Abstract
The frontal pursuit area (FPA) contains neurons that are directionally selective for pursuit eye movements. We found that FPA neurons discriminate target from distracter too late to account for pursuit directional selection. Rather, the timing of neuronal discrimination is linked to pursuit onset, suggesting a role in motor execution. We also found buildup of activity of FPA neurons prior to pursuit onset that correlated with eye acceleration. These results show that the FPA is unlikely to be involved in selection of initial pursuit direction, but could be involved in motor preparation by increasing pursuit gain prior to pursuit onset.
Keywords: smooth pursuit, frontal eye fields, target selection, decision making, macaque
Introduction
Primates use smooth eye movements to track moving objects in the world, and these eye movements are driven by target motion (Rashbass, 1961). Most models of the pursuit system (e.g. Lisberger, Morris, & Tychsen, 1987; Robinson, Gordon, & Gordon, 1986) account for pursuit of single targets, but primates are quite capable of selecting a moving target in the presence of distracter motion (Ferrera & Lisberger, 1995,Ferrera & Lisberger, 1997). The neural mechanisms involved in the selection of a pursuit direction are not yet known.
Spatial target selection in the primate brain is fairly well understood. A series of recording, microstimulation, and inactivation experiments have demonstrated that activity in the superior colliculus is important for the selection of targets for saccades (Horwitz & Newsome, 1999; McPeek & Keller, 2002; Krauzlis & Dill, 2002; Carello & Krauzlis, 2004; Dorris, Olivier, & Munoz, 2007) and smooth pursuit (Krauzlis & Dill, 2002; Carello & Krauzlis, 2004). The frontal eye fields (FEF) have also been implicated in saccadic target selection. Firing rate variability in FEF movement neurons is correlated with the latency of saccades (Hanes & Schall, 1996). Additionally, FEF neurons can discriminate a salient visual target from a distracter in the absence of a saccade, suggesting that they, like the superior colliculus, may play a more generalized role in target selection (Schall, Hanes, Thompson, & King, 1995).
Spatial target selection yields information about a location of interest. But the function of smooth pursuit is to match eye motion to target motion (Rashbass, 1961; Krauzlis & Lisberger, 1994; Lisberger et al., 1987; Robinson et al., 1986). Although the primate SC and the FEF contain neurons responsive to motion (Barborica & Ferrera, 2003; Krauzlis, 2004), neither structure appears to contain a representation of motion direction sufficient to account for pursuit selectivity. Several groups have tested the hypothesis that areas MT or MST could underlie pursuit selection. Ferrera and Lisberger (1997) found that most MT and MST neurons were not modulated by directional choice in a target-selection task, and concluded that the activity they observed probably could not account for pursuit selectivity. Recanzone and Wurtz (1999 Recanzone and Wurtz (2000) found correlates between pursuit selectivity and MT/MST responses, but these modulations were largely confined to the situation where both targets were in a single receptive field. Selection between two distant objects is common, but the activity in MT/MST could only weakly contribute to this phenomenon. These results, taken together, cast doubt on the hypothesis that areas MT or MST could account the directional selectivity of pursuit.
An alternate structure that could contribute to the selection of a pursuit direction is the frontal pursuit area (FPA). The FPA is located on the anterior bank and fundus of the arcuate sulcus, directly adjacent and posterior to the frontal eye fields. There are several reasons why the FPA is a likely candidate to underlie pursuit directional selection. First, neurons in this area exhibit strong directional selectivity during pursuit regardless of stimulus location (MacAvoy, Gottlieb, & Bruce, 1991; Tanaka & Lisberger, 2002b). This selectivity is often complete; cells only fire for pursuit in their preferred direction. Second, disrupting activity in the FPA causes directionally selective impairments of pursuit. Lesions, transient inactivations, and transcranial magnetic stimulation of the area have been shown to decrease pursuit velocity and acceleration (Lynch, 1987; Shi, Feldman, & Bruce, 1998; Drew & Van Donkelaar, 2007) and diminish predictive and anticipatory pursuit (Keating 1991, 1993; MacAvoy et al., 1991; Gagnon, Paus, Grosbras, Pike, & O’Driscoll, 2006). Deficits tend to be more pronounced for pursuit ipsiversive to the lesion or inactivation. Stimulation of the FPA can elicit smooth pursuit eye movements from fixation. These evoked eye movements have short latencies, typically within 20–40 ms (Gottlieb, Bruce, & MacAvoy, 1993; Tanaka & Lisberger, 2002a), and typically move the eyes towards the stimulated side.
Another reason to suspect that the FPA may be involved in directional selection for pursuit is its role in gain control. Subjects exhibit enhanced sensitivity to velocity changes during pursuit, and these changes in sensorimotor gain can be directionally specific (Schwartz & Lisberger, 1994; Keating & Pierre, 1996). The gain of the pursuit system can also be increased by the expectation of upcoming pursuit (Keating & Pierre, 1996; Kodaka & Kawano, 2003; Tabata, Miura, & Kawano 2005; Tabata, Miura, & Kawano 2008; Tabata, Miura, Taki, Matsuura, & Kawano, 2006). There is reason to believe that the FPA might underlie these modulations of pursuit gain. Tanaka and Lisberger (2001 Tanaka and Lisberger (2002a) stimulated the FPA during small target velocity perturbations and found that the eye velocity responses to the perturbations were enhanced during FPA stimulation, similar to the enhancement observed during ongoing pursuit. In humans, transcranial magnetic stimulation of the frontal eye fields disrupts pursuit gain control (Nuding, Kalla, Muggleton, Buttner, Walsh, & Glasauer, 2009). More generally, the processes of target selection and gain control appear to be linked (Lisberger, 1998; Gardner & Lisberger, 2001, 2002) and in their concluding remarks, Tanaka and Lisberger (2001) suggested that the frontal pursuit area could be “involved in voluntary functions, such as decision making, or selection of targets”. This untested hypothesis has persisted; in a recent effort to model the process of visuomotor selection, the FPA was granted the role of “decision-making for smooth pursuit eye movements” (Srihasam, Bullock, & Grossberg, 2009).
This study explores the role of the FPA in pursuit selection and gain control. We first explicitly test the hypothesis that the FPA could be involved in target selection for pursuit. We recorded from single cells in the FPA while monkeys performed a two-alternative pursuit-choice task and determined when the neuronal activity discriminates target from distracter and thus could contribute to the selection process. Second, we explore a new facet of the claim that frontal pursuit area activity is important for setting the gain of pursuit. In particular, we analyze the substantial modulations that occur before the motor response has begun and ask how this elevation of activity might facilitate pursuit. Finally, we examine how these two properties, discrimination time and buildup activity, vary as a function of pre-established FPA neuronal subtypes.
Methods
Subjects and Data acquisition
We collected data from two (J and T) adult rhesus monkeys (Macaca mulatta). Each monkey had a titanium chamber placed over the left frontal eye fields and frontal pursuit area; the chamber location was placed stereotaxically at coordinates determined by locating the arcuate sulcus in a high-resolution structural MRI. All experimental protocols for the monkeys were approved by the Institutional Animal Care and Use Committee and complied with U.S. Public Health Service policy on the humane care and use of laboratory animals.
The experiments were controlled by a computer using the Tempo software package (Reflective Computing), and a second computer running the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) of Matlab (MathWorks) acted as a server device for presenting the visual stimuli. Stimuli were presented with a video monitor (75 Hz; ~20 pixels/°) at a viewing distance of 41 cm. Eye movements were recorded using scleral search coils (Judge, Richmond, & Chu, 1980) and the electromagnetic induction technique (Fuchs & Robinson, 1966) using standard phase detector circuits (Riverbend Instruments). All data and events related to the onset of stimuli were stored on disk during the experiment (1 kHz sampling rate) for additional analysis.
Behavioral tasks
Our monkeys were presented with a display consisting of a small white spot (82 cd/m2) over a uniform gray background (7 cd/m2). Once they fixated this spot, an experimental trial began.
The target-selection task
The primary behavioral measure in all of our experiments was a target-selection task (Figure 1A). The task began with the monkey directing its gaze at a central fixation point. After a delay, the fixation point was briefly replaced by a color cue. The cue was a green or red isoluminant circular dot (19 cd/m2). The subject had to remember the cue color, as it was soon replaced by the fixation point. After a variable delay, the fixation point was extinguished and the choice stimulus was displayed. At this point, the trial structure branched into three different conditions that were pseudo-randomly interleaved.
Figure 1.
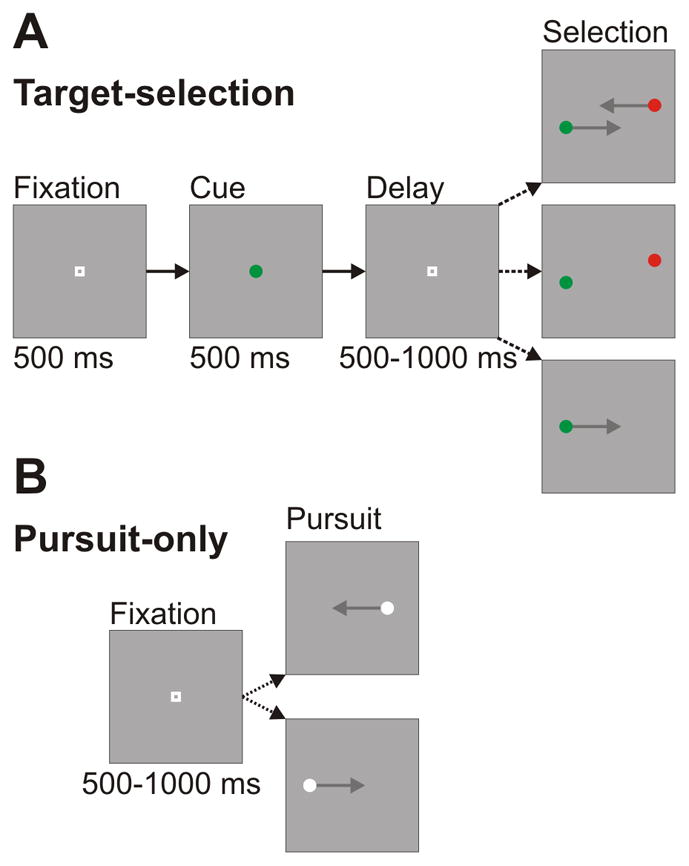
Sequence and timing of trial events. A: Target-selection task. Trials began with subjects directing gaze to a central fixation point. The fixation point transiently turned into a color cue. After a randomized delay, the fixation point was extinguished and the stimuli appeared. If the stimuli were moving (top panel), the monkey had to smoothly pursue the target that matched the color cue. If they were stationary (middle panel), the monkey had to make a saccade to the correct target. In the presence of a single dot (bottom panel), the monkey was rewarded for pursuing the target. B: Pursuit-only task. Trials began with subjects directing gaze to a central fixation point. After a randomized delay, a single target appeared and moved in a step-ramp fashion toward the fixation point. The monkey was rewarded for smoothly pursuing the target.
The first possible condition was smooth pursuit target-selection, and typically comprised 50% of the total target-selection trials. In this condition, after the second fixation point disappeared, two choice stimuli appeared offset from fixation and moved in parallel but opposite directions towards the center of the screen. The axis of motion was tailored to match the preferred and anti-preferred directions of the cell. The speed of both stimuli was 16 °/s. Their location was slightly perpendicularly offset from the fixation point so that they did not occlude each other as they passed the center of the display. The sizes of the steps were independently adjusted to minimize saccades, but they did not differ by more than 0.25°. The monkey’s task was to smoothly pursue the stimulus that matched the color of the cue. The color identity and direction of motion of the correct stimulus was chosen pseudo-randomly on each trial. If the monkey failed to choose a stimulus or if its initial eye movement was a saccade, the trial was aborted and the monkey was given a timeout. The monkeys were given a grace period of 250 ms to enter the correct window. We trained the monkeys to minimize saccades during the first several hundred milliseconds of pursuit, so our recording data during pursuit initiation was saccade-free.
The second possible condition was saccade target-selection, and typically comprised 25% of the total target-selection trials. In this condition, after the second fixation point disappeared, two choice stimuli appeared offset from fixation and remained stationary. The sizes of the offsets were identical to the steps in the pursuit target-selection condition. The monkey was required to make a saccade to one of the two choice spots within 500 ms. The color identity and location of the correct stimulus was chosen pseudo-randomly on each trial. If the monkey failed to choose a stimulus within this time period, or if the monkey failed to fixate the chosen stimulus for 500 ms, the trial was aborted.
The third possible condition was single-stimulus pursuit, and typically comprised 25% of the total trials in the target-selection block. This condition was only included for 65 of our 88 cells. After the second fixation point disappeared, only a single spot appeared and moved in a step-ramp fashion in one of the two potential directions. The color of the spot matched the color of the earlier cue. The stimulus speed was 16 °/s, and the size of the step was adjusted to minimize catch-up saccades.
The unbalanced frequency of the different conditions in this task (50% pursuit target-selection, 25% saccade target-selection, 25% single-stimulus pursuit) was prompted by the need to obtain additional trials in the pursuit target-selection condition to compensate for the presence of error trials and to ensure that we had sufficient trials to support the ROC analyses of neuronal discrimination.
The pursuit-only task
In this task (Figure 1B), the monkey engaged exclusively in standard step-ramp pursuit (Rashbass, 1961). After a random fixation interval of 500–1000 ms, the central fixation point disappeared and a white circular spot (luminance: 82 cd/m2) appeared offset from fixation. The speed and step-size in this task were identical to the parameters used in the single-stimulus pursuit condition in the target-selection task. The direction of the stimulus was chosen pseudo-randomly on each trial, and the monkeys were required to stay within a 2° window of the target. The trials from this condition were run as a block of trials separate from the target-selection task so that we would have a data set that was more directly comparable to data from previous studies of pursuit-related activity in the FPA (e.g., Ono & Mustari, 2009).
Recording
We used tungsten microelectrodes with impedances of 900 k to 3.0 M to record extracellular action potentials of individual neurons in the frontal pursuit area. We probed the anterior bank and the fundus of the arcuate sulcus for neurons with pursuit-related responses. To confirm that we were in the FPA, we evoked pursuit at every recording site by microstimulating with currents less than 50μA (MacAvoy et al. 1991). Since FPA cells are directionally tuned, but the tuning tends to be randomly and uniformly distributed throughout the area (MacAvoy et al. 1991; Gottlieb, MacAvoy, & Bruce, 1994; Tanaka & Lisberger, 2002b), we used circular pursuit as a search stimulus to identify neurons with pursuit-related activity. In this task, after a brief fixation, the monkey pursued a small white dot which moved along a circular trajectory (radius: 4°) around the screen. Only well-isolated single-units that were held for the duration of the experiment were included in our population. After isolating a neuron, we first ran the monkey on a block of pursuit-only trials in the preferred and non-preferred directions, followed by the target-selection task.
Data Analysis
Eye Movement Analysis
We detected saccades using velocity and acceleration criteria (Krauzlis & Miles, 1996). During tracking, these thresholds were applied relative to the average eye velocity and acceleration to avoid erroneously flagging periods of smooth tracking with nonzero velocity as saccades (de Brouwer, Misaal, Barnes, & Lefevre, 2002). All detected saccades were manually verified. Pursuit trials with saccades within 200 ms of pursuit onset were excluded from all analyses. Saccades during ongoing pursuit were excised from velocity traces.
We measured latency on each pursuit trial by fitting a “hinge” to the velocity data (Adler, Bala, & Krauzlis, 2002). The hinge is a combination of two conjoined lines, one horizontal and the other angled to fit the initial portion of the pursuit response. We allowed the angle and placement of the hinge to vary to minimize the mean-squared error of the model’s fit to the data. We then visually confirmed that the model had appropriately identified pursuit onset.
Single-neuron recordings
Acceptance pulses of spikes from isolated neurons were convolved with replicas of a post-synaptic potential (Hanes & Schall, 1996) and averaged to construct spike density functions. We chose to use post-synaptic potentials instead of a Gaussian convolution because we were concerned with the precise timing and latency of our signals and Gaussian kernels extend the effect of each spike back in time.
ROC Analysis
The time at which neural activity discriminated between target and distracter was determined with an analysis from signal detection theory (Green & Swets, 1966) comparing neuron-antineuron pairs (Britten, Shadlen, Newsome, & Movshon, 1992). We calculated the area under the ROC curve comparing the firing rates for trials where the target moved in the preferred versus the non-preferred direction. To determine when this ROC area became significant, we constructed confidence intervals at each millisecond with a bootstrapped permutation test (Britten, Newsome, Shadlen, Celebrini, & Movshon, 1996; Horwitz & Newsome, 2001). We deemed the ROC signal significant when it crossed this confidence interval and remained above it for 100 ms.
To investigate the interplay between neural discrimination timing and pursuit latency, we followed the procedure of Thompson, Hanes, Bichot, and Schall (1996) and divided our data into quick, middle, and slow pursuit latency epochs. We then performed an ROC analysis on the firing rate signals in the preferred and non-preferred direction for each of the three groups. In these analyses, our effective trial count was reduced by a factor of three. To obtain an accurate measurement of discrimination timing from noisier signals, we fit a cumulative Gaussian curve to the ROC area. When this cumulative Gaussian crossed a fixed ROC area of 0.75, we deemed it significant. This technique, which also follows Thompson et al. (1996), produced more consistent, albeit more conservative, estimates of neural discrimination latencies. We could then compare the discrimination latencies in each group to the pursuit latency of that group. We performed this analysis with our data aligned both to the stimulus onset and pursuit onset.
In addition to measuring the latency of the discrimination of target and distracter, we also measured the discrimination time of the activity related to pursuit versus saccades. For this analysis, we collapsed our data across saccade directions, since none of our cells showed any selectivity for saccades based on direction. We used the same techniques as described above for calculating confidence intervals and discrimination latencies. We confined our analysis to pursuit in the preferred direction.
FPA neuron subtypes
Recent work has demonstrated that there exist physiologically separable types of FPA cells (Ono & Mustari, 2009). These categories can roughly be classified as acceleration- and velocity-driven neurons. For each of our cells, we fit the firing rate with a combination of eye acceleration, eye velocity, and eye position signals to determine what component of the eye movement most strongly correlated with the pattern of FPA spiking. We used mean firing rate data from pursuit in the preferred direction during target-selection pursuit trials to identify coefficients in the following model:
In the equation described above, FR(t) is the estimated value of the spike density function at time t. A, B, C, and D are coefficients of the model. The value of t1 can also vary and represents the latency of the neural response with respect to pursuit onset. We minimized the squared difference between the model and the experimental data by varying the four coefficients and the latency of the response. The goodness of fit was determined by calculating a coefficient of determination (CD) or the square of the cross-correlation coefficient between the firing-rate and the estimated model. We then calculated partial r2 values for acceleration, velocity, and position components. Thus, for each neuron we acquired acceleration, velocity, and position CDs that characterize their firing pattern relative to the subjects’ eye movements.
We note that this model is a simplified version of the model primarily used by Mustari and colleagues – it omits the retinal error signals. Although we acknowledge that some FPA cells do show responses to retinal input, we find that, for our data, the simplified model does a good job of categorizing firing rates as having or lacking an acceleration transient. We have tested our data with the extended model, which includes three additional error components, and the results were qualitatively similar. The overall fits were marginally improved, but we found that the contributions of the different components were more unpredictable, and did a slightly inferior job of segregating the rest of our data.
Statistical tests
Unless otherwise indicated, all statistical comparisons were made with a two-sided t-test. P-values less than 0.05 were considered significant.
Results
We recorded from 88 cells from two monkeys (J and T). We first describe the timing of neural discrimination of FPA neurons in the pursuit target-selection task. Second, we describe buildup of FPA neuron activity prior to pursuit onset and the behavioral correlates of this buildup activity. Finally, we describe how neural discrimination times and pre-pursuit buildups cluster with respect to neuronal subtype.
FPA direction discrimination does not substantially precede pursuit onset
Direction selectivity during pursuit is one of the most salient features of FPA neurons. We found that, during a target-selection task, the timing of FPA direction discrimination is roughly coincident with the onset of pursuit, as illustrated by an example neuron (Figure 2). We first marked the latency for each trial. This was done with a two-part hinge model, and visually confirmed for each trial. Next, we aligned the spike-density function to pursuit onset. The spike density functions for pursuit trials in the preferred (solid trace) and non-preferred (dashed trace) directions are plotted in Figure 2A. The cell began to increase its firing rate well before the onset of pursuit, but this elevation of activity was initially nonselective with respect to direction. This directionally nonselective increase in firing rate was a common property of many of our cells, as discussed below, and was followed by directionally selective activity related to the choice of pursuit motion.
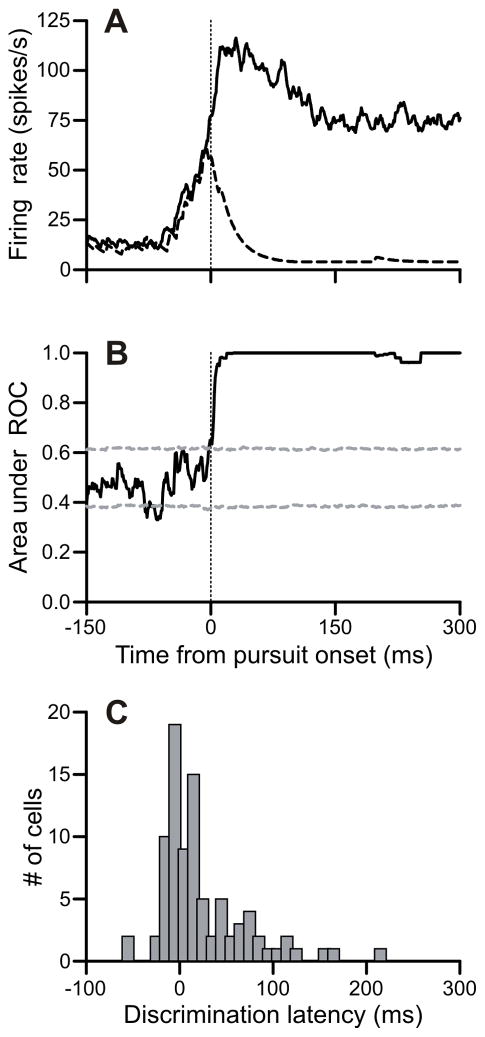
Figure 2.
FPA neurons discriminated target from distracter near pursuit onset in the target-selection pursuit task. A: Firing rates aligned on pursuit onset for trials in the preferred (solid) and non-preferred (dashed) directions in an example neuron. B: ROC analysis of this example neuron. Confidence intervals are derived from a shuffled bootstrap procedure. The discrimination latency for this neuron was 2 ms prior to pursuit onset. C: Histogram of discrimination latencies relative to pursuit onset for neuron population (n = 88).
To determine when the neuron discriminated the preferred from non-preferred direction, we used a receiver operating characteristic (ROC) analysis like that used in several previous studies (e.g. Britten et al., 1992; Britten et al., 1996; Horwitz & Newsome, 2001) and briefly described in Methods. The neuron’s directional discriminability was described by the ROC area at each millisecond (Figure 2B). The neuronal discrimination time was defined as the time point when the ROC area exceeded the 95% confidence interval (gray dashed lines) and remained above it for 100 ms, which occurred 2 ms before pursuit onset in the example cell.
The majority of our cells discriminated target from distracter coincident with or after the onset of pursuit. The median discrimination time across our sample of 88 neurons was 12 ms after the onset of pursuit (Figure 2C). A minority of neurons (33/88) had discrimination times prior to pursuit onset, and only 4 of the cells had discrimination times preceding pursuit onset by at least 20 ms. Another prominent feature of the distribution of discrimination times is the long tail containing neurons (14/88) that discriminated target from distracter more than 75 ms after pursuit onset. These neurons typically did not respond at all to pursuit until well after the onset of movement and were evidently not involved in determining the direction for pursuit.
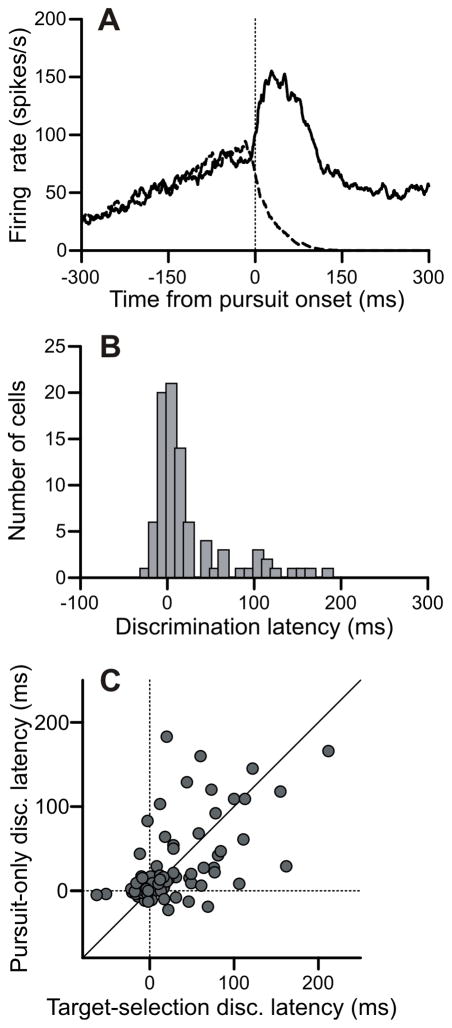
We found a similar pattern of results in the discrimination latencies during the pursuit-only condition, as illustrated by an example neuron (Figure 3A) during pursuit in the preferred (solid trace) and non-preferred direction (dashed trace) and the distribution of discrimination times from our sample of neurons (Figure 3B). Discrimination times in the pursuit-only condition were again tightly centered on pursuit onset (Figure 3B). The median discrimination latency was 9 ms after pursuit onset. Only one cell discriminated more than 20 ms prior to pursuit onset, and the majority of neurons (61/88) had discrimination times clustered within 20 ms of pursuit onset. Direction comparison of the discrimination times in the target-selection and the pursuit-only conditions (Figure 3C) showed no significant difference (p = 0.82). In both conditions, there was a distinct cluster of neurons that discriminated target from distracter direction within 20 ms of pursuit onset. Within this cluster, discrimination was as likely to happen after pursuit onset as before pursuit onset, casting doubt on the hypothesis that the neural discrimination of direction in these cells could be causally involved in choosing a pursuit direction.
Figure 3.
FPA neurons discriminated target from distracter near pursuit onset in the pursuit-only task. A: Firing rates aligned on pursuit onset for trials in the preferred (solid) and non-preferred (dashed) directions in an example neuron. The discrimination latency for this neuron was 1 ms prior to pursuit onset. B: Histogram of discrimination latencies relative to pursuit onset for pursuit-only task (n = 88). C: Comparison of discrimination latencies between the target-selection pursuit task and the pursuit-only task.
Neural discrimination of direction was linked to the motor output
The distribution of discrimination times (Figure 3C) indicates that FPA cells tended to discriminate target from distracter near the time of pursuit onset. This suggests that the discrimination of FPA neurons is related to the pursuit motor output and not the visual motion input. To test this, we determined whether neural discrimination times were fixed or variable relative to pursuit onset. If discrimination times were invariant with respect to pursuit latencies, it would be evidence that the FPA lies downstream of the presumed selection-related variability in eye movement reaction times (e.g., Thompson et al. 1996).
Because it was impractical to assess when neurons discriminated the preferred from non-preferred direction on single trials, we adopted the approach of grouping our trials based on pursuit latency, following the technique used previously to study saccade target selection in the frontal eye fields (Thompson et al., 1996). We exploited the variability of pursuit latencies in the target selection task and divided trials into three groups: short, middle, and long latency trials. We then compared the average firing rates between pursuit in the preferred and non-preferred directions for each of the three groups. We calculated the ROC area from these paired data sets to determine how directional discrimination time varied with pursuit latency.
It is worth noting that we used a slightly different, more conservative estimate for discrimination latency in this analysis to compensate for the lower trial counts resulting from subdividing the data into three latency groups, as in Thompson et al. (1996). We fit a cumulative Gaussian to the ROC area and measure when it crossed a fixed threshold (ROC area = 0.75). This yielded later absolute latencies than in the previous analysis, where we constructed confidence intervals for the ROC area. This analysis is more useful for comparing relative latencies between groups and less useful for assessing absolute discrimination latencies.
The results of this analysis applied to one FPA neuron show that discrimination time was fixed to pursuit onset (Figure 4A, blue traces show data for preferred direction, red traces for non-preferred direction). When aligned to the onset of the visual motion, the firing rates from the quick pursuit latency trials (solid lines) diverged after 130 ms and the firing rates from the slow pursuit latency trials (dashed lines) diverged after 149 ms. Trials with mid-range pursuit latencies are excluded from this plot for clarity, but the neural discrimination time for those trials fell between the two extremes at 136 ms. On the other hand, when the firing rates were aligned to pursuit onset (Figure 4D), the neural discrimination times are within three milliseconds of each other. Thus, for this cell, the timing of neural discrimination was linked to the timing of the pursuit output, and not the timing of the visual input.
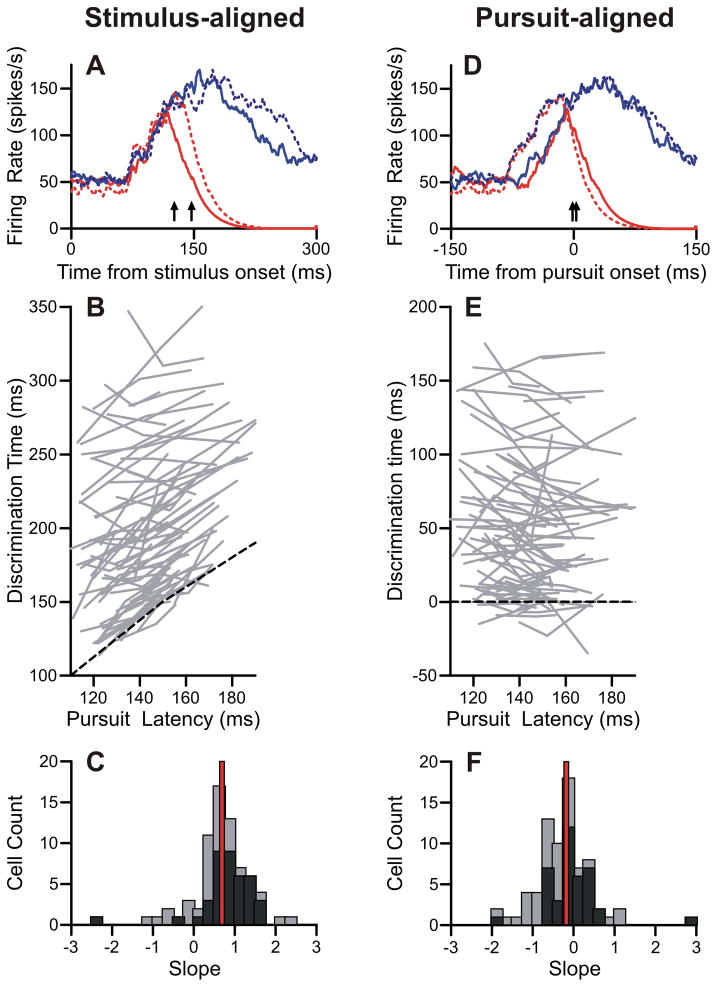
Figure 4.
Discrimination times varied with pursuit onset. A: Firing rates aligned on stimulus onset for an example neuron. Blue and red traces represent preferred and non-preferred trials, respectively. Solid and dotted traces represent trials with lower-third (quick) and upper-third (slow) pursuit latencies, respectively. Arrows indicate discrimination times for quick (130 ms) and slow (149 ms) pursuit latency trials. B: Population data of discrimination times aligned on stimulus onset. Each gray line contains three points from the three pursuit latency groups. Pursuit latency is the mean pursuit latency from all trials in that group. Black dashed line represents equality. C: Slopes of regression lines from stimulus-aligned data in B. Black bars are cells with an overall discrimination time less than median. Gray bars are plotted above, not behind, black bars. Red line is the median slope. D: Firing rates aligned on pursuit onset. Plotting conventions are the same as A. Discrimination times for quick (2 ms) and slow (−1 ms) pursuit latency trials are marked with arrows. E: Population data aligned on pursuit onset. Black dashed line has a slope of zero. Other conventions are the same as B. F: Slopes of regression lines from pursuit-aligned data. Same plotting conventions as C.
Many of the cells in our population showed a similar dependency of neural discrimination time on pursuit onset. We compared the discrimination latency with average pursuit latency for the three groups for each neuron. For the visual-onset aligned analysis (Figure 4B), there was a positive linear relationship between pursuit latency and discrimination time. We fit regression lines to each of these sets of three data points. The median slope of the regression fit was 0.69 (Figure 4C). On the other hand, when we compared pursuit latencies with discrimination times aligned to pursuit-onset (Figure 4E), discrimination time did not vary much as a function of pursuit latency. These regression lines had a median slope of −0.17 (Figure 4F). Both of these trends are consistent with the interpretation that FPA cells discriminated target from distracter at times that were tightly coupled to the pursuit motor output.
Not all of our cells’ discrimination times correlated with pursuit latency. We noted, though, that many of the outliers tended to have very late discrimination times. We have highlighted cells with average neural discrimination times less than the median in black (Figures 4C and 4F). The data from these cells form a tighter cluster than that from cells with longer latencies. There was a significant difference between the median slopes from the early-discriminating and the late-discriminating neurons, when the data were aligned to either visual onset (early: .9, late: 0.54 p < .01) or pursuit onset (early: −.07, late: −0.49, p < .01). Thus, it was primarily the neurons with discrimination times near pursuit onset that showed this dependency on pursuit latency.
Buildup activity in the frontal pursuit area
The previous section analyzed the timing and behavioral correlate of the discrimination of pursuit in the preferred direction from pursuit in the non-preferred direction in the frontal pursuit area. In addition, we noted that many of our cells showed significant modulations of activity prior to the directional discrimination (e.g., Figure 2A). We next address the properties of this buildup activity.
Buildup in the FPA is linked to the motion onset
We first tested whether the buildup activity was linked to the visual input or the motor output. We again took advantage of the natural variability in reaction times in the target-selection pursuit condition to probe the behavioral correlates of buildup activity. We first devised a way to identify the timing of the onset of buildup activity. We chose to use the discrimination of the firing rate during pursuit trials in the preferred direction from the firing rate during saccade trials as a proxy for the onset of buildup. Our cells showed no substantial response to saccades except a slight suppression of activity. The cells did not show any selectivity for saccade direction, so we collapsed our analysis across saccade trials. We excluded activity in the non-preferred direction for pursuit trials because their discrimination from saccade trials was transient and produced an ROC area that could not be well fit with a cumulative Gaussian.
The results of this analysis applied to one FPA neuron suggested that discrimination time was related to the onset of the visual stimulus (Figure 5A). This figure illustrates the similar time course of buildup activity for trials with quick pursuit latencies (solid black) and slow pursuit latencies (dashed black). The quick, middle, and slow trials began to increase their activity after 110, 116, and 111 milliseconds respectively. On the other hand, when the firing rates were aligned to pursuit onset (Figure 5D), buildup times were inversely correlated with pursuit latencies. Thus, for this cell, the timing of neural buildup was linked to the timing of the visual input and not the pursuit output.
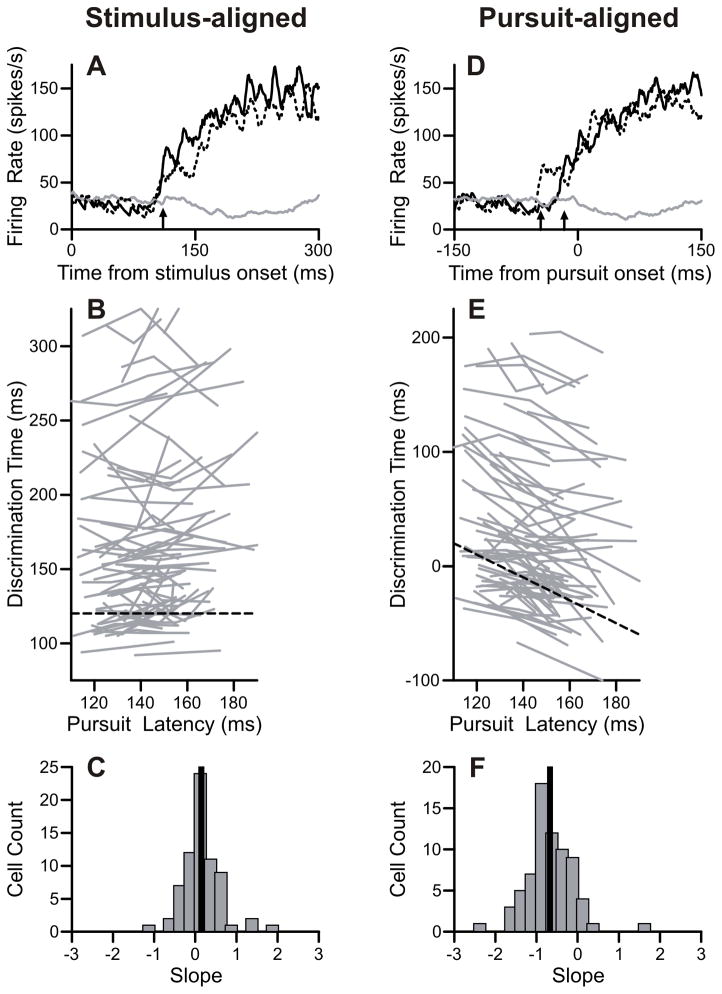
Figure 5.
Buildup times varied with stimulus onset. A: Firing rates aligned on stimulus onset for an example neuron. Target-selection pursuit trials directed in the preferred direction (black) and target-selection saccade trials (gray). Target-selection pursuit trials were divided into three pursuit latency groups; quick (solid black) and slow (dotted black) trials are plotted here. Buildup onsets were identified as the discrimination time between pursuit and saccade trials, and are indicated with arrows for quick (110 ms) and slow (111 ms) pursuit latency trials. B: Population data of buildup onset times aligned on stimulus onset. Each gray line contains three points from the three pursuit latency groups. Black dashed line has a slope of zero. C: Slopes of regression lines from stimulus-aligned data. Black line is the median slope. D: Firing rates aligned on eye movement. Plotting conventions are the same as A. Buildup onsets for quick (-16ms) and slow (−43 ms) pursuit latency groups are indicated with arrows. E: Population data aligned on pursuit onset. Black dashed line has a slope of −1. Other conventions are the same as B. F: Slopes of regression lines from pursuit-aligned data. Same plotting conventions as C.
The population analysis revealed a similar pattern of results. Aligned on the stimulus onset, the timing of the buildup tended to be invariant with respect to pursuit latency with a median slope of 0.15 (Figure 5B-C). Aligned on eye movement, the timing of the buildup was earlier when pursuit latency was later (median slope: −0.67) (Figure 5E-F). This pattern of results is consistent with the proposition that the initial buildup of activity was primarily dependent on the visual input, contrary to the discrimination of pursuit direction, which was dependent on the pursuit output. There was no significant difference in this analysis between cells with early versus late discrimination times.
Buildup in the FPA was not strictly visual
The previous analysis suggested that the buildup of activity of FPA neurons might be driven by visual motion. However, we next describe results that show that the buildup activity was not a response to motion per se, but to the increased probability that the subject would be engaged in pursuit.
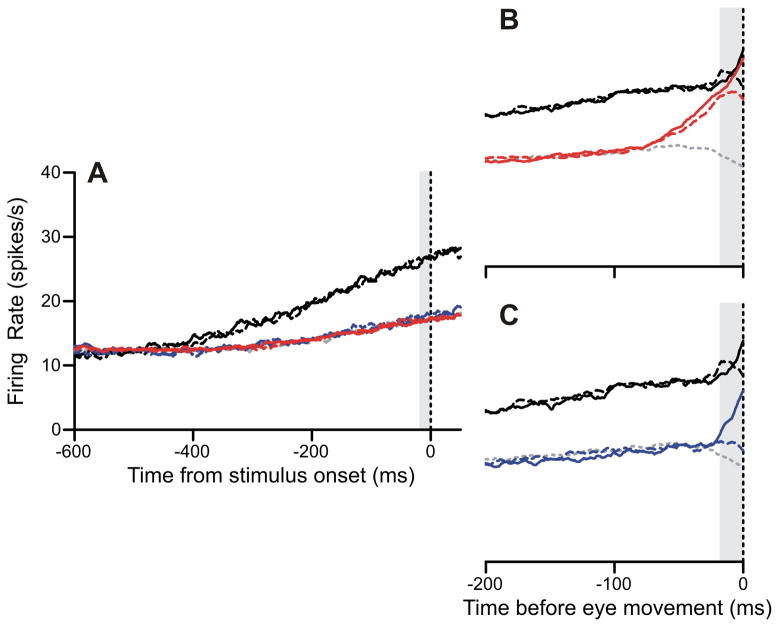
Tanaka and Fukushima (1998) first reported buildup of activity in the frontal pursuit area in anticipation of motion onset, and we found similar modulations of activity. Prior to stimulus onset, FPA neurons exhibited much larger buildups of activity during the pursuit-only block (black traces, Figure 6A) compared to the target-selection block (colored traces, Figure 6A). However, in the target-selection pursuit condition, activity begins to build up 75 ms prior to the onset of pursuit and nearly matches the activity in the pursuit-only block by the time of pursuit onset (Figure 6B). It is worth noting that this buildup of activity was initially directionally nonselective, but did not occur for saccade trials. FPA cells also elevated their activity prior to pursuit in the target-selection single-dot pursuit condition (Figure 6C), but only when the spot moved in the preferred direction. The buildup began later in this final plot because the single-dot pursuit latencies were shorter.
Figure 6.
FPA buildup activity was dependent on task context. Plotting conventions: red is target-selection pursuit, blue is target-selection single-dot, and black is pursuit-only. Dashed lines are trials in the non-preferred direction, solid lines are trials in the preferred direction. Saccades (gray dotted) are included for reference, and are collapsed across direction. Gray vertical bars are 20 ms measurement intervals. A: Firing rates aligned on stimulus onset for population data. Target-selection task conditions are all overlapping. B: Eye-movement aligned firing rates for pursuit-only task (black) and target-selection pursuit condition (red). C: Eye-movement aligned firing rates for pursuit-only task (black) and target-selection single-dot pursuit condition (blue).
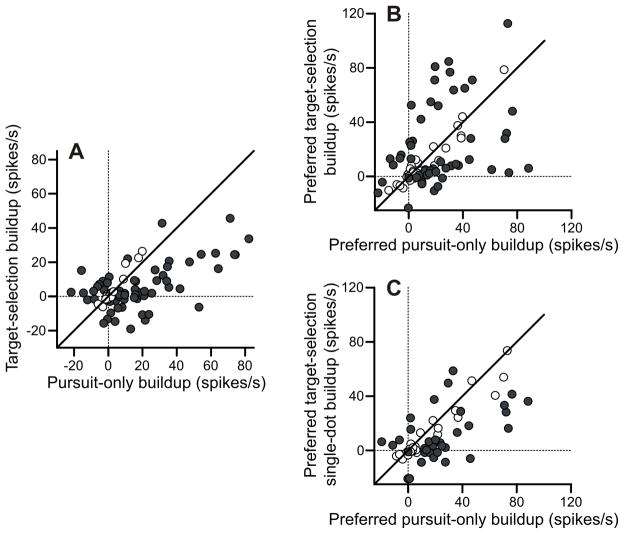
We have quantified the level of buildup for each cell in our population (Figure 7). We defined the “buildup” of activity as the difference between activity in a 20-ms interval prior to motion onset or pursuit onset to a baseline period 1000–500 ms before the visual onset. The buildup of activity prior to motion onset was greater in the pursuit-only task than the target selection task (Figure 7A). In this plot, the data were collapsed across the conditions in each task. On average, FPA cells emitted 10 spike/s more activity in the pursuit-only task. This difference was significant (p < 10−5). Interestingly, the cells that showed significantly greater target-selection buildup, with only two exceptions, were cells that actually show inhibition of activity prior to the onset of motion in the pursuit-only case.
Figure 7.
Analysis of buildup activity. For all plots, filled dots are statistically significant. A: Pre-stimulus buildup of activity for target-selection versus pursuit-only conditions. Target-selection trials are collapsed across condition. All activity is collapsed across direction. Baseline is from 1000–500 ms prior to stimulus onset. Measurement interval is from 20-0 ms prior to stimulus onset. B: Pre-pursuit buildup of activity for pursuit-only versus target-selection pursuit conditions. Only preferred direction is used. Baseline is from 1000–500 ms prior to stimulus onset. Measurement interval is from 20-0 ms prior to pursuit onset. C: Pre-pursuit buildup of activity for pursuit-only versus target-selection single-dot conditions. Only preferred direction is used. Baseline is from 1000–500 ms prior to stimulus onset. Measurement interval is from 20-0 ms prior to pursuit onset.
Activity in the target-selection task and the pursuit-only task reached similar levels near pursuit onset (Figure 7B). Overall, there was no significant pre-motion difference between the buildup in the pursuit-only preferred condition and the target-selection preferred condition (p = 0.95). Although this suggests the presence of a fixed-activity threshold for pursuit, several observations indicate that this was not the case. First, many individual neurons showed preferences for either the target-selection condition or the pursuit-only condition. If there were a set threshold that each cell reached prior to pursuit onset, one would expect the firing rate prior to pursuit onset to be the same regardless of condition. Second, the pre-pursuit activity in the target-selection single-dot preferred condition was significantly less than the activity in the pursuit-only preferred condition (average difference 9.2 spikes/s, p < .001) (Figure 7C). If there was a set level of activity that triggered pursuit, the activity prior to pursuit onset should have been the same regardless of the task context.
The behavioral correlates of buildup activity in the frontal pursuit area
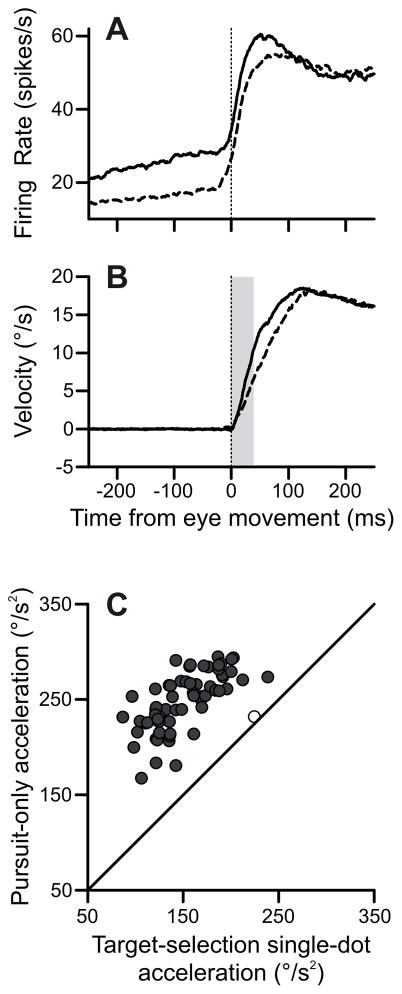
We next tested whether the degree of buildup activity was related to the metrics of pursuit eye movements. The pursuit-only condition produced higher firing rates (solid trace, Figure 8A) than the target-selection single-dot condition (dashed trace, Figure 8A) in FPA cells, despite the similarity in stimuli configuration between these two conditions. We found that this difference in activity correlated with a difference in pursuit metrics. The subjects’ initial eye velocities were elevated in the pursuit-only task (solid trace, Figure 8B) relative to the target-selection single-dot condition (dashed trace, Figure 8B). This figure collapses across pursuit direction, although the results were the same if we confined our analysis to the preferred direction. We measured eye acceleration for the first 40 ms of the pursuit response, and found that the pursuit-only condition produced consistently (64/65) higher initial accelerations (Figure 8C). The median difference between the accelerations in the two conditions was 95°/s2, which was highly significant. These effects were still significant when we extended our analysis window to the first 100 ms of acceleration. In that case, 51 out of the 65 days showed significant differences in acceleration; the population also showed a significant difference of, on average, 23°/s2.
Figure 8.
Pursuit-only task showed enhanced pursuit acceleration. A: FPA neurons were more active in the pursuit-only task. Pursuit-only trials (solid) and target-selection single-dot trials (dashed) in the preferred direction are plotted here. B: Eye velocity from pursuit-only trials (solid) and target-selection single-dot trials (dashed) aligned on pursuit onset. Leftward or downward conditions had their eye velocities inverted to be positive. Gray vertical bar is measurement interval from 0–40 ms after pursuit onset. C: Scatter plot of initial (0–40 ms) accelerations in pursuit-only versus target-selection single-dot conditions.
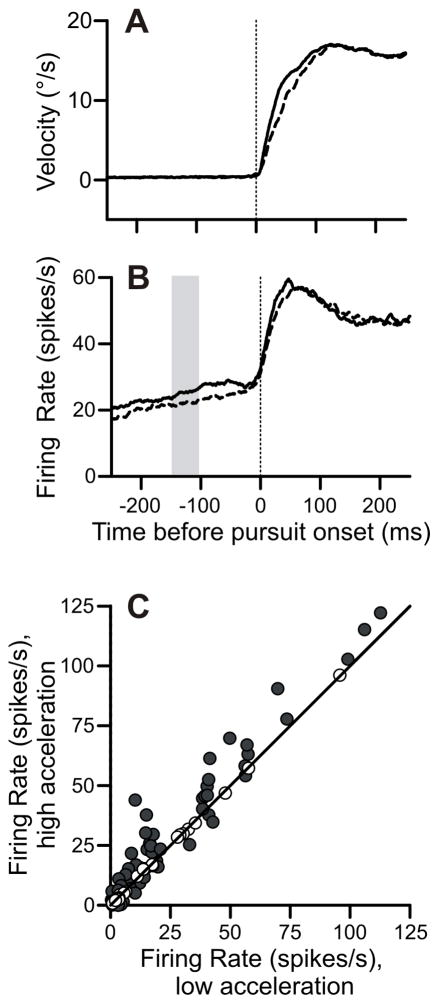
Next, we explored how variations in firing rate correlated with pursuit metrics within a single condition (Figure 9). We found that trials with higher accelerations were associated with higher firing rates. We used the pursuit-only task for this analysis. All illustrated data is from pursuit in the preferred direction. For every cell, we separated the trials based on whether they displayed initial acceleration greater or less than the median (Figure 9A). We found that trials with high acceleration displayed elevated firing rates many hundreds of milliseconds before pursuit onset (Figure 9B). The difference in firing rates was sustained until approximately 50 ms after pursuit onset.
Figure 9.
Within-task variations of acceleration correlated with differences in firing rate buildup. A: Eye velocity trace for the pursuit-only task in the preferred direction aligned on pursuit onset. Solid and dashed lines represent trials with initial accelerations greater or less than the median, respectively. B: Firing rates corresponding to the high-acceleration (solid) and low-acceleration (dashed) groups of trials in preferred direction. Vertical gray bar is the measurement interval 150–100 ms prior to pursuit onset. C: Scatter plot of firing rates in the high-acceleration versus low-acceleration trials. Preferred direction only. Solid dots are significantly different than equality.
We quantified the difference in preferred-direction firing rates in an interval from 150 to 100 ms before pursuit onset (Figure 9C). This interval was early enough not to be directly affected by pursuit. During this interval, the firing rate in high-acceleration trials averaged 3.2 spikes/s more than low-acceleration trials (p < 10−4). There was a small, non-significant trend towards lower firing rates for high acceleration trials in the cell’s non-preferred direction (difference: −0.74 spikes/s, p = 0.26, not pictured).
Acceleration units had early discrimination times and pre-motion buildup
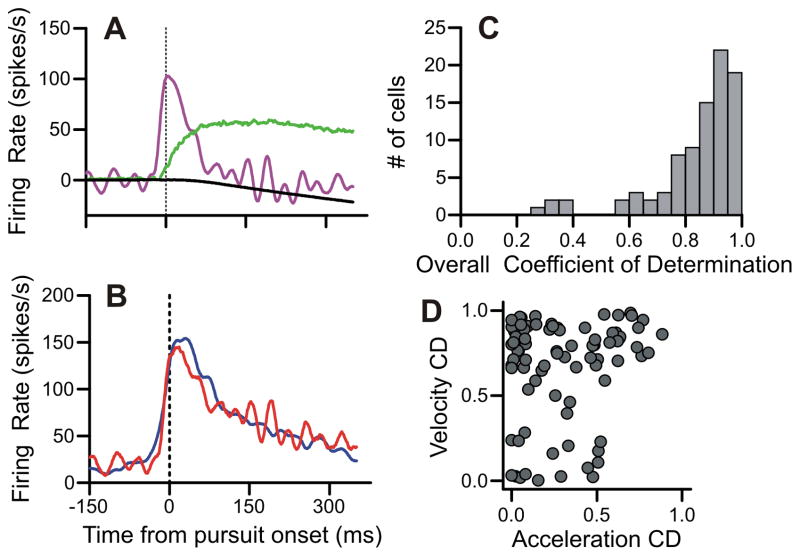
Our final analysis tested whether our measurements of discrimination times and buildup activity correlated with the subcategories of FPA cells (Ono & Mustari, 2009). Mustari and colleagues found that some FPA cells have a transient response that mimics eye acceleration, while other FPA cells have a more sustained response that correlates well with eye velocity.
We largely followed Ono and Mustari (2009) in identifying the relative contributions of velocity and acceleration to FPA responses (Figures 10A-B). We deviated slightly from the technique of the Mustari lab by not using the retinal error components. Including these components gave qualitatively similar results, but the identification of acceleration units was less consistent. The example unit (Figure 10A-B) had a large transient of activity that was well-fit by the acceleration component and a maintained level of activity that was fit largely by the velocity component. The acceleration coefficient of determination (CD) for this example was 0.8 and the velocity CD was 0.74. The overall CD was 0.88.
Figure 10.
Methodology for modeling firing rates with pursuit metrics A: Model parameters for an example cell. Eye acceleration (purple), velocity (green), and position (black) are plotted. B: Model fit (red) and experimental data (blue) for the example cell are illustrated. The equation for this model is FR(t) = 19.9 – 4.2*Position(t-14) + 3.2*Velocity(t-14) + 0.35*Acceleration(t-14). The acceleration, velocity, and position CDs for this model are 0.8, 0.74, and 0.45 respectively. The overall CD is 0.88. C: Histogram of overall CDs for all our neurons. D: Scatter plot of the velocity and acceleration CDs for all our cells. Position CDs are not used in our analysis and not plotted here.
Our modeling procedure produced good overall fits (Figure 10C) with values in line with those found by Mustari and colleagues. The vast majority of our cells had large CDs for eye velocity; our median velocity CD was 0.85 (Figure 10D). This was expected, since most FPA neurons had elevated activity during sustained pursuit. Cells with low CDs for eye velocity tended to be very late responding cells that were fit best by the eye position parameter. The most robust segregator of our data was the presence or absence of an eye acceleration component. Our median acceleration CD was 0.25 (Figure 10D), and we found that differences in the eye acceleration CDs were correlated with differences in other aspects of our data.
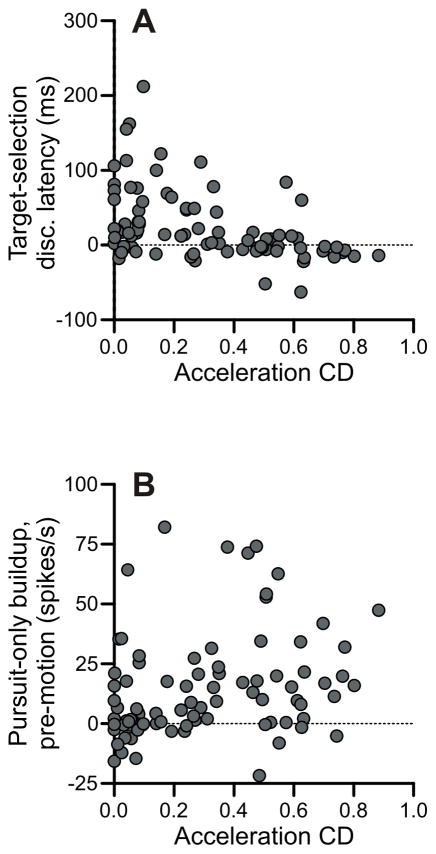
We found that FPA neurons with a large eye acceleration component tended to discriminate target from distracter near pursuit onset and elevated their activity prior to motion onset (Figure 11). First, we compared the acceleration CD for each cell with the neural discrimination latency in the target-selection paradigm, and we found a significant inverse correlation (r = −0.437, p < 10−4) (Figure 11A). We also compared the acceleration CD for each cell with the pre-motion buildup of activity in the pursuit-only condition and found a significant positive correlation (r = 0.284, p < .01) (Figure 11B).
Figure 11.
Acceleration units had short latencies and large buildups. A: Acceleration CDs plotted against discrimination latencies relative to pursuit onset in target-selection pursuit task. B: Acceleration CDs plotted against buildup of activity prior to stimulus onset in the pursuit-only task.
Discussion
We recorded from single neurons in the frontal pursuit areas (FPA) of two monkeys as they engaged in a smooth pursuit discrimination task and, as a control, smooth pursuit of a single stimulus. FPA neurons increase their firing rate prior to pursuit onset and show directionally selective activity as the pursuit response begins. The manner and timing of these spiking modulations in different trial conditions implicates the FPA in priming the subject for pursuit and executing the behavior with a high gain, but our results indicate that FPA neurons are unlikely to be responsible for determining the direction of pursuit.
Frontal pursuit area unlikely to underlie target selection for pursuit
The most salient feature of the cells in the FPA is that they are highly directionally selective for pursuit. When the cells become selective, as a general rule, the firing rate on trials in the preferred direction begins to increase and the firing rate on trials in the non-preferred direction begins to decrease. The timing of this directionally selective activity is tightly linked to the latency of the pursuit response; for many cells, especially cells that discriminated near pursuit onset, discrimination times were strongly linearly related to the onset of the eye movement.
Could the directional discrimination by FPA neurons determine the direction of pursuit? The FPA’s proximity to the FEF and its established role in gain control suggested this area could be responsible for pursuit direction selection. However, our data provides evidence against this hypothesis. The vast majority of FPA cells do not discriminate target from distracter early enough to be responsible for the selection of the initial velocity output of pursuit. Fifty-five of the 88 cells we recorded (62.5%) did not distinguish between target and distracter until after the onset of pursuit. These neurons cannot contribute to the initial selection of a pursuit direction, because their firing rates were largely identical until after pursuit had already started.
Previous reports have found that stimulation of the FPA typically evokes smooth pursuit 20–40 ms after stimulation onset (Gottlieb et al., 1993; Tanaka & Lisberger, 2002a). Our own stimulation results confirm this timing. Thus, it seems that signal conduction requires at least 20 ms to get from the frontal cortex and exert an effect on eye velocity. Only 4 of our 88 cells (4.5%) had neural discrimination times of more than 20 ms before the initiation of the eye movement in the target-selection task. These few cells may be accidental outliers, because in the pursuit-only block of trials they discriminated the preferred direction almost coincident with pursuit onset. Moreover, it would be surprising if such a small minority of cells were responsible for the subjects’ selection of pursuit direction.
Could some of these cells nonetheless contribute to the selection of a pursuit direction? Our recording experiment does not wholly rule out the possibility. Indeed, microstimulation is a relatively crude tool that evokes eye movements via poorly understood mechanisms. It is possible that in natural behavioral conditions, when the system is primed and ready to deploy pursuit, a signal from the FPA could influence pursuit direction in less than 20 ms. Indeed, the response to microstimulation is slightly faster when the animal is actively pursuing, although the latency in that case is still at least 18 ms, with a median response time of 21 ms (Tanaka and Lisberger 2002a). There is also uncertainty and some degree of subjectivity inherent in our techniques for measuring neural discrimination times and pursuit latencies. To definitively settle the question, it will be necessary to conduct causal manipulations of FPA activity and assess whether pursuit selection is affected. Nonetheless, our present recording data cast serious doubt on the hypothesis that the FPA is responsible for pursuit selection.
If pursuit directional selection is not mediated by the FPA, then how is it accomplished? One possibility is that all selection could be inherently spatial. Although it is classically known for its role in saccade execution and selection, there is good evidence that the superior colliculus is involved in the selection of pursuit targets (Krauzlis & Dill, 2002; Carello & Krauzlis, 2004). A second possibility is that the selection of a pursuit direction simply occurs elsewhere. For example, one possible candidate is the supplementary eye field (SEF). While the SEF seems less involved in setting the precise metrics of pursuit than the FPA (Tehovnik, Sommer, Cou, Slocum, & Schiller, 2000), its activity strongly reflects predictions about upcoming pursuit directions (Heinen, 1995; Heinen & Liu, 1997), and stimulating the SEF enhances the anticipatory pursuit response (Missal & Heinen, 2001,Missal & Heinen, 2004). More recently, the SEF has been shown to be necessary for the memory of visual motion and the decision-making process for pursuit in a delayed-response selection task (Shichinohe, Akao, Kurkin, Fukushima, Kaneko, & Fukushima, 2009). Other candidate cortical areas include VIP, LIP, 7a, and FST, all of which contain directionally selective activity during pursuit (Bremmer, Distler, & Hoffmann, 1997; Erickson & Dow, 1989; Schlack, Hoffmann, & Bremmer, 2003), but have not yet been tested in target-selection paradigms.
Buildup activity suggests a role for pursuit preparation
Some neurons in the FPA build up their activity prior to motion onset (Tanaka & Fukushima, 1998; Ilg & Their, 2008), and this activity seems to be related to the expectation of pursuit. Our data provide additional evidence for this hypothesis. We found substantial differences in the timing of buildup depending on experimental condition. In the pursuit-only block of trials, when the monkeys presumably knew they would be engaging in pursuit, many FPA neurons exhibited substantial increases in activity prior to motion onset. In the target-selection block of trials, when the monkeys likely were less certain about the motor output, these same neurons exhibited much weaker buildup. On the subset of target-selection trials when the monkeys were eventually asked to pursue, FPA neurons rapidly and nonselectively increased their firing rate prior to the initiation of pursuit. This pre-pursuit buildup of activity was tightly linked to motion onset. This new phenomenon is strong additional evidence that buildup in FPA is caused by the expectation of pursuit.
What is the functional significance of this buildup of activity? One hypothesis is that FPA activity builds up to a threshold level that defines the trigger for pursuit, but this idea is contradicted by our results. FPA activity at pursuit onset in the target-selection single-dot condition is significantly lower than activity in the pursuit-only condition. Also, the level of buildup activity just prior to pursuit onset for each neuron differs depending on the experimental condition. Thus, it seems that the triggering of pursuit is not strictly linked to the level of activity in the FPA. This is consistent with our neural discrimination data, which casts doubt on the FPA as the site of the directional selection for pursuit. The most concrete explanation of FPA activity in the literature is that it sets the gain of pursuit (Tanaka & Lisberger, 2001,Tanaka & Lisberger, 2002a). Our results confirm and extend this hypothesis. Two parallel lines of evidence in our data suggest that not only does the FPA modulate the gain during pursuit, it also prepares the gain of pursuit in the preferred direction prior to movement onset. First, we compared pursuit metrics from a condition with low buildup activity (target-selection single-dot) and a condition with high buildup activity (pursuit-only) and found that the latter condition was associated with a larger initial eye acceleration. Second, we found that within the pursuit-only task, trials with lower eye acceleration had less activity prior to and during pursuit onset. This latter effect was limited to pursuit in the preferred direction. These findings extend our understanding of the role of the FPA by suggesting that FPA neurons exert their influence before pursuit onset and even before the presence of visual motion. FPA neurons appear to pre-set the gain of the pursuit system in anticipation of the appearance of visual motion that the subject intends to track.
One unresolved point is whether the buildup of activity on FPA neurons is itself directionally selective. Tanaka and Fukushima (1998) concluded that the buildup of activity in the FPA was related to the expectation of pursuit generally, not pursuit in the preferred direction. In contrast, our results include two pieces of evidence that the buildup may be specifically related to the expectation of pursuit in the preferred direction. First, in the target-selection single-dot condition, there is only a pre-pursuit buildup of activity for trials in the preferred direction. If the activity were truly nonspecific, we would expect an initial nonselective elevation of activity. The second piece of evidence is that elevated activity only seems to facilitate pursuit in the preferred direction. Elevated activity in an FPA neuron has no effect on pursuit in the cell’s non-preferred direction. This is in line with the observation that stimulation of the frontal pursuit area during pursuit initiation primarily enhances eye velocity of pursuit ipsiversive to the stimulation site (Tanaka and Lisberger 2002a). Buildup activity also seems to be stronger prior to anticipatory pursuit in the preferred direction than the non-preferred direction (Ilg and Thier 2008). These findings are not conclusive, but they are sufficient to keep open the question of whether the buildup activity in FPA neurons facilitates pursuit generally or only in their preferred directions.
One final related point is that our results could account for the behavioral observation that subjects exhibit an increase in pursuit gain during fixation when they expect that they will be pursuing soon (Keating & Pierre, 1996; Kodaka & Kawano, 2003; Tabata et al., 2005, 2006, 2008). In these experiments, the expectation of upcoming pursuit increases the gain of the pursuit system, similar to the increase in gain that is observed during pursuit itself (Schwartz & Lisberger, 1994). We observe elevated pre-motion activity when pursuit is certain, and we find that this elevated activity is associated with higher initial pursuit accelerations. We consider it likely that this same activity is also responsible for the increased pursuit gain, as reported by responses to target perturbations, observed by other labs when subjects anticipate upcoming pursuit.
Acceleration cells tend to exert their effect near pursuit onset
Not all cells in the FPA appear to be involved in pursuit preparation; it is primarily acceleration-driven units that serve this function. One useful way of subdividing FPA cells is based on the presence or absence of an initial phasic burst of activity near the onset of pursuit (Ono & Mustari, 2009). Mustari and colleagues have characterized these cells as acceleration-driven units to distinguish them from velocity-driven units. The former category of cells projects preferentially to the NRTP in the pons, which also contains acceleration-dominated neurons. The latter group projects primarily to the DLPN, whose activity mirrors that of eye velocity (Ono, Das, Economides, & Mustari, 2005).
We measured the contribution of eye acceleration to the firing rate of our FPA neurons and find that cells with stronger acceleration components tend to discriminate target from distracter relatively early, near pursuit onset. These are the same neurons whose discrimination times are tightly linked to the pursuit latency. Additionally, these acceleration units tend to have greater pre-motion buildups of activity. Thus, it seems that acceleration-driven units preferentially serve the functions of preparing the subject to pursue and facilitating the execution of pursuit at its onset.
Summary
The frontal pursuit area is a structure in the frontal cortex that contains neurons that are strongly directionally selective for pursuit. The area seems to be primarily involved in the motor output of pursuit, but the majority of its neurons do not discriminate the upcoming pursuit direction early enough to be causally involved in actually selecting the pursuit target. The neurons are also probably not responsible for triggering pursuit, as the onset of pursuit is independent of the absolute level of activity in the FPA. Rather, the area’s output facilitates a higher-gain pursuit response to target motion. The frontal pursuit area seems to serve as a pillar of support for the execution of a rapid pursuit response. If the subject anticipates pursuit in the near future, FPA activity, especially those neurons with connections to the NRTP, increase their activity prior to the onset of pursuit, and this activity is related to the initial gain of the pursuit response. However, the frontal pursuit area is probably not responsible for the choice of pursuit direction or the decision of when to initiate the motor output.
Acknowledgments
This research was funded by the National Institutes of Health (Grant EY012212) and by a National Science Foundation Graduate Research Fellowship to SM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler SA, Bala J, Krauzlis RJ. Primacy of spatial information in guiding target selection for pursuit and saccades. J Vis. 2002;2(9):627–644. doi: 10.1167/2.9.5. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat Neurosci. 2003;6:66–74. doi: 10.1038/nn990. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Bremmer F, Distler C, Hoffmann KP. Eye position effects in monkey cortex. II. Pursuit- and fixation-related activity in posterior parietal areas LIP and 7A. J Neurophysiol. 1997;77(2):962–977. doi: 10.1152/jn.1997.77.2.962. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13(1):87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12(12):4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43(4):575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, Lefevre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol. 2002;87(4):1772–1780. doi: 10.1152/jn.00621.2001. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci. 2007;27(19):5053–5062. doi: 10.1523/JNEUROSCI.4212-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew AS, van Donkelaar P. The contribution of the human FEF and SEF to smooth pursuit initiation. Cereb Cortex. 2007;17(11):2618–2624. doi: 10.1093/cercor/bhl169. [DOI] [PubMed] [Google Scholar]
- Erickson RG, Dow BM. Foveal tracking cells in the superior temporal sulcus of the macaque monkey. Exp Brain Res. 1989;78(1):113–131. doi: 10.1007/BF00230691. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. Attention and target selection for smooth pursuit eye movements. J Neurosci. 1995;15(11):7472–7484. doi: 10.1523/JNEUROSCI.15-11-07472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. The effect of a moving distractor on the initiation of smooth-pursuit eye movements. Vis Neurosci. 1997;14(2):323–338. doi: 10.1017/s0952523800011457. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21(3):1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Paus T, Grosbras MH, Pike GB, O'Driscoll GA. Transcranial magnetic stimulation of frontal oculomotor regions during smooth pursuit. J Neurosci. 2006;26(2):458–466. doi: 10.1523/JNEUROSCI.2789-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci. 2001;21(6):2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Serial linkage of target selection for orienting and tracking eye movements. Nat Neurosci. 2002;5(9):892–899. doi: 10.1038/nn897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69(3):786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994;72(4):1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274(5286):427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Heinen SJ. Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp Brain Res. 1995;104(2):357–361. doi: 10.1007/BF00242022. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis Neurosci. 1997;14(5):853–865. doi: 10.1017/s0952523800011597. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284(5417):1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol. 2001;86(5):2543–2558. doi: 10.1152/jn.2001.86.5.2543. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Thier P. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain Cogn. 2008;68(3):229–240. doi: 10.1016/j.bandc.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res. 1991;86(2):311–323. doi: 10.1007/BF00228954. [DOI] [PubMed] [Google Scholar]
- Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res. 1993;53(1–2):91–104. doi: 10.1016/s0166-4328(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Keating EG, Pierre A. Architecture of a gain controller in the pursuit system. Behav Brain Res. 1996;81(1–2):173–181. doi: 10.1016/s0166-4328(96)89078-4. [DOI] [PubMed] [Google Scholar]
- Kodaka Y, Kawano K. Preparatory modulation of the gain of visuo-motor transmission for smooth pursuit in monkeys. Exp Brain Res. 2003;149(3):391–394. doi: 10.1007/s00221-003-1375-y. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Activity of rostral superior colliculus neurons during passive and active viewing of motion. J Neurophysiol. 2004;92:949–958. doi: 10.1152/jn.00830.2003. [DOI] [PubMed] [Google Scholar]
- Krauzlis R, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron. 2002;35(2):355–363. doi: 10.1016/s0896-6273(02)00756-0. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J Comput Neurosci. 1994;1(4):265–283. doi: 10.1007/BF00961876. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol. 1996;76(5):2822–2833. doi: 10.1152/jn.1996.76.5.2822. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1998;79(4):1918–1930. doi: 10.1152/jn.1998.79.4.1918. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res. 1987;68(2):437–441. doi: 10.1007/BF00248811. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1(1):95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88(4):2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol. 2001;86(5):2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol. 2004;92(2):1257–1262. doi: 10.1152/jn.01255.2003. [DOI] [PubMed] [Google Scholar]
- Nuding U, Kalla R, Muggleton NG, Buttner U, Walsh V, Glasauer S. TMS evidence for smooth pursuit gain control by the frontal eye fields. Cereb Cortex. 2009;19(5):1144–1150. doi: 10.1093/cercor/bhn162. [DOI] [PubMed] [Google Scholar]
- Ono S, Das VE, Economides JR, Mustari MJ. Modeling of smooth pursuit-related neuronal responses in the DLPN and NRTP of the rhesus macaque. J Neurophysiol. 2005;93(1):108–116. doi: 10.1152/jn.00588.2004. [DOI] [PubMed] [Google Scholar]
- Ono S, Mustari MJ. Smooth pursuit-related information processing in frontal eye field neurons that project to the NRTP. Cereb Cortex. 2009;19(5):1186–1197. doi: 10.1093/cercor/bhn166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Shift in smooth pursuit initiation and MT and MST neuronal activity under different stimulus conditions. J Neurophysiol. 1999;82(4):1710–1727. doi: 10.1152/jn.1999.82.4.1710. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol. 2000;83(2):777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern. 1986;55(1):43–57. doi: 10.1007/BF00363977. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15(10):6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. J Physiol. 2003;551(Pt 2):551–561. doi: 10.1113/jphysiol.2003.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JD, Lisberger SG. Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Vis Neurosci. 1994;11(3):411–424. doi: 10.1017/s0952523800002352. [DOI] [PubMed] [Google Scholar]
- Shichinohe N, Akao T, Kurkin S, Fukushima J, Kaneko CR, Fukushima K. Memory and decision making in the frontal cortex during visual motion processing for smooth pursuit eye movements. Neuron. 2009;62(5):717–732. doi: 10.1016/j.neuron.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihasam K, Bullock D, Grossberg S. Target selection by the frontal cortex during coordinated saccadic and smooth pursuit eye movements. J Cogn Neurosci. 2009;21(8):1611–1627. doi: 10.1162/jocn.2009.21139. [DOI] [PubMed] [Google Scholar]
- Tabata H, Miura K, Kawano K. Anticipatory gain modulation in preparation for smooth pursuit eye movements. J Cogn Neurosci. 2005;17(12):1962–1968. doi: 10.1162/089892905775008643. [DOI] [PubMed] [Google Scholar]
- Tabata H, Miura K, Kawano K. Trial-by-trial updating of the gain in preparation for smooth pursuit eye movement based on past experience in humans. J Neurophysiol. 2008;99(2):747–758. doi: 10.1152/jn.00714.2007. [DOI] [PubMed] [Google Scholar]
- Tabata H, Miura K, Taki M, Matsuura K, Kawano K. Preparatory gain modulation of visuomotor transmission for smooth pursuit eye movements in monkeys. J Neurophysiol. 2006;96(6):3051–3063. doi: 10.1152/jn.00412.2006. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80(1):28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Regulation of the gain of visually guided smooth-pursuit eye movements by frontal cortex. Nature. 2001;409(6817):191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J Neurophysiol. 2002;87(2):802–818. doi: 10.1152/jn.00409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol. 2002;87(6):2684–2699. doi: 10.1152/jn.2002.87.6.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32(2–3):413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76(6):4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]