Systemic mastocytosis (SM) is a heterogeneous disorder characterized by the proliferation and accumulation of atypical mast cells (MC) in tissues, principally in the bone marrow (BM) and skin. The diagnosis of systemic mastocytosis requires the presence of multifocal dense mast cell infiltrates in one or multiple extra-cutaneous organs, mostly bone marrow (major criterion), plus at least one of the following minor criteria: i) abnormal morphology of extra-cutaneous mast cells (spindle-shaped cells); ii) increased serum tryptase level (>20 ng/ml); iii) abnormal expression of CD2 and/or CD25 on bone marrow mast cells; iv) detection of a KIT mutation at codon 816 in extra-cutaneous organs.1 Diagnosis of systemic mastocytosis can also be made in the presence of at least 3 minor criteria. According to the World Health Organization (WHO) systemic mastocytosis was grouped into 4 major clinical variants: 1) indolent systemic mastocytosis; 2) systemic mastocytosis with an associated clonal, hematologic, non-mast cell lineage disease; 3) aggressive systemic mastocytosis; and 4) mast cell leukemia based on distinct clinico-pathological features. On the basis of the presence of two or more B-findings, indolent systemic mastocytosis can be subclassified as smoldering systemic mastocytosis (SSM).2
A lack of skin lesions has been described in cases of aggressive systemic mastocytosis and mast cell leukemia, but also in bone marrow mastocytosis (BMM), a subvariant of indolent systemic mastocytosis recognized by the 2008 WHO classification.2 Diagnosis of systemic mastocytosis in the absence of typical skin involvement presents a challenge for clinicians. It should be considered in the differential diagnosis for a number of clinical conditions, such as unexplained anaphylaxis, osteoporosis of unknown etiology, etc. Moreover, indolent systemic mastocytosis without skin lesions has been frequently reported in patients with systemic allergic reaction to hymenoptera venom and raised basal tryptase.3
We report the characteristics of 99 consecutive indolent systemic mastocytosis patients, diagnosed or revised according to the 2008 WHO classification2 from January 2005 to December 2009 at the Multidisciplinary Outpatient Clinic for Mastocytosis in Verona, Italy. These patients were among 145 patients referred from a regional network of Allergists and Dermatologists, following the appearance of classic skin lesions, or in the case of unexplained/recurrent anaphylaxis or severe allergic reactions to hymenoptera sting with persistent raised tryptase. They had complete blood cell count, routine biochemistry, abdominal ultrasonography, bone densitometry evaluation with Dual-energy X-ray Absorptiometry and, in selected cases, skeletal X-ray. All patients had a bone marrow evaluation with bone marrow histology/cytology, flow cytometric analysis, and detection of KIT mutation, performed as previously described.4 Anaphylaxis was defined according to the European Academy of Allergology and Clinical Immunology and World Allergy Organization criteria (2003).5 Approval for this study was obtained from the Azienda Ospedaliera Universitaria Integrata of Verona’s institutional review board.
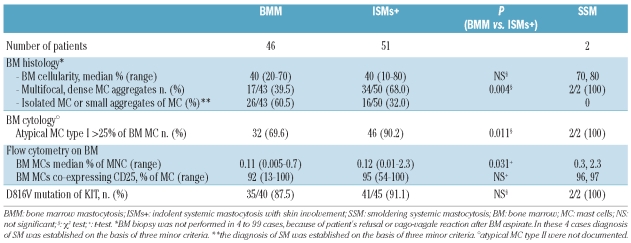
Sixty-one patients showed classic skin lesions: a diagnosis of indolent systemic mastocytosis (ISMs+) was made in 51 of 61 subjects, 2 patients were diagnosed with smoldering systemic mastocytosis and 8 were classified as cutaneous mastocytosis. Isolated bone marrow mastocytosis was diagnosed in 46 of 84 patients (54.7%) referred for unexplained/recurrent anaphylaxis or severe allergic reactions to hymenoptera sting without skin lesions. Another 13 patients fulfilled less than 3 minor criteria but displayed mast cell clonality markers and were considered as having monoclonal mast cell activation syndrome.6,7 The major diagnostic criterion and at least one minor criterion were fulfilled on bone marrow evaluation in 68.0% of indolent systemic mastocytosis with skin involvement and in 39.5% of bone marrow mastocytosis patients (Table 1).
Table 1.
Bone marrow evaluation of 99 indolent systemic mastocytosis patients.
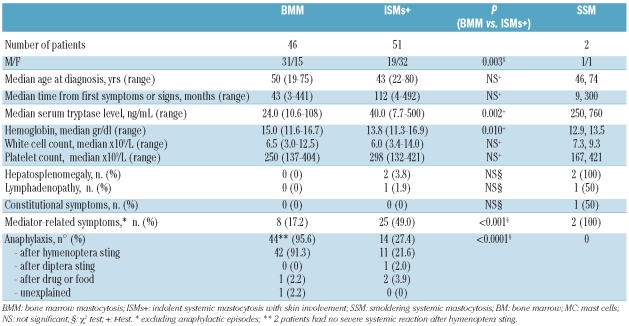
Clinical and biological characteristics of 46 bone marrow mastocytosis, 51 indolent systemic mastocytosis with skin involvement and 2 smoldering systemic mastocytosis patients are summarized in Table 2. There were 51 males and 48 females (median age 47 years, range 19–80). After a median of 26 months from diagnosis (range 6–104) all patients were alive with stable disease and constant serum tryptase levels; 2 bone marrow mastocytosis patients presented a severe disability following cerebral hypoxia due to a severe reaction to hymenoptera venom. Bone marrow mastocytosis patients were predominantly males and had a lower bone marrow mast cell burden, as evaluated by flow-cytometry, serum tryptase level and incidence of mediator-related symptoms other than anaphylaxis, than indolent systemic mastocytosis cases with skin involvement. Lower hemoglobin level documented in indolent systemic mastocytosis patients with skin involvement was related to a higher percentage of female patients in this group.
Table 2.
Clinical and laboratory characteristics of 99 indolent systemic mastocytosis patients.
Pardanani and Tefferi have recently demonstrated that bone marrow mastocytosis is not as rare as previously believed.8 Indeed 39 out of 159 (23%) indolent systemic mastocytosis patients referred to their institution during a period of about 20 years had bone marrow mastocytosis and 86% of them had history of anaphylactoid reactions; they were prevalently men, had lower tryptase levels, and a lower incidence of B-findings and constitutional symptoms than other types of indolent systemic mastocytosis. However, in a proposal to revise the systemic mastocytosis classification, they suggest that this subvariant can be removed due to its limited relevance.9
In our opinion, the incidence of indolent systemic mastocytosis limited to bone marrow has been frequently underestimated. Although it is a rare condition, close collaboration between different specialists could improve detection of this disease. In our experience a high number of bone marrow mastocytosis patients was detected in a relatively short period of time due to the creation of a multidisciplinary regional network and the availability of very sensitive diagnostic procedures. Because bone marrow mastocytosis could underlie unexplained anaphylaxis or severe allergic reactions even with normal tryptase levels,10 it could be hypothesized that the actual frequency of bone marrow mastocytosis is still underestimated. Evaluation of clinical characteristics of the allergic reactions, i.e. the presence of syncope in the absence of urticaria/angioedema, could be helpful to identify those subjects who are likely to have bone marrow mastocytosis before a bone marrow study, as recently proposed by Alvarez-Twose et al.11
We believe that early recognition of bone marrow mastocytosis is essential to reduce potential life-threatening events or severe skeletal complications. Moreover, patients with systemic reactions to hymenoptera venom should be considered for life-long venom immunotherapy.4 Our data and the series presented by Alvarez-Twose et al.11 suggest that indolent systemic mastocytosis limited to the bone marrow could represent a distinct disease subtype with a strong association with anaphylactic reactions and little or no probability of a progressive course. Nevertheless, collaborative longitudinal studies are needed to better evaluate these conditions and to establish common definitions.
Acknowledgments
We thank physicians in Italian institutes for their precious collaboration: Dr. A. Guella, Dr. S. Cerù, UO di Ematologia and Dr. G. Recchia, UO di Dermatologia, Ospedale S. Chiara, Trento; Dr. L. Castellani, UO di Dermatologia and Dr. L. Lenzi, UO di Medicina, Ospedale di Rovereto, Trento; Dr. L. Simioni, UO di Medicina, Ospedale Civile di Feltre, Belluno; Dr. I. Romano, UO di Dermatologia; Dr. G. Marcer, Dr A. Crivellaro, UO di Medicina del Lavoro and Prof. R. Mantero, UO di Endocrinologia, Università di Padova; Dr. F. Chieco-Bianchi, Servizio di Allergologia, ULSS 7, Conegliano, Treviso; Dr. M. Franchini, UO di Allergologia, Ospedale di Jesolo Lido, Venezia; Dr. A. Scarpa, Dr. M. Busa, UO di Dermatologia, Azienda ULSS 13, Mirano, Venezia; Dr A. Angheben, Centro per le Malattie Tropicali, Ospedale Sacro Cuore di Negrar, Verona; Dr. A.R. Trolese, U.O. di Oncologia, Ospedale “Mater Salutis” di Legnago, Verona; Dr. M.T. Costantino, UO di Allergologia, Ospedale Carlo Poma, Mantova; Dr. M. Pagani, Servizio di Allergologia e Oncologia, Ospedale di Asola, Mantova; Dr. C. Tosoni and Dr. F. Lodi-Rizzini, UO di Allergologia and Dr. M. D’Adda, UO di Ematologia, Spedali Civili di Brescia.
Footnotes
Funding: this work was supported by Associazione Italiana Leucemie e Linfomi (AIL).
References
- 1.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th ed. Lyon (France): IARC Press; 2008. pp. 54–63. [Google Scholar]
- 3.Bonadonna P, Zanotti R, Müller U. Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. 2010;10(4):347–53. doi: 10.1097/ACI.0b013e32833b280c. [DOI] [PubMed] [Google Scholar]
- 4.Bonadonna P, Perbellini O, Passalacqua G, Caruso B, Colarossi S, Dal Fior D, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009;123(3):680–6. doi: 10.1016/j.jaci.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113 (5):832–6. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 6.Sonneck K, Florian S, Müllauer L, Wimazal F, Födinger M, Sperr WR, Valent P. Diagnostic and subdiagnostic accumulation of mast cells in the bone marrow of patients with anaphylaxis: Monoclonal mast cell activation syndrome. Int Arch Allergy Immunol. 2007;142(2):158–64. doi: 10.1159/000096442. [DOI] [PubMed] [Google Scholar]
- 7.Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, Metcalfe DD. Demonstration of an aberrant mast cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110(7):2331–3. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardanani A, Lim KH, Lasho TL, Finke CM, McClure RF, Li CY, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115(1):150–1. doi: 10.1182/blood-2009-10-249979. [DOI] [PubMed] [Google Scholar]
- 9.Pardanani A, Tefferi A. Proposal for a revised classification of systemic mastocytosis. Blood. 2010;115(13):2720–1. doi: 10.1182/blood-2009-12-259085. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe DD, Schwartz LB. Assessing anaphylactic risk? Consider mast cell clonality. J Allergy Clin Immunol. 2009;123(3):687–8. doi: 10.1016/j.jaci.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Twose I, González de Olano D, Sánchez-Muñoz L, Matito A, Esteban-López MI, Vega A, et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J Allergy Clin Immunol. 2010;125(5):1269–78. doi: 10.1016/j.jaci.2010.02.019. [DOI] [PubMed] [Google Scholar]