Abstract
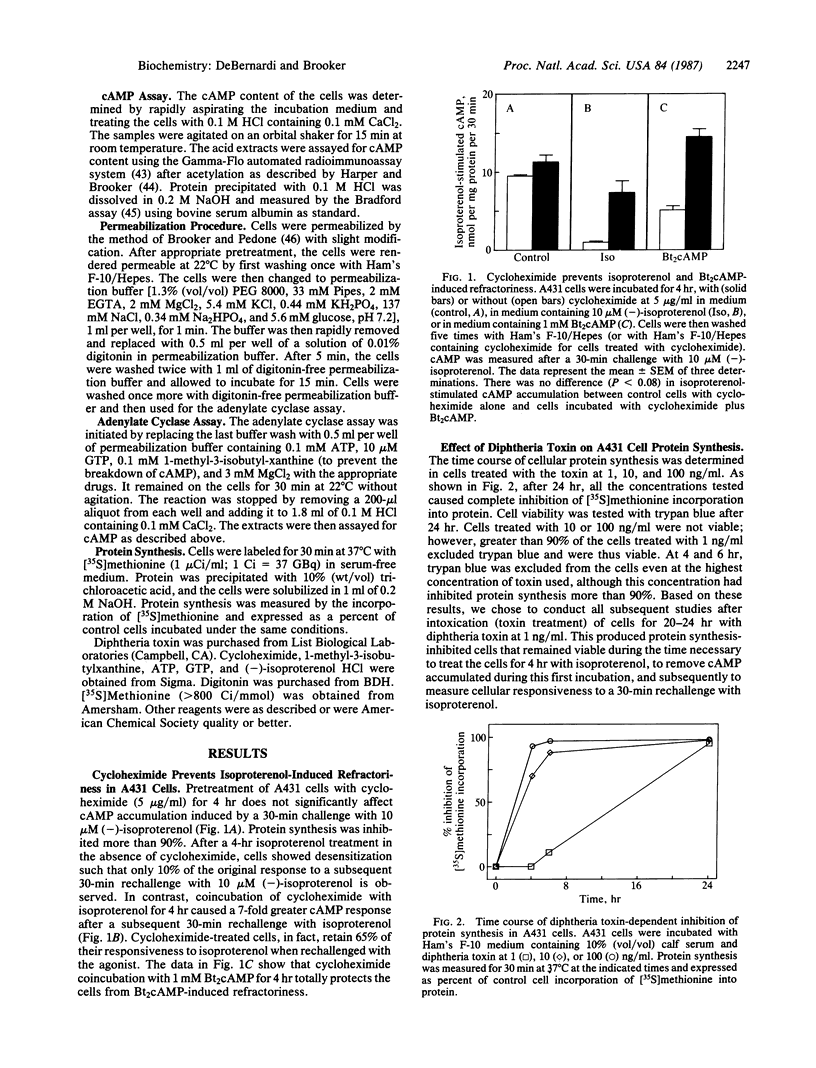
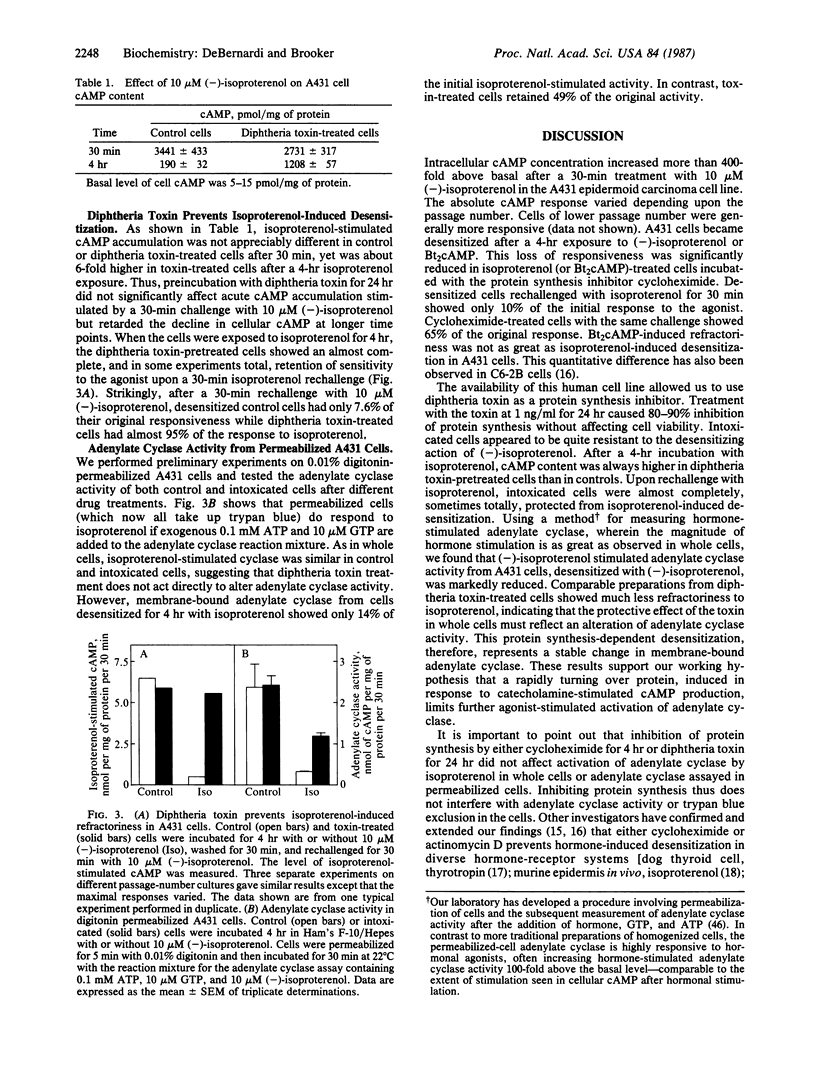
We proposed that a rapidly turning over protein, induced in response to catecholamine stimulation of C6-2B rat astrocytoma cells, inhibits subsequent hormonal activation of adenylate cyclase. Studies upon which our hypothesis is based and confirmatory work in a variety of other cell lines and in vivo have utilized actinomycin D and cycloheximide to inhibit RNA and protein synthesis, respectively. These inhibitors, however, are not specific and have been reported also to interfere with other cellular processes. Diphtheria toxin is a specific protein synthesis inhibitor that acts only by ADP-ribosylating elongation factor 2, thus preventing peptide chain elongation. We thus tested whether diphtheria toxin could prevent catecholamine-induced desensitization in A431 human epidermoid carcinoma cells. The toxin inhibited protein synthesis and altered the time course of isoproterenol-stimulated cAMP accumulation as did the less-specific protein synthesis inhibitor cycloheximide. Cellular cAMP content after a 30-min exposure to isoproterenol was similar in control and in toxin-treated cells. However, after 4 hr of treatment with isoproterenol, toxin-treated cells accumulated up to six times more cAMP than controls. When cells or cell-free adenylate cyclase preparations were rechallenged with agonists, toxin-mediated inhibition of protein synthesis prevented desensitization. These results show that diphtheria toxin, a specific inhibitor of protein synthesis, can interfere with the normal physiological regulation of cAMP metabolism in eukaryotic cells and provide compelling evidence that catecholamine stimulation of adenylate cyclase promotes the synthesis of a protein(s) that, in some way, inhibits hormone-stimulated adenylate cyclase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balinsky J. B., Shambaugh G. E., 3rd, Cohen P. P. Glutamate dehydrogenase biosynthesis in amphibian liver preparations. J Biol Chem. 1970 Jan 10;245(1):128–137. [PubMed] [Google Scholar]
- Barovsky K., Pedone C., Brooker G. Forskolin-stimulated cyclic AMP accumulation mediates protein synthesis-dependent refractoriness in C6-2B rat glioma cells. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(3):181–189. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh C., Ahrén K. Prolongation of the effects of gonadotrophins and prostaglandin E2 on ovarian cyclic AMP formation by inhibitors of protein synthesis. Acta Endocrinol (Copenh) 1980 Jun;94(2):251–258. doi: 10.1530/acta.0.0940251. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brooker G., Pedone C. Maintenance of whole cell isoproterenol and forskolin responsiveness in adenylate cyclase of permeabilized cells. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(2):113–121. [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Caron M. G., Cerione R. A., Benovic J. L., Strulovici B., Staniszewski C., Lefkowitz R. J., Codina-Salada J., Birnbaumer L. Biochemical characterization of the adrenergic receptors: affinity labeling, purification, and reconstitution studies. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:1–12. [PubMed] [Google Scholar]
- Cerione R. A., Sibley D. R., Codina J., Benovic J. L., Winslow J., Neer E. J., Birnbaumer L., Caron M. G., Lefkowitz R. J. Reconstitution of a hormone-sensitive adenylate cyclase system. The pure beta-adrenergic receptor and guanine nucleotide regulatory protein confer hormone responsiveness on the resolved catalytic unit. J Biol Chem. 1984 Aug 25;259(16):9979–9982. [PubMed] [Google Scholar]
- DeVellis J., Brooker G. Reversal of catecholamine refractoriness by inhibitors of RNA and protein synthesis. Science. 1974 Dec 27;186(4170):1221–1223. doi: 10.1126/science.186.4170.1221. [DOI] [PubMed] [Google Scholar]
- Dix C. J., Cooke B. A. Effect of lutropin and cycloheximide on lutropin receptors and cyclic AMP production in Leydig tumour cells in vitro. Biochem J. 1981 Jun 15;196(3):713–719. doi: 10.1042/bj1960713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Ernest M. J. Regulation of tyrosine aminotransferase messenger ribonucleic acid in rat liver. effect of cycloheximide on messenger ribonucleic acid turnover. Biochemistry. 1982 Dec 21;21(26):6761–6767. doi: 10.1021/bi00269a022. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. The elongation factor 2 content of mammalian cells. Assay method and relation to ribosome number. J Biol Chem. 1973 Jan 25;248(2):654–658. [PubMed] [Google Scholar]
- Green D. A., Clark R. B. Adenylate cyclase coupling proteins are not essential for agonist-specific desensitization of lymphoma cells. J Biol Chem. 1981 Mar 10;256(5):2105–2108. [PubMed] [Google Scholar]
- Grossman A., Mavrides C. Studies on the regulation of tyrosine aminotransferase in rats. J Biol Chem. 1967 Apr 10;242(7):1398–1405. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Harper J. F. Desensitization in rat parotid to beta-adrenergic agonists and counteracting effects of forskolin are conserved in membrane and detergent-solubilized adenylate cyclase catalyst activity. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(3):167–176. [PubMed] [Google Scholar]
- Hedin L., Ahrén K. Involvement of cyclic AMP and protein synthesis in the desensitization of the prepubertal rat ovary. Mol Cell Endocrinol. 1983 Mar;29(3):335–347. doi: 10.1016/0303-7207(83)90021-7. [DOI] [PubMed] [Google Scholar]
- Hertel C., Coulter S. J., Perkins J. P. A comparison of catecholamine-induced internalization of beta-adrenergic receptors and receptor-mediated endocytosis of epidermal growth factor in human astrocytoma cells. Inhibition by phenylarsine oxide. J Biol Chem. 1985 Oct 15;260(23):12547–12553. [PubMed] [Google Scholar]
- Hildebrandt J. D., Sekura R. D., Codina J., Iyengar R., Manclark C. R., Birnbaumer L. Stimulation and inhibition of adenylyl cyclases mediated by distinct regulatory proteins. Nature. 1983 Apr 21;302(5910):706–709. doi: 10.1038/302706a0. [DOI] [PubMed] [Google Scholar]
- Hirayu H., Magnusson R. P., Rapoport B. Studies on the mechanism of desensitization of the cyclic AMP response to TSH stimulation in a cloned rat thyroid cell line. Mol Cell Endocrinol. 1985 Aug;42(1):21–27. doi: 10.1016/0303-7207(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Hoebeke J., Durieu O., Delavier C., Schmutz A., Strosberg A. D. Biochemical and immunological studies of beta-adrenergic receptors on various cell types. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:73–80. [PubMed] [Google Scholar]
- Honig G. R., Rabinovitz M. Actinomycin D: inhibition of protein synthesis unrelated to effect on template RNA synthesis. Science. 1965 Sep 24;149(3691):1504–1506. doi: 10.1126/science.149.3691.1504. [DOI] [PubMed] [Google Scholar]
- Jomain-Baum M., Garber A. J., Farber E., Hanson R. W. The effect of cycloheximide on the interaction between mitochondrial respiration and gluconeogenesis in guinea pig and rat liver. J Biol Chem. 1973 Mar 10;248(5):1536–1543. [PubMed] [Google Scholar]
- Laszlo J., Miller D. S., McCarty K. S., Hochstein P. Actinomycin D: inhibition of respiration and glycolysis. Science. 1966 Feb 25;151(3713):1007–1010. doi: 10.1126/science.151.3713.1007. [DOI] [PubMed] [Google Scholar]
- Marks F. Induction of catecholamine refractoriness by isoproterenol via a cycloheximide-sensitive reaction in mouse epidermis in vivo. FEBS Lett. 1980 May 5;113(2):206–210. doi: 10.1016/0014-5793(80)80592-8. [DOI] [PubMed] [Google Scholar]
- May D. C., Ross E. M., Gilman A. G., Smigel M. D. Reconstitution of catecholamine-stimulated adenylate cyclase activity using three purified proteins. J Biol Chem. 1985 Dec 15;260(29):15829–15833. [PubMed] [Google Scholar]
- Mitranic M., Sturgess J. M., Moscarello M. A. The effect of cycloheximide on galactosyltransferase of rat liver Golgi membranes. Biochim Biophys Acta. 1976 Feb 24;421(2):272–279. doi: 10.1016/0304-4165(76)90293-2. [DOI] [PubMed] [Google Scholar]
- Nickols G. A., Brooker G. Induction of refractoriness to isoproterenol by prior treatment of C6-2B rat astrocytoma cells with cholera toxin. J Cyclic Nucleotide Res. 1979 Dec;5(6):435–447. [PubMed] [Google Scholar]
- Nilsson H., Ahrén B., Gustafson A., Hedner P. Effect of inhibition of protein synthesis on thyroid cyclic AMP accumulation in vitro. Acta Endocrinol (Copenh) 1981 Jun;97(2):202–206. doi: 10.1530/acta.0.0970202. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Pastan I., Friedman R. M. Actinomycin D: inhibition of phospholipid synthesis in chick embryo cells. Science. 1968 Apr 19;160(3825):316–317. doi: 10.1126/science.160.3825.316. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Pfeuffer E., Dreher R. M., Metzger H., Pfeuffer T. Catalytic unit of adenlyate cyclase: purification and identification by affinity crosslinking. Proc Natl Acad Sci U S A. 1985 May;82(10):3086–3090. doi: 10.1073/pnas.82.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport B., Adams R. J. Induction of refractoriness to thyrotropin stimulation in cultured thyroid cells. Dependence on new protein synthesis. J Biol Chem. 1976 Nov 10;251(21):6653–6661. [PubMed] [Google Scholar]
- Reddy P., Miller D., Peterkofsky A. Stimulation of Escherichia coli adenylate cyclase activity by elongation factor Tu, a GTP-binding protein essential for protein synthesis. J Biol Chem. 1986 Sep 5;261(25):11448–11451. [PubMed] [Google Scholar]
- Reel J. R., Kenney F. T. "Superinduction" of tyrosine transaminase in hepatoma cell cultures: differential inhibition of synthesis and turnover by actionomycin D. Proc Natl Acad Sci U S A. 1968 Sep;61(1):200–206. doi: 10.1073/pnas.61.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robishaw J. D., Russell D. W., Harris B. A., Smigel M. D., Gilman A. G. Deduced primary structure of the alpha subunit of the GTP-binding stimulatory protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1251–1255. doi: 10.1073/pnas.83.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambaugh G. E., 3rd, Balinsky J. B., Cohen P. P. Synthesis of carbamyl phosphate synthetase in amphibian liver in vitro. The effect of thyroxine. J Biol Chem. 1969 Oct 10;244(19):5295–5308. [PubMed] [Google Scholar]
- Smigel M. D. Purification of the catalyst of adenylate cyclase. J Biol Chem. 1986 Feb 5;261(4):1976–1982. [PubMed] [Google Scholar]
- Sullia S. B., Griffin D. H. Inhibition of DNA synthesis by cycloheximide and blasticidin-S is independent of their effect on protein synthesis. Biochim Biophys Acta. 1977 Mar 2;475(1):14–22. doi: 10.1016/0005-2787(77)90334-3. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G., de Vellis J., Inglish D., Hsu C. Y., Moylan R. D. Involvement of cyclic amp and protein synthesis in catecholamine refractoriness. Adv Cyclic Nucleotide Res. 1978;9:33–52. [PubMed] [Google Scholar]
- Timberlake W. E., Griffin D. H. Direct inhibition of the uptake of proline by cycloheximide. Biochem Biophys Res Commun. 1973 Sep 5;54(1):216–221. doi: 10.1016/0006-291x(73)90910-8. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Hagen G., Griffin D. H. Rat liver DNA-dependent RNA polymerase I is inhibited by cycloheximide. Biochem Biophys Res Commun. 1972 Aug 21;48(4):823–827. doi: 10.1016/0006-291x(72)90681-x. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Griffin E. E. Reduction by cycloheximide of lysosomal proteolytic enzyme activity and rate of protein degradation in organ-cultured hearts. Biochim Biophys Acta. 1976 Sep 24;444(2):519–524. doi: 10.1016/0304-4165(76)90395-0. [DOI] [PubMed] [Google Scholar]


