Summary
Early stressful events can increase vulnerability for psychopathology, although knowledge on the effectors is still limited. In this report we describe the characterization of a single nucleotide polymorphism (SNP) in rhesus macaques, which results in a Val to Met transition in the pro-BDNF domain, similar to a well described variant in the human gene. Further, we tested the hypothesis that peripheral levels of BDNF, which is involved in the response to stress and in the pathophysiology of anxiety and depression, might be differentially affected in a non-human primate model of early adverse rearing in a genotype-dependent manner. Males and females rhesus macaques reared either with their mothers (MR), in peer-only groups (PR), or in a “surrogate/peer-reared” (SPR) condition with limited peer interactions, were used as experimental subjects. BDNF levels were determined at baseline on postnatal days (PND) 14, 30 and 60 by means of specific ELISA procedure. Data indicate that BDNF levels were increased as a result of peer-rearing and that this increase was moderated by the presence of the SNP. Overall these data indicate that a SNP, which results in a Val to Met transition in the pro-BDNF domain, is present in rhesus macaques and is able to affect BDNF peripheral levels, thus making this primate model a fundamental tool to study gene by environment interactions involving the BDNF gene.
Keywords: Brain-derived neurotrophic factor (BDNF), polymorphism, stress, depression, non-human primates
1. Introduction
Early adverse experiences in humans are associated with an increased risk for developing psychiatric disorders such as anxiety and major depression (Kaufman et al., 2000; McEwen, 2000; Heim and Nemeroff, 2001), although little is known of the neurobiological mediators. Brain-Derived Neurotrophic Factor (BDNF), a neurotrophin which is affected by stress and plays a fundamental role in brain functions and neuroprotection, is a good candidate for mediating the effects of adverse life events into changes in brain functions (Smith et al., 1995; Thoenen, 1995; Duman et al., 1997; Martinowich et al., 2007). Neurotrophins are also produced by cells outside the nervous system, thus being in a position to integrate neural, immune and endocrine responses to stress (Aloe et al., 1986; Nisoli et al., 1996; Nockher and Renz, 2005).
Both genetic and experiential factors can contribute to the development of psychopathology (Yehuda et al., 1997; Heim and Nemeroff, 1999). In one of the first studies involving gene-environment interactions, Caspi and coworkers (Caspi et al., 2003) showed that a functional polymorphism in the promoter region of the serotonin transporter gene (5-HTT-LPR) moderates the influence of stressful life events on vulnerability to depression, i.e. provided the first evidence for gene × environment (G × E) interaction in psychiatric disorders. Individuals with one or two copies of the 5-HTT ‘short’ allele exhibited more depressive symptoms, diagnosable depression and suicidality following stressful life events than individuals with two copies of the ‘long’ allele (Caspi et al., 2003). A considerable number of replication studies have been published since that initial paper; although one meta-analysis did not provide unequivocal evidence of 5-HTT × E (Risch et al., 2009), critical appraisal of all pertinent data (Uher and McGuffin, 2008; Huezo-Diaz et al., 2009; Caspi et al. 2010) suggests that 5-HTT-LPR indeed has a role in translating environmental adversities to human behavior, and non-human primate studies have been shown to have predictive validity for examining GxE interactions (Barr et al., 2004; Caspi et al., 2010). Although 5-HTT-LPR genotype may explain a portion of GxE effects, other genes, most likely, have to play a role as well.
An increasing number of studies use serum BDNF levels as a potential indicator for central nervous system alterations as changes in peripheral levels of this neurotrophin have been associated with mood disorders, also in interaction with early trauma (Aloe et al., 1994; Hadjiconstantinou et al., 2001; Karege et al., 2002; Karege et al., 2005; Kaufman et al., 2006; Castren et al., 2007; Kauer-Sant'Anna et al., 2007; Mitoma et al., 2008). In addition, recent data on a rat model of electroconvulsive treatment has provided substantial evidence that it can be justified to measure serum BDNF levels as indices of central activity, although changes in neurotrophin levels might show a different time course in the brain and periphery (Sartorius et al., 2009).
Haploinsufficiency of BDNF goes along with decreased peripheral BDNF levels as well as childhood-onset obesity (Han et al., 2008). Although this genetic variant is rare, as are several other coding region variants (Licinio et al., 2009), there is also a frequent non-synonymous single-nucleotide polymorphism (SNP), which results in an aminoacid substitution in the pro-BDNF domain (rs6265, Val66Met). Met allele carriers have attenuated intracellular trafficking and secretion of BDNF and show comparatively lower hippocampal gray matter and poorer cognitive performance (Egan et al., 2003; Chen et al., 2004). Not surprisingly, this SNP has been tested for association with a wide range of psychiatric disorders. Rs6265 has been associated with substance abuse, eating disorders and schizophrenia (Verhagen et al., 2010). G × E interactions might add a further level of complexity, as it was shown that the Met allele interacts with severe life events thereby causing psychiatric symptoms (Kaufman et al., 2000; Savitz et al., 2007; Elzinga et al., 2010), also in interaction with 5-HTT-LPR (Kaufman et al., 2006; Wichers et al., 2008).
In line with the notion that the Met allele is implicated in the etiopathology of depression, it was shown that Met allele carriers, irrespective of diagnosis, have a decreased hippocampal volume (Frodl et al., 2007), which might also provide a neuroanatomical basis for G × E effects as Met allele carriers have a smaller hippocampal volume only in the presence of severe life events (Gatt et al., 2009). A similar mechanism has already been proposed for post-traumatic stress disorder (Gross and Hen, 2004).
Taken together, there is thus ample evidence that rs6265 (a) predicts peripheral BDNF levels and (b) interacts with life stress to increase the risk for depression, making this SNP a prime biomarker for susceptibility to psychopathology in humans, including depression. The precise interplay between rs6265 and life stress however cannot readily be tested in humans and strategies, such as variant BDNF mouse models (BDNF Met/Met) that reproduce phenotypic hallmarks characterizing humans with the variant allele (Chen et al., 2006), although of great advantage, bear the disadvantage of suboptimal behavioral repertoires which do not mirror well human psychopathology.
We have previously shown in a non-human primate model that maternal deprivation with some form of social contact, such as access to peers, leads to important emotional and social disturbances and behavioral abnormalities, such as motor stereotypies (Suomi, 1991; Champoux et al., 2002; Barr et al., 2003). Peer-reared macaques develop inadequate social skills, are highly reactive and aggressive and, as adults, show increased voluntary alcohol consumption, and typically are low-ranking in mixed social groups (Suomi, 1991; Barr et al., 2003). When peer interactions are limited in time, the effects are even more pronounced. We have recently shown that peripheral levels of BDNF are able to track these behavioral changes (Cirulli et al., 2009b). In particular, we have shown that a selective increase in BDNF peripheral levels occurs in response to early adversity caused by peer-rearing.
In this paper we report for the first time that a polymorphism of the BDNF gene (Val66Met) interacts with early social rearing adversity and results in lower peripheral BDNF levels in rhesus macaques.
2. Methods
2.1. Animals and rearing procedures
Subjects of these studies were 19 males and 18 females rhesus monkey infants (Macaca mulatta) born between 2003 and 2005 in the Laboratory of Comparative Ethology, NICHD breeding facility at the NIH Animal Center near Poolesville (MD, USA). Thirteen subjects were “mother-reared” (MR), raised in social groups either by their biological mothers or by an unrelated multiparous foster mother. Thirteen others infants were reared without adults, but with constant access to age-mate peers in a “peer-only reared” (PR) condition, while an additional 11 infants were reared with inanimate surrogates and limited peer interactions in a “surrogate/peer-reared” (SPR) condition. Rearing conditions were randomly assigned at the time of birth balancing the number of males and females in each rearing condition.
MR infants remained with their mothers in a stable social group of 8-10 adults and peers. Infants assigned to the PR and SPR conditions were separated from their mothers at birth, and were subsequently hand-reared in a neonatal nursery. During the first 37 days of their life, PR and SPR infants were treated in an identical manner. For the first 14 days, they were kept in an incubator and hand-fed. Each cage contained a blanket and a terry cloth-covered rocking “surrogate” covered by a heating pad. The infant could see and hear, but did not experience any physical contact with the other infants. From day 15 until day 38, infants were moved with their surrogate in individual nursery cages. At 38 days of age all nursery-reared infants were placed into their final condition (PR or SPR). PR infants were placed in permanent social groups of 3-4 age-mates, similarly-reared peers, with whom they had continuous contact. SPR monkeys differed from PR monkeys only in the amount of time that they were allowed to interact with their age-mates each day. SPR infants continued to be housed individually with their surrogates, being provided with only limited peer contact (2 hours/day, 5 days/week). This procedure allowed the infants to socialize in the absence of a mother, without allowing them to become overly attached to one another (cf. Shannon et al., 1998) for additional details regarding these respective rearing procedures).
Protocols for the use of experimental animals were approved by the Institutional Animal Care and Use Committee of the NICHD. All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. DNA extraction and genotyping
In order to test whether the human polymorphisms rs6265 is also present in rhesus monkeys, DNA from the above subjects was amplified by PCR using the forward primer CAAACATCCGAGGACAAGGT and the reverse primer AGAAGAGGAGGCTCCAAAGG, thereby generating an amplicon of 250 bp covering rs6265 in humans, as tested in four previously genotyped human control subjects, and rhesus monkeys based on the UCSC database (Jan. 2006 [MGSC Merged 1.0/rheMac2] assembly). PCR was carried out with 50 mM KCl, 0.025% Tween20, 1.5mM MgCl2. 200 μM dNTPs, 0.2 μM primers dissolved in 10 mM Tris-HCl (pH=8.3). PCR conditions were 2 min at 96°C, and 30 cycles of 20 sec at 96°C, 20 sec at 58°C, and 30 sec at 72°C. Amplicons were treated with exonuclease and phosphatase (ExoSapIT, Amersham) and sequenced thereafter using the QuickStart Sequencing-Kit (Beckman-Coulter, Krefeld, Germany) on a Beckman CEQ8000 sequencer (Beckman-Coulter, Krefeld, Germany).
2.3. BDNF measurement
On days 14 and 30 after standardized behavioral testing (Schneider et al., 1991), animals were anesthetized with ketamine hydrochloride (intramuscularly, 10 mg/kg), and blood was drawn from the femoral vein in EDTA-coated tubes. After centrifugation, a 500 μl aliquot of plasma was placed into a plastic vial and stored at −70°C until assay. On PND 60 another baseline measure was taken. The same sampling and processing procedures were used for all groups.
Plasma was assayed for levels of BDNF by highly sensitive immunoenzymatic assays, following the procedure suggested by the manufacturer (Emaxtm ImmunoAssay System number G6891, Promega, Madison, WI, USA; Aloe et al., 1994). A monoclonal anti-mouse-BDNF antibody and a polyclonal anti-human-BDNF antibody were used. BDNF concentration was determined from the regression line for the BDNF standard curve (ranging from 7.8 to 500 pg/ml-purified mouse BDNF) incubated under similar conditions in each assay. The sensitivity of the assay is about 15 pg/ml of BDNF and the cross-reactivity with other related neurotrophic factors (NGF, NT-3, and NT-4) is considered nil.
Our sampling methodology (which was the same for all experimental groups) can be considered not to be biased by platelet reactivity and reflects circulating levels of BDNF (see also Cirulli et al., 2009b) for more details).
2.4. Statistical analysis
StatView 5.0.1 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. Mixed design, repeated measures ANOVAs were performed on data to assess the effect of genotype, age and rearing condition, but not gender, and their interactions. Since only three cases analyzed were homozygous for the BDNF gene variant described, these were considered together with heterozygous cases. Tukey tests were used for post-hoc comparisons.
3. Results
3.1. Characterization of a novel SNP in the BDNF gene in rhesus macaques
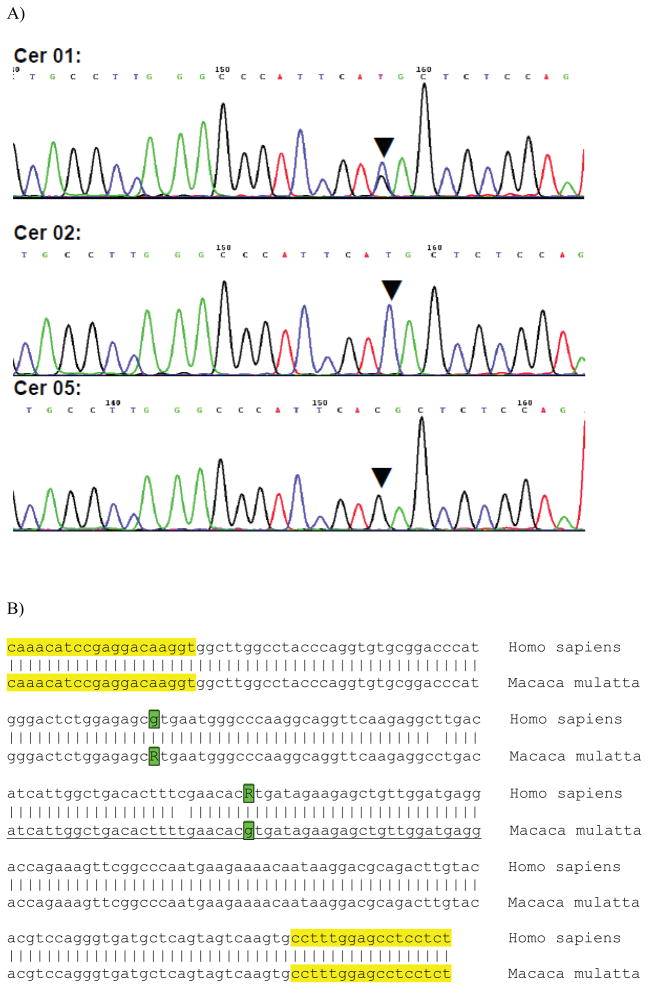
Although SNPs are not generally conserved between rhesus and humans, there are instances in which functionally similar variants occur in the two species (see for example Miller et al., 2004; Barr et al., 2008). To determine whether there were any SNPs in macaques that were functionally similar to the human Val66Met SNP, we sequenced the 250 bp genomic region flanking the orthologous site in rhesus monkeys. Sequencing revealed that all cases were homozygous for the G allele at position 196, arguing against the presence of a polymorphic variant at this site. However, we detected a polymorphic site at p.136 (G>A; Fig. 1A and B), which results in a Val to Met transition in the BDNF pro-domain at codon 46.
Figure 1.
A) Spherograms of rhesus DNA heterozygous at p.196 (Cer01), homozygous for the A allele (Cer02) and homozygous for the G allele (Cer05). B) Sequence alignment of human and rhesus DNA at the BDNF locus. Primer sequences are highlighted in yellow, and the polymorphic variants at p.136 and p.196 (G>A transitions) are printed in bold, boxed, and highlighted in green. Both variants result in a Val > Met transition at position 46 or 66, respectively, of the resulting transcript.
3.2. Effects of age, genotype and rearing condition on plasma BDNF levels of rhesus monkey infants
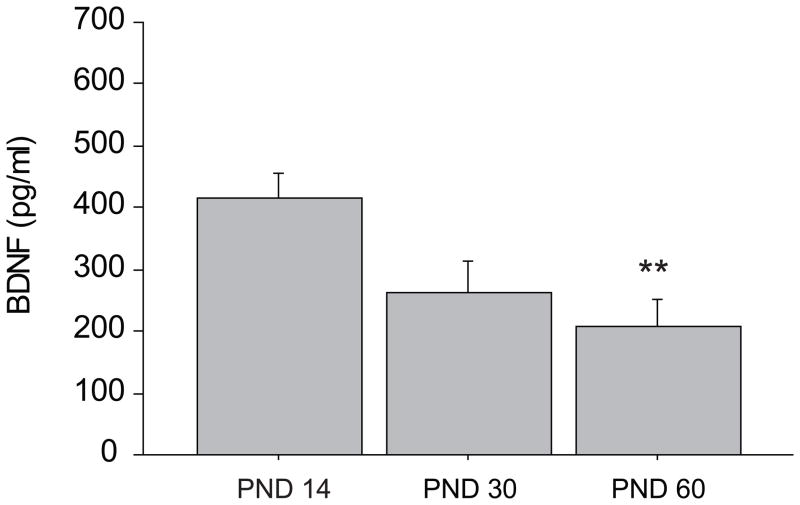
Regardless of genotype and rearing condition, age affected levels of BDNF (main effect on age, F (2,46) = 5.236, p = 0.0089). In particular post-hoc comparisons showed that subjects in the PND 14 group were characterized by higher levels of BDNF, this neurotrophin decreasing over time from PND14 to PND 60 (post-hoc comparisons: PND 14 vs PND 30 and PND 60, p < 0.01) (Figure 2).
Figure 2.
Main effect of age on BDNF plasma levels in rhesus macaques. BDNF levels decreased significantly with age being higher on PND 14 compared to both PND 30 and PND 60 (** p <0.01). In the graph each represented group of age is averaged over the rearing conditions and the genotype groups. Data are expressed as means (+ SEM). N = 29 subjects in each final group
3.3. Effects of genotype and rearing condition in rhesus monkey infants on plasma BDNF levels
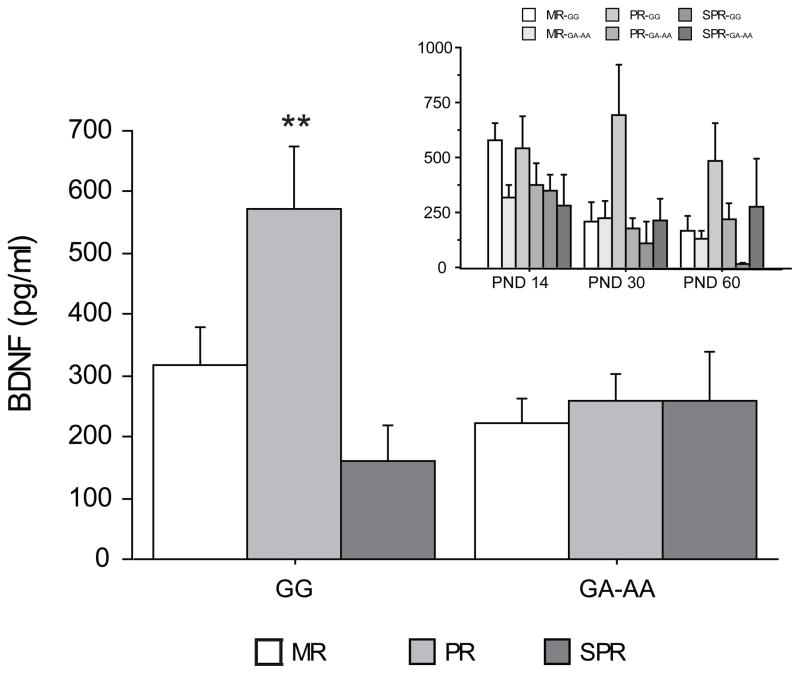
Genotype and rearing conditions per se also affected BDNF levels (main effects of genotype: F (1,23) = 4.472, p = 0.0455 and of rearing condition: F (2,23) = 6.071, p = 0.0076). In particular: the GG group showed higher BDNF levels than the GA-AA, while PR subjects were characterized by higher BDNF levels when compared both to the MR and the SPR groups (post-hoc comparisons: p < 0.01).
A significant interaction between genotype and rearing condition was also found (F (2,23) = 5.299, p = 0.0128). In particular PRGG showed higher BDNF levels when compared to MRGG, SPRGG and PRAA-AG (post-hoc comparisons: p < 0.01; see Figure 3). Genotype and age did not significantly interact between each other to affect BDNF levels (F (2,46) = 0.852, p = 0.4333).
Figure 3.
Genotype interacts with rearing condition to affect BDNF protein levels in the peripheral circulation. In the PR group significantly higher BDNF levels were found in subjects with the GG genotype. This difference was abated in the AA-AG group (Tukey: PRGG vs MRGG, SPRGG and PRAA-AG p < 0.01; ** p <0.01). In the inset data are split by age. Data are expressed as means (+ SEM) of BDNF values. N = 18 (MRGG); 12 (PRGG); 9 (SPRGG); 21 (MRAA-AG) 18 (PRAA-AG) 9 (SPRAA-AG) values in each final group.
4. Discussion
This study describes for the first time a rhesus macaque SNP that produces a Val to Met transition in the pro-BDNF domain. This polymorphism affects peripheral BDNF levels in a G × E manner, with Met allele carriers which were peer-reared displaying significantly lower peripheral levels of this neurotrophin as compared to Val allele carriers raised the same way.
Animal models have provided supportive evidence that neurotrophins are sensitive to manipulations of the mother-infant relationship and, more in general, of the rearing environment (Cirulli et al., 1998; Cirulli, 2003a; Branchi et al., 2006; Cirulli et al., 2007). In a previous study we have shown that peer-rearing leads to increased peripheral BDNF levels in rhesus macaques, especially in females (Cirulli et al. 2009). In the present study, a further rearing condition was used, the surrogate/peer-reared condition or SPR. Subjects raised under this condition did not show an elevation in BDNF levels as the PR animals did. We hypothesize that the lack of a BDNF increase in the SPR group, compared to the peer-reared, could be a marker of unsuccessful coping, as we know that they show suppressed neuroendocrine activity (Shannon et al., 1998). Indeed, as compared to peer-rearing, the SPR condition involves reduced peer interactions and can thus be considered to be more adverse for the infants, who nonetheless grow up and establish social relationships, although often ranking at the bottom of the social hierarchy at adulthood (Bastian et al., 2003). It is possible to hypothesize that, while moderate adversity will activate a coping response (higher BDNF levels; see Cirulli et al., 2009b), a more severe situation might be characterized by an inability to mount a stress response, or even result in a suppression of BDNF gene expression, testifying an inability to face the stress of being reared in the SPR group. Since PR subjects are characterized by a greater amount of social interactions between peers than SPR, this result indirectly suggests that social relationships might represent an important buffer for highly stressful situations early during postnatal life.
Even more interesting, however, is the finding that the presence of the Met allele prevented any elevation in BDNF levels in the PR group. These data appear to be in line with previous reports indicating attenuated intracellular trafficking and secretion of BDNF in human Met allele carriers and further indicate that the effects of the gene variant might depend upon expression levels, which may be altered following stress. These data are in line with results from a variant BDNF mouse model in which the presence of the Met allele does not affect basal BDNF secretion but results in a 30% deficit in activity-dependent release of BDNF-Met from neurons (Chen et al., 2006).
Since an increase in BDNF levels is an index of a coping response to stress, we believe that the presence of the Met allele might thwart such a response, thus endangering plasticity processes (see Cirulli and Alleva, 2009 for a review). Integrating neurobiological variables (such as changes in BDNF levels) with behavioural data in subjects carrying the SNP, and exposed to different contexts, will clarify the functional significance of the BDNF polymorphism here described. Indeed the ultimate effect of a gene variant will depend upon the environmental constraints characterizing the individual. In a broader and comparative perspective, however, it is worth mentioning the positive value of genetic variation which has allowed humans and some non-human primates, such as rhesus macaques, to successfully colonize different ecological niches (Suomi, 2006).
Integrating the function of early life stress might account for inconsistencies found in the literature when investigating candidate genes or performing genome-wide association studies for affective disorders as studies examining the role of the BDNF Val66Met polymorphism on major depression are characterized by mixed results. Differences between studies may be due to unmeasured environmental variables, including exposure to stressful stimuli (Krishnan and Taylor, 2009; Elzinga et al., 2010). Although genetic differences contribute to the vulnerability and progression of stress-related neuropsychiatric disorders, environmental factors are also important and there is evidence in humans showing that vulnerability to depression and anxiety disorders are markedly increased by early trauma (Heim and Nemeroff, 2001). It has been recently confirmed in a large epidemiological study that human Val66Met is indeed relevant for an early life GXE interaction (Elzinga et al., 2010). These authors investigated, in subjects with lifetime major depressive disorder, the impact of childhood abuse and recent life events on serum BDNF levels and examined whether BDNF Val66Met polymorphism might moderate the impact of such events. Results indicate that Met carriers are particularly sensitive to early stressful events (Elzinga et al., 2010). Data from the present study confirm in a non-human primate model these findings and suggest that a functional effect of BDNF gene variants may become manifest early on only in interaction with moderately adverse events.
Increased levels of BDNF indeed characterize acute responses to stressful events - including maternal separation - early during postnatal life, while decreased expression is more reliably found at adulthood and following chronic stress exposure (Roceri et al., 2004; Savitz et al., 2007; Cirulli et al., 2010). These data are in line with the notion that in humans, BDNF concentrations in the blood can be considered a biomarker of depression, with reduced levels of this neurotrophin characterizing depressed patients, also in interaction with early trauma (Aloe et al., 1994; Hadjiconstantinou et al., 2001; Karege et al., 2002; Karege et al., 2005; Kaufman et al., 2006; Castren et al., 2007; Kauer-Sant'Anna et al., 2007; Grassi-Oliveira et al., 2008; Mitoma et al., 2008). While animal models have provided evidence that neurotrophins are sensitive to manipulations of the mother-infant relationship and, more in general, of the rearing environment (Cirulli et al., 1998; Cirulli, 2003a; Branchi et al., 2006; Cirulli et al., 2007), early adversity, as represented by maternal separation, has been shown to increase, rather than to decrease levels of BDNF in the hippocampus of rodents as well as peripheral BDNF levels in rhesus macaques (Roceri et al., 2004). Results obtained in animal models thus suggests that changes in neurotrophin levels following early stressful events can not directly be related with neurotrophin levels in adulthood (Roceri et al., 2004).
Changes in BDNF levels following stress indicate “allostatic” processes activated to coordinate brain and body responses to specific external challenges (Chaldakov et al., 2004; McEwen, 2007; Cirulli and Alleva, 2009). In addition to acting on processes regulating neuronal growth and connectivity, neurotrophins could counteract the negative impact of stress hormones on selected brain regions, such as the hippocampus, as well as on other body organs (Thoenen, 1995). In particular, peripheral BDNF levels could mediate the response to stress through activation of peripheral tissues (Chaldakov et al., 2004; Cirulli and Alleva, 2009). However, an effect of peripheral BDNF on the central nervous system cannot be excluded since peripheral and central BDNF levels are closely related (Karege et al., 2002).
A mechanistic link between lifelong changes in behavioural traits and the establishment of permanent modifications of key genes following adverse events during the perinatal period is still lacking. Identifying a clear causal relationship between these events will require an in-depth understanding of the players and mechanisms involved, including changes taking place in chromatin and in transcriptional networks (Dulac, 2010). Among the mechanisms that have been proposed to mediate stable behavioural differences we can enlist the effect of modifications in the mother-infant relationship causing selective changes in the expression of neurotrophin genes affecting brain development in rodents and non-human primates during appropriate developmental windows (Cirulli et al., 2009a). Data presented in this paper add to these findings suggesting that such changes might depend upon the genetic background of the individual.
One of the limitations of this study is that we could not discriminate whether the G × E effect is different in females, compared to males, due to the small sample size. Indeed, it has been previously shown that gender may play an important role in GxE interactions, as rs6265 has been selectively associated with depression in males (Verhagen et al., 2010). We are currently increasing our sample size to address this point. Indeed, based upon previous studies it is possible to hypothesize that the effect of a Val to Met transition might be more pronounced in females (Cirulli and Alleva, 2009).
The BDNF polymorphism described in this report makes this primate model a fundamental tool to study gene by environment interactions involving the BDNF gene. These studies bear important implications in order to unravel the role of this functional BDNF variation in mental health and for the characterization of the early determinants of psychopathology.
Acknowledgments
Funding for this study was provided by the ISS-NIH Collaborative Project to FC and EA (530F/51), by the Italian Ministry of Health (Ricerca Finalizzata ex art. 12-2006) and by the Deutsche Forschungsgemeinschaft (DFG; SFB-TRR-58 Z02 to AR; Grant KFO 125 to AR; DE357/4-1 to AR; RE1632/5 to AR) and the BMBF (Panic-Net, to AR; details see webpage http://www.paniknetz.de/netzwerk.html). This research was also supported by funds from the Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health; the NIH and the Italian Ministry of Health had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. We are grateful for excellent technical support of T. Töpner and Veronica Bellisario.
Footnotes
Conflict of interest
All authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloe L, Alleva E, Bohm A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci U S A. 1986;83:6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Bracci-Laudiero L, Alleva E, Lambiase A, Micera A, Tirassa P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc Natl Acad Sci U S A. 1994;91:10440–10444. doi: 10.1073/pnas.91.22.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley D, Heilig M, Suomi SJ, Goldman D. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian ML, Sponberg AC, Sponberg AC, Suomi SJ, Higley JD. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta) Dev Psychobiol. 2003;42:44–51. doi: 10.1002/dev.10091. [DOI] [PubMed] [Google Scholar]
- Branchi I, D'Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and "depression"-like behavior. J Neurosci Res. 2006;83:965–973. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chaldakov GN, Fiore M, Stankulov IS, Manni L, Hristova MG, Antonelli A, Ghenev PI, Aloe L. Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: a role for NGF and BDNF in cardiovascular disease? Prog Brain Res. 2004;146:279–289. doi: 10.1016/S0079-6123(03)46018-4. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Alleva E. The NGF saga: from animal models of psychosocial stress to stress-related psychopathology. Front Neuroendocrinol. 2009;30:379–395. doi: 10.1016/j.yfrne.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Micera A, Alleva E, Aloe L. Early maternal separation increases NGF expression in the developing rat hippocampus. Pharmacol Biochem Behav. 1998;59:853–858. doi: 10.1016/s0091-3057(97)00512-1. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Capone F, Bonsignore LT, Aloe L, Alleva E. Early behavioural enrichment in the form of handling renders mouse pups unresponsive to anxiolytic drugs and increases NGF levels in the hippocampus. Behav Brain Res. 2007;178:208–215. doi: 10.1016/j.bbr.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci Biobehav Rev. 2009a;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, Alleva E. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009b;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Bonsignore LT, Capone F, D'Andrea I, Aloe L, Branchi I, Alleva E. Early life influences on emotional reactivity: evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress and central BDNF levels. Neurosci Biobehav Rev. 2010;34:808–820. doi: 10.1016/j.neubiorev.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Alleva E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev. 2003a;27:73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Molendijk ML, Oude Voshaar RC, Bus BA, Prickaerts J, Spinhoven P, Penninx BJ. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val(66)Met. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Moller HJ, Meisenzahl EM. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biol Psychiatry. 2008;64:281–285. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, McGuire L, Duchemin AM, Laskowski B, Kiecolt-Glaser J, Glaser R. Changes in plasma nerve growth factor levels in older adults associated with chronic stress. J Neuroimmunol. 2001;116:102–106. doi: 10.1016/s0165-5728(01)00278-8. [DOI] [PubMed] [Google Scholar]
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A, Mors O, Maier W, Hauser J, Souery D, Placentino A, Zobel A, Larsen ER, Czerski PM, Gupta B, Hoda F, Perroud N, Farmer A, Craig I, Aitchison KJ, McGuffin P. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry. 2009;195:30–38. doi: 10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Kauer-Sant'Anna M, Tramontina J, Andreazza AC, Cereser K, da Costa S, Santin A, Yatham LN, Kapczinski F. Traumatic life events in bipolar disorder: impact on BDNF levels and psychopathology. Bipolar Disord. 2007;9(Suppl 1):128–135. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Taylor WD. Neurobiological pathways that link gene and environment: early life stress disorder. Mol Psychiatry. 2009;14:648–649. doi: 10.1038/mp.2009.27. [DOI] [PubMed] [Google Scholar]
- Licinio J, Dong C, Wong ML. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry. 2009;66:488–497. doi: 10.1001/archgenpsychiatry.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Mitoma M, Yoshimura R, Sugita A, Umene W, Hori H, Nakano H, Ueda N, Nakamura J. Stress at work alters serum brain-derived neurotrophic factor (BDNF) levels and plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) levels in healthy volunteers: BDNF and MHPG as possible biological markers of mental stress? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:679–685. doi: 10.1016/j.pnpbp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/endo.137.2.8593794. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins in clinical diagnostics: pathophysiology and laboratory investigation. Clin Chim Acta. 2005;352:49–74. doi: 10.1016/j.cccn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry. 2004;55:708–714. doi: 10.1016/j.biopsych.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270–276. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Stein DJ, Solms M, Ramesar R. Genotype and childhood sexual trauma moderate neurocognitive performance: a possible role for brain-derived neurotrophic factor and apolipoprotein E variants. Biol Psychiatry. 2007;62:391–399. doi: 10.1016/j.biopsych.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Suomi SJ, Champoux M. Laboratory assessment of temperament and environmental enrichment in rhesus monkey infants (Macaca mulatta) Am J Primatol. 1991;25:137–155. doi: 10.1002/ajp.1350250302. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found Symp. 1991;156:171–183. doi: 10.1002/9780470514047.ch11. discussion 183–178. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann N Y Acad Sci. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15:260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, van Os J. The BDNF Val(66)Met x 5-HTTLPR x child adversity interaction and depressive symptoms: An attempt at replication. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:120–123. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Schmeidler J, Siever LJ, Binder-Brynes K, Elkin A. Individual differences in posttraumatic stress disorder symptom profiles in Holocaust survivors in concentration camps or in hiding. J Trauma Stress. 1997;10:453–463. doi: 10.1023/a:1024845422065. [DOI] [PubMed] [Google Scholar]