Abstract
Detection of autoantibodies associated with neurological disease typically involves immunoprecipitation of radioactively labeled native proteins. We explored whether single receptor subunits, fused to Renilla luciferase (Ruc), could detect patient autoantibodies in Luciferase Immunoprecipitation Systems. Myasthenia Gravis patient sera were tested for conformational autoantibodies to only the α1-subunit of the nicotinic acetylcholine receptor (AChR). Using a panel of 10 AChR-α1 fragments, AChR-α1-Δ5-Ruc demonstrated the highest immunoreactivity with a conformational-specific antibody and the highest sensitivity in a pilot cohort. Testing a larger cohort with AChR-α1-Δ5-Ruc demonstrated 21% sensitivity and 97% specificity. A point mutation within Ruc increased the diagnostic performance of AChR-α1-Δ5 (32% sensitivity, 97% specificity). The 125I-α-bungarotoxin multi-subunit AChR assay demonstrated 63% sensitivity and 97% specificity. These findings highlight the difficulty in detecting Myasthenia Gravis conformational epitopes across assay formats and lay the foundation for detecting autoantibodies to defined recombinant chains of the AChR and potentially other neurotransmitter receptors.
Keywords: Myasthenia Gravis, Autoantibodies, Autoantigens, AChR, Luciferase Immunoprecipitation Systems (LIPS)
Introduction
Autoantibodies directed at neurotransmitter receptors and ion channels are pathogenic and/or biomarkers in several autoimmune neurological diseases [1]. For example, autoantibodies directed against voltage-gated potassium channels, the P/Q-type (α1A) voltage-gated calcium channel and the α3 ganglionic nicotinic acetylcholine receptor (AChR) are found in limbic encephalitis, Lambert-Eaton Myasthenic Syndrome (LEMS) and autoimmune autonomic neuropathy, respectively [2; 3; 4]. Sensitive detection of autoantibodies to these neurological receptors is critical to understanding disease pathogenesis, diagnosis and treatment. Detection of these autoantibodies using recombinant proteins in ELISAs is usually not useful because these tests miss important conformational epitopes on these multi-subunit receptors. The most widely used quantitative immunoassay involves using immunoprecipitation of native receptors labeled with radioactive ligands [5]. Alternatively, one can test patient serum by immunohistochemistry with cell lines expressing a given receptor [6; 7; 8].
In Myasthenia Gravis (MG), approximately 85% of patients produce pathogenic autoantibodies which bind and cross link muscle endplate derived nicotinic acetylcholine receptor (AChR). These autoantibodies induce AChR receptor internalization and degradation [2; 9; 10; 11] and/or can activate complement at the cell membrane resulting in cell lysis and destruction of the muscle endplate [12; 13]. Defining the exact targets of autoantibodies in MG has been complicated by the fact that the AChR is composed of five homologous glycoprotein subunits arranged around a central ion pore in the stoichiometry α2βεδ (adult) or α2βγδ (fetal). The four subunits composing the AChR share the same topological structure and all have an N-terminal extracellular domain (~200 amino acids), four transmembrane segments (~20 amino acids each) and a short extracellular tail (~10 amino acids). While the transmembrane segments mediate subunit assembly, the large extracellular domains of the α1, δ and ε (γ in the fetus) subunits contribute to the formation of the two acetylcholine binding sites within the receptor [14]. Lindstrom's group generated a library of monoclonal antibodies derived from animals immunized against the entire AChR or against chain-specific peptides and used these monoclonal antibodies and radioiodinated AChR to map antigenic determinates on the receptor [15]. One of the most immunodominant regions on the receptor was a 10 amino acid peptide sequence within the extracellular domain of the AChR-α1 subunit, designated the Main Immunogenic Region (MIR) [15; 16]. Competition experiments with these defined monoclonal antibodies also demonstrated that a significant number of MG patients had autoantibodies against the MIR [17]. Additionally, passive transfer of antibodies directed at the MIR also induces experimental autoimmune myasthenia gravis in rats [18]. Although the AChR-α1 subunit appears to be the major target of autoantibodies in MG, it is possible that other chains alone or in combination with the α1 chain are required for detecting some MG patient autoantibodies.
Clinical detection of MG usually involves immunoprecipitation of native receptor labeled with radioactive α-bungarotoxin (α-BTX), a snake venom-derived toxin that binds the AChR outside the ligand binding domain with high affinity (KD=2.6 × 10−10M) [19]. In these immunoassays, native pentameric AChR from human sources is first partially purified, radioactively labeled with 125I-α-bungarotoxin and then used in radioimmunoprecipitation assay (RIA) to measure patient autoantibodies [20]. Immunohistochemical analysis using cell lines expressing the various AChR receptor subunits has been successfully used to detect patient autoantibodies [8].
Immunoassays using peptides or recombinant AChR proteins have also been tried [21; 22]. Recombinant, bacterially-expressed AChR proteins react poorly with MG patient sera, suggesting that most MG patient autoantibodies recognize conformational rather than linear epitopes within the extracellular domain of the AChR-α1 subunit. Other assay formats employing non-radioactive labeling of the receptor require cumbersome purification methods [23; 24].
Luciferase Immunoprecipitation Systems (LIPS), which utilizes recombinant antigens fused to the enzyme reporter Renilla luciferase (Ruc) to detect patient antibodies, provides a unique platform to investigate antibodies directed against a variety of antigenic targets [25]. Previously, LIPS has been used to efficiently evaluate autoantibody responses in several autoimmune diseases mainly targeting soluble human autoantigens [25]. Here we investigated whether LIPS could be used to evaluate autoantibodies associated with the single AChR-α1 chain in MG. Using a series of deletion mutants, the antigenicity of the AChR-α1 subunit was systematically studied with an anti-AChR-α1 monoclonal antibody, control and MG patient serum samples. From these studies, statistically significant levels of autoantibodies against the AChR-α1 subunit were detected in 32% of MG patients. This approach, employing single subunits of the AChR to detect patient autoantibodies may provide a potentially useful method of evaluating patient autoantibodies to other neurotransmitter receptors and ion channels in other autoimmune neurological diseases.
Materials and Methods
Subjects and Samples
Patient sera were obtained from the Neuromuscular Disease Section, Johns Hopkins Hospital (Baltimore, MD) under IRB-approved protocols. The cohort consisted of 29 controls, 42 disease controls (25 ALS, 4 myositis and 10 neuropathy) and 63 MG patients previously diagnosed by either radioimmunoassay or EMG. Sera were stored at −80 °C prior to testing, then diluted 1:10 in buffer A (50 mM Tris, 100 mM NaCl, 50 mM MgCl2 and 1% Triton X-100).
Antibodies
Mouse monoclonal anti-AChRα1 (D6 clone) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and was used at a 1:100 dilution in each assay.
Generation of Ruc-antigen fusion constructs
pREN3S, a mammalian Ruc expression vector, was used to generate N-terminal antigen fusions. Human cDNA clones were amplified by PCR specific linker-primer adapters. For each construct, including deletion mutants, the N-terminal signal sequence was included. The primer adapter sequences used to clone full length AChR-α1 (457 amino acids) were 5′-GAGGGATCCATGGAGCCCTGGCCTCTC-3′ and 5′-GAGGAATTCTCCTTGCTGATTTAATTC-3′. Amino acid 1 in the following deletion fragment nomenclature refers to the start methionine. The following protein fragments were tested: AChR-α1-Δ1 (spanning amino acid residues 1–232), AChR-α1-Δ2 (spanning amino acid residues 1–260), AChR-α1-Δ3 (spanning amino acid residues 1–290), AChR-α1-Δ4 (spanning amino acid residues 1–373), AChR-α1-Δ5 (spanning amino acids 1–384), AChR-α1-Δ6 (spanning amino acid residues 1–394), AChR-α1-Δ7 (spanning amino acid residues 1–408), AChR-α1-Δ8 (spanning amino acids 1–412), AChR-α1-Δ9 (spanning amino acids 1–428) and AChR-α1-Δ10 (spanning amino acids 1–432). For each of these deletion mutants, the primer adapter sequences used to clone each fragment were 5′-GAGGGATCCATGGAGCCCTGGCCTCTC-3′ and one of the following: 5′-GAGGAATTCGAGGGGCAGGCGCTGCAT-3′ (Δ1), 5′-GAGGAATTCCCCTGAGTCTGTGGGCAG-3′ (Δ2), 5′-GAGGAATTCACTGGACGTGGAGGGGAT -3′(Δ3), 5′-GAGGAATTCTCCAGAAATGTCAGAGAT-3′ (Δ4), 5′-GAGGAATTCAGAGTGGAAGCCCATGGG-3′ (Δ5), 5′-GAGGAATTCACTTTTGACCTCGAAATG-3′ (Δ6), 5′-GAGGAATTCTGACTTCATGGTCTCTGC-3′(Δ7), 5′-GAGGAATCCAGACTCCTGGTCTGACTT-3′ (Δ8), 5′- GAGGAATTCGTGGTCCATCACCATTGC-3′ (Δ9) or 5′-GAGGAATTCTCCGAGGAGTATGTGGTC-3′ (Δ10). All clones were verified by sequencing.
Site Directed Mutagenesis
To generate the Ruc-C124A mutant, site-directed mutagenesis was performed by PCR using the primers 5′-CATGATTGGGGTGCTGCTTTGGCATTTCATTATAG-3′ and 5′-CTATAATGAAATGCCAAAGCAGCACCCCAATCATG-3′. Phusion High Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA) was used under the following conditions: for 20 cycles, denaturation 98 °C, 10 s; annealing 66 °C, 30 s; extension 72 °C, 3 min. The last cycle was performed under the same conditions except the extension time was increased to 10 min. PCR products were digested for 1 hr at 37 °C with Dpn1, then used for bacterial transformation. The correct point mutants were confirmed by DNA sequencing.
Cell culture, transfection and LIPS analysis
Cos1 cells were cultured at 5% CO2, 37°C with DMEM supplemented with 10% FCS and L-glutamine. Transfections were carried out using FuGene6 according to the manufacturer's instructions (Roche, Indianapolis, IN). Cell extracts were obtained 48 h post-transfection in lysis buffer (50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100, a mixture of protease inhibitors (Complete Mini protease inhibitor cocktail tablets, Roche Diagnostics, Indianapolis, IN) and 50% glycerol. The lysates were centrifuged twice at 12,500 g, supernatants collected and then stored at −80°C until use. The activities of the lysates (light units (LU)/μl) were determined using a single tube luminometer (20/20 from Turner Scientific) with a coelenterazine substrate mix (Promega, Madison, WI).
The LIPS assay was performed in a 96-well plate format as previously described with slight modification [26]. Briefly, 10 μL diluted patient sera (1μL equivalent) and Ruc-antigen Cos1 cell extract, both diluted in assay buffer A (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100), were added to each well of a polypropylene plate and incubated for 1 h on an orbital shaker. Note that in the standard LIPS format, an input of 1 × 107 LU is used, but here because of technical limitations and the less than optimal Ruc activity with many of the AChR-α1-Ruc fusions, a much lower input was used. Five μL of a 30% suspension of Ultralink protein A/G beads (Pierce Biotechnology, Rockford, IL) in phosphate-buffered saline was added to the bottom of each well of a 96-well filter HTS plate (Millipore, Bedford, MA). To this filter plate, the 100 μL antigen-antibody reaction mixture was transferred and incubated for 1 h on an orbital shaker. The amount of fusion protein immunoprecipitated was measured using a Berthold LB 960 Centro microplate luminometer (Berthold Techonologies, Bad Wilbad, Germany). All LU data represent the average of at least two independent experiments and are representative of four determinations. For data analysis, the GraphPad Prism software (San Diego, CA) was used.
Radioimmunoprecipitation
Patient and control sera were tested for anti-AChR autoantibodies using a commercial in vitro diagnostic Acetylcholine Receptor Antibody radioimmunoassay kit (Kronus, Star, ID), according to the manufacturer's instructions. In these experiments, 5 μL of sera were incubated with 100,000 cpm of fetal and adult detergent-solubilized AChR labeled with 125I-α-BTX for 2 h at room temperature. Next, 50 μL of anti-human IgG was added to each sample and incubated an additional 2 h at 4°C. Samples were then washed twice with 1 mL of wash buffer (PBS and 0.01% surfactant), centrifuged at 1,500 g at 4°C and the supernatant decanted. Each tube was counted for 1 min using a gamma counter set for 125I. Data represent the average of two independent experiments.
Results
Characteristics of AChR-α1-Ruc fusion proteins
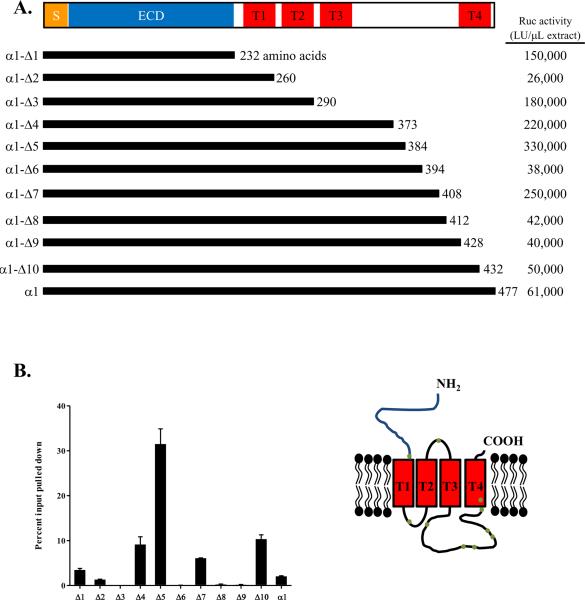
Using the LIPS technology, we evaluated whether the single AChR-α1 subunit could detect autoantibodies in MG patients. Fusions between the C-terminus of the AChR-α1 chain and the N-terminus of Renilla luciferase (Ruc) were used [25] because the AChR-α1 chain is a cell surface receptor and contains an N-terminal signal sequence. Since the fusion of the Ruc reporter to the C-terminus of AChR-α1 subunit might alter folding and/or solubility, 10 truncation mutants were also generated, each containing the N-terminal extracellular domain of the AChR-α1 subunit and variable lengths of its C-terminus. Following transfection of each of these AChR-α1 Ruc constructs into Cos1 cells, cell extracts were prepared and tested for expression using luciferase activity as a surrogate marker for production of the AChR-α1 protein. Overall the 10 deletion fragments and full length AChR-α1 showed significant differences in Ruc activity ranging from 26,000 to 330,000 light units (LU)/μL cell extract (Fig. 1A). Compared to Ruc-antigen fusions for other proteins, the AChR-α1-Ruc fusions showed relatively poor expression. Three fusion proteins with the highest Ruc activity, AChR-α1-Δ4, -Δ5 and -Δ7, all terminated in the cytosolic loop between the third and fourth transmembrane domains of the AChR-α1 subunit. However, among the other AChR-α1 truncation mutants, there was no correlation between the size of the truncation mutant and the level of luciferase activity. The variable recovery of luciferase activity is likely due to altered folding and/or solubility of the different AChR-α1-Ruc fusion proteins.
Figure 1. Recombinant AChR-α1 proteins used in LIPS.
(A) The full length and 10 deletion mutants of the α1 subunit of the AChR were cloned into the pREN3S vector to generate N-terminal fusions with Renilla luciferase. SP: signal peptide; ECD: extracellular domain; T: transmembrane segment. Typical recoverable Ruc activity for each construct is shown to the right as LU/μL extract. (B) The D6 monoclonal antibody, directed at the AChR-α1 subunit was used to immunoprecipitate the AChR-α1-Ruc fusion proteins. The amount of protein immunoprecipitated is expressed as a percentage of the total input for each assay. The inset shows a schematic of the AChR-α1 subunit within the plasma membrane; the dots on the figure denote the approximate location of each truncation mutant.
Immunoprecipitation by a monoclonal antibody recognizing conformational epitopes
Using a known monoclonal antibody, D6, that recognizes conformational epitopes within the AChR-α1 subunit [27], we tested the immunoreactivity of each of the 11 different AChR-α1-Ruc fusion proteins. Due to poor activity of the AChR-α1-Ruc antigens, a lower input of light units, ranging between 0.8 × 106 and 6.6 × 106 LU, was added per sample versus the normal activity of 1 × 107 LU typically used in LIPS (See Material and Methods). Despite the decreased input, several of the fusion proteins were significantly immunoprecipitated by the D6 monoclonal antibody. In particular, the AChR-α1-Δ4, -Δ5 and -Δ10 Ruc antigen fusion proteins showed 9%, 32% and 10% of the input immunoprecipitated by this monoclonal antibody, respectively (Fig. 1B). Several other AChR-α1-Ruc recombinant proteins reacted quite poorly with the D6 monoclonal, including AChR-α1-Δ3, -Δ6, -Δ8 and -Δ9, (<1% immunoprecipitated) (Fig. 1B). Since each of the AChR-α1 protein fragments tested contained the N-terminal extracellular region for immunoreactivity with the D6 monoclonal antibody, these results suggest that conformational epitopes can differ markedly between these different fusion proteins.
Testing a panel of AChR-α1 Ruc fusion proteins with a pilot serum cohort
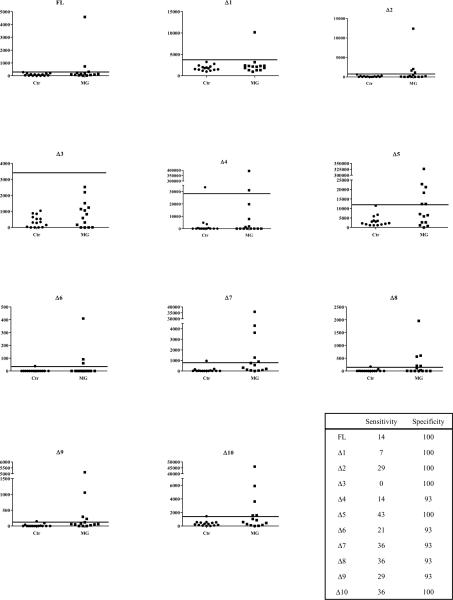
Next we tested whether MG patient autoantibodies could be detected using the panel of AChR-α1-Ruc antigen protein fragments with LIPS. A pilot set of serum samples consisting of 14 MG patients and 15 controls was used. Despite the less than optimal input, several of the AChR-α1-Ruc protein extracts showed high levels of immunoreactivity with some of the MG patient serum samples (Fig. 2). To determine sensitivity and specificity, a cutoff derived from the mean plus three standard deviations of the controls was used. The AChR-α1-Δ5-Ruc fusion protein detected autoantibodies in 43% of the MG patients in the pilot cohort and displayed the greatest sensitivity of the fusion proteins in the panel (Fig. 2). The AChR-α1-Δ5-Ruc fusion protein also displayed the largest dynamic range of detection, measuring over 320,000 LU in one patient sample versus 4,000 LU in several of the controls. Other protein fragments corresponding to AChR-α1-Δ7, -Δ8 and -Δ10 demonstrated 36% sensitivity in detecting MG patient autoantibodies and had a narrower dynamic range (Fig. 2). Even less immunoreactivity (29% sensitivity) was observed with the AChR-α1-Δ2 and -Δ9 proteins (Fig 2). In contrast, full length AChR-α1 and the remaining truncation mutants demonstrated markedly lower immunoreactivity. AChR-α1-Δ6 demonstrated only 21% sensitivity, while full length AChR-α1 and AChR-α1-Δ4 demonstrated 14% sensitivity (Fig. 2). AChR-α1-Δ1 demonstrated 7% sensitivity. Lastly, one truncation mutant, AChR-α1-Δ3, which terminated within the first cytoplasmic loop of the subunit, failed to detect any autoantibodies within the cohort. The specificity of the panel of recombinant AChR-α1 proteins in the small number of control serum samples ranged from 93% to 100% (Fig. 2, inset). These findings demonstrate that the diagnostic performance for detecting autoantibodies to the AChR-α1 subunit can vary greatly within this panel of protein fragments.
Figure 2. Detection of MG patient autoantibodies by a panel of AChR-α1 truncation mutants.
A cohort of MG patients (N=14) and controls (N=15) was screened for autoantibodies using full length AChR-α1 and 10 deletion mutants. Each point represents the average amount of fusion protein immunoprecipitated in two independent experiments by each patient in LU. The long solid line in each graph represents the cutoff derived from the average plus three standard deviations of the control group.
Autoantibodies against the AChR-α1-Δ5 protein fragment in a larger cohort of MG patients
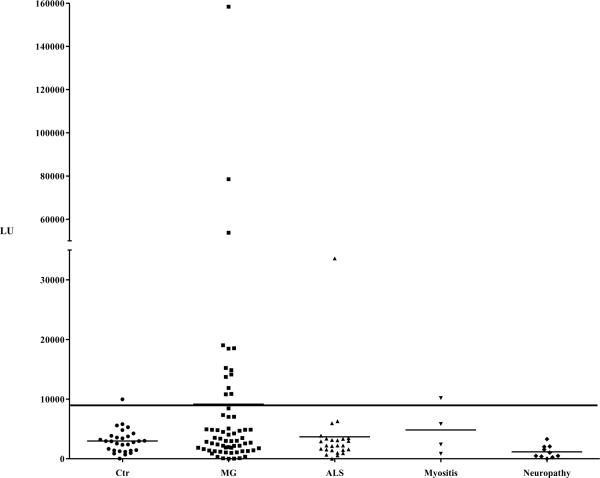
Based on the highest diagnostic performance of the AChR-α1-Δ5-Ruc fusion protein in the pilot serum samples, a larger cohort of 63 MG patients, 39 disease controls (25 ALS, 4 myositis and 10 neuropathy patients), and 29 healthy controls were tested. Based on a cutoff derived from the mean plus three standard deviations of the controls (9,041 LU), the LIPS assay using the AChR-α-Δ5 extract demonstrated 21% sensitivity (13/63) and 97% specificity (Fig. 3) Within the disease controls, one ALS patient and one myositis patient had autoantibody titers above the cutoff. These findings suggest that the AChR-α1-Δ5 LIPS assay can detect a number of MG patients with autoantibodies, and that this test is relatively specific because it generally does not detect seropositivity with samples from healthy and other disease controls.
Figure 3. Detection of MG patient autoantibodies using AChR-α1-Δ5.
A larger cohort of MG patients (N=63) and controls (N=29) and disease controls (N=39) were screened using AChR-α1-Δ5. Each point represents the average amount of fusion protein immunoprecipitated in two independent experiments by each patient in LU. In each panel, the long solid line shows a cutoff based on the average of the control group plus three standard deviations. The shorter solid lines indicate the average of each group.
A modified Ruc reporter shows enhanced LIPS antibody detection
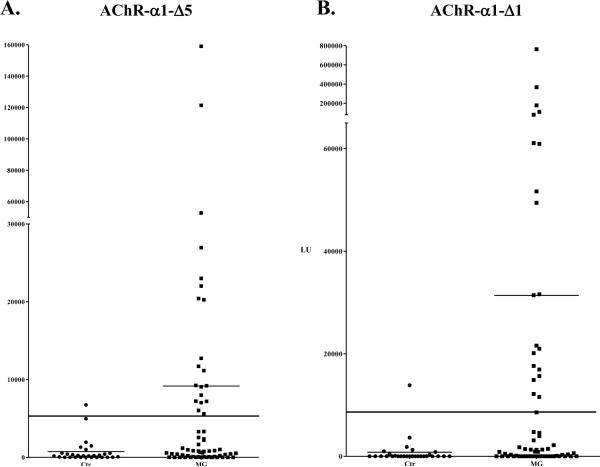
Due to the less than optimal level of luciferase activity associated with the different AChR-α1-Ruc fusion proteins, we attempted to increase the luciferase activity by mutagenesis of the Ruc enzyme. Since previous studies demonstrated enhanced luciferase activity of a Ruc-C124A point mutant [28; 29], site-directed mutagenesis was used to generate this corresponding mutant in three Ruc-AChR-α1 fusion proteins: full length AChR-α1, AChR-α1-Δ1 and -Δ5. Although this amino acid substitution had no effect on the enzymatic activity of the full length AChR-α1-Ruc-C124A protein (data not shown), the luciferase activity of the AChR-α1-Δ1-Ruc-C124A and AChR-α1-Δ5-Ruc-C124A extracts increased approximately 5-fold. Testing of the AChR-α1-Δ5-Ruc-C124A protein fragment in the LIPS assay with MG and control samples demonstrated an increased sensitivity of 32% and still showed 97% specificity (Fig 4A). A dramatic increase in sensitivity was observed with the AChR-α1-Δ1-C124A extracellular domain protein fragment, which previously showed 7% sensitivity when fused to the unmodified Ruc backbone, but now showed 32% sensitivity with 97% specificity (Fig. 4B). This protein fragment showed the highest dynamic range, attaining a titer value as high as 760,000 LU in one MG patient compared to the mean antibody titer in the controls of 803 LU. All of the MG patients detected by AChR-α1-Δ1 and Δ5 fused to the native Ruc reporter fusions were detected by the corresponding truncations fused to Ruc-C124A. These results demonstrate that this subtle improvement in enzymatic activity of the Ruc reporter can further increase sensitivity of some AChR-α1 constructs for detecting MG patient autoantibodies. Based on both sensitivity and dynamic range, these two protein fragments showed the best diagnostic performance for detecting autoantibodies against the AChR-α1 subunit in MG.
Figure 4. Effect of Ruc-C124A reporter on the detection of patient autoantibodies.
The larger cohort of MG patients (N=63) and controls (N=29) was revaluated using (A) AChR-α1-Δ5 and (B) AChR-α1-Δ1 fused to the Ruc-C124A mutant reporter. Each point represents the average amount of fusion protein immunoprecipitated in two independent experiments by each patient in LU. The long solid line shows a cutoff based on the average plus three standard deviations of the controls. The shorter solid lines indicate the average of each group.
Comparison of LIPS single chain AChR-α1 assay with 125I-α-BTX radioimmunoprecipitation
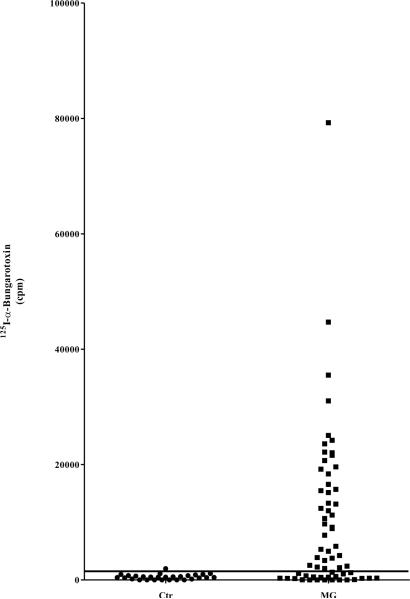
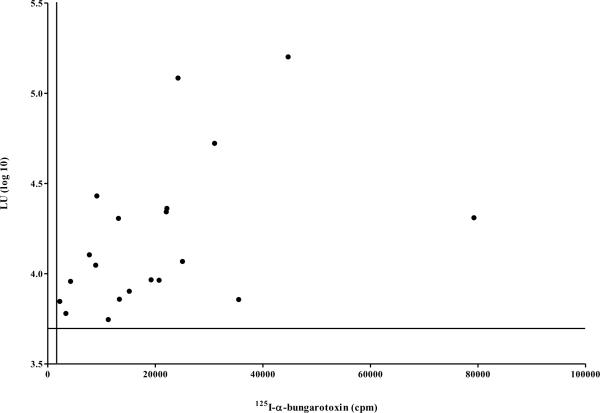
The results of the single chain AChR-α1 LIPS assay were compared with the established clinical immunoprecipitation assay that employs 125I-α-BTX with the native receptor. Based on a cutoff derived from the mean plus three standard deviations of the control group (1,844 LU), the 125I-BTX assay had 63% sensitivity (40/63) and 97% specificity for detection of MG autoantibodies in this cohort (Fig. 5A). Comparison of the results from the α-BTX RIA and LIPS showed several interesting findings. The α-BTX RIA, employing a native pentameric receptor, had much greater sensitivity (63%), essentially showing two times better performance than LIPS (32%). In the 20 MG patients copositive in the 125I- BTX assay and LIPS, we were interested if the values from the two assays correlated with each other. Comparison of the AChR-α1-Δ5-C124A antibody titer values with the results from the α-BTX RIA, as shown in Fig. 5B, revealed that the antibody titers in these selected samples tracked each other well (Spearman Rank coefficient=0.53; P=0.02). Of note, all of the MG patients detected by LIPS using the AChR-α1-Δ5 or AChR-α1-Δ1 protein fragments were also detected by the 125I-α-BTX immunoprecipitation assay. While LIPS had a greater dynamic range of detection, it still missed many of the samples detected by the α-BTX RIA. The present results identify a subpopulation of patients who display prominent autoimmunity to the single chain AChR-α1 subunit and suggest further possibilities for clinical evaluation. These results using LIPS are promising, and suggest that further improvements are needed to match the overall performance of the α-BTX RIA.
Figure 5. Measurement of MG patient autoantibodies by 125I-α-BTX RIA.
(A) MG patients and normal controls were also evaluated for anti-AChR autoantibodies using the standard RIA. Each point represents the average amount of 125I-α-BTX immunoprecipitated (cpm) by each serum in two independent experiments. The long solid line represents the cutoff (1,728 cpm) based on the average plus three standard deviations of the controls. (B) The correlation of antibody titers for the 20 MG patients who were co-positive in the AChR-α1-Δ5-Ruc-C124A LIPS assay and the α-BTX RIA were analyzed. LU data for each patient sample screened by AChR-α1-Δ5-Ruc-C124A was log10-transformed and plotted on the y-axis. Each sample's corresponding RIA result is plotted on the x-axis in cpm. The solid horizontal line represents the log10-transformed cutoff for the LIPS data (3.7 LU), and the solid vertical line represents the RIA cutoff (1,728 cpm).
Discussion
Antibodies directed at neurotransmitter receptors and ion channels have been recognized in several autoimmune channelopathies, including LEMS, paraneoplastic cerebellar ataxia, limbic encephalitis and MG [1]. To date, immunoprecipitation assays using native receptor or ion channels, radiolabeled with specific ligands, remain the primary sensitive method of detecting patient autoantibodies in the clinical setting. Here we have used the LIPS assay, employing single chain receptor antigen fusions, to study autoantibodies in MG. From screening a panel of recombinant Ruc-AChR-α1 fusions, the different AChR fusion extracts showed a large range of immunoreactivity (0–43%), despite the fact that they all contained the immunogenic extracellular domain. The findings that some AChR-Ruc fusions showed high levels of MG patient immunoreactivity while other did not, highlight the variable and unpredictable nature of recapitulating the needed conformational epitopes associated with this receptor chain. This reductionistic approach provides a route to isolating important factors that shape the immune responses in MG and possibly the clinical course of disease.
Comparison of the single AChR-α1 chain LIPS assay with the established RIA employing 125I-α-BTX-labeled native receptor revealed that the RIA showed 63% sensitivity, while LIPS showed 32% sensitivity. One possible reason for the markedly higher sensitivity of the RIA compared to LIPS is that RIA uses the native receptor containing three additional subunits (β, δ, and γ/ε subunits) besides the alpha chain. It is likely that these other AChR subunits are required for optimal folding and detection of conformational autoantibodies that are missed by LIPS. Consistent with this idea, a chimera in which the MIR of the AChR-α1 subunit was incorporated into the AChR-α7 subunit and which was immunoprecipitated by monoclonal antibodies directed at the MIR, failed to directly bind autoantibodies in MG patient sera, suggesting the importance of specific contributions from the other subunits of the muscle derived AChR in the detection of autoantibodies in some patients [30]. Another possibility accounting for the lower sensitivity of the LIPS assay compared to the α-BTX RIA is that the AChR-α1 protein fusions expressed in the LIPS assay may still not fold optimally. This may be because the AChR-α1 chain is normally a transmembrane protein and its artificial expression as a fusion with Ruc may disrupt its native conformation. Along these lines, autoantibody immunoreactivity toward the NMDA receptor was originally thought to involve multiple receptor subunits, but was later shown to be directed toward a single subunit [6; 7; 31]. These studies of the AChR and NMDAR highlight the complexity in identifying and recapitulating conformational epitopes directed at neurotransmitter receptors to detect patient autoantibodies and give further incentive to develop a method to systematically evaluate epitopes to specific receptor subunits.
Comparatively, cell-based assays appear to be the most sensitive method to detect patient autoantibodies directed at neurotransmitter receptors. Leite et al. demonstrated that many α-BTX RIA seronegative myasthenic patients do have autoantibodies against the AChR when a highly sensitive immunofluorescence-based assay was used for detection [8]. In this immunofluorescence-based assay, all four AChR subunits, along with rapsyn, are coexpressed in HEK cells to mimic the tightly clustered pentameric receptor found embedded in the plasma membrane at the neuromuscular junction. Although the specific antigenic epitopes on these other AChR chains have not been extensively investigated, it is possible that the β, δ, ε/γ subunits of AChR are direct targets of MG autoantibodies [21; 22]. Future LIPS studies using co-expression of other AChR subunits and/or additional proteins (e.g. rapsyn) may also be helpful for improving the sensitivity of detection of MG patient autoantibodies and understanding the relative contribution in immunoreactivity regarding these other subunits.
Despite our inability to directly predict in advance which subunit fragment might be the most useful in LIPS for detecting autoantibodies to the AChR-α1 chain, these studies yield some important insights. Based on this empirical data using multiple protein fragments, LIPS detection of AChR-α1 autoantibodies is strongly influenced by protein folding. Nevertheless, once established for AChR-α1 chain or likely other receptors, LIPS could be a powerful, reproducible tool for evaluating patient autoantibodies. Another finding that emerged from these studies was that the single amino acid change (C124A) in the Ruc reporter resulted in increased detection of immunoreactivity from 7% to 32% for the AChR-α1-Δ1 fragment. There are two possible explanations for the increased sensitivity of the Ruc-C124A fusion proteins. First, the Ruc-C124A fusions demonstrated increased enzymatic activity compared to the native Ruc-antigen fusions, providing an increased input for the assay. Second, it is possible that subtle amino acid changes within Ruc outside the target antigen can alter conformational folding of the antigen. Since this enhanced Ruc enzyme contains a substitution of alanine for cysteine, it is also possible that intramolecular disulfide bonds between the single cysteine in the extracellular domain of the AChR and Ruc are now minimized, thereby enhancing the native conformation of the receptor. Further modification of the Ruc reporter may also enhance immunoreactivity of some of the AChR-α1 fusion proteins.
While the clinical α-BTX RIA demonstrates markedly greater sensitivity than the LIPS assay described here, LIPS still provides highly quantitative detection of autoantibodies to the AChR in a subset of MG patients. For many of the samples, a much wider dynamic range of antibody titers was found by LIPS compared to the RIA. For example, by the LIPS assay, one MG patient showed titers 88-fold higher than the cutoff compared to only 22-fold for the α-BTX RIA. The ability to detect a larger dynamic range by LIPS assay may be clinically useful for determining whether MG patient autoantibody titers change in response to treatment. The studies outlined here lay the groundwork for additional diagnostic improvements and for evaluating immunoreactivity to likely other neurotransmitter receptors.
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health and by the Biomarkers Subsection of the Center of Regenerative Medicine (CNRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vincent A, Lang B, Kleopa KA. Autoimmune channelopathies and related neurological disorders. Neuron 52. 2006:123–38. doi: 10.1016/j.neuron.2006.09.024. [DOI] [PubMed] [Google Scholar]
- [2].Vincent A, Newsom-Davis J. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results in 153 validated cases and 2967 diagnostic assays. J Neurol Neurosurg Psychiatry 48. 1985:1246–52. doi: 10.1136/jnnp.48.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lennon VA, Kryzer TJ, Griesmann GE, O'Suilleabhain PE, Windebank AJ, Woppmann A, Miljanich GP, Lambert EH. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med 332. 1995:1467–74. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- [4].Kleopa KA, Elman LB, Lang B, Vincent A, Scherer SS. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain 129. 2006:1570–84. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- [5].Vincent A. Autoantibodies in neuromuscular transmission disorders. Ann Indian Acad Neurol 11. 2008:140–5. doi: 10.4103/0972-2327.42932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dalmau J, Bataller L. Limbic encephalitis: the new cell membrane antigens and a proposal of clinical-immunological classification with therapeutic implications. Neurologia 22. 2007:526–37. [PubMed] [Google Scholar]
- [7].Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7. 2008:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, Beeson D, Willcox N, Vincent A. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain 131. 2008:1940–52. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lefvert AK, Bergstrom K, Matell G, Osterman PO, Pirskanen R. Determination of acetylcholine receptor antibody in myasthenia gravis: clinical usefulness and pathogenetic implications. J Neurol Neurosurg Psychiatry 41. 1978:394–403. doi: 10.1136/jnnp.41.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heinemann S, Bevan S, Kullberg R, Lindstrom J, Rice J. Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci U S A 74. 1977:3090–4. doi: 10.1073/pnas.74.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med 298. 1978:1116–22. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- [12].Vincent A, Willcox N, Hill M, Curnow J, MacLennan C, Beeson D. Determinant spreading and immune responses to acetylcholine receptors in myasthenia gravis. Immunol Rev 164. 1998:157–68. doi: 10.1111/j.1600-065x.1998.tb01217.x. [DOI] [PubMed] [Google Scholar]
- [13].Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and C3) at the motor endplate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc 52. 1977:267–80. [PubMed] [Google Scholar]
- [14].Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40. 2000:431–58. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- [15].Tzartos SJ, Lindstrom JM. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A 77. 1980:755–9. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tzartos S, Langeberg L, Hochschwender S, Lindstrom J. Demonstration of a main immunogenic region on acetylcholine receptors from human muscle using monoclonal antibodies to human receptor. FEBS Lett 158. 1983:116–8. doi: 10.1016/0014-5793(83)80688-7. [DOI] [PubMed] [Google Scholar]
- [17].Tzartos SJ, Seybold ME, Lindstrom JM. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A 79. 1982:188–92. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loutrari H, Kokla A, Tzartos SJ. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur J Immunol 22. 1992:2449–52. doi: 10.1002/eji.1830220939. [DOI] [PubMed] [Google Scholar]
- [19].Sine SM. Functional properties of human skeletal muscle acetylcholine receptors expressed by the TE671 cell line. J Biol Chem 263. 1988:18052–62. [PubMed] [Google Scholar]
- [20].Lindstrom J, Criado M, Ratnam M, Whiting P, Ralston S, Rivier J, Sarin V, Sargent P. Using monoclonal antibodies to determine the structures of acetylcholine receptors from electric organs, muscles, and neurons. Ann N Y Acad Sci 505. 1987:208–25. doi: 10.1111/j.1749-6632.1987.tb51293.x. [DOI] [PubMed] [Google Scholar]
- [21].Kostelidou K, Trakas N, Zouridakis M, Bitzopoulou K, Sotiriadis A, Gavra I, Tzartos SJ. Expression and characterization of soluble forms of the extracellular domains of the beta, gamma and epsilon subunits of the human muscle acetylcholine receptor. Febs J 273. 2006:3557–68. doi: 10.1111/j.1742-4658.2006.05363.x. [DOI] [PubMed] [Google Scholar]
- [22].Psaridi-Linardaki L, Mamalaki A, Remoundos M, Tzartos SJ. Expression of soluble ligand- and antibody-binding extracellular domain of human muscle acetylcholine receptor alpha subunit in yeast Pichia pastoris. Role of glycosylation in alpha-bungarotoxin binding. J Biol Chem 277. 2002:26980–6. doi: 10.1074/jbc.M110731200. [DOI] [PubMed] [Google Scholar]
- [23].Ricny J, Simkova L, Vincent A. Determination of anti-acetylcholine receptor antibodies in myasthenic patients by use of time-resolved fluorescence. Clin Chem 48. 2002:549–54. [PubMed] [Google Scholar]
- [24].Hewer R, Matthews I, Chen S, McGrath V, Evans M, Roberts E, Nute S, Sanders J, Furmaniak J, Smith BR. A sensitive non-isotopic assay for acetylcholine receptor autoantibodies. Clin Chim Acta 364. 2006:159–66. doi: 10.1016/j.cccn.2005.05.035. [DOI] [PubMed] [Google Scholar]
- [25].Burbelo PD, Ching KH, Bush ER, Han BL, Iadarola MJ. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev Vaccines 9. 2010:567–78. doi: 10.1586/erv.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burbelo PD, Leahy HP, Groot S, Bishop LR, Miley W, Iadarola MJ, Whitby D, Kovacs JA. A Four Antigen Mixture Containing V-Cyclin for Serological Screening of Human Herpesvirus 8 (HHV-8) Infection. Clin Vaccine Immunol. 2009 doi: 10.1128/CVI.00474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jacobson L, Beeson D, Tzartos S, Vincent A. Monoclonal antibodies raised against human acetylcholine receptor bind to all five subunits of the fetal isoform. J Neuroimmunol 98. 1999:112–20. doi: 10.1016/s0165-5728(99)00086-7. [DOI] [PubMed] [Google Scholar]
- [28].Liu J, Escher A. Improved assay sensitivity of an engineered secreted Renilla luciferase. Gene 237. 1999:153–9. doi: 10.1016/s0378-1119(99)00314-5. [DOI] [PubMed] [Google Scholar]
- [29].Loening AM, Fenn TD, Wu AM, Gambhir SS. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 19. 2006:391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- [30].Lindstrom J, Luo J, Kuryatov A. Myasthenia gravis and the tops and bottoms of AChRs: antigenic structure of the MIR and specific immunosuppression of EAMG using AChR cytoplasmic domains. Ann N Y Acad Sci 1132. 2008:29–41. doi: 10.1196/annals.1405.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133. 2010:1655–67. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]