Abstract
Within a cohort of 1,915 consecutive patients with myeloproliferative neoplasm followed for a median time of 5.2 years (range 0–33.3), we investigated the occurrence of lymphoid neoplasm with the aim of defining this risk and to investigate the role of genetic predisposing factors. We identified 22 patients with myeloproliferative neoplasm who developed lymphoid neoplasm over their lifetime. We found that the risk of developing lymphoid neoplasm was 2.79-fold higher (95% CI, 1.80–4.33; P<0.001) than that of the general Italian population. A tag SNP surrogate for JAK2 GGCC haplotype was used to clarify a potential correlation between lymphoid-myeloid neoplasm occurrence and this genetic predisposing factor. As we did not find any difference in GGCC haplotype frequency between patients with both myeloid and lymphoid neoplasm and patients with myeloid neoplasm, JAK2 GGCC haplotype should not be considered a genetic predisposing factor. No difference in familial clustering was observed between the two groups.
Keywords: polycythemia, thrombocythemia, myelofibrosis, lymphoid, familial
Introduction
Myeloproliferative neoplasm (MPN), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF) are rare disorders with an annual incidence rate of 2.1 per 100,000 person-years.1 The course of myeloproliferative neoplasm is complicated mainly by vascular events and transformation to myelofibrosis or leukemia; secondary malignancies may occur with a low incidence.2 A previous study demonstrated that patients with myeloproliferative neoplasm have a 3.44-fold higher risk of developing lymphoid neoplasm (LPN) than the general population.3 On this basis, a common genetic susceptibility may be taken into account. In a familial-MPN setting, first-degree relatives of patients with myeloproliferative neoplasm have a 5- to 7-fold higher risk of developing these diseases,4 and an increased risk of secondary malignancies.5 Likewise, first-degree relatives of patients with chronic lymphocytic leukemia or lymphoplasmocytic lymphoma are at increased risk for lymphoid neoplasm.6,7
Inherited genetic factors with a role in the development of sporadic myeloproliferative neoplasm have been recently defined.8–10 More than 80% of JAK2 (V617F) mutations are acquired on a particular JAK2 gene haplotype, the GGCC or 46/1 haplotype. This sequence variant of the JAK2 gene confers susceptibility to JAK2-mutated myeloproliferative neoplasm8–10 and to JAK2-wild type myeloproliferative neoplasm.11
To define the association of myeloproliferative neoplasm and lymphoid neoplasm, we explored our myeloproliferative neoplasm database to identify those patients with a well defined diagnosis of lymphoid neoplasm. In addition, we investigated whether GGCC JAK2 haplotype and familial clustering are overrepresented in LPN-MPN patients.
Design and Methods
Study population
This study includes 1,915 consecutive patients with polycythemia vera, essential thrombocythemia and primary myelofibrosis, post-essential thrombocythemia and post-polycythemia vera myeloid fibrosis recorded in our myeloproliferative neoplasm database. Patients with diagnosis of myeloproliferative neoplasm not otherwise specified were excluded from the study. All patients were followed at the Division of Hematology, Fondazione I.R.C.C.S. Policlinico San Matteo, University of Pavia, Italy from 1970 to 2009. Diagnosis of myeloproliferative neoplasm was made according to criteria in use at the time of first observation.12–17 The International Working Group on MPNs Research and Treatment (IWG-MRT) criteria were used to define post-essential thrombocythemia and post-polycythemia vera myeloid fibrosis.18 Clinical charts of patients recorded to have lymphoid neoplasm were reviewed to obtain a final diagnosis in agreement with the WHO criteria. Among 1,915 myeloproliferative neoplasm cases, information on familial history of myeloproliferative neoplasm was obtained in 1,050 (54.8%) patients available for the interview. The study was approved by the Institute Review Board of the Fondazione I.R.C.C.S. Policlinico San Matteo and the procedures followed were in accordance with the 1975 Declaration of Helsinki, as revised in 2000. Samples for molecular analysis were obtained following patients’ informed written consent.
JAK2 (V617F) analysis and SNP genotyping
The JAK2 (V617F) mutational status was assessed on peripheral blood granulocytes using a quantitative real-time polymerase chain reaction-based allelic discrimination assay.19 A tag SNP (rs10974944) surrogate for the GGCC haplotype (GG or GC = GGCC haplotype; CC=not GGCC haplotype) was genotyped on T-lymphocyte DNA.20 The commercially available C_31941696 Taqman assay (Applied Biosystems) was used on the Rotor-Gene™ 6000 real-time PCR instrument in a final volume of 10 μL according to the manufacturer’s procedure. We genotyped 694 (36%) patients and 203 controls: 43 healthy subjects, 126 with JAK2 (V617F)-negative secondary erythrocytosis and 34 with JAK2 (V617F)-negative secondary leukocytosis.
Analysis of VH gene usage and mutations
The analysis of immunoglobulin heavy chain gene variable, diversity and joining (IGHV-D-J) genes was performed on mononuclear cells obtained from peripheral blood or bone marrow samples after isolation on a Ficoll gradient, as previously described.21 We studied 8 of 10 patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and chronic B lineage lymphoproliferative disorder (B-LPL) for whom DNA was available in order to evaluate whether CLL/SLL/B-LPL associated to myeloproliferative neoplasms display a particular repertoire of IGHV genes.
Statistical analysis
For the aims of this study, only lymphoid neoplasms occurring after the diagnosis of myeloproliferative neoplasm were considered. The period at risk was defined for each patient from the date at diagnosis of myeloproliferative neoplasm to the date of occurrence of lymphoid neoplasm, date of death, or date of the last follow up, whichever came first. The relative risk of lymphoid neoplasms was expressed as a standardized incidence ratio (SIR), defined as the ratio between observed and expected cases. The expected number of lymphoid neoplasm cases was estimated using sex and age incidence rates reported by AIRTUM (Associazione Italiana Registri Tumori) for Northern Italy in the period 2000–2003, applied to the corresponding person-years at risk. When investigating the effect of familial predisposition and GGCC JAK2 haplotype, all lymphoid neoplasm cases, i.e. occurring either before or after myeloproliferative neoplasm, were taken into account.
Results and Discussion
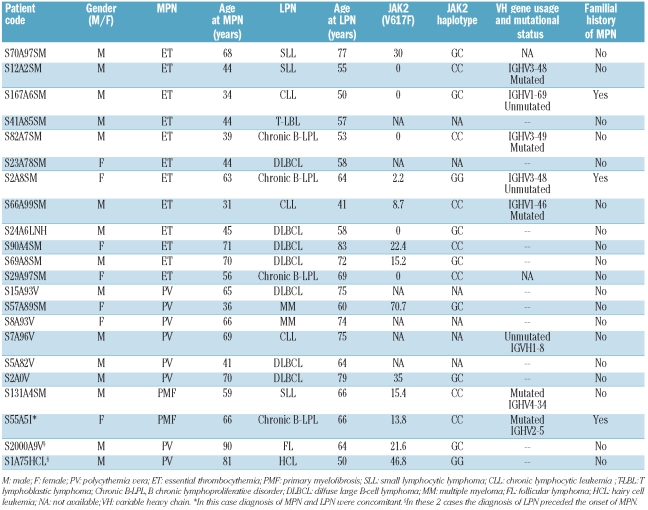
The myeloproliferative neoplasm database included 1,915 patients with myeloproliferative neoplasm: 651 (34%) polycythemia vera, 848 (44%) essential thrombocythemia, 334 (18%) primary myelofibrosis and 82 (4%) secondary myelofibrosis. Among these, 22 (1.1%) patients developed lymphoid neoplasm over their lifetime: 20 had lymphoid neoplasia after myeloproliferative neoplasm and 2 before. Clinical characteristics of patients with LPN-MPN are reported in Table 1. All but 3 patients received cytoreductive therapy: hydroxyurea alone (n=8), pipobroman alone (n=6), or more than one cytoreductive treatment (n=5). After a median follow up of 10.2 years (range 0.1–23.5 years), 8 patients died because of progressive lymphoid neoplasm (n=4), blastic phase of myeloproliferative neoplasm (n=1) or unknown causes (n=3). In lymphoid neoplasm studies, variable region genes were mutated in 5 of 8 cases. Although no biased IGVH gene usage was observed, 2 patients carried IGHV1–69 and IGHV4–34, belonging to the immunoglobulin repertoire overrepresented in chronic lymphocytic leukemia.22
Table 1.
Clinical characteristics of the 22 patients with myeloid and lymphoid neoplasm (LPN-MPN).
The median follow up of 1,915 patients was 5.2 years (range 0–33.3 years). Overall, the risk of myeloproliferative neoplasm patients developing lymphoid neoplasm was 2.79-fold higher (95% CI: 1.80–4.33; P<0.001) than in the general population. This risk was higher than expected both in males (SIR 3.23, 95% CI: 1.88–5.57; P<0.001) and in females (SIR 2.23, 95% CI: 1.06–4.68; P=0.029).
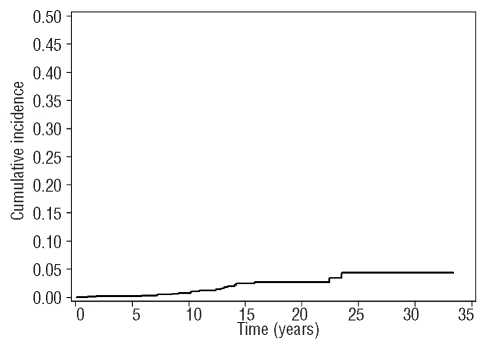
Considering the effect of age on SIR, we found that the SIR was 6.16 (95% CI: 3.2–11.84; P<0.001) in patients aged under 50 and 1.93 (95% CI: 1.07–3.49; P=0.026) in patients aged over 50. The median time from myeloproliferative neoplasm diagnosis to lymphoid neoplasm occurrence was 10.5 years (range 0–23.4 years). Cumulative incidence of lymphoid neoplasm was calculated considering death for other causes as a competing risk (Figure 1). Considering data available in the literature, we may state that, at least in Italy, the 10-year risk of lymphoid neoplasm in myeloproliferative neoplasm patients ranges from 0.7% (this study) to 2.96%.3 Heterogeneity of the two Italian cohorts including age (55.6 years in this study and 59 years in the Vannucchi study)3 may explain this discrepancy.
Figure 1.
Cumulative incidence of LPN. The figure shows the cumulative incidence of LPN considering the death for other causes as a competing risk.
The JAK2 (V617F) mutational status was available in 16 (73%) of 22 patients with LPN-MPN: 11 (69%) carried the mutation and 5 (31%) were wild type. There was no significant difference in occurrence of lymphoid neoplasm between JAK2 (V617F)-positive and JAK2 (V617F)-negative patients, even after stratification for diagnosis. This is in contrast with a prior study3 that did not report lymphoid neoplasms among patients with JAK2 wild-type myeloproliferative neoplasm. Our finding on a large cohort implies that susceptibility genes other than JAK2 (V617F) might favor the genetic instability predisposing to both lymphoid neoplasm and myeloproliferative neoplasm.
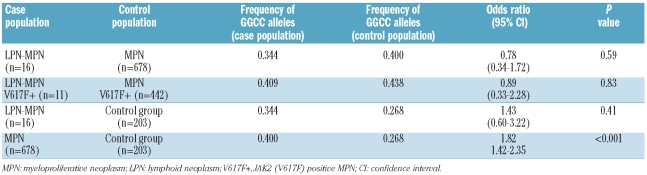
Recently, a specific constitutional JAK2 haplotype, the GGCC or 46/1 haplotype, has been demonstrated to confer susceptibility to JAK2 (V617F)-positive myeloproliferative neoplasm8–10 and JAK2-wild type myeloproliferative neoplasm.11 To evaluate whether the JAK2 GGCC haplotype might represent the genetic predisposition to develop both myeloproliferative neoplasm and lymphoid neoplasm, we compared the frequency of the high-risk G allele among 16 LPN-MPN patients for whom DNA was available: 678 myeloproliferative neoplasm patients and 203 control subjects (Table 2). No difference was observed between patients with LPN-MPN and patients with myeloproliferative neoplasm (OR 0.78; 95% CI: 0.34–1.72; P=0.59), even when considering only the JAK2-mutated patients (OR 0.89; 95% CI: 0.33–2.28; P=0.83). Thus, the risk of developing lymphoid neoplasm is not associated with the JAK2 GGCC haplotype.
Table 2.
Genotyping results for the JAK2 GGCC tag SNP rs10974944.
The GGCC haplotype was more frequent in myeloproliferative neoplasm patients than in the control population (OR 1.82; 95% CI: 1.42–2.35; P<0.001), confirming that the JAK2 GGCC haplotype is overrepresented in myeloproliferative neoplasm patients in agreement with recent literature.8–11
To look for clinical arguments in favor of an inherited susceptibility to hematologic neoplasia we focused on the 1,050 myeloproliferative neoplasm patients interviewed about familial history.
We compared the prevalence of familial cases reported in the 22 patients with LPN-MPN and that observed in the remaining 1,028 patients without lymphoid neoplasm. The presence of another relative affected with myeloproliferative neoplasm within the same pedigree was referred in 3 out of the 22 (13.6%) patients with LPN-MPN and in 65 out of 1,028 (6.3%) patients with myeloproliferative neoplasm. No difference in terms of familial clustering was observed between LPN-MPN and myeloproliferative neoplasm (OR 2.33; 95% CI: 0.43–8.25; P=0.16). Further studies comparing the total occurrence of secondary malignancies between familial and sporadic myeloproliferative neoplasm might clarify whether this 2.33-fold higher Odds Ratio reaches a statistical significance and whether it’s reasonable to suppose a genetic predisposition to somatic mutagenesis. The prevalence of familial cases among myeloproliferative neoplasm patients without occurrence of lymphoid neoplasm is consistent with the 7.6% previously published.23
In 2 patients the diagnosis of lymphoid neoplasm preceded the onset of myeloproliferative neoplasm. They developed JAK2 (V617F)-positive polycythemia vera three decades after the initial diagnosis of hairy cell leukemia and of follicular non-Hodgkin’s lymphoma, respectively. If a genetic susceptibility exists, one should hypothesize a random order of onset of the myeloid or of the lymphoid neoplasm and then should expect an equal frequency of patients with myeloproliferative neoplasm who develop lymphoid neoplasm or vice versa. The low number of myeloproliferative neoplasm patients with a previous lymphoid neoplasm reported in our study may be due to a more aggressive behavior of lymphoid neoplasms, so that patients die before myeloproliferative neoplasm develops. The distribution of myeloproliferative neoplasm types among patients with LPN-MPN supports this hypothesis. The highest number of lymphoid neoplasm was observed among patients with essential thrombocythemia (n=12), whose survival is generally longer due to the good prognosis of this disease.2 Conversely, the lowest number of lymphoid neoplasm was observed in patients with primary myelofibrosis (n=2), whose life expectancy is not long enough to develop secondary lymphoid neoplasms.24
In conclusion, patients with myeloproliferative neoplasm have a higher risk of developing lymphoid neoplasm than the general population. No difference in terms of familial clustering was observed between LPN-MPN and myeloproliferative neoplasm. We did not find a statistical association between the JAK2 GGCC haplotype and LPN-MPN. These results are based on a low number of patients with both myeloproliferative neoplasm and lymphoid neoplasm, due to the rare occurrence of both disorders. Moreover familial history and JAK2 haplotype were available only in a subgroup of patients. Further studies of larger patient cohorts, including thorough investigation into possible familial history and genotyping for JAK2 haplotype, are needed to definitively rule out an association.
Footnotes
Funding: this study was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan) Special Program Molecular Clinical Oncology “5 per mille” and by Fondazione Cariplo, Milan, Italy.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 2.Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117(10):755–61. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Vannucchi AM, Masala G, Antonioli E, Chiara Susini M, Guglielmelli P, Pieri L, et al. Increased risk of lymphoid neoplasms in patients with Philadelphia chromosome-negative myeloproliferative neoplasms. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2068–73. doi: 10.1158/1055-9965.EPI-09-0353. [DOI] [PubMed] [Google Scholar]
- 4.Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Bjorkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112 (6):2199–204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemminki K, Sundquist J, Bermejo JL. Associated cancers in parents and offspring of polycythaemia vera and myelofibrosis patients. Br J Haematol. 2009;147(4):526–30. doi: 10.1111/j.1365-2141.2009.07874.x. [DOI] [PubMed] [Google Scholar]
- 6.Kristinsson SY, Bjorkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112 (8):3052–6. doi: 10.1182/blood-2008-06-162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood. 2004;104(6):1850–4. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- 8.Olcaydu D, Harutyunyan A, Jager R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41(4):450–4. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- 9.Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41(4):455–9. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41(4):446–9. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tefferi A, Lasho TL, Patnaik MM, Finke CM, Hussein K, Hogan WJ, et al. JAK2 germline genetic variation affects disease susceptibility in primary myelofibrosis regardless of V617F mutational status: nullizygosity for the JAK2 46/1 haplotype is associated with inferior survival. Leukemia. 24(1):105–9. doi: 10.1038/leu.2009.225. [DOI] [PubMed] [Google Scholar]
- 12.Berk PD, Goldberg JD, Donovan PB, Fruchtman SM, Berlin NI, Wasserman LR. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin Hematol. 1986;23(2):132–43. [PubMed] [Google Scholar]
- 13.Murphy S, Iland H, Rosenthal D, Laszlo J. Essential thrombocythemia: an interim report from the Polycythemia Vera Study Group. Semin Hematol. 1986;23(3):177–82. [PubMed] [Google Scholar]
- 14.Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34(1):29–39. [PubMed] [Google Scholar]
- 15.Barosi G, Ambrosetti A, Finelli C, Grossi A, Leoni P, Liberato NL, et al. The Italian Consensus Conference on Diagnostic Criteria for Myelofibrosis with Myeloid Metaplasia. Br J Haematol. 1999;104(4):730–7. doi: 10.1046/j.1365-2141.1999.01262.x. [DOI] [PubMed] [Google Scholar]
- 16.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 17.Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110(4):1092–7. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 18.Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, et al. Proposed criteria for the diagnosis of post-poly-cythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–8. doi: 10.1038/sj.leu.2404914. [DOI] [PubMed] [Google Scholar]
- 19.Passamonti M, Ghiselli F. Doubly uni-parental inheritance: two mitochondrial genomes, one precious model for organelle DNA inheritance and evolution. DNA Cell Biol. 2009;28(2):79–89. doi: 10.1089/dna.2008.0807. [DOI] [PubMed] [Google Scholar]
- 20.Olcaydu D, Skoda RC, Looser R, Li S, Cazzola M, Pietra D, et al. The ‘GGCC’ hap-lotype of JAK2 confers susceptibility to JAK2 exon 12 mutation-positive polycythemia vera. Leukemia. 2009;23(10):1924–6. doi: 10.1038/leu.2009.110. [DOI] [PubMed] [Google Scholar]
- 21.Arcaini L, Pascutto C, Passamonti F, Bruno R, Merli M, Rizzi S, et al. Bayesian models identify specific lymphoproliferative disorders associated with hepatitis C virus infection. Int J Cancer. 2009;124(9):2246–9. doi: 10.1002/ijc.24162. [DOI] [PubMed] [Google Scholar]
- 22.Murray F, Darzentas N, Hadzidimitriou A, Tobin G, Boudjogra M, Scielzo C, et al. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: implications for the role of antigen selection in leukemogenesis. Blood. 2008;111(3):1524–33. doi: 10.1182/blood-2007-07-099564. [DOI] [PubMed] [Google Scholar]
- 23.Rumi E, Passamonti F, Della Porta MG, Elena C, Arcaini L, Vanelli L, et al. Familial chronic myeloproliferative disorders: clinical phenotype and evidence of disease anticipation. J Clin Oncol. 2007;25(35):5630–5. doi: 10.1200/JCO.2007.12.6896. [DOI] [PubMed] [Google Scholar]
- 24.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]