplasmic Skp2 expression and different clinicopathologic characters. As shown in Figure 3A and Table 1, high expression of cytoplasmic Skp2 was detected in 33% of melanomas at AJCC stage I compared to only 55–58% of melanomas at AJCC II, III and IV (P<0.001, χ2 test); however, no significant difference was found in Skp2 expression between AJCC stage II and III or IV, indicating that increased cytoplasmic Skp2 expression may be involved in the melanoma development from stage I to II. In addition, we found that the cytoplasmic Skp2 expression was correlated with Ki67 expression, the proliferative index (Supporting Information Figure S3), and inversely correlated with nuclear p27 expression, a cyclin-dependent kinase inhibitor for cell cycle progression (Supporting Information Figure S4). These data suggest that cytoplasmic Skp2 regulates melanoma progression may through promoting melanoma cell cycle progression.
Figure 3. Cytoplasmic Skp2 correlates with melanoma AJCC stages, tumor thickness and ulceration.
(A) Tumors in AJCC stages II, III and IV have a higher percentage of high cytoplasmic Skp2 expression compared with tumors in stage I (P<0.001, χ2 test). (B) Tumors thicker than 2.0 mm have a higher percentage of high cytoplasmic Skp2 expression compared with tumors ≤2.0 mm thick (P<0.001, χ2 test). (C) Increased cytoplasmic Skp2 expression is correlated with ulceration of melanoma (P = 0.005, χ2 test).
There was no correlation between cytoplasmic Skp2 expression and age or sex in all melanoma patients (Table 1). In primary melanoma, high cytoplasmic Skp2 expression was found in 58% of tumors with thickness >2.00 mm, compared to 33% of melanomas with thickness ≤2.00 mm (P<0.001, χ2 test; Figure 3B). Increased cytoplasmic Skp2 expression was correlated with ulceration of melanoma (P = 0.005, χ2 test; Table 1). We did not find significant correlations between cytoplasmic Skp2 expression and other variables (Table 1). In addition, cytoplasmic Skp2 expression was not correlated with age or sex in metastatic melanoma (Table 1).
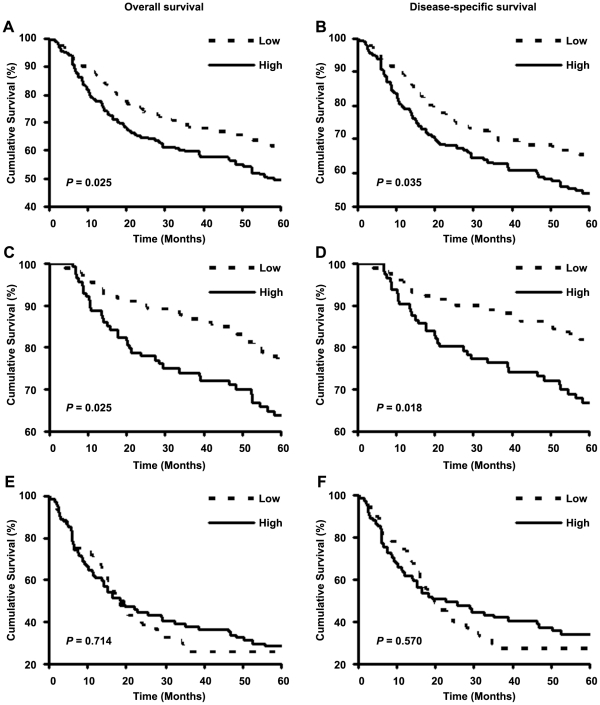
Increased cytoplasmic Skp2 expression is associated with poor patient survival
A total of 392 patients had complete follow-up and clinical information (Figure 1). Demographic and clinical characteristics of the patients were listed in Table 2 and Supporting Information Table S1 and S2. The Kaplan-Meier curve and log-rank test analyses revealed that increased cytoplasmic Skp2 expression was associated with poor overall (P = 0.025) or disease-specific five-year survival (P = 0.035) (Figure 4A and B). Next, we separated all melanoma (n = 392) into primary melanoma (n = 259) and metastatic melanoma (n = 133) and found cytoplasmic Skp2 expression was associated with overall (P = 0.025) and disease-specific five-year survival (P = 0.018) in primary melanoma patients (Figure 4C and D). However, cytoplasmic Skp2 expression was not associated with overall and disease-specific five-year survival in metastatic melanoma patients (P>0.05 for both) (Figure 4E and F). Since previous study showed that nuclear Skp2 expression was associated with melanoma patient survival [14], we also examined the correlation between nuclear Skp2 expression and patient survival. The results indicate that nuclear Skp2 expression is not associated with overall and disease-specific five-year survival in all melanoma, primary melanoma or metastatic melanoma patients (Supporting Information Figure S5).
Table 2. Univariate Cox proportional regression analysis on overall and disease-specific 5-year survival of 259 primary melanoma patients.
| Variable | Patients (%) | Overall survival | Disease-specific survival | ||||||
| Deaths | Death Rate | HR (95% CI) | P † | Deaths | Death Rate | HR (95% CI) | P † | ||
| Age | |||||||||
| ≤60 | 124 (47.9%) | 21 | 16.9% | 2.65 (1.58–4.42) | <0.001 | 21 | 16.9% | 2.14 (1.26–3.65) | 0.005 |
| >60 | 135 (52.1%) | 48 | 35.6% | 39 | 28.9% | ||||
| Sex | |||||||||
| Male | 138 (53.3%) | 37 | 26.8% | 1.00 (0.62–1.61) | 0.994 | 34 | 24.6% | 0.89 (0.53–1.48) | 0.645 |
| Female | 121 (46.7%) | 32 | 26.4% | 26 | 21.5% | ||||
| Thickness (mm) | |||||||||
| ≤2.00 | 152 (58.7%) | 22 | 14.5% | 4.32 (2.60–7.19) | <0.001 | 17 | 11.2% | 5.09 (2.89–8.94) | <0.001 |
| >2.00 | 107 (41.3%) | 47 | 43.9% | 43 | 40.2% | ||||
| Ulceration | |||||||||
| Absent | 211 (85.3%) | 42 | 19.9% | 4.64 (2.84–7.59) | <0.001 | 37 | 17.5% | 4.43 (2.61–7.51) | <0.001 |
| Present | 48 (14.7%) | 27 | 56.3% | 23 | 47.9% | ||||
| Site* | |||||||||
| Sun-protected | 196 (75.7%) | 53 | 27.0% | 1.00 (0.57–1.76) | 0.990 | 44 | 22.4% | 1.21 (0.68–2.14) | 0.515 |
| Sun-exposed | 63 (24.3%) | 16 | 25.4% | 16 | 25.4% | ||||
| Cytoplasmic Skp2 | |||||||||
| Low staining | 145 (56.0%) | 30 | 20.7% | 1.71 (1.07–2.76) | 0.027 | 25 | 17.2% | 1.84 (1.10–3.08) | 0.020 |
| High staining | 114 (44.0%) | 39 | 34.2% | 35 | 30.7% | ||||
| Nuclear Skp2 | |||||||||
| Low staining | 60 (23.2%) | 21 | 35.0% | 0.65 (0.39–1.09) | 0.100 | 19 | 31.7% | 0.62 (0.36–1.06) | 0.080 |
| High staining | 199 (76.8%) | 48 | 24.1% | 41 | 20.6% | ||||
*Sun-protected sites: trunk, arm, leg and feet; Sun-exposed sites: head and neck.
Log-Rank test. Abbreviations: HR, hazard ratio; CI, confidence interval.
Figure 4. Cytoplasmic Skp2 expression is associated with five-year survival of all melanoma and primary melanoma patients.
Kaplan-Meier curves analyses for the correlation between cytoplasmic Skp2 expression and overall or disease-specific five-year survival in all melanoma patients (A,B), primary melanoma patients (C,D) and metastatic melanoma patients (E,F).
Next, we used univariate Cox proportional hazards regression model to estimate the crude hazard ratios (HRs) of Skp2 expression or each clinicopathologic variable on patient survival. The log-rank test and univariate Cox regression analyses revealed that both AJCC stages and cytoplasmic Skp2 expression were significantly associated with overall or disease-specific survival in all melanoma patients (Supporting Information Table S1). In primary melanoma patients, age, tumor thickness, ulceration and cytoplasmic Skp2 expression were all significantly associated with both overall and disease-specific survival (Table 2). However, the nuclear expression was not associated with either overall or disease-specific survival outcomes (Table 2). In metastatic melanoma patients, both cytoplasmic and nuclear expressions were not associated with either overall or disease-specific survival (Supporting Information Table S2).
Cytoplasmic Skp2 expression is not an independent prognostic marker for primary melanoma
Since cytoplasmic Skp2 was associated with melanoma patient survival, we next examined whether cytoplasmic Skp2 expression is an independent prognostic marker for melanoma patient survival by multivariate Cox proportional hazard analysis. Age, sex, tumor thickness, ulceration and location and cytoplasmic Skp2 expression were included in the regression model. The results indicated that cytoplasmic Skp2 expression was not associated with both overall and disease-specific survival (P>0.05 for both, Table 3). As expected, cytoplasmic Skp2 expression was not associated with survival in all melanoma patients by adjustment with AJCC stages, age and sex (Supporting Information Table S3). These data indicate that cytoplasmic Skp2 staining is not independent prognostic factor for five-year survival of melanoma patients.
Table 3. Multivariate Cox regression analysis on overall and disease-specific 5-year survival of 259 primary melanoma patients.
| Variables* | Overall survival | Disease-specific survival | ||||||||
| β† | SE | HR | 95% CI | P ‡ | β† | SE | HR | 95% CI | P ‡ | |
| Age | 0.544 | 0.278 | 1.74 | 1.01–3.02 | 0.046 | 0.282 | 0.289 | 1.33 | 0.75–2.34 | 0.329 |
| Sex | 0.012 | 0.254 | 1.01 | 0.62–1.66 | 0.964 | –0.060 | 0.276 | 0.94 | 0.55–1.62 | 0.828 |
| Thickness | 1.035 | 0.295 | 2.82 | 1.58–5.02 | <0.001 | 1.241 | 0.324 | 3.46 | 1.83–6.53 | <0.001 |
| Ulceration | 0.908 | 0.282 | 2.48 | 1.43–4.31 | 0.001 | 0.884 | 0.301 | 2.42 | 1.34–4.37 | 0.003 |
| Location | –0.091 | 0.292 | 0.91 | 0.52–1.62 | 0.755 | 0.100 | 0.301 | 1.11 | 0.61–2.00 | 0.740 |
| Cytoplasmic Skp2 | 0.124 | 0.258 | 1.13 | 0.68–1.88 | 0.629 | 0.177 | 0.277 | 1.19 | 0.69–2.05 | 0.523 |
*Coding of variables: Age was coded as 1 (≤60 years) and 2 (>60 years). Sex was coded as 1 (male) and 2 (female). Thickness was coded as 1 (≤2.00 mm) and 2 (>2.00 mm). Ulceration was coded as 1 (absent) and 2 (present). Location was coded as 1 (sun-protected) and 2 (sun-exposed). Cytoplasmic Skp2 was coded as 1 (low staining) and 2 (high staining).
β: regression coefficient.
Log-Rank test.
Abbreviations: SE, standard error of β; HR, hazard ratio; CI, confidence interval.
Discussion
Melanocytes are located in the skin, eyes and other epithelial tissues and are the precursors of benign nevus and melanoma [18], [19]. The highly differentiated benign nevi have a low proliferation potential and retain melanin production, whereas malignant melanoma show increased proliferation and may have partly or fully lost the ability to synthesize melanin, in parallel with loss of differentiation [19]. Constitutive activation of cell proliferation signaling, including mitogen-activated protein kinase (MAPK) pathway, induces malignant transformation of melanocytes to melanoma [20]. Due to their relatively small size, variable criteria for histological diagnosis, controversial terminology, and difficulty in establishing in vitro cultures, melanocytic dysplastic nevi have hardly been studied [21]. The limited studies on dysplastic nevi mainly focus on some genetic changes [22]. In addition to genetic changes, recent studies indicate that the alterations of tumor suppressor, onco-proteins and growth factors contribute to development of dysplastic nevi from normal nevi and suggest that evolution of some dysplastic nevi may result in primary melanoma [23], [24]. Our study showed that cytoplasmic Skp2 expression was gradually increased from normal nevi, dysplastic nevi to melanoma tissues, indicating that increased cytoplasmic Skp2 expression may be involved in the development of dysplastic nevi and melanoma. Skp2 has oncogenic potential and is overexpressed in human cancers [25]. To our knowledge, this study for the first time showed that cytoplasmic Skp2 plays a key role in both melanoma initiation and progression.
Mutations in BRAF, a regulator of the mitogen-activated protein-kinase kinase (MAPKK)-ERK1/2 pathway, are associated with approximately 70% of melanomas; the most common mutants are BRAFV599E and BRAFV600E [26], [27]. In melanoma cells, mutation in BRAF constitutively activates BRAF signaling, which regulates Cks1/Skp2-mediated p27 degradation and controls G1 cell cycle event [28], [29]. In human thyroid carcinomas, constitutive signaling of the MAP kinase cascade contributes to the development of thyroid cancer promoted by activated RAS and BRAF onco-proteins and that this occurs, at least in part, by compromising the Skp2-dependent degradation pathway [30]. Our study showed that cytoplasmic Skp2 expression was increased in melanoma, which may be due to BRAF mutation. In addition, increased copy number at the Skp2 locus might also be associated with overexpression of Skp2 protein in human metastatic melanoma tissues [31].
In primary melanoma, we found a correlation between the depth of tumor invasion (thickness), ulceration and cytoplasmic Skp2 expression in the primary melanoma, which suggests a role of Skp2 in tumor invasion. It has been reported that overexpression of Skp2 correlated with metastasis in different cancers including colorectal tumors, lung cancers, oral and esophageal squamous cell carcinoma, human gastric carcinoma and pancreatic ductal adenocarcinoma [32], [33], . Skp2 regulates invasive ability of melanoma cells [40] and overexpression of Skp2 correlates with advanced melanoma [31]. Li et al. found that Skp2 phosphorylation by Akt triggers SCF complex formation and E3 ligase activity, promotes cell proliferation and tumorigenesis [41]. Akt-mediated phosphorylation triggers 14-3-3 beta-dependent Skp2 relocalization to the cytosol and positively regulates cell migration. Furthermore, high levels of activation of Akt correlate with the cytosolic accumulation of Skp2 in human colonic adenocarcinoma. In addition, Skp2 overexpression induces the expression of matrix metalloproteinase (i.e. MMP-2, MMP-9) and invasion of lung cancer cells [42]. Thus, increased cytoplasmic Skp2 expression contributes to melanoma invasion possibly through multiple pathways since both Akt and MMP proteins play import roles in melanoma invasion and metastases [16], [43], [44].
Cytoplasmic but not nuclear Skp2 expression was associated with five-year overall and disease-specific survival in primary melanoma patients. This finding was consistent with the study by Li et al. that high cytoplasmic but not nuclear Skp2 expression predicted worse 10-year overall survival in primary melanoma [13]. However, Woenckhaus et al. found that increased nuclear Skp2 expression correlates with a reduced survival time in melanoma [14]. The controversial findings may be due to a relative small number of patients (n = 25) for the survival analysis in the study by Woenckhaus et al. Recent study by Rose et al. showed that high Skp2 expression (>25% Skp2 staining) predicted worse seven-year post-recurrence survival when comparing low Skp2 expression (≤25% Skp2 staining) in 93 cases of metastatic melanoma patients [31]. In our study, neither nuclear nor cytoplasmic Skp2 expression was associated with five-year survival in 133 cases of metastatic melanoma patients. The clarification on the correlation between Skp2 expression and survival in metastatic melanoma patients awaits future study with a longer follow-up time.
Although surgical removal has been reported with cure rate over 80% for stage I melanoma and as high as 98% for melanoma-in-situ [45], [46], there is no effective treatment for advanced melanoma, It is important to understand the molecular mechanisms of melanoma development and provides a necessary basis to enable the generation of more effective therapeutic modalities. We found that cytoplasmic Skp2 expression is increased in melanoma and the increased cytoplasmic Skp2 expression correlated with melanoma development. In melanoma, Skp2 plays a key role in promoting cell cycle progression [47], [48], [49], [50]. Katagiri et al. reported that knockdown of Skp2 inhibited the melanoma cell growth in vitro and suppressed tumor proliferation in vivo, suggesting that gene silencing of Skp2 can be a potential tool of cancer gene therapy in malignant melanoma [51]. Another study by Sumimoto et al. revealed that the combined suppression of BRAFV599E and Skp2 inhibited cell growth and attenuated the invasive potential of melanoma cell lines in vitro prompting speculation regarding the possibility of combination therapy targeting BRAF and Skp2 [40]. Thus, targeting Skp2 in gene therapy may hold promise for melanoma treatment.
Supporting Information
Nuclear Skp2 expression does not correlate with human melanoma progression.
(DOCX)
Skp2 staining was consistent when probed by two different anti-Skp2 antibodies.
(DOCX)
Cytoplasmic Skp2 expression was positively correlated with Ki67 expression.
(DOCX)
Cytoplasmic Skp2 expression was inversely correlated with nuclear p27 expression.
(DOCX)
Nuclear Skp2 expression is not associated with melanoma patient survival.
(DOCX)
Univariate Cox proportional regression analysis on overall and disease-specific 5-year survival of all 392 melanoma patients.
(DOC)
Univariate Cox proportional regression analysis on overall and disease-specific 5-year survival of 133 patients with metastic melanoma.
(DOC)
Multivariate Cox regression analysis on overall and disease-specific 5-year survival of all 392 melanoma patients.
(DOC)
Acknowledgments
We thank Dr. L. Fazli, E. Li and J. Liew for the technical assistance in TMA construction, and S. Kwong for TMA photography.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work is supported by Canadian Institutes of Health Research (MOP-84559 and MOP-93810) and Canadian Dermatology Foundation (G.L.). G.C. is a recipient of Postdoctoral Trainee Award from Michael Smith Foundation for Health Research jointly funded with VGH & UBC Hospital Foundation. Y.C. is a recipient of Canadian Institutes of Health Research Skin Research Training Centre PhD Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000-2006) and future directions. J Am Acad Dermatol. 2007;57:555–572; quiz 573-556. doi: 10.1016/j.jaad.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 4.Houghton AN, Polsky D. Focus on melanoma. Cancer Cell. 2002;2:275–278. doi: 10.1016/s1535-6108(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 5.Trinh VA. Current management of metastatic melanoma. Am J Health Syst Pharm. 2008;65:S3–8. doi: 10.2146/ajhp080460. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 9.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 10.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 11.Skaar JR, D'Angiolella V, Pagan JK, Pagano M. SnapShot: F Box Proteins II. Cell. 2009;137:1358, 1358 e1351. doi: 10.1016/j.cell.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415–1424. doi: 10.1002/cncr.23317. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Murphy M, Ross J, Sheehan C, Carlson JA. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J Cutan Pathol. 2004;31:633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 14.Woenckhaus C, Maile S, Uffmann S, Bansemir M, Dittberner T, et al. Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of the skin and its relation to clinical outcome. Histol Histopathol. 2005;20:501–508. doi: 10.14670/HH-20.501. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Cheng Y, Martinka M, Li G. Cul1 expression is increased in early stages of human melanoma. Pigment Cell Melanoma Res. 2010;23:572–574. doi: 10.1111/j.1755-148X.2010.00725.x. [DOI] [PubMed] [Google Scholar]
- 16.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 17.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 18.Herlyn M, Clark WH, Rodeck U, Mancianti ML, Jambrosic J, et al. Biology of tumor progression in human melanocytes. Lab Invest. 1987;56:461–474. [PubMed] [Google Scholar]
- 19.Houghton AN, Real FX, Davis LJ, Cordon-Cardo C, Old LJ. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med. 1987;165:812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, et al. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J Biol Chem. 2003;278:9790–9795. doi: 10.1074/jbc.M212929200. [DOI] [PubMed] [Google Scholar]
- 21.Barnhill RL, Cerroni L, Cook M, Elder DE, Kerl H, et al. State of the art, nomenclature, and points of consensus and controversy concerning benign melanocytic lesions: outcome of an international workshop. Adv Anat Pathol. 2010;17:73–90. doi: 10.1097/PAP.0b013e3181cfe758. [DOI] [PubMed] [Google Scholar]
- 22.Friedman RJ, Farber MJ, Warycha MA, Papathasis N, Miller MK, et al. The “dysplastic” nevus. Clin Dermatol. 2009;27:103–115. doi: 10.1016/j.clindermatol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim N, Haluska FG. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol. 2009;4:551–579. doi: 10.1146/annurev.pathol.3.121806.151541. [DOI] [PubMed] [Google Scholar]
- 24.Crowson AN, Magro C, Miller A, Mihm MC., Jr The molecular basis of melanomagenesis and the metastatic phenotype. Semin Oncol. 2007;34:476–490. doi: 10.1053/j.seminoncol.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 27.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt KV, Spofford LS, Aram G, McMullen M, Pumiglia K, et al. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;24:3459–3471. doi: 10.1038/sj.onc.1208544. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt KV, Hu R, Spofford LS, Aplin AE. Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene. 2007;26:1056–1066. doi: 10.1038/sj.onc.1209861. [DOI] [PubMed] [Google Scholar]
- 30.Motti ML, De Marco C, Califano D, De Gisi S, Malanga D, et al. Loss of p27 expression through RAS–>BRAF–>MAP kinase-dependent pathway in human thyroid carcinomas. Cell Cycle. 2007;6:2817–2825. doi: 10.4161/cc.6.22.4883. [DOI] [PubMed] [Google Scholar]
- 31.Rose AE, Wang G, Hanniford D, Monni S, Tu T, et al. Clinical relevance of SKP2 alterations in metastatic melanoma. Pigment Cell Melanoma Res. doi: 10.1111/j.1755-148X.2010.00784.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tosco P, La Terra Maggiore GM, Forni P, Berrone S, Chiusa L, et al. Correlation between Skp2 expression and nodal metastasis in Stage I and II oral squamous cell carcinomas. Oral Dis. doi: 10.1111/j.1601-0825.2010.01713.x. In press. [DOI] [PubMed] [Google Scholar]
- 33.Wang XC, Wu YP, Ye B, Lin DC, Feng YB, et al. Suppression of anoikis by SKP2 amplification and overexpression promotes metastasis of esophageal squamous cell carcinoma. Mol Cancer Res. 2009;7:12–22. doi: 10.1158/1541-7786.MCR-08-0092. [DOI] [PubMed] [Google Scholar]
- 34.Salon C, Merdzhanova G, Brambilla C, Brambilla E, Gazzeri S, et al. E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene. 2007;26:6927–6936. doi: 10.1038/sj.onc.1210499. [DOI] [PubMed] [Google Scholar]
- 35.Einama T, Kagata Y, Tsuda H, Morita D, Ogata S, et al. High-level Skp2 expression in pancreatic ductal adenocarcinoma: correlation with the extent of lymph node metastasis, higher histological grade, and poorer patient outcome. Pancreas. 2006;32:376–381. doi: 10.1097/01.mpa.0000220862.78248.c4. [DOI] [PubMed] [Google Scholar]
- 36.Ma XM, Liu Y, Guo JW, Liu JH, Zuo LF. Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J Gastroenterol. 2005;11:6716–6721. doi: 10.3748/wjg.v11.i42.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada K, Supriatno, Kawaguchi S, Kawashima Y, Itashiki Y, et al. High expression of S-phase kinase-associated protein 2 (Skp2) is a strong prognostic marker in oral squamous cell carcinoma patients treated by UFT in combination with radiation. Anticancer Res. 2005;25:2471–2475. [PubMed] [Google Scholar]
- 38.Yokoi S, Yasui K, Mori M, Iizasa T, Fujisawa T, et al. Amplification and overexpression of SKP2 are associated with metastasis of non-small-cell lung cancers to lymph nodes. Am J Pathol. 2004;165:175–180. doi: 10.1016/S0002-9440(10)63286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JQ, Wu F, Masaki T, Kubo A, Fujita J, et al. Correlation of Skp2 with carcinogenesis, invasion, metastasis, and prognosis in colorectal tumors. Int J Oncol. 2004;25:87–95. [PubMed] [Google Scholar]
- 40.Sumimoto H, Hirata K, Yamagata S, Miyoshi H, Miyagishi M, et al. Effective inhibition of cell growth and invasion of melanoma by combined suppression of BRAF (V599E) and Skp2 with lentiviral RNAi. Int J Cancer. 2006;118:472–476. doi: 10.1002/ijc.21286. [DOI] [PubMed] [Google Scholar]
- 41.Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung WC, Tseng WL, Shiea J, Chang HC. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer Lett. 2010;288:156–161. doi: 10.1016/j.canlet.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann UB, Houben R, Brocker EB, Becker JC. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87:307–314. doi: 10.1016/j.biochi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, McClay EF. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol. 2002;29:413–426. doi: 10.1053/sonc.2002.35237. [DOI] [PubMed] [Google Scholar]
- 47.Chen G, Wang Y, Garate M, Zhou J, Li G. The tumor suppressor ING3 is degraded by SCF(Skp2)-mediated ubiquitin-proteasome system. Oncogene. 2010;29:1498–1508. doi: 10.1038/onc.2009.424. [DOI] [PubMed] [Google Scholar]
- 48.Chen G, Li G. Increased Cul1 expression promotes melanoma cell proliferation through regulating p27 expression. Int J Oncol. 2010;37:1339–1344. doi: 10.3892/ijo_00000786. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, Yamauchi H. p27-Associated G1 arrest induced by hinokitiol in human malignant melanoma cells is mediated via down-regulation of pRb, Skp2 ubiquitin ligase, and impairment of Cdk2 function. Cancer Lett. 2009;286:240–249. doi: 10.1016/j.canlet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 50.Hu R, Aplin AE. Skp2 regulates G2/M progression in a p53-dependent manner. Mol Biol Cell. 2008;19:4602–4610. doi: 10.1091/mbc.E07-11-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katagiri Y, Hozumi Y, Kondo S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J Dermatol Sci. 2006;42:215–224. doi: 10.1016/j.jdermsci.2005.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nuclear Skp2 expression does not correlate with human melanoma progression.
(DOCX)
Skp2 staining was consistent when probed by two different anti-Skp2 antibodies.
(DOCX)
Cytoplasmic Skp2 expression was positively correlated with Ki67 expression.
(DOCX)
Cytoplasmic Skp2 expression was inversely correlated with nuclear p27 expression.
(DOCX)
Nuclear Skp2 expression is not associated with melanoma patient survival.
(DOCX)
Univariate Cox proportional regression analysis on overall and disease-specific 5-year survival of all 392 melanoma patients.
(DOC)
Univariate Cox proportional regression analysis on overall and disease-specific 5-year survival of 133 patients with metastic melanoma.
(DOC)
Multivariate Cox regression analysis on overall and disease-specific 5-year survival of all 392 melanoma patients.
(DOC)