Abstract
Recombination with single-strand DNA oligonucleotides (oligos) in E. coli is an efficient and rapid way to modify replicons in vivo. The generation of a nucleotide alteration by oligo recombination provides novel assays for studying cellular processes. Single-strand exonucleases inhibit oligo recombination, and by mutating all four known exonucleases recombination is increased. Increasing the oligo concentration or addition of non-specific carrier oligo titrates out the exonucleases. In a model for oligo recombination, λ Beta protein anneals the oligo to complementary single-strand DNA at the replication fork. Mismatches are created and the methyl-directed mismatch repair (MMR) system acts to eliminate the mismatches inhibiting recombination. Three ways to evade MMR through oligo design include, in addition to the desired change 1) a C~C mismatch six bp from that change, 2) four or more adjacent mismatches, or 3) mismatches at four or more consecutive wobble positions. The latter proves useful for making high frequency changes that alter only the target amino-acid sequence and even allows modification of essential genes. Efficient uptake of DNA is important for oligo-mediated recombination. Uptake of oligos or plasmids is growth media-dependent and is 10,000-fold reduced for cells grown in minimal vs rich medium. Genome-wide engineering technologies utilizing recombineering will benefit from both optimized recombination frequencies and a greater understanding of how biological processes such as DNA replication and cell division impact recombinants formed at multiple chromosomal loci. Recombination events at multiple loci in individual cells are described here.
Keywords: mismatch repair, lambda Red, genome engineering, targeted mutagenesis, DNA transformation
INTRODUCTION
In the past decade, recombineering, recombination-mediated genetic engineering, has become the method of choice to genetically modify large DNA molecules. Recombineering is highly efficient,1; 2 is targeted by short (~50 base) DNA homologies,3-5 is not limited by availability of restriction sites,5 and can be automated to rapidly make genome wide alterations.6 With large DNA molecules such as BACs or bacterial genomes, traditional genetic engineering techniques fail since finding convenient, unique restriction sites proves impossible and the physical manipulation of large DNA molecules is difficult. Recombineering has enabled researchers working on a wide range of organisms to precisely modify large genetic constructs contained on a BAC, PAC, virus or plasmid in E. coli, before moving back into their organism of choice. Recently, genome-wide engineering technologies that utilize recombineering have been developed, including multiplex automated genome engineering (MAGE)6 and trackable multiplex recombineering, or TRMR.7 In addition to advantages in genetic engineering, oligo recombination provides novel assays for studying cellular processes such as DNA uptake, methyl-directed mismatch repair (MMR), DNA replication, and chromosome segregation. Lessons learned from these experiments are providing new insights about such processes as well as ways to maximize oligo-mediated recombination frequencies. These methods are useful for functional genomic studies in E. coli, for techniques such as MAGE, and as a starting point for developing recombineering in new organisms.
Recombineering utilizes bacteriophage-encoded recombination systems, the most commonly used being λ Red3; 8-10 or E. coli RecET,5 however, several new systems are being developed.11-14 The λ Red system includes three proteins, Exo, Beta and Gam, and recombines both double-stranded (dsDNA)3; 8-10 and single-stranded (ssDNA) DNA.1; 2; 15 Beta, a single-strand annealing protein, is the only λ function required for efficient recombination with ssDNA oligos.2 Oligo recombination can be used to make single or clustered base substitutions, deletions up to ~45 kb6 or small (20-30 base) insertions.6; 16; 17 Beta-like functions from other bacterial species and phage have been isolated and shown to be functional.11; 12
Most homologous recombination depends on RecA or a RecA-like function.18; 19 In E. coli, numerous recombination functions (Figure 1) have been categorized as being part of the RecBCD and/or RecF recombination pathways. Both the RecBCD and RecF pathways require RecA protein and thus, mutation of the recA gene results in a severe recombination defect as neither pathway is functional.19 Hence it is significant that Red recombination using either dsDNA20; 21 or oligos1; 2; 15; 22 does not require the E. coli RecA protein. This proves useful, as after briefly expressing the Red functions to allow recombination, expression can be shut-off preventing further homology-dependent rearrangements in recA mutant cells.10
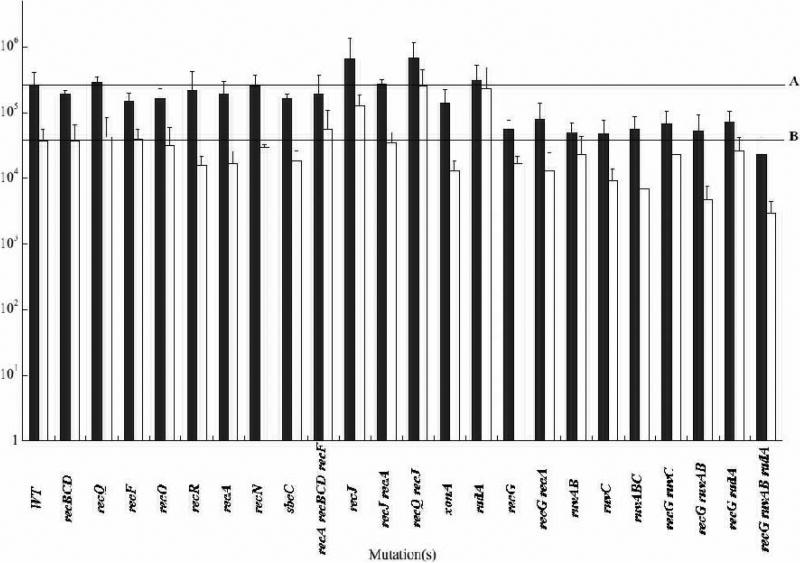
Figure 1. Oligo recombination in E. coli recombination mutant strains.
All strains are derivatives of HME6 and contain a knockout of the indicated genes. Recombination frequencies in this and all tables and figures are normalized per 108 viable cells, which is approximately the number of survivors in each electroporation. (~) Recombination with lagging-strand oligo 100. (~) Recombination with leading-strand oligo 101. The recombination frequency in wild type cells with the lagging strand is shown by line “A”, the leading strand by line “B”. Important note: the bias between recombinants obtained with the lagging vs the leading strand is variable in these experiments as some of the host mutations affect MMR. Additionally, the strand bias is directly affected by MMR because the mismatch on the lagging strand and the leading strand are different and thus repaired at different efficiencies.
Previous studies have established several parameters that affect oligo-mediated recombination.1; 2; 6; 15; 22 One critical factor is the direction of DNA replication through the target sequence. Of the two complementary oligos that can be used at any locus, the one corresponding in sequence to the lagging strand is more efficient at recombination.1; 2; 15; 22 This observation supports the model that during DNA replication the Beta protein anneals the oligo to complementary single-stranded DNA at the replication fork.1; 2; 15; 23-25 An annealed oligo containing at least one base different from the chromosome creates a mismatch, which targets the oligo-mediated recombination events to be repaired by the MMR system. Mismatch repair reduces recombination between diverged sequences.26 Costantino and Court1 found that avoidance of the MMR system is key for high frequency recombination, as MMR can eliminate more than 99% of the recombinants from an oligo-mediated recombination. Mutation of the MMR genes increased oligo-mediated recombination by ~100-fold with most mismatches, and nearly 400-fold for the well-repaired G-G mismatch.1 Temporary inhibition of the MMR system by addition of 2-aminopurine increases oligo recombination 10-fold.1 Unfortunately, both of these techniques to reduce MMR cause uncontrolled generalized DNA mutagenesis.
Evading mismatch repair in cells with a functional MMR system is desirable for oligo recombination as it avoids generalized mutagenesis. A method we devised1 is to design the recombining oligo such that the mismatch created during recombination is not recognized by the MutS protein, which binds mismatched base pairs and initiates the repair process. An oligo that creates a C~C mismatch at the targeted change1 generates high numbers of recombinants since MutS binds poorly to the C~C mismatch.27 This method is highly effective but limited in general applicability. It has been reported27 that the MMR system efficiently repairs the other seven single base mismatches as well as mismatches with up to three adjacent unpaired bases caused by either an insertion or deletion on one strand of the DNA.28 Less is known, however, about the correction of multiple nearby or consecutive mismatches and context effects on individual mismatches.29 In vitro, MutS protein has been shown to recognize multiple nearby mismatches, although at a reduced affinity as compared to a similar single mismatch.30 In vivo, Yang and Sharan17 obtained a high frequency of recombination when 6-20 consecutive nucleotides were altered around the desired change; probably because the MMR system is unable to bind and repair these multiple alterations.16 Here we determine the effect of several types of multiple mismatches on recombination frequencies.
Technologies such as MAGE6 rely on the fact that oligo recombination can be targeted to several loci simultaneously with the goal of achieving multiple changes on a single chromosome. With MAGE, however, it is difficult to tell precisely when and how each recombination event occurred. We analyzed the recombinants generated when two loci were simultaneously targeted in order to better understand this process. Further, we examined oligo recombination in cells grown in minimal medium, which contain fewer sister chromosomes.31
RESULTS
Oligo-mediated recombination does not require any known E. coli recombination function
Red-mediated oligo recombination has been shown to occur in the absence of RecA.1; 2; 15; 22 We asked whether other known E. coli recombination functions are involved in oligo recombination. Recombination frequencies were determined by enumerating the number of Gal+ recombinants obtained when a 70 base, leading-strand (oligo 101) or lagging-strand oligo (oligo 100) was used to correct the galKtyr145am mutation.1; 2 In this experiment, the MMR system was functional, thus limiting recombination levels in order to allow detection of any increase or decrease in frequencies.
Recombination frequencies were determined for strains containing single or multiple defects in E. coli recombination functions. Our results are consistent with and extend previously published data.32 We saw no major effect on the number of oligo-mediated recombinants in any of the mutant backgrounds (Figure 1). The largest effect seen, about a 10-fold decrease, occurred when four genes involved in the resolution of Holliday junctions, radA, recG, ruvA, and ruvB were simultaneously mutated; smaller effects were seen with single mutants. Paradoxically, a radA single mutation increased recombination six-fold but only with the leading strand oligo. The recJ and recQ recJ mutant strains showed increased recombination for both leading and lagging strands. We have determined that these effects are due to RecJ's role in MMR (data not shown).33
Concentration of oligo used in recombination
We routinely use 5 pmoles of oligo for recombination,1; 34; 35 which is approximately 3000 oligos per bacterial cell electroporated under our conditions. Increasing the number of oligos per cell 10- or 100-fold (Table 1 and data not shown) does not increase the number of recombinants, indicative of saturation. Table 1 also compares results obtained when the oligo was diluted in either sterile dH2O or with a “carrier” oligo. When present, carrier was added at 3000 oligos per cell. The carrier oligo contains no homology to any sequence within the recombining cells, however, like the galK oligo, it can be bound by Beta and other single-strand DNA binding proteins including single-strand exonucleases. Reducing the number of galK oligos per cell results in fewer recombinants as expected (Table 1). Although the first 10-fold dilution of galK oligo in dH2O (3000→300) decreased recombination only ~2-fold, a further 10-fold dilution (300→30 oligos/cell) decreased recombination more than 80-fold. However, by diluting into carrier oligo, the dilution from 300→30 galK oligos/cell results in only a 6-fold drop in recombination frequency. Thus, adding carrier oligo can have a positive effect on recombinant frequencies when the recombining oligo becomes limiting.
Table 1.
The effects of oligo concentration, non-specific carrier oligo, and single-strand exonucleases.
| # of Gal+ oligos/cell | Gal+/108 : Dilute in H2O | Gal+/108 : Dilute in Oligo3 | ||
|---|---|---|---|---|
| 1Exo+ | 2Exo- | 1Exo+ | 2Exo- | |
| 30000 | 1.4×107 | |||
| 3000 | 1.6×107 | 3.9×107 | ||
| 300 | 7.3×106 | 1.3×107 | 1.3×107 | 9.3×106 |
| 30 | 8.7×104 | 1.5×106 | 2.2×106 | 1.5×106 |
| 3 | 6.4×103 | 3.9×105 | 2.2×105 | 3.7×105 |
Recombination with oligo 144 in HME6 cells.
Recombination with oligo 144 in XTL74, which is deleted for the Exo I, Exo VII, Exo X and RecJ exonucleases. All data throughout the table are the average of three or more experiments. 3000 oligos/cell is 5 pmoles of oligo per reaction. Experiments in XTL74 cells used 2-fold higher oligo concentration than indicated in column 1.
Carrier oligo (LT217) was included at 3000 oligos/cell.
Do the host single-strand exonucleases affect recombination?
Oligonucleotides may be sensitive to degradation by single-strand exonucleases within the cell.36 E. coli encodes four such exonucleases: ExoI, ExoVII, ExoX and RecJ. We deleted all four of these exonucleases in order to determine how their absence affected Red-mediated recombination with an oligo. Table 1 shows that in the absence of the single-strand exonucleases, up to 40-fold more recombinants are recovered at low oligo concentrations (3 or 30 oligos/cell). Addition of carrier oligo did not result in an increase in recombination frequency for exonuclease mutant cells; in other words, the presence of carrier oligo has the same effect as mutating the four exonucleases. At our standard oligo concentration, the absence of these exonucleases does not affect recombination frequencies, again indicating that recombination is saturated at an oligo concentration of 3000 molecules/cell.
Varying oligo length affects recombinant yield
Ellis et al.2 found that decreasing the oligo length resulted in decreased recombinant formation, however, those experiments were performed with an oligo that was subject to mismatch repair.1 Here we expand on previous experiments by examining a wider range of oligo lengths and using oligos that create a C~C mispair and thus, are immune to the MMR system. Figure 2 shows the frequency of Gal+ recombinants obtained with oligos of various lengths. Oligos of 40-70 bases yield nearly the same number of recombinants, although a 60-mer or 70-mer generates the highest level. Reducing the length of the oligo from 40 to 23 bases decreases recombinant yield about 20-fold in an exponential fashion. There is a dramatic, 10-fold drop in recombinant yield when an oligo is reduced from 23 to 22 bases in length. The low level of residual recombination with oligos 15-20 bases in length is independent of the λ Red functions (data not shown).37
Figure 2. Effect of oligo length on oligo recombination.
The oligo will create a C~C mispair, avoiding the MMR system, when annealed to the target. The oligos are of variable length with the correcting “C” always centered. The host cells were HME6 and the selection was for Gal+. All values represent the average of three or more experiments and are normalized per 108 viable cells.
In a similar experiment where the MMR system was functional and the oligo-generated recombinant was susceptible to it, recombination was reduced approximately 100-fold for each oligo length tested (data not shown). Thus, the general effect of oligo length on recombination frequencies is independent of the MMR system.
Altering the position of the mismatch within the oligo affects recombinant yield
Figure 3 shows the yield of Gal+ recombinants obtained with a series of 70 base oligos that contain the same correcting “T” base but the position of the correcting base within the oligo varies. The correcting base is denoted as 1 through 70 along the x-axis, assigned in a 5’→3’ direction. Assays were performed in strain HME63 in which the MMR system is inactive. If the correcting base was from positions 9 to 61, the recombinant yield was similarly high, yielding approximately 1-3×107 Gal+ recombinants/108 viable cells. If the correcting base was outside of this interval, recombinant yield dropped off precipitously. An oligo with the correcting base at the extreme 5’ end, position 1, yielded recombinants well above background but five orders of magnitude lower than when the correcting base was in the middle. Recombination with the correcting base at the extreme 3’ end, position 70, generated 104 recombinants/108 viable cells, about 50-fold higher than the 5’ end. The low level of recombination observed when the correcting base was on either end was completely dependent on the λ Red functions (data not shown). Experiments using control oligos located such that they end just 5’ or 3’ of the base to be corrected, i.e. they do not contain the correcting base, and experiments in which no oligo was added had the same very low level of spontaneous Gal+ colonies (Figure 3 legend). These results indicate that oligo recombination in the immediate vicinity of the galK mutation does not affect the natural reversion frequency found in the absence of oligos.
Figure 3. Effect of the position of the correcting base within an oligo.
Each data point shows the position of the correcting base within that oligo. All oligos are 70 bases in length and create a T~C mispair when annealed to the target. To avoid MMR, recombination was done in the mutS mutant strain, HME63. All values represent the average of two or more experiments and are normalized per 108 viable cells. Control oligos were used that did not overlap the galKam, but ended either three bases upstream or started one base downstream. The Gal+ frequency for these oligos was ~3×101/108 viable cells; the same as was seen with no oligo added.
A C~C mismatch protects a region from mismatch repair
Figure 4 shows the sequence flanking the galKTYR145UAG mutation. Recombination with an oligo that substitutes a “T” for the boldface “G” in the stop codon of the amber mutation yields Gal+ recombinants at an average frequency of 3.5×105/108 viable cells (oligo 100). However, if the oligo creates a C~C mismatch (oligo 144), MMR is avoided and high recombination levels, 2×107/108, are achieved.1 Since it is impossible to make all alterations using a C~C mismatch, we asked whether a second, unselected C~C mismatch near a galK T~C correcting mismatch, which is sensitive to the MMR system, can confer high frequency recombination (Figure 4). The level of recombination increased 30-fold if an additional alteration that results in a C~C mismatch is made six bases from the correcting “T” (Oligos 254 and 176), while a C~C mismatch further away than six bases from the correcting base had little or no effect. Inhibition of correction of a mismatch by a nearby C~C mispair has been seen previously in Streptococcus pneumoniae.38
Figure 4. A nearby c~C mismatch can increase the recombination frequency of a correcting base.
The double-strand sequence of the region around the galKam mutation in HME6 is shown at the top where the upper sequence denotes the lagging-strand and the “G” of the amber codon is shown in bold. Oligos listed below are identical in sequence to the lagging-strand except for the indicated bases. All oligos are 70 bases in length and only the relevant changes are shown. Recombination values obtained from these oligos are an average of three or more experiments and are normalized per 108 viable cells. HME6 has a functional MMR system.
MMR cannot repair multiple adjacent mismatches
As large heterologies are not recognized by the MMR system,1 we reasoned that if we extended the mismatch length base by base, at some point the mismatch distortion would prevent MMR because MutS would be unable to bind. At this length, high level recombination should result. Figure 5 shows experiments with multiple consecutive changes in an oligo, none of which creates a C~C mispair. In all cases when four consecutive bases were altered, and in certain cases with only three base changes, up to 20% of the cells became recombinant indicating avoidance of the MMR system. Surprising exceptions exist; for example, oligo 282 creates only a single T~T mispair (Figure 5A) that is resistant to MMR in its native context. Adding an additional alteration next to this T~T mispair reduces recombination (oligo 283). The same oligo 282 gives a lower recombination frequency when two mismatches are created (Figure 5B), again demonstrating the importance of sequence context. Such contextual effects have been previously demonstrated.29
Figure 5. The effect of multiple changes on oligo recombination frequencies.
The double-strand sequence of the region around the galK mutations in HME6 (panel “A”) and HME58 (panel “B”) is shown at the top where the upper sequence denotes the lagging-strand and the stop codon is shown in bold. Oligos listed below are identical in sequence to the lagging-strand of the strain above except for the indicated bases. All oligos are 70 bases in length and only the relevant changes are shown. Recombination values obtained from these oligos are an average of three or more experiments and are normalized per 108 viable cells. Both HME6 and HME58 have a functional MMR system.
Evasion of the MMR system by consecutive wobble base alterations
We examined whether changing only the wobble (3rd) position in several nearby codons, while retaining wild type amino acid sequence, could also result in high levels of recombination. As shown in Figure 6, changing the wobble position of three consecutive codons increased the frequency of recombination up to 25-fold more than having changes in two consecutive wobble positions. When four or five consecutive codons were altered in their wobble position, the highest levels of recombination were obtained. Thus, the MMR system can be avoided not only by consecutive changes but also by altering multiple wobble positions of successive codons. The wobble method of MMR-avoidance allows efficient targeted mutagenesis of genes without amino acid changes in an encoded protein except for the desired change.
Figure 6. Changing the wobble position of multiple codons to increase oligo recombination frequencies.
See Figure 5 legend for details.
Recombination with two different oligos
Techniques such as MAGE6 use several different oligos to target multiple loci simultaneously with recombineering technology. However, as MAGE involves automated repeating rounds of recombination, the details of what happens in a given round are unclear. Can more than one locus be successfully recombined during one round of recombineering and how often does the recombination occur on the same sister chromosome? We addressed these questions with two 70 base lagging-strand oligos in a malKam galKam mutS strain (HME82). The malK and galK markers are located on the same arm of the replicon approximately 1.2 megabases apart (Figure 7). In this experiment, immediately after electroporation cells were plated on L agar, incubated overnight and independent colonies patched to MacConkey galactose and MacConkey maltose plates to screen for “red patches” indicating recombinants. Of 490 colonies tested, 24% (118) were Mal+ Gal-, 18% (90) were Mal- Gal+ and 9% (42) had both Mal+ and Gal+ properties. The remaining 240 colonies were white on both indicator plates and thus non-recombinant. The 42 Mal+ Gal+ patches were further tested by suspending the original L agar colony in buffer, diluting and plating for single colonies on MacConkey maltose or MacConkey galactose plates, allowing determination of the genetic makeup of individual cells within each of the 42 colonies. During this assay, all red colonies, either Mal+ or Gal+, were tested on the other sugar indicator. Only two of the original 42 colonies contained a single type of recombinant cell, Mal+ Gal+. Thirty-one of the 42 colonies contained two recombinant cell types: Mal+ Gal- or Mal- Gal+. The remaining nine colonies each contained three recombinant cell types, Mal+ Gal-, Mal- Gal+, and Mal+ Gal+. All of these mixed phenotypes are likely the result of multiple recombination events and are elaborated on in the Discussion. Consistent with Wang et al,6 we conclude that oligo recombination can occur at more than one locus in the same cell. Most of the cells are competent for oligo uptake and recombination as demonstrated by the >50% recombination frequency. However, the generation of two alterations on one chromosome was rare. We saw only 11/490 (2.2%) colonies in which cells had become genetically malK+ galK+.
Figure 7. Diagram of potential routes to create multiple recombinant cell types in an individual colony.
I) One copy of the double-stranded E. coli chromosome is denoted as linear for simplicity. The DNA replication origin, oriC, is shown as a green box. Relative positions of the malK and galK genes are shown. This diagram is not to scale. Throughout the diagram, DNA strands are labeled on the left end for identification with newly made strands receiving new labels. Newly synthesized DNA is dashed when on the lagging-strand and solid when on the leading strand, but both are solid when they replicate again. The purple “+” signs indicate an oligo recombination event on that DNA strand. From panel “I” to “II”, DNA replication and oligo recombination with both malK () and galK (ℑ) oligos occurs. III) The replication origins in “II” have initiated another round of DNA replication and there are two more recombination events; one at malK () and one at galK (℘) on different strands. It is important to note that under our growth conditions, about 66% of cells will have four origins, the rest having eight,39 so the events depicted can potentially happen in a single cell before any cell division. Continued DNA replication and cell division will occur as the cell generates a colony that will contain Mal+ Gal+, Mal+ Gal-, and Mal- Gal+ recombinant cell types in addition to the Mal- Gal- parental type.
Can more than one sister chromosome recombine in a cell?
When E. coli grows on a rich medium such as LB, its division time is less than the time required to replicate the full genome. In order to grow this fast, E. coli initiates new rounds of replication before the first round has completed. Therefore, in these cells galK is present in several copies while the terminus is represented by only one copy until just prior to cell division. We examined whether our high oligo concentrations could target more than one copy of galK during oligo recombination.
As in the previous experiment, we plated for colonies on L agar immediately after the electroporation, thus before allele segregation. Within a recombinant colony, some cells will be recombinant and some will be parental with the exact percentage of recombinant cells dependent on the chromosomal position of the targeted gene, the number of targets recombined, and how early in the cell cycle the recombination occurs, ie. in a newly divided “baby” cell vs an “old” dividing cell. A newly divided cell has half as many targets as a cell actively undergoing division and a recombinant chromosome in a baby cell will likely replicate before cell division increasing the percentage of recombinant cells in the resulting colony. We expect four or eight replicating copies of the chromosome with our growth conditions.39 As more targets within a cell are modified and then replicate, the recombinant cell frequency within the colony should increase. If recombination occurred at every target on all sister chromosomes then the maximum percentage would be 50%. By a similar calculation, if eight chromosomes are being generated and only one recombines, then there should be ~6% (1/16 of the DNA strands) recombinant cells in the resultant colony. To address these predictions we determined the percentage of Gal+ cells in individual recombinant colonies taken from L agar plates. Table 2 demonstrates that the percentage of Gal+ recombinant cells within a recombinant colony varies but that these percentages sort into restricted groups or bins, suggesting that various numbers of the targeted gene had recombined in the different groups. The major groups I to V (Table 2) consisted of colonies with ~6%, ~13%, ~19%, ~25%, and ~31% recombinants in accord with our predictions. Other colonies with 37 to ~50% Gal+ recombinants were found less frequently.
Table 2.
Percentage of recombinant cells within a colony.
| Group | Expected results if 8 copies of galK are present when recombination occurs1 | Observed % Gal+ found in individual colonies2 | |
|---|---|---|---|
| # of galK recombined | % of Gal+ cells expected | ||
| I. | 1 | 6.25 | 5, 5, 6, 6, 6, 6, 7, 7 |
| II. | 2 | 12.5 | 10,10,10,11,12,12,13,14 |
| III. | 3 | 18.75 | 17,17,17,17,18,18,20,20 |
| IV. | 4 | 25 | 23,24,24,25,27 |
| V. | 5 | 31.25 | 29,30,30,33 |
| VI. | 6 | 37.5 | 37 |
| VII. | 7 | 43.75 | 40,41 |
| VIII. | 8 | 50 | 52,52 |
This population of cells is not synchronized so when recombination occurs, there is a variable number of sister chromosomes present. However, under our growth conditions cells have four or eight sister chromosomes.38
Analysis of variance (ANOVA) of these data, assuming 1/16, 2/16, 3/16 etc. of the DNA strands have recombined with the oligo confirm the model is supported by the data. The p-value was a highly significant p < 0.001 for all groups but 37.5 and 43.75 where the number of data points in the group was limiting.
Effects of growth medium on oligo recombination
Theoretically, the generation of recombinants at multiple loci on the same chromosome should be easier with cells grown in minimal medium as the cells contain fewer sister chromosomes due to the reduced growth rate.31 Accordingly, we determined how the choice of growth medium affects oligo recombination. All experiments used oligo 144, which creates a C~C mismatch and thus, should give the highest possible recombination frequency.1 When cells were grown in M63 glucose medium, however, recombination levels decreased nearly 1000-fold from levels seen with cells grown in LB, yielding only 1×104 recombinants per 108 viable cells (Table 3). Supplementing the minimal medium with all 20 amino acids did not increase recombination (data not shown). Either adding a small amount of yeast extract (0.2%) to M63 glucose medium, or using MOPS defined medium40 to grow cells increased recombination to similar levels, approximately 100-fold over levels seen in M63 glucose medium but still 10-fold lower than LB. Recombination frequencies were normal if cells were grown in LB even when the recovery medium was M63 glucose. Thus, the medium used for cell growth determines recombination level rather than the recovery or plating medium.
Table 3.
Transformation and recombination efficiencies with cells grown in either minimal or LB medium:
| Growth medium | Gal+/108 viable1 | Plasmid Transformants/108 Viable2 | Gal+ Recombinants/plasmid transformant |
|---|---|---|---|
| M63 glucose LB | 1.1×104 | 3.0×103 | 4.1% |
| 8.7×106 | 1.4×107 | 34% | |
Values are the average of three or more experiments throughout the table. Recombination was with oligo 144 in HME6.
Transformation was with 20 ng of plasmid pLT60.
What causes poor oligo-mediated recombination in cells grown in minimal medium? We asked whether recombination itself is inhibited or if cells grown in minimal medium are defective for DNA uptake. If the latter is true and recombinants are scored amongst cells that have been successfully transformed with a plasmid, the relative recombination frequency might be improved. Table 3 shows that transformation of a supercoiled plasmid is reduced ~10,000-fold with cells grown in M63 glucose medium. However, if only plasmid transformants are sampled, recombinants on the chromosome are readily found (4% of transformants). Thus, poor uptake of plasmids and presumably oligos via electroporation of cells grown in minimal medium appears to be the main reason that recombination appears defective in these cells. The normalized recombination among plasmid transformants is about 10-fold less efficient in minimal medium vs LB grown cells. This lower level may reflect in part fewer replicating copies of the target gene per cell in the more slowly growing bacteria.31
The effect of recovery time on recombinant yield
To find a recombinant generated by oligo recombination, one must either select for or against a function, or screen for the desired mutation. The length of time that elapses between addition of LB after electroporation and plating of the recombinant mix, previously referred to as “outgrowth time”16; 34; 35, impacts cell survival and cell growth thereby affecting recombinant frequency.
Cell survival following electroporation was determined by plating on L agar after various amounts of time (Table 4). If cells are plated immediately after electroporation, they suffer a 10-fold loss in viability but recover with a 15-30 min recovery period. Recombinant colonies formed after various recovery times were assayed by plating on MacConkey galactose plates, where both recombinants and non-recombinants can grow. We scored red sectored colonies as Gal+ (Figure 8). Table 4 shows that recombination levels, like survival, are inhibited by plating immediately after electroporation. Optimal recombination is seen with a 15-30 minute recovery.
Table 4.
Effect of recovery/outgrowth time on oligo recombination.
| 32° outgrowth (min.) | Viability1 | % Recombinants2 |
|---|---|---|
| 0 | 9.0×105 | 7.6 |
| 15 | 7.1×106 | 25.2 |
| 30 | 7.8×106 | 17.2 |
| 45 | 9.7×106 | 18.8 |
| 60 | 1.2×107 | 22.2 |
| 75 | 2.0×107 | 22.7 |
| 90 | 1.4×107 | 22.8 |
| 120 | 3.3×107 | 18.2 |
Titers determined on L plates at the time shown after electroporation.
Percentage of recombinant colonies on MacConkey galactose plates. Sectored red/white colonies were scored as recombinants. Recombination was with oligo 144 in HME6 cells. Values are an average of three or more experiments throughout the table.
Figure 8. Photograph of optimized oligo recombination of galKam.
Strain HME6 was recombined with the lagging-strand, 70 base oligo 144, which creates a C~C mispair when annealed to the target and thus avoid the MMR system. After a 30 minute recovery, cells were diluted and plated on a MacConkey galactose plate. Approximately 70% of the colonies contain red, ie. Gal+ cells. Most of the Gal+ colonies are sectored, consistent with multiple copies of the galK locus being present under these growth conditions.
DISCUSSION
Parameters affecting recombination
We find that host recombination functions have little or no effect on oligo recombination (Figure 1). However, several oligo parameters can greatly affect recombination. At any locus, of the two complementary oligos that can be used for recombination, the oligo identical in sequence to the lagging-strand is always more efficient,1; 2; 15; 22 however, the level of bias varies.1; 2; 13; 15 At least some of this variability may be due to the concentration of oligo used. With our standard conditions, the lagging/leading-strand bias is approximately 15- to 20-fold,1 however if less oligo is used, the bias increases dramatically (our unpublished results). We believe that the bias arises because the length of the available single-stranded region on the leading strand is limiting and thus, more restrictive when oligo concentration is also limiting.
With either a leading- or lagging-strand oligo, if the oligo concentration is too low, recombination is adversely affected by single-strand exonucleases. At our standard oligo concentration, mutation of all four single-strand exonucleases present in E. coli has little effect (Table 1), however, at 100-fold lower oligo concentrations, the mutations enhance recombination levels indicating that the exonucleases are degrading oligos. Thus, the optimal oligo concentration of 3000 molecules per cell avoids issues with single-strand exonucleases and yields consistently high recombination levels. If one uses less than optimal amounts of targeting oligo, carrier oligo can be added to titrate the inhibitory exonuclease activities.
Oligos from 40 to 70 bases in length showed similar recombinant frequencies (Figure 2). Others have reported maximum recombination with oligos of 90 or ~120 bases, respectively,6; 22 however, these frequencies were less than two-fold higher than with 70 base oligos, and since the synthesis of these longer oligos is more expensive and more mistakes are generated, we consider 70 bases to be optimal. Even with 70 base oligos, the recombinants should be sequenced as errors may have occurred during oligo synthesis.41 Note that recombination with oligos longer than ~120 bases occurs at reduced frequencies (our unpublished results),6; 22 possibly due to oligo secondary structure and/or increased mistakes in synthesis. Recombination drops off exponentially as oligo length is decreased from 40 to 23 bases, consistent with in vitro studies showing Beta binding poorly to oligos shorter than 36 bases.42 For oligos shorter than 23 bases, a very low level of recombination is found. The level of recombination seen with 21 and 22 base oligos appears to be partially Beta-dependent as this frequency of recombination is not reached in the absence of Beta at this oligo concentration.37 Recombination with oligos 15 to 20 bases in length is independent of the Red proteins as described by Swingle et. al,37 possibly because Beta cannot anneal such short oligos. Finally, the MMR system reduces recombination frequencies for all oligo lengths.
The position of the correcting base along the length of a 70 base oligo has little effect on recombination frequency as long as it is not less than 9 bases from an end. Once the change is less than 9 bases from an end, recombination frequencies decrease significantly. This effect could be largely due to degradation of the marker by exonucleases when the change is too close to an end (our unpublished results), although oligos with the correcting base at the extreme 5’ or 3’ end still generate recombinants. We have determined that the low levels of recombination seen with the correcting bases at the extreme 5’ or 3’ ends of the oligo are Red-dependent. It was previously reported that if four unpaired changes were on the 5’ or 3’ end, recombination did not occur,22 however, we find that an oligo with a single alteration at the last base of the 3’ end yields 104 recombinants per 108 viable cells. Thus, we presume that some DNA polymerase(s) is able to utilize, albeit poorly, this unpaired 3’ end as a substrate for DNA synthesis and repair. Likewise, an oligo with the correcting base on the 5’ end generates recombinants but at an even lower level, 102/108 viable cells. Here, to get a recombinant, DNA ligase must join, albeit inefficiently, the mismatched 5’ end base to an adjoining upstream DNA molecule.
Evading mismatch repair by oligo design
Although much is known about MMR in E. coli, oligo recombination provides a powerful new tool to examine behavior of the system in vivo. It was previously shown that eliminating MMR by mutation can increase oligo recombination more than 100-fold.1 Using a MMR mutant strain to increase recombination can be useful, but a drawback is the accompanying accumulation of mutations throughout the genome.27 Here we have identified three ways to design oligos that increase recombination in a MMR-proficient host and thus avoid generalized mutagenesis. The galKtyr145am model system is excellent for studying mismatch repair since amino acid substitutions in this region of the GalK protein are well tolerated without affecting the Gal+ phenotype (Figure 5).
Mismatch repair does not recognize C~C mismatches in vitro,27 and we have previously shown that an oligo creating a C~C mispair when annealed to its target greatly increases recombination in vivo.1 We show here that creating a C~C mismatch located six bases from a desired change leads to higher levels of recombination, indicating that the MMR system does not recognize this double mispair. In contrast, if the C~C mismatch is nine bases or farther away, recombination is not enhanced (Figure 4). This indicates a C~C mismatch may create a region of six bases to either side that is refractory to mismatch repair. On the other hand, a correcting C~C mismatch can be repaired resulting in lower recombination frequencies if an additional repairable mismatch is 9 bases from the C~C mismatch (data not shown). In this case efficient co-repair of the C~C mismatch with the repairable mismatch has likely occurred.
Previously it has been shown that the MMR system does not recognize a five base insertion/deletion on one strand and poorly recognizes a four base insertion/deletion.28 Consistent with these results, we found that substituting four or more consecutive bases (Figure 5 and data not shown) also escapes MMR. However, we observed exceptional cases where a single T~T mismatch or three alterations in a row resulted in high levels of recombination (Figure 5). These “exceptions” are likely due to sequence context effects. Although useful, methods using a C~C mismatch or altering four or more consecutive bases have limitations. Creation of a C~C mismatch is not always feasible, and changing four consecutive bases of a gene may alter coding sequence and function. A powerful new way to avoid both of these problems is to alter four or more wobble positions of adjacent codons in addition to the desired alteration (Figure 6). This configuration of mismatches evades the MMR system allowing high frequency targeted mutagenesis without additional undesired changes to the encoded protein. This method is of general utility and is particularly useful for mutating essential genes with high efficiency in a single recombination reaction. It has been used to generate over 40 mutations in the E. coli RNA polymerase gene, rpoB (our unpublished results). Although the amino acid sequence need not be altered due to the wobble changes, it should be kept in mind that infrequently, synonymous substitutions lead to altered protein expression; such altered expression is often due to mRNA stability43 rather than codon bias. If this is a concern, an additional recombination step can be included to revert the wobble positions to wild type, leaving the desired mutation intact. This altered wobble position procedure should yield high efficiency targeted modification within any protein-coding sequence, allowing high throughput alterations of genomes. For either consecutive or wobble position changes, to be safe we suggest changing five or more bases. Having five changes allows specific detection of mutations by colony PCR. These advances in recombineering technology should be readily applicable to other organisms where MMR exists and oligo recombination has been established such as Salmonella, Shigella, Yersinia, and Pseudomonas.
Growth considerations
Bacteria grown in LB contain several replicating copies (4-8) of the chromosome,39 such that recombination might occur on any one strand of any sister chromosome.1 However, even if recombination occurred at every target on all sister chromosomes, at most, 50% of the DNA strands can be recombinant. Thus, if cells are plated immediately after electroporation, the colony that forms will always be a mixture of parental and recombinant cells (Figure 8). Pure recombinant colonies are obtained only if the recombined cells are allowed several generations of outgrowth (about three hrs at 30°C) permitting DNA replication and cell division. During the outgrowth, the recombinant and parental chromosomes segregate away from each other and into daughter cells. DNA replication and cell division reduce the frequency of cells containing recombinants as much as 8- to 16-fold for E. coli growing in LB.39 If the recombinant cell has a growth disadvantage and the recombination mix is allowed to recover for extended periods, recombinants will be greatly diluted out by cell growth and division.
We note a loss of viability of electroporated cells when allowed to recover less than 15 minutes. Thus, we recommend that the minimal length of recovery should be 30 minutes. This allows for full recovery from the electroporation yet avoids segregation and dilution of the recombinant chromosome, which make it harder to screen for the recombinant. This short recovery is also ideal for selecting for growth on a non-toxic medium (e.g. selecting Gal+), or screening for recombinants non-selectively by diluting and plating on L agar plates after the recovery period. Screening techniques such as PCR and colony hybridization16; 44 can be used to identify recombinants.
Cells grown in minimal medium take up DNA poorly, yielding very low recombination levels with oligos and poor transformation with plasmids. However, among cells selected as plasmid transformants when co-electroporated with oligos, the relative frequency of oligo recombination increased 100-fold (Table 3). Similar techniques to find recombinants have proven useful in Saccharomyces cerevisiae45 and Mycobacterium tuberculosis13 and should be considered when moving oligo recombination technology to organisms where cells must be grown in minimal medium or DNA uptake may be an issue. To our knowledge, poor uptake of DNA by electrotransformation of cells grown in minimal medium has not been extensively studied but consistent with our findings, it was previously shown that phage DNA transfection by electroporation into E. coli is less efficient when cells were grown in minimal medium.46
Recombination with multiple oligos
We have shown that two different oligos can be used to alter simultaneously two genes in an individual cell with a single round of recombination. We expect that numerous different oligos can be used to modify single or multiple loci simultaneously, with the limiting factors being how much DNA can be introduced into a cell and having effective screens or selections to find the changes.6 The presence of several DNA replication forks in rapidly dividing E. coli provides multiple targets for oligo recombination, and explains our results with co-electrotransformation by two different oligos. Within a single recombinant colony (plated without any recovery period), we found cells that were Mal+ Gal-, Mal- Gal+, Mal+ Gal+, as well as the parental Mal- Gal-. One of the recombinant classes observed, Mal+ Gal+, can be explained by a recombination event occurring when a replication fork reaches malK, and a second recombination event on that same DNA strand after the fork travels another 1.2 megabases to the galK locus. In the original recombinant cell, two additional recombination events involving other forks on sister chromosomes are likely to have occurred to generate the Mal+ Gal- and Mal- Gal+ recombinants found. Thus, in this cell, we speculate that three separate DNA forks and four oligo recombination events were involved in generating the recombinants seen in a single colony (Figure 7). The recombination events could occur concurrently in one cell as described or, if Beta and the oligo persist, additional recombination events might occur even after the cell starts to divide to form the colony. A much more extensive study of recombinants in a single colony is warranted. For example, having the two loci on opposite sides of the origin of DNA replication or one near the origin and one near the terminus should prove fruitful for better understanding multiple oligo recombination events with the process of DNA replication.
For cells grown in LB, four or eight copies of the genome can be present.39 As seen in Figure 7, during DNA replication one or more of the target lagging-strands may be recombined. In the four chromosome example shown, anywhere from 1/8 to 1/2 of the total DNA strands (lagging plus leading) can be recombined for a given marker, resulting in 12-50% of the cells in the colony being recombinant. If there are eight copies of the genome in a targeted cell, then 1/16 to 1/2 of the DNA strands can be modified. Initial analysis of individual cells from recombinant colonies has demonstrated recombinant frequencies from different colonies with this non-overlapping pattern, ie. 1/16, 1/8, 3/16, 1/4, 5/16, 3/8, or 1/2 of the cells being recombinant (Table 2). This pattern would be expected when as many as eight copies of galK are present. Some cells, however, would have had four chromosomes when recombination occurred yielding a subset of these groups (1/8, 1/4, 3/8, 1/2). Here again a broader study is required to understand the targeting and frequency of oligo recombination within a single cell.
Key results to consider when using recombineering as a technique
Recombination with an oligo is a precise, rapid and simple way to modify both large and small replicons and can be used to create point mutations as well as deletions and small insertions. General guidelines for optimizing oligo recombination include: 1) avoid the MMR system 2) use a lagging-strand oligo 3) use saturating oligo concentrations 4) use a 70 base oligo and 5) locate the altered bases >9 bases from an end. Figure 8 shows E. coli recombinants obtained where conditions are optimal. Avoiding the mismatch repair system through careful oligo design can maximize the frequency of oligo recombination in cells wild type for MMR. These techniques avoid the generalized mutagenesis associated with the absence of mismatch repair and are of general utility for both automated systems such as MAGE6 and researchers using standard recombineering procedures.
MATERIALS AND METHODS
Baterial Strains
HME6 is W3110 galKtyr145UAG ΔlacU169 [λ cI857 Δ(cro-bioA)].2 Strains shown in Figure 1 are derivatives of HME6. All mutations in the recombination genes are complete gene replacements where the coding sequence was replaced with a drug-resistance cassette by dsDNA recombineering.10 HME63 is HME6 mutS<>amp. Strain XTL74 is HME6 recJ<>amp xonA<>kan xseA<>tet exoX<>spec. HME58 is HME6 but with galKtyr145UAA instead of an amber mutation. HME82 is HME6 malKtyr84UAG mutS<>amp. Details of strain constructions are available upon request.
Oligonucleotides and plasmids
Oligos were purchased from IDT as salt-free but otherwise unpurified. Oligos 100, 101, 144 and the malK oligo were as described previously.1 The “carrier” oligo was LT217, an oligo with homology to a plasmid not in the cells of this experiment.47 The DNA sequences of all oligos are available upon request. Plasmid pLT60 is an amp-resistant pUC-derivative with a kantyr39UAG allele.47
Recombination assay
Unless otherwise noted, the recombination assay for all experiments was as described in Costantino and Court.1 Cells were prepared for recombination by standard methods.34; 35 Five pmoles of oligo was electrotransformed into the cells, 1 ml of LB was added and cells were allowed to recover at 30°C with shaking. The length of recovery time varies from experiment to experiment and is indicated in the figures, tables, and text. Cells were diluted in TMG or M9 salts and plated on M63 minimal galactose plates with biotin to select for Gal+ recombinants.1 Cells were also diluted and plated on L plates to determine total viable cells or to screen for recombinants among the total. For the experiments in minimal medium, cells were grown to an OD600 of 0.4 and prepared for recombineering by the standard protocol.34 Other relevant methods are described in table and figure legends.
Proper mixing is important for optimized recombination levels
Pipette the electro-competent cells into the chilled microfuge tubes or cuvettes. Add the 0.5-1 μl of salt free oligo. Using a 200 μl pipette tip, pipette up and down several times to mix. At no time should electro-competent cells be vortexed. Use care to keep the cells in the electotransformation chamber of the cuvette before electrotransformation. Once electrotransformation is complete, quickly add the 1 ml of LB for recovery, pipetting up and down several times before finally transferring the entire volume immediately to an recovery tube. Never electrotransform without prior mixing of the DNA and cells.
Media
LB, L agar, M63 galactose with biotin, M63 glucose, TMG, MOPS,40 MacConkey galactose, MacConkey maltose are as described previously.1; 4; 34 For the plasmid transformation experiment, ampicillin was added at 100 μg/ml.
Acknowledgements
We thank Xiaomei Zhou for helpful discussions and Matthew Fivash for statistical assistance. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and in part by a Trans National Institutes of Health/Food and Drug Administration Intramural Biodefense Program Grant of National Institutes of Allergy and Infectious Disease (to D.L.C.). This project has been also partly funded with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- MMR
methyl-directed mismatch repair
- MAGE
multiplex automated genome engineering
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costantino N, Court DL. Enhanced levels of λ Red-mediated recombinants in mismatch repair mutants. Proc. Natl. Acad. Sci. USA. 2003;100:15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis HM, Yu D, DiTizio T, Court DL. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proc. Natl. Acad. Sci. USA. 2001;98:6742–6746. doi: 10.1073/pnas.121164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muyrers JP, Zhang Y, Testa G, Stewart AF. Rapid modification of bacterial artificial chromosomes by ET- recombination. Nucleic Acids Res. 1999;27:1555–1557. doi: 10.1093/nar/27.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu D, Sawitzke JA, Ellis H, Court DL. Recombineering with overlapping single-stranded DNA oligonucleotides: testing a recombination intermediate. Proc. Natl. Acad. Sci. USA. 2003;100:7207–7212. doi: 10.1073/pnas.1232375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 6.Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, Forest CR, Church GM. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warner JR, Reeder PJ, Karimpour-Fard A, Woodruff LB, Gill RT. Rapid profiling of a microbial genome using mixtures of barcoded oligonucleotides. Nat. Biotechnol. 2010;28:856–862. doi: 10.1038/nbt.1653. [DOI] [PubMed] [Google Scholar]
- 8.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy KC. Use of bacteriophage λ recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 1998;180:2063–2071. doi: 10.1128/jb.180.8.2063-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta S, Costantino N, Zhou X, Court DL. Identification and analysis of recombineering functions from Gram-negative and Gram-positive bacteria and their phages. Proc. Natl. Acad. Sci. USA. 2008;105:1626–1631. doi: 10.1073/pnas.0709089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kessel JC, Hatfull GF. Recombineering in Mycobacterium tuberculosis. Nat. Methods. 2007;4:147–152. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- 13.van Kessel JC, Hatfull GF. Efficient point mutagenesis in mycobacteria using single-stranded DNA recombineering: characterization of antimycobacterial drug targets. Mol. Microbiol. 2008;67:1094–1107. doi: 10.1111/j.1365-2958.2008.06109.x. [DOI] [PubMed] [Google Scholar]
- 14.Swingle B, Bao Z, Markel E, Chambers A, Cartinhour S. Recombineering using RecTE from Pseudomonas syringae. Appl. Environ. Microbiol. 2010;76:4960–4968. doi: 10.1128/AEM.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XT, Costantino N, Lu LY, Liu DP, Watt RM, Cheah KS, Court DL, Huang JD. Identification of factors influencing strand bias in oligonucleotide-mediated recombination in Escherichia coli. Nucleic Acids Res. 2003;31:6674–6687. doi: 10.1093/nar/gkg844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharan SK, Thomason LC, Kuznetsov SG, Court DL. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Prot. 2009;4:206–223. doi: 10.1038/nprot.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Sharan SK. A simple two-step, ‘hit and fix’ method to generate subtle mutations in BACs using short denatured PCR fragments. Nucleic Acids Res. 2003;31:e80. doi: 10.1093/nar/gng080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 1999;63:751–813. doi: 10.1128/mmbr.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark AJ, Sandler SJ. Homologous genetic recombination: the pieces begin to fall into place. Crit. Rev. Microbiol. 1994;20:125–142. doi: 10.3109/10408419409113552. [DOI] [PubMed] [Google Scholar]
- 20.Brooks K, Clark AJ. Behavior of λ bacteriophage in a recombination-deficient strain of Escherichia coli. J. Virol. 1967;1:283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shulman MJ, Hallick LM, Echols H, Signer ER. Properties of recombination-deficient mutants of bacteriophage λ. J. Mol. Biol. 1970;52:501–520. doi: 10.1016/0022-2836(70)90416-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Muyrers JP, Rientjes J, Stewart AF. Phage annealing proteins promote oligonucleotide-directed mutagenesis in Escherichia coli and mouse ES cells. BMC Mol. Biol. 2003;4:1. doi: 10.1186/1471-2199-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu LY, Huen MS, Tai AC, Liu DP, Cheah KS, Huang JD. Highly efficient deletion method for the engineering of plasmid DNA with single-stranded oligonucleotides. BioTechniques. 2008;44:217–20. 222, 224. doi: 10.2144/000112684. [DOI] [PubMed] [Google Scholar]
- 24.Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat. Rev. Genet. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 25.Murphy KC, Marinus MG. RecA-independent single-stranded DNA oligonucleotide-mediated mutagenesis. F1000 Biology Reports. 2010;2:56. doi: 10.3410/B2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayssiguier C, Thaler DS, Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 27.Modrich P. Mechanisms and biological effects of mismatch repair. Annu. Rev. Genet. 1991;25:229–253. doi: 10.1146/annurev.ge.25.120191.001305. [DOI] [PubMed] [Google Scholar]
- 28.Parker BO, Marinus MG. Repair of DNA heteroduplexes containing small heterologous sequences in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1992;89:1730–1734. doi: 10.1073/pnas.89.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones M, Wagner R, Radman M. Repair of a mismatch is influenced by the base composition of the surrounding nucleotide sequence. Genetics. 1987;115:605–610. doi: 10.1093/genetics/115.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi A, Rao BJ. MutS recognition: multiple mismatches and sequence context effects. J. Biosci. (Bangalore) 2001;26:595–606. doi: 10.1007/BF02704758. [DOI] [PubMed] [Google Scholar]
- 31.Brendler T, Sawitzke J, Sergueev K, Austin S. A case for sliding SeqA tracts at anchored replication forks during Escherichia coli chromosome replication and segregation. EMBO J. 2000;19:6249–6258. doi: 10.1093/emboj/19.22.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huen MS, Li XT, Lu LY, Watt RM, Liu DP, Huang JD. The involvement of replication in single stranded oligonucleotide-mediated gene repair. Nucleic Acids Res. 2006;34:6183–6194. doi: 10.1093/nar/gkl852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burdett V, Baitinger C, Viswanathan M, Lovett ST, Modrich P. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc. Natl. Acad. Sci. USA. 2001;98:6765–6770. doi: 10.1073/pnas.121183298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawitzke JA, Thomason LC, Costantino N, Bubunenko M, Datta S, Court DL. Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 2007;421:171–199. doi: 10.1016/S0076-6879(06)21015-2. [DOI] [PubMed] [Google Scholar]
- 35.Thomason L, Court DL, Bubunenko M, Costantino N, Wilson H, Datta S, Oppenheim A. Recombineering: Genetic engineering in bacteria using homologous recombination. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 1. John Wiley & Sons, Inc.; Hoboken, N.J.: 2007. pp. 1–24. pp. Unit 16. 6 vols. [DOI] [PubMed] [Google Scholar]
- 36.Dutra BE, Sutera VA, Jr., Lovett ST. RecA-independent recombination is efficient but limited by exonucleases. Proc. Natl. Acad. Sci. USA. 2007;104:216–221. doi: 10.1073/pnas.0608293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swingle B, Markel E, Costantino N, Bubunenko MG, Cartinhour S, Court DL. Oligonucleotide recombination in Gram-negative bacteria. Mol. Microbiol. 2010;75:138–148. doi: 10.1111/j.1365-2958.2009.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gasc AM, Sicard AM, Claverys JP. Repair of single- and multiple-substitution mismatches during recombination in Streptococcus pneumoniae. Genetics. 1989;121:29–36. doi: 10.1093/genetics/121.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sergueev K, Court D, Reaves L, Austin S. E. coli cell-cycle regulation by bacteriophage lambda. J. Mol. Biol. 2002;324:297–307. doi: 10.1016/s0022-2836(02)01037-9. [DOI] [PubMed] [Google Scholar]
- 40.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J. Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oppenheim AB, Rattray AJ, Bubunenko M, Thomason LC, Court DL. In vivo recombineering of bacteriophage λ by PCR fragments and single-strand oligonucleotides. Virology. 2004;319:185–189. doi: 10.1016/j.virol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Mythili E, Kumar KA, Muniyappa K. Characterization of the DNA-binding domain of β protein, a component of phage λ Red-pathway, by UV catalyzed cross-linking. Gene. 1996;182:81–87. doi: 10.1016/s0378-1119(96)00518-5. [DOI] [PubMed] [Google Scholar]
- 43.Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli. Science. 2009;324:255–258. doi: 10.1126/science.1170160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swaminathan S, Ellis HM, Waters LS, Yu D, Lee E-C, Court DL, Sharan SK. Rapid engineering of bacterial artificial chromosomes using oligonucleotides. Genesis. 2001;29:14–21. doi: 10.1002/1526-968x(200101)29:1<14::aid-gene1001>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Moerschell RP, Wakem LP, Komar-Panicucci S, Sherman F. Strand-specificity in the transformation of yeast with synthetic oligonucleotides. Genetics. 1992;131:811–819. doi: 10.1093/genetics/131.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taketo A. Properties of electroporation-mediated DNA transfer in Escherichia coli. J. Biochem., Tokyo. 1989;105:813–817. doi: 10.1093/oxfordjournals.jbchem.a122750. [DOI] [PubMed] [Google Scholar]
- 47.Thomason LC, Costantino N, Shaw DV, Court DL. Multicopy plasmid modification with phage λ Red recombineering. Plasmid. 2007;58:148–158. doi: 10.1016/j.plasmid.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]