Abstract
Background
The prognostic value of cytogenetic findings in chronic myelomonocytic leukemia is unclear. Our purpose was to evaluate the independent prognostic impact of cytogenetic abnormalities in a large series of patients with chronic myelomonocytic leukemia included in the database of the Spanish Registry of Myelodysplastic Syndromes.
Design and Methods
We studied 414 patients with chronic myelomonocytic leukemia according to WHO criteria and with a successful conventional cytogenetic analysis at diagnosis. Different patient and disease characteristics were examined by univariate and multivariate methods to establish their relationship with overall survival and evolution to acute myeloid leukemia.
Results
Patients with abnormal karyotype (110 patients, 27%) had poorer overall survival (P=0.001) and higher risk of acute myeloid leukemia evolution (P=0.010). Based on outcome analysis, three cytogenetic risk categories were identified: low risk (normal karyotype or loss of Y chromosome as a single anomaly), high risk (presence of trisomy 8 or abnormalities of chromosome 7, or complex karyotype), and intermediate risk (all other abnormalities). Overall survival at five years for patients in the low, intermediate, and high risk cytogenetic categories was 35%, 26%, and 4%, respectively (P<0.001). Multivariate analysis confirmed that this new CMML-specific cytogenetic risk stratification was an independent prognostic variable for overall survival (P=0.001). Additionally, patients belonging to the high-risk cytogenetic category also had a higher risk of acute myeloid leukemia evolution on univariate (P=0.001) but not multivariate analysis.
Conclusions
Cytogenetic findings have a strong prognostic impact in patients with chronic myelomonocytic leukemia.
Keywords: chronic myelomonocytic leukemia, CMML, cytogenetic
Introduction
Chronic myelomonocytic leukemia (CMML) is a rare clonal hematologic disorder, with a heterogeneous clinical and morphological expression, sharing features of both myelodysplastic syndromes (MDS) and chronic myeloproliferative disorders. Classification of chronic myelomonocytic leukemia is controversial. It was considered a myelodysplastic syndrome disorder in the original FAB (French–American–British) classification.1 In 1994, the FAB group distinguished two subtypes of chronic myelomonocytic leukemia depending on the absolute leukocyte count at diagnosis (lower and equal or greater than 13×109/L):2 a myelodysplastic syndrome type (CMML–MD) and a myeloproliferative disorder variant (CMML–MP). Taking this into account, chronic myelomonocytic leukemia cases with an absolute leukocyte count greater than 12×109/L were excluded from the cooperative study that resulted in the development of the International Prognostic Scoring System (IPSS).3 More recently, the World Health Organization’s (WHO) panel of experts decided to include chronic myelomonocytic leukemia in a new category of mixed myeloproliferative/myelodysplastic neoplasms and to segregate chronic myelomonocytic leukemia cases into two categories, CMML-1 and CMML-2, depending on the proportion of blasts in peripheral blood (PB) and bone marrow (BM).4,5
Certain chromosomal abnormalities have a strong impact on the outcome for patients with myelodysplastic syndrome and are used for risk stratification in both the IPSS and the WHO classification-based prognostic scoring system (WPSS).3,6 However, the prognostic value of chromosomal abnormalities in chronic myelomonocytic leukemia is unclear. The only series assessing the potential impact of cytogenetics in chronic myelomonocytic leukemia was unable to find an independent relationship between cytogenetic results and outcomes.7
The aims of this study were to evaluate the incidence and independent prognostic value of cytogenetic abnormalities in a large series of 414 patients from the Spanish MDS Registry diagnosed with chronic myelomonocytic leukemia and with a successful conventional cytogenetic study available.
Design and Methods
Data collection
The Spanish MDS Registry is a database of the Spanish MDS cooperative group, which was constituted in 2005 to promote collaborative research among Spanish institutions working on myelodysplastic syndromes. The database includes retrospective and prospective clinical and biological data from patients diagnosed with myelodysplastic syndromes at the participating institutions. On completion of this report the database contained complete information on 564 patients with chronic myelomonocytic leukemia. All data were verified and updated by the institution's physicians and data managers, and double-checked for avoiding duplicate cases.
Patients
A total of 414 patients diagnosed with chronic myelomonocytic leukemia between 1980 and 2008, with a successful conventional cytogenetic analysis at diagnosis, and included in the database of the Spanish MDS Registry constitute the basis of the present report (participating institutions are listed in Appendix 1). Diagnosis of chronic myelomonocytic leukemia was made according to WHO criteria.4,5 In keeping with the guidelines of the Declaration of Helsinki, this retrospective non-interventional study was conducted with the approval of both the internal review board of Bioethics and Medical Research at the University Hospital La Fe and the Spanish MDS Registry. All patients or legal guardians provided informed consent.
Cytogenetics
Bone marrow samples for cytogenetic analysis were obtained from all patients at the time of diagnosis and were processed after short-term culture (24 h) following standard procedures. The chromosomes were stained by G-banding and cytogenetic results were interpreted and reported according to the International System for Human Cytogenetic Nomenclature (ISCN, 2005)8 recommendations. The presence of less than 20 evaluable metaphases did not disqualify cases from study inclusion as long as 10 or more metaphases were examined in those patients with normal karyotypes. Most cytogenetic analyses were performed at nine reference laboratories participating in an external quality program of the Spanish Haematological Cytogenetics Working Group (Grupo Cooperativo Español de Citogenética Hematológica, GCECGH). The cytogenetic reports but not the original metaphase slides from all cases were reviewed centrally (JC and ES) to ensure they followed the ISCN 2005 nomenclature guidelines.8
Prognostic factors
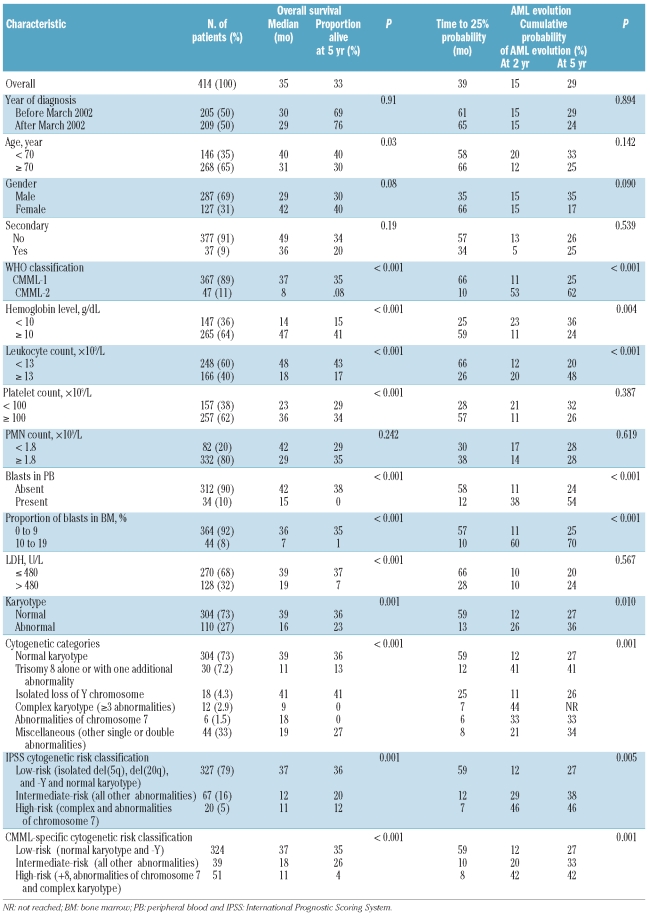
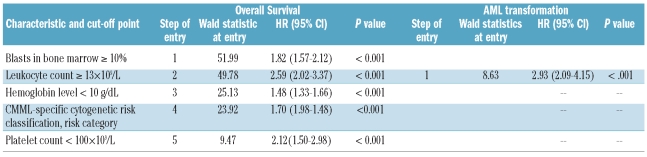
Different patient and disease characteristics, recorded at the time of diagnosis, were examined in the prognostic factor analysis to establish their possible relationship with overall survival (OS) and evolution to acute myeloid leukemia (AML). These include basic demographic data (age and sex), year of diagnosis, hematologic parameters (hemoglobin level, leukocyte count, polymorphonuclear cell (PMN) count, absolute platelet count, presence of blasts in peripheral blood), proportion of blasts in bone marrow, LDH level, and cytogenetics. For cytogenetic prognostic categorization, chromosomal abnormalities were classified into six different cytogenetic categories: normal karyotype, trisomy 8 (alone or with one additional abnormality), isolated loss of Y chromosome, complex karyotype (three or more abnormalities), anomalies of chromosome 7 (monosomy 7 or del(7q) alone or with one additional abnormality) and other miscellaneous abnormalities (other single or double chromosomal abnormalities). The IPSS cytogenetic risk classification was also considered.3 All characteristics were analyzed as dichotomous variables and the cut-off points selected for each one were those presented in Table 1. March 2002 was the date that divided the series in 2 groups with the same number of patients and was selected as cut-off point to evaluate a potential influence in the results of changes in the practical management of chronic myelomonocytic leukemia patients over time and exclude its possible association with other relevant prognostic variables.
Table 1.
Clinical and hematologic characteristics of patients with CMML related to survival and risk of AML evolution.
Statistical analysis
The χ2 and Fisher’s exact tests were used to analyze differences in the distribution of variables among patient subsets. Unadjusted time-to-event analyses were performed using the Kaplan-Meier estimate and log rank tests were used for comparisons. Multivariate analysis was performed using Cox’s proportional hazards regression model. Characteristics selected for possible inclusion in the multivariate model were those for which there was some indication of association with overall survival or acute myeloid leukemia evolution in univariate analysis (P<0.20). The forward stepwise procedure was stopped when the P value for entering an additional variable was above 0.05. Overall survival was measured from the time of diagnosis to the time of last follow up or death from any cause. Evolution to acute myeloid leukemia was measured from the time of diagnosis to the date of acute myeloid leukemia evolution (presence of more than 19% of blasts in bone marrow or peripheral blood). All patients received supportive care [red blood cell (RBC) and platelet transfusions, and antibiotics as required]. One hundred and seventy-three patients with transfusion-dependent anemia received erythroid stimulating agents and 65 received hydroxyurea to ameliorate hyperleukocytosis and/or symptoms related to splenomegaly. None of the patients had received azacitidine or decitabine. Patients undergoing hematopoietic allogeneic stem cell transplantation (n=4) or intensive AML-type chemotherapy (n=23) were considered censored data at the date of transplant or the date of starting chemotherapy. Patient follow up was updated on 15 January 2009, and all follow-up data were censored at that point. The statistical package SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. A two-sided P value below 0.05 was considered significant.
Results
Incidence and characteristics of chromosomal abnormalities
Main patients’ characteristics are shown in Table 1. The series included 287 (69%) males and 127 females (31%). Median age was 72 years (range 17–99). According to FAB criteria,2 248 patients (60%) had CMML-MD and 166 (40%) had CMML-MP. Morphological subtypes according to the WHO classification were CMML-1 in 367 (89%) and CMML-2 in 47 (11%). Karyotype was normal in 304 patients (73%) and abnormal in 110 (27%). The most frequent cytogenetic abnormalities were trisomy 8 (n=30; isolated in 24 patients and with one additional abnormality in 6: del(5)(q31q33), +10, del(11)(q14), del(12)(p13), add(17)(p13.3), +19, and +21, respectively), isolated loss of Y chromosome (n=18), abnormalities of chromosome 7 (n=6; isolated in 4 patients and with one additional abnormality in 2; monosomy 7 in 5 and del(7q) in one), and complex karyotype (n=12). Other miscellaneous abnormalities were present in 44 patients. Abnormalities present in at least 3 patients were del(20q) (n=3; isolated in all instances), and del(5q) (n=3; isolated in 2 patients and with one additional abnormality in one). Other chromosomal abnormalities present in less than 3 patients were evident in 38 patients. More detailed information on the different chromosomal abnormalities encountered is available in Online Supplementary Figure S1.
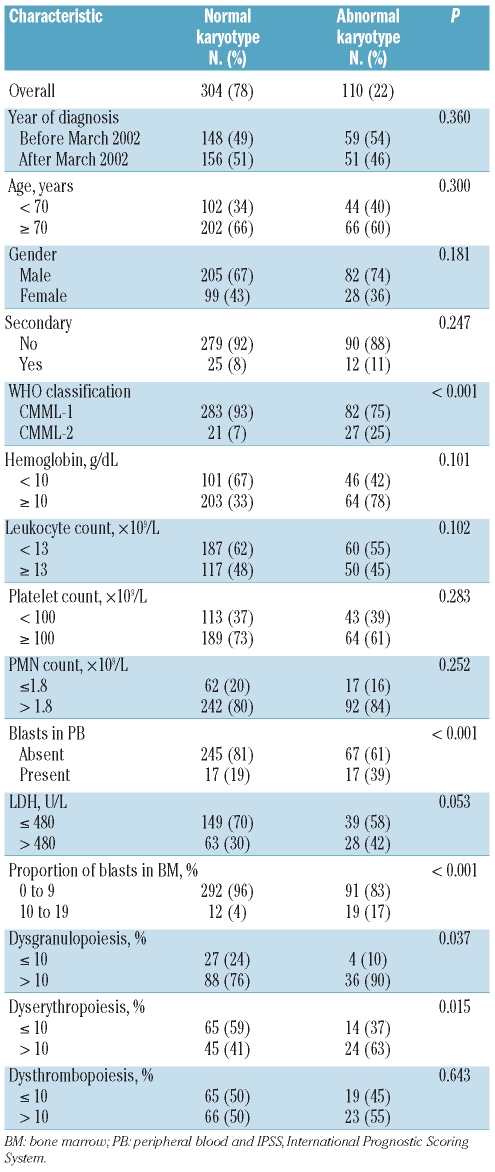
There were significant differences in the proportion of blasts in bone marrow, presence of blasts in peripheral blood, and consequently in WHO subgroup, and presence of dyserythropoiesis and dysgranulopoiesis between patients with normal and abnormal karyotypes (Table 2). Chromosomal abnormalities were more frequently found in patients with CMML-2 subtype (P<0.001), 10% or more blasts in bone marrow (P<0.001), presence of blasts in peripheral blood (P<0.001), dyserythropoiesis (P=0.015), and dysgranulopoiesis (P=0.037). There were no differences in the frequency and pattern of chromosomal abnormalities over time.
Table 2.
Comparative analysis of the characteristics of patients with CMML according to the presence or absence of chromosomal abnormalities.
Prognostic value of cytogenetic findings
With a median follow up of 22 months for surviving patients (range 1–157 months) the median survival was 35 months. Patients with normal karyotype had significantly better survival than patients with abnormal karyotype (P=0.001) and patients with isolated loss of Y chromosome also showed significantly longer survival than all the remaining patients with abnormal karyotype (P=0.020). By contrast, patients with trisomy 8 or complex karyotypes had a shorter survival than all the other patients (P<0.001 and P<0.001, respectively). Survival of patients with miscellaneous chromosomal abnormalities was significantly shorter than in patients with normal karyotype or isolated loss of Y chromosome (P=0.017) and also significantly longer than in patients with trisomy 8 or complex karyotype (P=0.007). No significant differences in survival were observed for any of the low-frequency single or double aberrations included in the group with miscellaneous abnormalities. The median survival of patients with anomalies of chromosome 7 was 18 months, shorter than that observed in the overall series of 414 patients (35 months). But there were no statistically significant differences in survival between this group of patients and any of the other cytogenetic groups of patients. It should be stressed that the outcome analyses of low-frequency chromosomal aberrations, including those of chromosome 7, were hampered by small sample size.
Fifty-nine patients developed acute myeloid leukemia, the median time to 25% probability of AML evolution was 59 months, and the cumulative probability of AML evolution at two and five years in the overall series of patients was 15% and 29%, respectively. Presentation of a complex karyotype was associated with a higher probability of developing acute myeloid leukemia (P<0.001) and patients with trisomy 8 also showed a trend towards a higher AML risk (P=0.081). In contrast, patients with normal cytogenetics present a lower probability of AML development (P=0.010). The median time to 25% probability of AML evolution of patients with abnormalities of chromosome 7 was six months and the cumulative probability of AML evolution at two years was 33% but, again, no statistically significant differences in AML evolution risk between these patients and those in the remaining cytogenetic groups were seen.
The prognostic impact of other clinical and hematologic characteristics on overall survival and risk of AML evolution in univariate analysis is shown in Table 1.
Development of a new CMML-specific cytogenetic risk classification
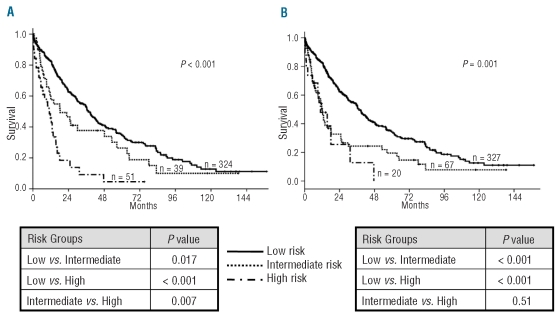
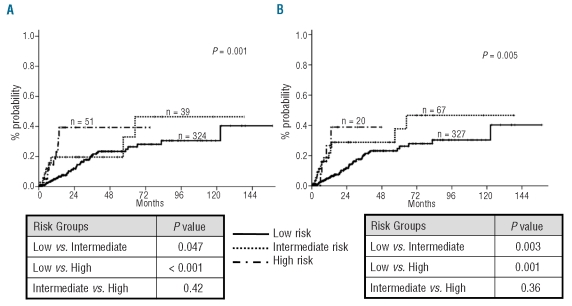
Taking into account the results observed in univariate analyses we developed a new CMML-specific cytogenetic risk classification. Patients were separated into three prognostic subgroups related to their cytogenetic pattern: low, intermediate, and high risk. Patients with normal karyotype and isolated loss of Y chromosome were assigned to the low risk category. Patients with trisomy 8, chromosome 7 abnormalities, and complex karyotype were considered as high risk. The assignment of patients with abnormalities of chromosome 7 to the high risk category despite the lack of statistical significance in univariate analysis was based on their particularly short overall survival and high risk of acute myeloid leukemia in the current series and in other reported studies of patients with chronic myelomonocytic leukemia,7 and in the IPSS cohort.3 The remaining patients with any other single or double abnormality were defined as intermediate risk. The proportion of patients surviving at five years in these three different cytogenetic risk categories was 35% for low risk, 26% for intermediate risk, and 4% for high risk (P < 0.001). The actuarial median survival of patients within these three cytogenetic subgroups was 37, 18, and 11 months, respectively (Table 1 and Figure 1A). The new CMML-specific cytogenetic risk classification was compared with the IPSS cytogenetic risk classification which, in contrast, includes normal karyotype, and isolated loss of Y chromosome, del(5q) or del(20q) in the low risk group, complex karyotype (three or more abnormalities), or chromosome 7 abnormalities in the high risk group, and all other karyotypic abnormalities in the intermediate risk group. The IPSS cytogenetic risk classification also showed a statistically significant association with survival on univariate analysis (P=0.001), but was only capable of segregating two risk groups, survival probabilities being very similar for patients in the intermediate and high risk categories (median survival 37, 12, and 11 months for patients in the low, intermediate and high risk categories; Figure 1B). There was a significant relationship between the new CMML-specific cytogenetic risk classification defined above and probability of AML evolution (overall P=0.001, Figure 2A). The actuarial risk of AML evolution at two years was 40% for patients in the high risk group, 20% for patients in the intermediate risk group, and 12% for patients in the low risk group. Patients in the low risk group had a statistically significantly lower risk of AML evolution than patients in the intermediate group (P=0.047). In contrast, no clear differences in risk of AML evolution were evident between patients in the intermediate and high risk categories (P=NS). As shown in Figure 2B, the IPSS cytogenetic risk classification yielded similar results. This system was significantly associated with rates of AML evolution (overall P=0.005) but there was no clear difference in risk of AML evolution for patients between the intermediate and high risk categories (P=NS).
Figure 1.
Unadjusted probability of overall survival according to (A) the new CMML-specific and (B) the IPSS cytogenetic risk classifications.
Figure 2.
Unadjusted risk of AML evolution according to (A) the new CMML-specific and (B) the IPSS cytogenetic risk classifications.
Both the IPSS and the new CMML-specific cytogenetic risk classifications were also significantly associated with overall survival and AML evolution when the analyses were restricted to patients with less or more than 12×109 leukocytes/L (Online Supplementary Table S1). However, none of them was able to clearly segregate three risk groups for overall survival or AML risk in patients with CMML-MD or with CMML-MP. While the shape of the survival curves with the new CMML-specific cytogenetic risk classification in patients with CMML-MP was as expected that was not the case with the IPSS cytogenetic risk classification, where the survival curve for patients in the intermediate risk category was below the survival curve for patients in the high risk category (Online Supplementary Figure S2).
Multivariate analysis confirmed that the new CMML-specific cytogenetic risk classification had a strong and independent prognostic impact on overall survival (P<0.001) but not on AML evolution. The IPSS cytogenetic risk categorization did not demonstrate a statistically significant value once the new CMML-specific cytogenetic risk classification was entered into the overall survival regression model (P=0.076 when forced to enter the regression model) and did not show an independent impact on AML evolution risk. The main results of multivariate analyses of prognostic factors for both overall survival and AML evolution are shown in Table 3.
Table 3.
Results of the multivariate analysis of survival and risk of AML evolution.
Discussion
The present study shows, for the first time, that cytogenetic findings have a clear and independent relationship with overall survival and, to a lesser extent, with the risk of acute leukemic evolution in patients with chronic myelomonocytic leukemia.
Based on the survival analysis, we were able to define three cytogenetic risk categories, low risk (normal karyotype and loss of chromosome Y as a single anomaly), high risk (trisomy 8 alone or with one additional abnormality, abnormalities of chromosome 7 alone or with one additional abnormality and complex karyotype), and intermediate risk (all other single or double abnormalities), with clearly different probabilities of survival in univariate and multivariate analyses. Patients in the high risk cytogenetic subgroup also had a significantly higher risk of AML evolution in univariate, but not multivariate, analyses.
The frequency of abnormal karyotype and specific chromosomal abnormalities in series of patients with chronic myelomonocytic leukemia has varied greatly, largely because of small numbers, inclusion criteria (WHO or FAB criteria), and referral patterns. Overall, the incidence of chromosomal abnormalities is close to 25% (range 11–42%; 27% in the current study) and the karyotypic aberrations encountered are not specific for chronic myelomonocytic leukemia and are commonly found in other myelodysplastic syndrome subtypes and acute myeloid leukemia.7,9–13 The most frequently reported chromosomal abnormalities in patients with chronic myelomonocytic leukemia are trisomy 8, complex karyotype, and abnormalities of chromosome 7.7,9,10 Other less frequent aberrations include del(12p)11, del(20q)12, and hypodiploid karyotype13. Although trisomy 8, abnormalities of chromosome 7, and complex karyotype along with isolated loss of Y chromosome were also the most prevalent chromosomal abnormalities in our study, the frequency of abnormalities of chromosome 7 and complex karyotype was lower than in other reports. The lower frequency of chromosome 7 anomalies and complex karyotypes in the current study might be due to two different reasons. First, we used WHO criteria for chronic myelomonocytic leukemia definition and excluded patients with 20–30% blasts in bone marrow, in whom the incidence of those chromosomal abnormalities may be higher. Second, in contrast to other studies,7,10,14–16 karyotyping was assessed at diagnosis. Not unexpectedly, and as occurs in other MDS subtypes,11,12 the current study showed for the first time that the presence of chromosomal abnormalities is more common in patients with CMML-2 WHO subtype, which is characterized by 5–19% blasts (including promonocytes) in blood or 10–19% in the marrow, or when Auer rods are present irrespective of the blast plus promonocyte count.10 Our study also found a higher incidence of chromosomal abnormalities in patients with more frequent morphological findings of dyserythropoiesis and dysgranulopoiesis, which could suggest a potential relationship between chromosomal aberrations and the presence of myelodysplastic features. These findings need confirmation in other series.
The natural course of chronic myelomonocytic leukemia is highly variable, with reported life expectancy ranging from months to several years. In a meta-analysis of reported series of chronic myelomonocytic leukemia median survival was 20 months, ranging from seven to 60 months.10 In the current series median survival was 35 months. Reasons for this longer median survival are unclear. Although it could just be explained by the heterogeneity of the disorder, it might also be partly due to an inadvertent selection bias, with cytogenetic analysis being performed and yielding successful results more frequently in patients with a better prognosis (data not shown). The wide variation in the clinical course of patients with chronic myelomonocytic leukemia has provided the stimulus for investigation into factors predictive of outcome.
The only published study that had previously evaluated the potential impact of cytogenetic results in chronic myelomonocytic leukemia showed shorter survival in patients with chromosomal abnormalities, but was unable to confirm by multivariate analysis the independent prognostic impact of this finding on outcome.7 Furthermore, the small number of patients with specific chromosomal aberrations made it impossible to identify a subpopulation of patients with unfavorable karyotypes among the abnormal karyotype group.7 Our study, performed in a cohort of CMML patients who homogenously received supportive treatment and were considered censored for statistical analysis at the moment they switched to intensive therapy, confirmed the strong association between abnormal karyotype and shorter survival, and showed a trend for a greater risk of evolution to acute myeloid leukemia. Additionally, in the current study, patients with trisomy 8 and with complex karyotype had a very poor outcome and could be included in the high risk cytogenetic subgroup. In contrast, patients with normal karyotype or isolated loss of Y chromosome had better survival and were stratified in the low risk cytogenetic category. As reported elsewhere,7 patients with abnormalities of chromosome 7 experienced a short survival and a high risk of AML evolution. These results are in line with the universally accepted poor outcome of abnormalities of chromosome 7 (monosomy 7/del(7q)) in other myeloid malignancies.3,9,15,18 For these reasons and despite their low number in the current series, patients with abnormalities of chromosome 7 were included in the high risk category of the new CMML-specific cytogenetic classification system. The small number of patients with other specific chromosomal abnormalities precluded a meaningful analysis of their potential association with survival or evolution to acute myeloid leukemia. Therefore, all these chromosomal aberrations were merged in a miscellaneous intermediate risk cytogenetic group.
As discussed above, this new CMML-specific cytogenetic risk classification showed a clear and independent association with survival length. Further, patients in the high risk cytogenetic category also showed a greater probability of developing acute myeloid leukemia on univariate analysis. Our data suggest that the prognostic impact of cytogenetic findings in patients with chronic myelomonocytic leukemia seems to diverge from that observed in patients with other MDS subtypes. In contrast to IPSS, where trisomy 8 is considered among the intermediate risk cytogenetic abnormalities, that aberration was included in the high risk cytogenetic category of the new CMML-specific cytogenetic risk classification. Furthermore, isolated del(5q) and del(20q), that are included in the low risk IPSS cytogenetic category, were assigned to the intermediate risk category of the new CMML-specific cytogenetic risk classification. We were able to show that the new CMML-specific cytogenetic classification system developed in the present study was more accurate than the IPSS cytogenetic risk classification for assessing the prognosis of patients with chronic myelomonocytic leukemia, most likely due to the grim outcome of patients with trisomy 8. The advantage of the CMML-specific cytogenetic risk classification was especially evident for predicting survival of patients with CMML-MP, a subset of CMML patients excluded from the original IPSS score. Nonetheless, it should be underlined that all these findings require validation in an independent cohort of CMML patients.
None of the published scoring systems specifically designed for evaluating prognosis in patients with chronic myelomonocytic leukemia has included the cytogenetic results.7,10,17,19 Our data suggest that the present CMML-specific cytogenetic risk classification could add significant prognostic information to that provided by other universally accepted prognostic variables in patients with chronic myelomonocytic leukemia. In accordance with previous series, our study also confirmed the independent prognostic value of the proportion of blasts in bone marrow,7,10,11,17,19 leukocyte count,17 hemoglobin level,7,10,11 and platelet count.10,11 Our current understanding of the molecular bases of chronic myelomonocytic leukemia and other myeloid neoplasms is evolving very quickly.20,21 In a recent paper, Kosmider et al. have elegantly shown that TET-2 gene mutations were present at diagnosis in 40% of patients with chronic myelomonocytic leukemia.22 Furthermore, in this study there was a trend towards shorter survival for mutated cases, which was statistically significant for 29 patients with CMML-1 WHO subtype. Thus, studies of prognostic factors incorporating clinical parameters, cytogenetic findings, and new molecular markers are critical to further advance our knowledge of chronic myelomonocytic leukemia.
To sum up, this study demonstrates the prognostic relevance of chromosomal abnormalities in patients with chronic myelomonocytic leukemia and proposes a new CMML-specific cytogenetic risk classification. Further studies including a substantial number of patients will be required to validate and potentially refine this cytogenetic risk classification before it can be used as a prognostic tool in the clinical management of patients with chronic myelomonocytic leukemia. In this sense, a large, multinational, cooperative effort would be essential. The primary aim of such a study should be to better define the prognosis of the different low-incidence specific chromosomal abnormalities, included in this study in the miscellaneous intermediate risk cytogenetic group.
Acknowledgments
The authors thank Luis Benlloch for data management.
Appendix
The following institutions and clinicians participated in the study: Hospital Universitario La Fe, Valencia: E. Such, J. Cervera, ML. Senent, and G. Sanz; Hospital Clinic, Barcelona: D. Costa and B. Nomdedeu; Hospital del Mar, Barcelona: F. Solé and M. Mallo; Hospital Universitario Vall D’Hebron, Barcelona: T. Vallespí; Hospital Central de Asturias, Oviedo: E. Luño; Hospital General de Valencia: R. Collado; Universidad de Navarra, Pamplona: MJ. Calasanz; Centro Nacional de Investigaciones Oncológicas, Madrid: J. Cruz and S. Alvarez; Hospital Txagorritxu; Vitoria: Ardanaz MT; Complejo Hospitalario, León: F. Ramos; Hospital Clínico Universitario, Valencia: M. Tormo; Hospital Arnau de Villanova, Valencia: R. Sancho-Tello; Hospital Universitario de Salamanca: JM Hernandez-Rivas and C. del Cañizo; Hospital La Princesa, Madrid: V. Gómez; Hospital Arnau de Vilanova, Lleida: V. Marco; Hospital Germans Trias i Pujol, Badalona: B. Xicoy; Hospital de la Ribera, Valencia: S. Bonanad; Hospital Dr. Peset, Valencia: R. Andreu; Hospital Severo Ochoa; Madrid: MJ Requena; ICO - D y R, Barcelona: Instituto Catalán de Oncología Durant y Reinals: R. Duarte. Hospital Carlos Haya, Málaga: A. Bailén; Hospital de Sagunto, Valencia: MJ. Arilla; Hospital. Morales Meseguer, Murcia: M.L. Amigo and F. Ortuño; Fundación Hospital de Alcorcón, Madrid: L. Villalón; Hospital. Clínico San Carlos, Madrid: A. Villegas; Hospital Universitario La Paz, Madrid: R. de Paz; Hospital General de Castellón: G.Cañigral.
Footnotes
Funding: this study was supported in part by research funding from the “Instituto de Salud Carlos III” grants RD06/0020/0031, RD07/0020/2004, CA08/00141 and grants from DIUE, Generalitat de Catalunya (2009 SGR 541).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–99. [PubMed] [Google Scholar]
- 2.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick H, et al. The chronic myeloid leukemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid and chronic myelomonocytic leukemia. Proposals by the French–American–British Cooperative leukemia group. Br J Haematol. 1994;87 (4):746–54. doi: 10.1111/j.1365-2141.1994.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 4.Vardiman JW, Pierre R, Bain B, Bennett JM, Imbert M, Brunning RD, et al. Chronic myelomonocytic leukaemia. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization. Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon; France: IARC press, World Health Organization; 2001. p. 49. [Google Scholar]
- 5.Orazi A, Bennett JM, Germing U, Brunning RD, Bain BJ, Thiele J. Chronic myelomonocytic leukaemia. In: Swerdlow S, Campos E, Lee Harris N, Jaffe E, Pileri S, Stein H, Thiele J, Vardiman J, editors. World Health Organization. Classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC press, World Health Organization; 2008. pp. 76–81. [Google Scholar]
- 6.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-Dependent Prognostic Scoring System for Predicting Survival and Leukemic Evolution in Myelodysplastic Syndromes. J Clin Oncol. 2007;25(23):3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 7.Onida F, Kantarjian HM, Smith TL, Ball G, Keating MJ, Estey EH, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99(3):840–9. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer LG, Slovak ML, Campbell LJ, editors. S. Karger; Basel: 2009. An International System for Human Cytogenetic Nomenclature. [Google Scholar]
- 9.Solé F, Espinet B, Sanz GF, Cervera J, Calasanz MJ, Luño E, et al. Incidence, characterization and prognostic significance of chromosomal abnormalities in 640 patients with primary myelodysplastic syndromes. Br J Haematol. 2000;108(2):346–56. doi: 10.1046/j.1365-2141.2000.01868.x. [DOI] [PubMed] [Google Scholar]
- 10.Germing U, Kündgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML) Leuk Lymphoma. 2004;45(7):1311–8. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 11.Groupe Français de Cytogénétique Hématologique. Chronic myelomonocytic leukemia: single entity or heterogeneous disorder? A prospective multicenter study of 100 patients. Cancer Genet Cytogenet. 1991;55(1):57–65. doi: 10.1016/0165-4608(91)90235-m. [DOI] [PubMed] [Google Scholar]
- 12.Ngo NT, Lampert IA, Naresh KN. Bone marrow trephine morphology and immunohistochemical findings in chronic myelomonocytic leukaemia. Br J Haematol. 2008;141(6):771–81. doi: 10.1111/j.1365-2141.2008.07117.x. [DOI] [PubMed] [Google Scholar]
- 13.Catalano L, Improta S, de Laurentiis M, Molica S, Majolino I, Musto P, et al. Prognosis of chronic myelomonocytic leukemia. Haematologica. 1996;81(4):324–9. [PubMed] [Google Scholar]
- 14.Solé F, Luño E, Sanzo C, Espinet B, Sanz GF, Cervera J, et al. Identification of novel cytogenetic markers with prognostic significance in a series of 968 patients with primary myelodysplastic syndromes. Haematologica. 2005;90:1168–78. [PubMed] [Google Scholar]
- 15.Haase D, Germing U, Schanz J, Pfeilstöcker M, Nösslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 16.Fenaux P, Beuscart R, Lai JL, Jouet JP, Bauters F. Prognostic factors in adult chronic myelomonocytic leukemia: an analysis of 107 cases. J Clin Oncol. 1998;6(9):1417–24. doi: 10.1200/JCO.1988.6.9.1417. [DOI] [PubMed] [Google Scholar]
- 17.González-Medina I, Bueno J, Torrequebrada A, López A, Vallespí T, Massagué I. Two groups of chronic myelomonocytic leukaemia: myelodysplastic and myeloproliferative. Prognostic implications in a series of a single center. Leuk Res. 2002;26(9):821–4. doi: 10.1016/s0145-2126(02)00021-8. [DOI] [PubMed] [Google Scholar]
- 18.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 19.del Cañizo MC, Sanz G, San Miguel JF, Vallespi T, Irriguible D, Torrabadella M, et al. Chronic myelomonocytic leukemia-clinicobiological characteristics: a multivariate analysis in a series of 70 cases. Eur J Haematol. 1989;42(5):466–73. doi: 10.1111/j.1600-0609.1989.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 20.Reiter A, Invernizzi R, Cross NC, Cazzola M. Molecular basis of myelodysplastic/myeloproliferative neoplasms. Haematologica. 2009;94(12):1634–8. doi: 10.3324/haematol.2009.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzola Mario. IDH1 and IDH2 mutations in myeloid neoplasms – Novel paradigms and clinical implications. Haematologica. 2010;95(10):1623–7. doi: 10.3324/haematol.2010.030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosmider O, Gelsi-Boyer V, Ciudad M, Racoeur C, Jooste V, Vey N, et al. TET2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica. 2009;94(12):1676–81. doi: 10.3324/haematol.2009.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]