Abstract
Drugs of abuse serve as cofactors to susceptibility to HIV infection and disease progression. Although clinical reports indicate association between HIV/AIDS and drug use, the molecular mechanism of infection susceptibility and disease progression remains unclear. Drugs such as cocaine exert their addictive effects in part by epigenetic mechanisms. Given that epigenetic modifications play an important role in HIV-1 life cycle, it is essential to unravel whether drug abuse-associated epigenetic changes may contribute to HIV/AIDS. In this article we will provide a prospective on the impact of epigenetic mechanisms on HIV-1 life cycle.
Overview of HIV-1 Replication
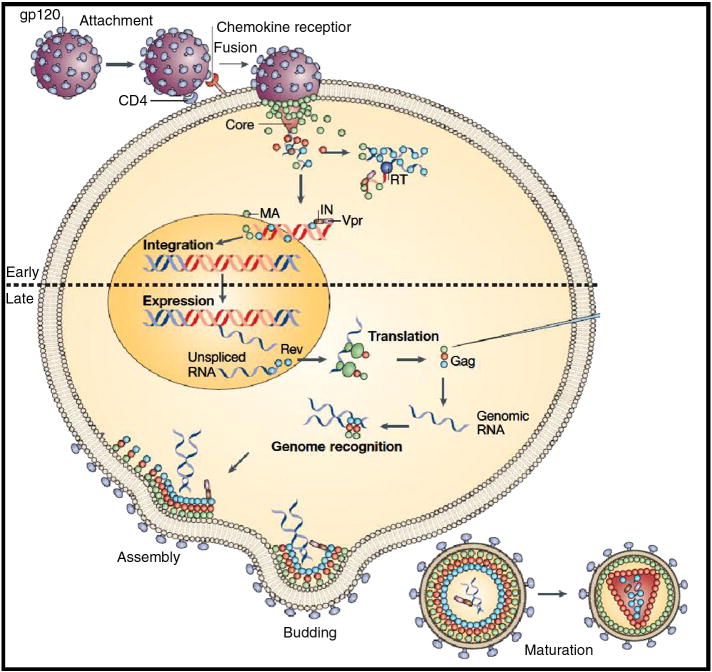
Human Immunodeficiency Virus-1 (HIV-1) is a retrovirus and the causative agent of acquired immunodeficiency syndrome (AIDS) (Telesnitsky and Goff 1997). HIV-1 replication can be broadly categorized into early and late events (Fig. 1). The early events include; entry, reverse transcription and integration, whereas the late events include, transcription, assembly, release and maturation. The first step in the HIV-1 life cycle is entry, which is facilitated by the binding of viral glycoproteins on to CD4 and chemokine receptors on the target cell. This is followed by fusion of the viral and cellular membranes and the release of the viral capsid into the cell (Chan and Kim 1998; Wyatt and Sodroski 1998). Subsequently, virally encoded reverse transcriptase enzyme converts the single-stranded RNA genome into double stranded proviral DNA via reverse transcription (Telesnitsky and Goff 1997). This proviral DNA along with several viral and cellular factors are transported into nucleus in the form of a large nucleoprotein complex called the preintegration complex (PIC). In the nucleus integration of the viral DNA into the host genome is carried out by viral enzyme integrase (IN). Following integration, the virus exploits the host cell machinery to transcribe viral RNAs that encode for essential viral proteins and unspliced genomic RNA (D’Souza and Summers 2005). The full-length unspliced RNA is assembled by the viral Gag protein at the plasma membrane and new virus particles are released. Following proteolytic cleavage of viral polyproteins by viral protease, the virus undergoes maturation before carrying out new infections.
Figure 1.
Overview of HIV-1 lifecycle as described by D’Souza and Summers 2005. The HIV-1 genome encodes nine open reading frames. Three of these encode the Gag, Pol, and Env polyproteins. Gag consists of MA (matrix), CA (capsid), NC (nucleocapsid), and p6 proteins. The two Env proteins; SU (surface or gp120) and TM (transmembrane or gp41), along with the Gag proteins make up the virion core and outer membrane envelope. The three Pol proteins; PR (protease), RT (reverse transcriptase), and IN (integrase) provide essential enzymatic functions and are also encapsulated within the particle. HIV-1 encodes six additional accessory proteins, three of which (Vif, Vpr, and Nef) are found in the viral particle. Two other accessory proteins, Tat and Rev, provide essential gene regulatory functions, and the last protein, Vpu, assists in assembly of the virion. Two genomic RNA molecules of ~ 9 kb are also packaged in the particle.
Epigenetic Modifications and HIV Life Cycle
“Epigenetics” refers to all heritable changes in gene expression that are not coded in the DNA sequence (Egger et al. 2004). Epigenetic regulators include DNA methylation, histone modifications and RNA-associated silencing. DNA methylation has long been recognized as an epigenetic regulator of fundamental importance for cell function (Holliday and Pugh 1975; Riggs 1975). Histone modifications such acetylation, phosphorylation, and methylation have also been defined as epigenetic modifiers, which play important roles in gene regulation (Strahl and Allis 2000; Luger and Richmond 1998). The highly condensed structure of chromatin, consisting of two copies each of H2A, H2B, H3, and H4 histones, wrapped around by DNA, provides unique control over gene expression (Rando and Chang 2009). RNA, in the form of antisense transcripts, noncoding RNAs, or RNA interference (RNAi), can lead to mitotically heritable transcriptional silencing (Egger et al. 2004). In addition, RNA might be a key trigger to direct histone modifications and DNA methylation (Egger et al. 2004). Epigenomic regulations have been implicated during integration and latency of HIV-1 life cycle (Coull et al. 2000; Williams et al. 2006; Jiang et al. 2007; Tyagi and Karn 2007; Bednarik et al. 1987; Bednarik et al. 1990; Harbers et al. 1981; Hu et al. 1984; Kauder et al. 2009; Brady et al. 2009; Wang et al. 2007; Mitchell et al. 2004; Berry et al. 2006; Pruss et al. 1994).
Epigenetics and HIV-1 Integration
Integration of the proviral DNA into the host genome is a defining feature for HIV-1 replication (Suzuki and Craigie 2007). After reverse transcription, the proviral DNA remains associated with IN and other viral/cellular proteins in the PIC (Fig. 2). The PIC travels to the nucleus and integration of the viral DNA into the host genome is carried out by the IN enzyme. It has been demonstrated in vitro that, in comparison to naked DNA, DNA wrapped in nucleosomes is favored for integration (Pryciak and Varmus 1992; Taganov et al. 2004). In addition, given that chromatin is a highly compact and ordered structure, structural features within the nucleosome core are known to influence HIV-1 integration (Pruss et al. 1994). Although different retroviral genera favor integration in different regions of the host genomes, HIV-1 integration has been proposed to be favored in active transcription units (Wang et al. 2007; Mitchell et al. 2004; Barr et al. 2005; Barr et al. 2006; Schroder et al. 2002). Genome wide sequencing data reveals a possible correlation between HIV-1 integration and epigenomic modifications in the nucleosome (Bushman et al. 2005). These include histone modifications and DNA methylation. Transcription-associated histone modifications that favor HIV-1 integration include H3 acetylation, H4 acetylation, and H3 K4 methylation (Bushman et al. 2005). HIV-1 integration is disfavored in regions rich in transcription-inhibiting modifications such as H3 K27 trimethylation and DNA CpG methylation. This is in contrast to Murine Leukemia Virus (MLV) integration that is favored by CpG islands (Lewinski et al. 2005; Lewinski et al. 2006). Previously, Coffin et al. have reported that in Avian Leukosis Virus (ALV) CpG methylation of target DNA created highly preferred targets within runs of alternative CpG islands for integration (Kitamura et al. 1992). It is important to point out that a direct correlation between epigenomic modifications and HIV-1 integration targeting is yet to be demonstrated biochemically. However, HIV-1 IN has been reported to bind to several chromatin-associated proteins (Kalpana et al. 1994; Peytavi et al. 1999). Furthermore, a family of related integrase enzymes encoded by yeast retrotransposons contains chromodomains which bind methylated histone tails (Hizi and Levin 2005). In addition, cellular proteins recruited by specific histone modifications may contribute to integration. These cumulative evidence points toward an important role of epigenetic mechanisms during retroviral integration and warrants further investigation.
Figure 2.
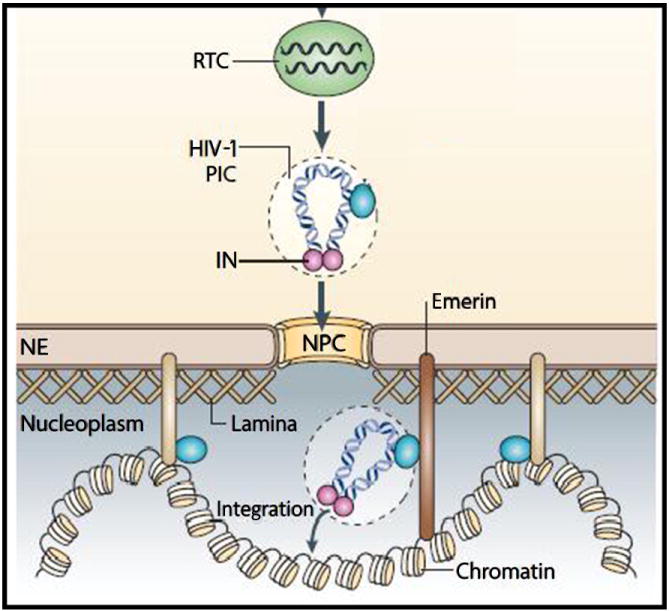
Schematics of retroviral integration as per Craigie, et al. The pre-integration complex (PIC) enters the nucleus via nuclear pore complex (NPC). The PIC gains access to chromatin and viral DNA is integrated by IN.
Epigenetics and HIV-1 Latency
Epigenetic control is thought to be involved in latent infection of HIV-1 in resting CD4+ T cells. Latently infected cells contain replication-competent integrated HIV-1 genomes that are blocked at the transcriptional level. CpG methylation has been implicated in silencing of the integrated provirus genome (Kauder et al. 2009). Demethylation induced by 5-Azacytidine (5-AzaC), an inhibitor of DNA methyltransferase, was shown to reactivate latent provirus (Niwa and Sugahara 1981). In vitro studies have shown that DNA methylation suppresses the promoter activity of the HIV- 1 long terminal repeat (LTR) (Harbers et al. 1981; Smith 2005; Schulze-Forster et al. 1990). In addition, histone deacetylation has been shown to be important for quiescence of HIV gene expression in infected resting CD4+ T lymphocytes (Kauder et al. 2009).
Transcriptional Regulation and HIV-1 Replication
HIV-1 exploits multiple host factors to complete a productive life cycle. These host factors play critical roles at different stages of HIV life cycle (Brass et al. 2008; Konig et al. 2008; Zhou et al. 2008). It is important to point out that transcriptional regulation of host factors contributes significantly to HIV-1 replication. For example, lens epithelium-derived growth factor (LEDGF/p75) is known to bind HIV-1 IN, plays a critical role in integration and is required for efficient HIV infection (Vandegraaff et al. 2006). When LEDGF/p75 expression is depleted from cells using RNA interference, HIV-1 integration was diminished (Engelman 2005), indicating importance of transcriptional regulation of host factors in HIV-1 replication. Since epigenetic modifications regulate transcription, we believe drug-abuse associated epigenomic modifications can regulate transcription of host factors, thereby influence HIV-1 replication.
How drug abuse-associated epigenetics may affect HIV-1 replication?
Illicit drug use remains the second most common mode of HIV infection and drugs such as amphetamines, cocaine, marijuana, and opiates serve as cofactors for susceptibility to HIV infection and disease progression (Goedert 1984; Siegel 1986; Donahoe and Falek 1998; Friedman 1996; Cabral 2006). Although clinical reports indicate an association between HIV/AIDS and use of illicit drugs (Duncan et al. 2007; Cook et al. 2008; Baum et al. 2009), and indirect effects are undoubtedly play a role, the molecular mechanism of infection susceptibility and disease progression remain unclear. HIV-1 infects peripheral blood mononuclear cells (PBMCs) such as macrophages and CD4+ T lymphocytes by binding to co-receptors such as CXCR4 and CCR5 (Telesnitsky and Goff 1997; Chan and Kim 1998). It is known that drugs of abuse modulate expression of chemokines in CD4+ lymphocytes (Nair et al. 2000). However, the underlying mechanism and the downstream signals by which drugs of abuse regulate expression of these chemokines are yet to be established. Although epigenetic modifications are known to regulate gene expression during drug addiction (Renthal and Nestler 2009), biochemical evidence on epigenetic mechanisms in CD4+ T cells and macrophages in response to drug exposure are lacking. Therefore, we hypothesize that drugs of abuse may regulate gene expression in CD4+ lymphocytes via epigenetic modifications. Based on this hypothesis, an effect of drug abuse-induced epigenetic changes can be envisioned at several steps of HIV life cycle. For example, drug abuse-associated epigenetic changes may have direct influence on integration events, because epigenetic modifications in the host genome modulate integration. In addition, these modifications may regulate expression of host factors, thereby influencing entry and post-entry events of HIV life cycle. Although, the literature on drug abuse-associated epigenetic changes in CD4+ T cells is still in infancy, it is important to point out that CD4+ T lymphocytes undergo extensive changes in chromatin structure via epigenomic modifications during cytokine production (Webster et al. 2007; Kaneko et al. 2007; Miyatake et al. 2000; Akimzhanov et al. 2007). Therefore, efforts to understand drug abuse-associated epigenetic modifications and their implications in HIV-1 replication will bridge a major gap in drug abuse and HIV/AIDS field.
Mother-to-Child-Transmission of HIV and Epigenetics
It has been well documented that HIV-infected women who use illicit drugs during pregnancy had a higher risk of transmitting HIV to their infants (Rodriguez et al. 1996; Ellis et al. 2003). Since maternal drug use during pregnancy has been shown to have profound structural and functional modifications in the epigenomic programs of neonatal mice (Novikova et al. 2008; Zhang et al. 2009; Meyer et al. 2009), similar epigenomic mechanisms cannot be ruled out in pregnant women drug users. If these epigenetic programs exist in humans, it may confound the biology associated with mother to child transmission of HIV by another level of complexity in this process. Therefore, it is critical that we gain insights into drug abuse associated-epigenetic changes in cells that are targets for HIV infection. This will certainly help us better understand whether drug abuse associated-epigenetic changes have any implications in mother to child transmission of HIV.
Conclusions
Epigenetic modifications play an important role in HIV life cycle. Co-incidentally epigenetic mechanisms play an important role in drug addiction. Since drugs of abuse serve as cofactors in HIV/AIDS, it is important to investigate the correlation between drug abuse-associated epigenetics and HIV/AIDS. This will help us bridge a major gap in the drug abuse and HIV/AIDS biology and will serve as the basis for a comprehensive analysis of effects of drug abuse-induced epigenomics on HIV pathogenesis.
Acknowledgments
Our laboratory is partly funded by National Institute on Drug Abuse, NIH (Grant# R00DA024558).
Due to page limitation, we could not cite all the relevant literature and we apologize to authors whose papers were not cited.
Footnotes
Conflict of Interest Statement: The authors declare that they have no financial interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. The Journal of Biological Chemistry. 2007;282(9):5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- Barr SD, Ciuffi A, Leipzig J, Shinn P, Ecker JR, Bushman FD. HIV integration site selection: Targeting in macrophages and the effects of different routes of viral entry. Molecular Therapy. 2006;14(2):218–225. doi: 10.1016/j.ymthe.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Barr SD, Leipzig J, Shinn P, Ecker JR, Bushman FD. Integration targeting by avian sarcoma-leukosis virus and human immunodeficiency virus in the chicken genome. The Journal of Virology. 2005;79(18):12035–12044. doi: 10.1128/JVI.79.18.12035-12044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack–Cocaine Use Accelerates HIV Disease Progression in a Cohort of HIV-Positive Drug Users. The Journal of Acquired Immune Deficiency Syndrome. 2009;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Bednarik DP, Cook JA, Pitha PM. Inactivation of the HIV LTR by DNA CpG methylation: evidence for a role in latency. The EMBO Journal. 1990;9(4):1157–1164. doi: 10.1002/j.1460-2075.1990.tb08222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik DP, Mosca JD, Raj NB. Methylation as a modulator of expression of human immunodeficiency virus. The Journal of Virology. 1987;61(4):1253–1257. doi: 10.1128/jvi.61.4.1253-1257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, Hannenhalli S, Leipzig J, Bushman FD. Selection of target sites for mobile DNA integration in the human genome. PLoS Computational Biology. 2006;2(11):1450–1462. doi: 10.1371/journal.pcbi.0020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady T, Lee YN, Ronen K, Malani N, Berry CC, Bieniasz PD, Bushman FD. Integration target site selection by a resurrected human endogenous retrovirus. Genes & Development. 2009;23(5):633–642. doi: 10.1101/gad.1762309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319(5865):921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, Hoffman C. Genome-wide analysis of retroviral DNA integration. Nature Review Microbiology. 2005;3(11):848–858. doi: 10.1038/nrmicro1263. [DOI] [PubMed] [Google Scholar]
- Cabral GA. Drugs of Abuse, Immune Modulation, and AIDS. Journal of Neuroimmune Pharmacology. 2006;1(3):280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Chan D, Kim P. HIV entry and its inhibition. Cell. 1998;93(5):681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Levine AM, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, Davie JR, Shi Y, Hansen U, Margolis DM. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. The Journal of Virology. 2000;74(15):6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza V, Summers MF. How retroviruses select their genomes. Nature Reviews Microbiology. 2005;3(8):643–655. doi: 10.1038/nrmicro1210. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Falek A. Neuroimmunomodulation by opiates and other drugs of abuse: relationship to HIV infection and AIDS. Advances in Biochemical Psychopharmacology. 1988;44:145–158. [PubMed] [Google Scholar]
- Duncan R, Shapshak P, Page JB, Chiappelli F, McCoy CB, Messiah SE. Crack cocaine: effect modifier of RNA viral load and CD4 count in HIV infected African American women. Frontiers in Biosciences. 2007;1(12):488–495. doi: 10.2741/2162. [DOI] [PubMed] [Google Scholar]
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. HIV Neurobehavioral Research Center Group. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. Journal of Infectious Diseases. 2003;188(12):1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Engelman A. The ups and downs of gene expression and retroviral DNA integration. The Proceedings of National Academy of Sciences of the United States of America. 2005;102(5):1275–1276. doi: 10.1073/pnas.0409587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. Drugs of abuse as possible co-factors in AIDS progression: summary of panel discussion. Advances in Experimental Medical Biology. 1996;402:225–228. doi: 10.1007/978-1-4613-0407-4_29. [DOI] [PubMed] [Google Scholar]
- Goedert JJ. Recreational drugs: relationship to AIDS. Annals of New York Academy of Sciences. 1984;437:192–199. doi: 10.1111/j.1749-6632.1984.tb37137.x. [DOI] [PubMed] [Google Scholar]
- Harbers K, Schnieke A, Stuhlmann H, Jahner D, Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. The Proceedings of National Academy of Sciences of the United States of America. 1981;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A, Levin HL. The integrase of the long terminal repeat-retrotransposon tf1 has a chromodomain that modulates integrase activities. The Journal of Biological Chemistry. 2005;280(47):39086–39094. doi: 10.1074/jbc.M506363200. [DOI] [PubMed] [Google Scholar]
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187(4173):226–232. [PubMed] [Google Scholar]
- Hu WS, Fanning TG, Cardiff RD. Mouse mammary tumor virus: specific methylation patterns of proviral DNA in normal mouse tissues. The Journal of Virology. 1984;49(1):66–71. doi: 10.1128/jvi.49.1.66-71.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Espeseth A, Hazuda DJ, Margolis DM. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. The Journal of Virology. 2007;81(20):10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpana GV, Marmon S, Wang W, Crabtree GR, Goff SP. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science. 1994;266(5193):2002–2006. doi: 10.1126/science.7801128. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Hosokawa H, Yamashita M, Wang CR, Hasegawa A, Kimura MY, Kitajiama M, Kimura F, Miyazaki M, Nakayama T. Chromatin remodeling at the Th2 cytokine gene loci in human type 2 helper T cells. Molecular Immunology. 2007;44(9):2249–2256. doi: 10.1016/j.molimm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathogens. 2009;5(6):1–15. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Lee YM, Coffin JM. Nonrandom integration of retroviral DNA in vitro: Effect of CpG methylation. The Proceedings of National Academy of Sciences of the United States of America. 1992;89(12):5532–5536. doi: 10.1073/pnas.89.12.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig R, Zhou Y, Elleder D, Diamond TL, Bonamy GM, Irelan JT, Chiang CY, Tu BP, De Jesus PD, Lilley CE, Seidel S, Opaluch AM, Caldwell JS, Weitzman MD, Kuhen KL, Bandyopadhyay S, Ideker T, Orth AP, Miraglia LJ, Bushman FD, Young JA, Chanda SK. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135(1):49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski M, Bisgrove D, Shinn P, Chen H, Verdin E, Berry CC, Ecker JR, Bushman FD. Genome-wide analysis of chromosomal features repressing HIV transcription. The Journal of Virology. 2005;79(11):6610–6619. doi: 10.1128/JVI.79.11.6610-6619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski MK, Yamashita M, Emerman M, Ciuffi A, Marshall H, Crawford G, Collins F, Shinn P, Leipzig J, Hannenhalli S, Berry CC, Ecker JR, Bushman FD. Retroviral DNA integration: Viral and cellular determinants of target-site selection. PLoS Pathogens. 2006;6:e60. doi: 10.1371/journal.ppat.0020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Richmond TJ. The histone tails of the nucleosome. Current Opinions in Genetics and Development. 1998;8(2):140–146. doi: 10.1016/s0959-437x(98)80134-2. [DOI] [PubMed] [Google Scholar]
- Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKC gene repression in the fetal rat heart. Journal of Molecular and Cellular Cardiology. 2009;47(4):504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RS, Beitzel BF, Schroder ARW, Shinn P, Chen H, Berry CC, Ecker JR, Bushman FD. Retroviral DNA Integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biology. 2004;2(8):1127–1137. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S, Arai N, Arai K. Chromatin remodeling and T helper subset differentiation. IUBMB Life. 2000;49(6):473–478. doi: 10.1080/15216540050166990. [DOI] [PubMed] [Google Scholar]
- Nair MP, Chadha KC, Hewitt RG, Mahajan S, Sweet A, Schwartz SA. Cocaine Differentially Modulates Chemokine Production by Mononuclear Cells from Normal Donors and Human Immunodeficiency Virus Type 1-Infected Patients. Clinical and Diagnostic Laboratory Immunology. 2000;7(1):96–100. doi: 10.1128/cdli.7.1.96-100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O, Sugahara T. 5-Azacytidine induction of mouse endogenous type C virus and suppression of DNA methylation. The Proceedings of National Academy of Sciences of the United States of America. 1981;78(10):6290–6294. doi: 10.1073/pnas.78.10.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova S, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal Cocaine Administration in Mice Alters DNA Methylation and Gene Expression in Hippocampal Neurons of Neonatal and Prepubertal Offspring. PLoS ONE. 2008;3(4):1–15. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peytavi R, Hong SS, Gay B, d’Angeac AD, Selig L, Benichou S, Benarous R, Boulanger P. HEED, the product of the human homolog of the murine eed gene, binds to the matrix protein of HIV-1. The Journal of Biological Chemistry. 1999;274(3):1635–1645. doi: 10.1074/jbc.274.3.1635. [DOI] [PubMed] [Google Scholar]
- Pruss D, Bushman FD, Wolffe AP. Human immunodeficiency virus integrase directs integration to sites of severe DNA distortion within the nucleosome core. The Proceedings of National Academy of Sciences of the United States of America. 1994;91(13):5913–5917. doi: 10.1073/pnas.91.13.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryciak PM, Varmus HE. Nucleosomes, DNA-binding proteins, and DNA sequence modulate retroviral integration target site selection. Cell. 1992;69(5):769–780. doi: 10.1016/0092-8674(92)90289-o. [DOI] [PubMed] [Google Scholar]
- Rando OJ, Chang HY. Genome-Wide Views of Chromatin Structure. Annual Reviews in Biochemistry. 2009;78:245–321. doi: 10.1146/annurev.biochem.78.071107.134639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Histone acetylation in drug addiction. Seminars in Cell and Developmental Biology. 2009;20(4):387–394. doi: 10.1016/j.semcdb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenetics and Cellular Genetics. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Mofenson LM, Chang BH, Rich KC, Fowler MG, Smeriglio V, Landesman S, Fox HE, Diaz C, Green K, Hanson IC. Association of maternal drug use during pregnancy with maternal HIV culture positivity and perinatal HIV transmission. AIDS. 1996;10(3):273–282. doi: 10.1097/00002030-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Schulze-Forster K, Gotz F, Wagner H, Kroger H, Simon D. Transcription of HIV-1 is inhibited by DNA methylation. Biochemical Biophysical Research Communications. 1990;168(1):141–147. doi: 10.1016/0006-291x(90)91685-l. [DOI] [PubMed] [Google Scholar]
- Siegel L. AIDS: relationship to alcohol and other drugs. Journal of Substance Abuse Treatment. 1986;3(4):271–274. doi: 10.1016/0740-5472(86)90039-5. [DOI] [PubMed] [Google Scholar]
- Smith SM. Valproic acid and HIV-1 latency: beyond the sound bite. Retrovirology. 2005;2:56. doi: 10.1186/1742-4690-2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Craigie R. The road to chromatin - nuclear entry of retroviruses. Nature Reviews in Microbiology. 2007;5(3):187. doi: 10.1038/nrmicro1579. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Cuesta I, Daniel R, Cirillo LA, Katz RA, Zaret KS, Skalka AM. Integrase-specific enhancement and suppression of retroviral DNA integration by compacted chromatin structure in vitro. The Journal of Virology. 2004;78(11):5848–5855. doi: 10.1128/JVI.78.11.5848-5855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesnitsky A, Goff SP. In: Retroviruses. Coffin JM, Hughes SH, Varmus HE, editors. Cold Spring Harbor Laboratory Press; Plainview, New York: 1997. pp. 121–160. [Google Scholar]
- Tyagi M, Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO Journal. 2007;26(24):4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegraaff N, Devroe E, Turlure F, Silver PA, Engelman A. Biochemical and genetic analyses of integrase-interacting proteins lens epithelium-derived growth factor (LEDGF)/p75 and hepatoma-derived growth factor related protein 2 (HRP2) in preintegration complex function and HIV-1 replication. Virology. 2006;346(2):415–426. doi: 10.1016/j.virol.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Wang GP, Ciuffi A, Leipzig J, Berry CC, Bushman FD. HIV integration site selection: Analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Research. 2007;17(8):1186–1194. doi: 10.1101/gr.6286907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. The Journal of Biological Chemistry. 2007;282(1):700–709. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO Journal. 2006;25(1):139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280(5371):1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- Zhang H, Meyer K, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biology of Reproduction. 2009;80(3):440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xu M, Huang Q, Gates AT, Zhang XD, Castle JC, Stec E, Ferrer M, Strulovici B, Hazuda DJ, Espeseth AS. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbes. 2008;4(5):495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]