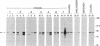Abstract
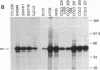
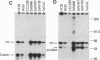
The tyrosine-specific protein kinase activity of pp60c-src molecules obtained from human colon carcinoma tissues and tumor-derived cell lines was found to be elevated over that from normal colon tissues or cultures of normal colon mucosal cells. The elevated pp60c-src protein kinase activity in tumor tissues and in cultured colon carcinoma cells does not appear to result solely from an increase in the abundance of the c-src-encoded protein, suggesting that the specific activity of the pp60c-src tyrosine phosphotransferase is enhanced. These results raise the possibility that activation of the pp60c-src protein kinase may contribute to the genesis of human colon tumors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K., Winqvist R., Lin C. C., de la Chapelle A., Schwab M., Bishop J. M. Aberrant expression of an amplified c-myb oncogene in two cell lines from a colon carcinoma. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4534–4538. doi: 10.1073/pnas.81.14.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S., DeSeau V., Reddy S., Shalloway D., Bolen J. B. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol Cell Biol. 1986 Jul;6(7):2305–2316. doi: 10.1128/mcb.6.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Lewis A. M., Jr, Israel M. A. Stimulation of pp60c-src tyrosyl kinase activity in polyoma virus-infected mouse cells is closely associated with polyoma middle tumor antigen synthesis. J Cell Biochem. 1985;27(2):157–167. doi: 10.1002/jcb.240270209. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Rosen N., Israel M. A. Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7275–7279. doi: 10.1073/pnas.82.21.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Kaczmarek L., Ming P. M., Au F., Ming S. C. Expression of c-myc and other cell cycle-dependent genes in human colon neoplasia. Cancer Res. 1985 Dec;45(12 Pt 1):6000–6004. [PubMed] [Google Scholar]
- Cartwright C. A., Hutchinson M. A., Eckhart W. Structural and functional modification of pp60c-src associated with polyoma middle tumor antigen from infected or transformed cells. Mol Cell Biol. 1985 Oct;5(10):2647–2652. doi: 10.1128/mcb.5.10.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard J. G., van Beek W. P., Janssen J. W., Schijven J. F. Transfection by human oncogenes: concomitant induction of tumorigenicity and tumor-associated membrane alterations. Int J Cancer. 1985 Feb 15;35(2):207–213. doi: 10.1002/ijc.2910350211. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman M. D., Rothberg P. G., Diehl R. E., Morse C. C., Spandorfer J. M., Astrin S. M. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol. 1985 Aug;5(8):1969–1976. doi: 10.1128/mcb.5.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallick G. E., Kurzrock R., Kloetzer W. S., Arlinghaus R. B., Gutterman J. U. Expression of p21ras in fresh primary and metastatic human colorectal tumors. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1795–1799. doi: 10.1073/pnas.82.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand P. H., Thor A., Wunderlich D., Muraro R., Caruso A., Schlom J. Monoclonal antibodies of predefined specificity detect activated ras gene expression in human mammary and colon carcinomas. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5227–5231. doi: 10.1073/pnas.81.16.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C., Rübsamen H. Expression of pp60c-src protein kinase in adult and fetal human tissue: high activities in some sarcomas and mammary carcinomas. Cancer Res. 1983 Apr;43(4):1696–1702. [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M. S., Bargmann C. I., Weinberg R. A. Human colon carcinoma Ki-ras2 oncogene and its corresponding proto-oncogene. Mol Cell Biol. 1984 Aug;4(8):1577–1582. doi: 10.1128/mcb.4.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The product of the protooncogene c-src is modified during the cellular response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7845–7849. doi: 10.1073/pnas.82.23.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Eisenman R. N., Leitch C. R. Identification of a Rous sarcoma virus transformation-related protein in normal avian and mammalian cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4479–4483. doi: 10.1073/pnas.76.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen N., Bolen J. B., Schwartz A. M., Cohen P., DeSeau V., Israel M. A. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986 Oct 15;261(29):13754–13759. [PubMed] [Google Scholar]
- Rothberg P. G., Spandorfer J. M., Erisman M. D., Staroscik R. N., Sears H. F., Petersen R. O., Astrin S. M. Evidence that c-myc expression defines two genetically distinct forms of colorectal adenocarcinoma. Br J Cancer. 1985 Oct;52(4):629–632. doi: 10.1038/bjc.1985.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Stewart J., Evan G., Watson J., Sikora K. Detection of the c-myc oncogene product in colonic polyps and carcinomas. Br J Cancer. 1986 Jan;53(1):1–6. doi: 10.1038/bjc.1986.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor A., Horan Hand P., Wunderlich D., Caruso A., Muraro R., Schlom J. Monoclonal antibodies define differential ras gene expression in malignant and benign colonic diseases. Nature. 1984 Oct 11;311(5986):562–565. doi: 10.1038/311562a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Williams A. R., Piris J., Spandidos D. A., Wyllie A. H. Immunohistochemical detection of the ras oncogene p21 product in an experimental tumour and in human colorectal neoplasms. Br J Cancer. 1985 Nov;52(5):687–693. doi: 10.1038/bjc.1985.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Tsunetsugu-Yokota Y., Battifora H., Le Fevre C., Cline M. J. Alterations of myc, myb, and rasHa proto-oncogenes in cancers are frequent and show clinical correlation. Science. 1986 Jan 17;231(4735):261–265. doi: 10.1126/science.3941898. [DOI] [PubMed] [Google Scholar]