Abstract
Clinical development of malaria vaccines progresses from trials in malaria naïve adults to malaria exposed adults followed by malaria exposed children. It is not well known whether immune responses in non-target populations are predictive of those in target populations, particularly in African children. Therefore humoral responses in three different populations (U.S. adults, Malian adults and Malian children) were compared in this study. They were immunized with 80 μg of Apical Membrane Antigen 1 (AMA1)/Alhydrogel on Days 0 and 28. Sera were collected on Days 0 and 42; antibody levels were determined by ELISA and the functionality of antibodies was evaluated by Growth Inhibition Assay. After immunization, there was no significant difference in antibody levels between the Malian children and the Malian adults, but U.S. adults showed lower antibody levels. Vaccination did not significantly change growth-inhibitory activity in Malian adults, but inhibition increased significantly in both U.S. adults and Malian children. Vaccine-induced inhibitory activity was reversed by pre-incubation with AMA1 protein, but pre-existing infection-induced inhibition was not. This study shows that humoral responses elicited by the AMA1 vaccine varied depending on the population, most likely reflecting different levels of previous malaria exposure. Thus predicting immune responses from non-target populations is not desirable.
Keywords: Apical Membrane Antigen 1, Phase 1 trial, Phase 2 trial, Humoral immunity, ELISA, Growth Inhibition Assay
1. Introduction
Malaria remains one of the biggest global health problems, and there are five species which are pathogenic in humans; Plasmodium falciparum, P. vivax, P. ovale, P malariae and P. knowlesi. Out of the five, P. falciparum is the most virulent and it is estimated that there were 451 million P. falciparum cases of in 2007 [1]. While a passive transfer study conducted in the1960's has shown that a gamma-globulin is a critical factor for the protection in blood-stage of P. falciparum malaria [2], the target antigen(s) and the mechanism(s) of protection have not yet been completely elucidated. An effective vaccine would have an enormous impact on malaria control and eventually eradication. One candidate for a blood-stage vaccine is apical membrane antigen 1 (AMA1), which is an essential protein for erythrocyte invasion, and a number of lines of evidence from preclinical studies and epidemiology studies suggest that a high level of AMA1 antibody is associated with a reduced risk of P. falciparum malaria (reviewed in [3]). We and other investigators have conducted multiple AMA1 Phase 1 trials [4–13] and two Phase 2 field trials [14, 15]. However, to date no significant effects have been shown in a target population of African children.
In view of regulatory and ethical concerns, usually a Phase 1 trial is conducted in malaria naïve adults first to establish safety, then in malaria exposed adults, followed by in malaria exposed children (or infants), who are the main target population of a blood-stage vaccine. While the main objective of a Phase 1 trial is to evaluate safety, immunological responses are an important secondary objective. However, in the case of a malaria vaccine, it is not well documented whether it is possible to predict the immunological responses induced by a vaccine in a target population (i.e., malaria exposed children) from the response in another population (i.e., malaria naïve adults or malaria exposed adults). For other vaccines, such as measles-mumps-rubella vaccine [16, 17] and meningococcal vaccine [18], it has been reported that ethnicity and age factors affect antibody responses. In addition, other factors, such as nutritional status and environmental infections, are also thought to modify the immune response in the vaccine recipients (reviewed in [19]).
To our knowledge, no study has been reported in malaria vaccine research where the immune responses elicited by the same vaccine formulation administered with the same regimen were compared head-to-head in different populations. In the current study, the quantity of antibody induced by an AMA1 vaccine in trials in three different populations (Phase 1 in U.S. adults, Phase 1 in Malian adults and Phase 2 in Malian children) was compared on the same scale by converting absorbance-based ELISA titer to mass concentration (μg/ml). In addition, for functional assessments of humoral responses, we conducted an in vitro Growth Inhibition Assay (GIA) and the specificity of the inhibition was evaluated by an antigen-reversal GIA. This is the first report of GIA response in children receiving an AMA1 vaccine. The results in the Mali pediatric trial were compared with those in the U.S. adult [6] and the Mali adult [5] trials. We found humoral immune responses elicited by the AMA1 vaccine varied depending on the population immunized.
2. Materials and methods
2.1. Clinical trials and data used in the current study
The details of the U.S. adult Phase 1 trial [6], Mali adult Phase 1 trial [5] and Mali pediatric Phase 2 trial [14] have been supplied elsewhere (NCT00344539, NCT00343005 and NCT00341250). In brief, volunteers were immunized on Days 0 and 28 with 80 μg of AMA1-C1 (a mixture of the recombinant AMA1-FVO and AMA1-3D7 proteins) formulated on 800 μg of Alhydrogel® and blood samples were collected on Days 0 and 42. The volunteers received three immunizations in U.S. adult and Mali adult trials and there were other groups (e.g., volunteers received a comparator vaccine, etc) in the three trials. However, only data to Day 42 from the groups receiving 2 doses of 80 μg of AMA1 on Alhydrogel® were used in this analysis to compare immunological responses in the three populations under the same vaccination conditions. Adults 18–45 years of age were enrolled in the two adult trials; in the Mali pediatric trial children were age 2–3 at enrollment. All of the participants in the three trials were healthy volunteers. In the U.S. adult Phase 1 trial, individuals with prior malaria infection, recent or planned travel to a malaria endemic country and recent use of malaria prophylaxis were excluded during the recruitment. The Mali adult Phase 1 trial was conducted in Donéguébougou where malaria transmission occurs mainly June to November and the second vaccination was completed by end of July [5]. The Mali pediatric Phase 2 trial was conducted in Bancoumana where the transmission also occurs June to November. The second vaccination of the trial was conducted in August and September [14]. Subjects who were missing either Day 0 or Day 42 data or who did not receive 2 does of vaccine were excluded from the analysis unless otherwise specified. The number of subjects included in this study are; n=27 (ELISA) or 25 (GIA) in U.S. adult trial, n=12 (ELISA and GIA) in Mali adult trial, and n=127 (ELISA) or 89 (GIA) in Mali pediatric trial.
All trials were conducted under Investigational New Drug Applications reviewed by the U.S. Food and Drug Administration, and all were reviewed and approved by the Institutional Review Boards at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and at the respective study sites.
2.2. ELISA
The standardized methodology for performing the ELISA has been described previously [20]. The absorbance of each test sample was converted into ELISA units using a standard curve generated by serially diluting the standard in the same plate. The ELISA units of each sample were then converted to μg/ml using a conversion factor as described elsewhere [21]. Because the ELISA response for the AMA1-FVO and AMA1-3D7 proteins were highly correlated in the three trials, the arithmetic average of the two was used as that subject's AMA1 antibody response. The minimal detection level of the AMA1 antibody in this study was 4.4 μg/ml, and all responses below that limit of detection were assigned a value of 2.2 μg/ml for the analysis.
2.3. GIA and antigen-reversal GIA
The standard methodology for the GIA has been described previously [4]. From 0.5–3 ml of individual serum (U.S. and Mali adults) or plasma (Mali children) was obtained, total IgG was purified using a protein G column, buffer exchanged to RPMI1640 and concentrated to a concentration of 20 to 40 mg/ml. The assay was performed with purified IgGs at a final concentration of 10 mg/ml against 3D7 strain parasites. For the antigen-reversal GIA experiment, a test IgG was pre-incubated with AMA1-3D7 antigen (2 μM) for 45 minutes before mixing with parasites. The final concentration of the culture had the same parasitemia and hematocrit levels as those in the standard GIA.
2.4. Statistical analysis
A comparison of antibody level or functional activity among the three groups was assessed by a Kruskal-Wallis test, and if significant, followed by the three pairwise Mann-Whitney tests. Adjusted p-values are given for the pairwise tests, defined as the maximum of the Kruskal-Wallis p-value and the Mann-Whitney p-value. A comparison of functional activity between two groups was assessed by a Mann-Whitney test. For a paired comparison in the same group, a Wilcoxon signed rank test was used. A Spearman rank correlation test was employed to assess correlation between the two data sets (e.g., Day 0 antibody level versus change of antibody level between Days 0 and 42, etc). For the Mali pediatric trial, the correlation between GIA data and biological impact was tested by a Spearman rank correlation test as described previously [14] but with Day 42 GIA data used as the immunological readout for this study rather than ELISA data in the previous study. In brief, for each individual, the biological impact was defined using the rate of episodes of P. falciparum infection with density >3000/μl/day at risk (P3000). Time at risk was the period of parasitologic follow up minus specified time periods (e.g., 28 days following malaria treatment, etc). The correlation between P3000 and Day 42 GIA was evaluated using the data from all children whose Day 42 GIA were available (n=130).
Data were analyzed using Prism 5 (GraphPad Software, Inc., CA, USA), SAS (SAS Institute Inc., NC, USA) or R software (R Core Development Team, Vienna Austria) and p values less than 0.05 were considered significant.
3. Results
3.1. Antibody level comparison in the three populations
In the previous manuscripts [5, 14], the level of anti-AMA1 antibody was expressed in ELISA units. To compare all three populations on the same scale, ELISA units were converted to μg/ml using the corresponding conversion factors. As reported previously, there was a significant increase of antibody levels in the AMA1-vaccinated group in each trial when Day 42 data were compared with the Day 0 data (Wilcoxon signed rank tests, p<0.001, for all three populations).
As shown in Figure 1, on Day 0 there was a significant difference in baseline antibody levels among the three populations (Kruskal-Wallis test, p<0.001). Although both the Malian children and the U.S. adults showed the same median of 2.2 μg/ml (i.e., at least 50% of the individuals in each population had undetectable level of antibody at baseline), the Malian children showed significantly higher antibody levels compared to the U.S. adults (adjusted p<0.001 by Mann-Whitney test), but Malian children levels were significantly lower than that of Malian adults (median 40.4 μg/ml, adjusted p=0.003 by Mann-Whitney test). On Day 42, there was a significant difference among the three populations (Kruskal-Wallis test, p<0.001), but there was no significant difference (p=0.863) between the Malian adults (median 111.8 μg/ml) and the Malian children (median 111.6 μg/ml). The U.S. adults showed significantly lower antibody levels after vaccination (median 7.8 μg/ml) compared to both the Malian adults and the Malian children (adjusted p<0.001 for both Mann-Whitney tests). Regardless of the population, group or the day of testing, the IgG1 subclass was the dominant subclass of the anti-AMA1 antibodies (data not shown).
Figure 1.
Anti-AMA1 antibody levels on Days 0 and 42 in the three populations. Box-whisker plots illustrate medians with 25th and 75th percentiles; whiskers denote the 10th and 90th percentiles. All responses below the limit of detection (4.4 μg/ml) were assigned a value of 2.2 μg/ml for the analysis. The dotted line represents that limit (2.2 μg/ml). All U.S. adults had measured values below the limit of detection on Day 0 and their data overlaps with the dotted line. Only adjusted p-values less than 0.05 (by Mann-Whitney test) are shown.
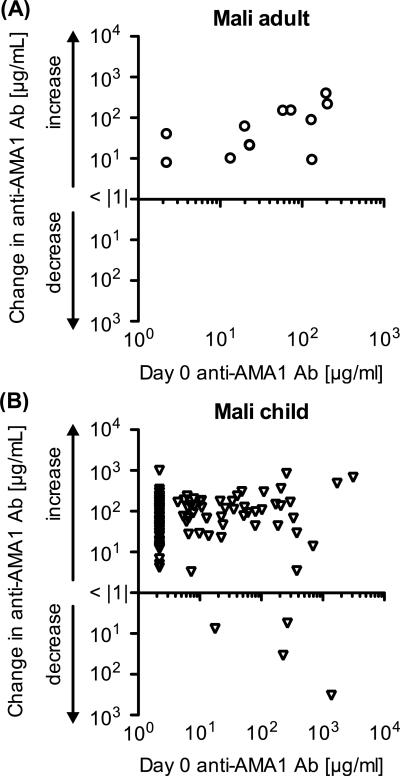
In the Mali adult trial, as we reported previously [5], there was a significant positive correlation between the antibody level on Day 0 and the increase in antibody level between Days 0 and 42 (Figure 2A: Spearman Rank test; p=0.035; ρs=0.620, 95% Confidence Interval, 0.052 to 0.885). To assess the phenomenon in Malian children, we compared the Day 0 antibody and the change of anti-AMA1 antibody (Figure 2B). There was no significant correlation between antibody level on Day 0 and the change in the Mali pediatric trial (Spearman Rank test; p=0.203; ρs=0.114, 95% CI, −0.067 to 0.287).
Figure 2.
Correlation between Day 0 antibody level and the change from Day 0 to Day 42. The data in the Mali adult trial (A) and in the Mali pediatric trial (B) are shown. The x-axis represents antibody levels on Day 0 and the y-axis represents the change of antibody level from Day 0 to Day 42 on a logarithmic scale. No volunteers showed less than 1 μg/ml change between Days 0 and 42.
3.2. Functional activity comparison in the three populations
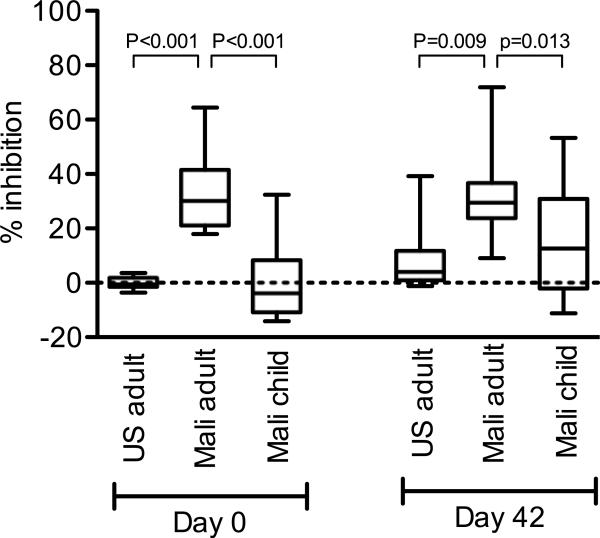
As shown in Figure 3, on Day 0, there was a significant difference among the three populations (Kruskal-Wallis test, p<0.001) in the GIA analysis, and Malian adults showed significantly higher activity (median 30.2% inhibition) than the other two populations (adjusted p<0.001 by Mann-Whitney test compared to both U.S. adults and to Malian children). The difference between the Malian children (median −3.9 % inhibition) and the U.S. adults (median −0.6 % inhibition) did not reach significance on Day 0 (adjusted p=0.095 by Mann-Whitney test). The Day 0 and 42 GIA data were then compared in each population. In the Mali adult trial, there was no significant change as previously reported [5]. However, both in the U.S. adult and the Mali pediatric trials, there was a small, but significant, increase of growth-inhibitory activity (U.S. adults, median 4.1 % inhibition on Day 42, Wilcoxon signed rank test, p<0.001; Malian children, median 12.6% inhibition on Day 42, p<0.001). On Day 42, there was a significant difference among the three populations (Kruskal-Wallis test, p=0.009): the Malian adults showed significantly higher activity compared to the other populations (Mann-Whitney test; adjusted p=0.009 to U.S. adults and 0.013 to Malian children), and there was no difference between the Malian children and the U.S. adults (adjusted p=0.312).
Figure 3.
The growth-inhibitory activity on Days 0 and 42 in the three populations. Box-whisker plots illustrate medians with 25th and 75th percentiles; whiskers denote the 10th and 90th percentiles. The dotted line represents zero % inhibition. Only adjusted p-values less than 0.05 (by Mann-Whitney test) are shown.
The effect of pre-existing growth-inhibitory activity on the change between Days 0 and 42 was analyzed (Figure 4). In the Mali adult trial, regardless of activity on Day 0, the change between Days 0 and 42 was less than 20% inhibition. In Malian children, similar to Malian adults, if the children showed more than 20% inhibition on Day 0, the change between Days 0 and 42 was less than 20%. However, out of 68 children with less than 10% inhibition on Day 0, 23 (34%) children had more than a 20% increase from Day 0 to Day 42.
Figure 4.
The correlation between Day 0 growth-inhibitory activity and the change from Day 0 to Day 42. The data in the Mali adult trial (A) and in the Mali pediatric trial (B) are shown. The x-axis represents % inhibition on Day 0 and the y-axis represents the change from Day 0 to Day 42.
3.3. Further analysis of functional activity in the Mali pediatric trial
The correlation between Day 42 GIA and biologic impact (P3000) was evaluated using the data from all AMA1-vaccinated children whose Day 42 GIA data were available (n=130). The correlation was not significant (Spearman rank correlation test, p=0.594). Growth-inhibitory activity was significantly higher at Day 0 (p=0.036 by Mann-Whitney test) but not Day 42 (p=0.106) in children who were parasitemic at those time points.
We have shown that growth-inhibitory activity is a function of anti-AMA1 antibody level in the case of malaria naïve adults [22] and there was a significant correlation between pre-vaccination anti-AMA1 antibody levels and the growth-inhibitory activity in Malian adults when all data were combined (i.e., data from all 54 adults enrolled in the trial) [5]. However, when Day 0 data only from AMA1-vaccinated Mali adults (n=12) was analyzed, the correlation did not reach significance (Figure 5; Spearman rank correlation, p=0.514). In the Malian children who were vaccinated with AMA1, there was a significant correlation on Day 0 (p<0.001, ρs=0.629). On Day 42, the correlation was preserved in the Malian children vaccinated with AMA1 (p<0.001, ρs=0.764) and no significant correlation in the Malian adults vaccinated with AMA1 (p=0.110).
Figure 5.
Correlation between anti-AMA1 antibody level and the growth-inhibitory activity in the three populations. The Day 0 (A) and Day 42 (B) data are shown. Because there was no pre-existing immunity to malaria in the U.S. adults on Day 0, the data are not shown in the Figure 5A. The x-axis represents the antibody level of a test IgG in GIA well and the y-axis represents the % inhibition of the sample.
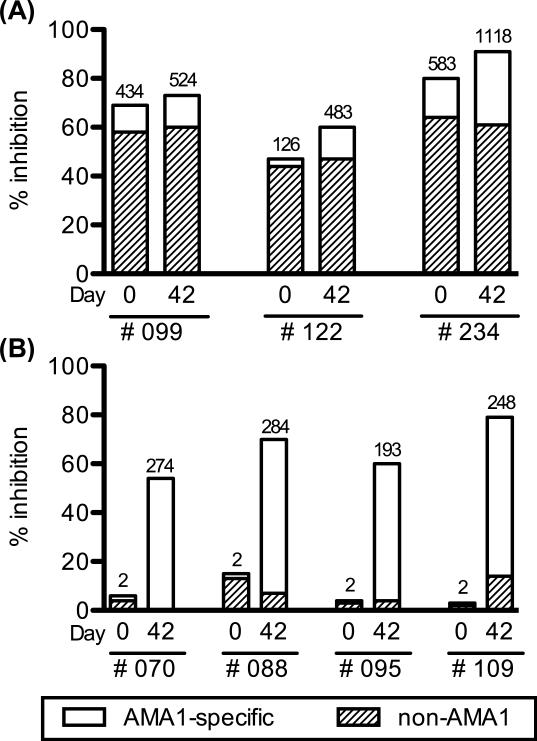
An antigen-reversal GIA was conducted to determine the specificity of the growth-inhibitory activity. As reported previously, the inhibitory activity induced by the AMA1 vaccine in the U.S. adults was completely reversed by the pre-incubation with specific antigen [6], but no or marginal change was observed in the Malian adults [5]. In the current study, we conducted the assay with samples from 7 AMA1-vaccinated Malian children who showed more than 50% inhibition on Day 42. There were two types of children among the 7 volunteers selected; 1) children who showed high activity on Day 0 and remained high on Day 42 (n=3), and 2) children who showed almost no activity on Day 0, but high activity on Day 42 (n=4). A smaller proportion of the inhibitory-activity was reversed by pre-incubation with AMA1 protein in the former group (Figure 6A) as compared to the latter group (Figure 6B).
Figure 6.
Antigen-reversal GIA with IgGs from the Mali pediatric trial. Each test IgG was incubated with 2 μM of AMA1 protein for 45 min before mixing with a malaria culture. (A) IgGs from children who showed high activity on Day 0 and remained high on Day 42 (B) IgGs from children who showed almost no activity on Day 0, but high activity on Day 42. For each IgG, the total height of the bar (AMA1-specific plus non-AMA1) represents the growth-inhibitory activity of the IgG without the pre-incubation, and the height of the open bar (AMA1-specific) represents the activity which was reversed by the antigen pre-incubation. The number on top of each bar represents the level of anti-AMA1 antibody in the GIA well judged by ELISA.
4. Discussion
In the present study, we present in vitro GIA results in children receiving AMA1 vaccination for the first time, and show that the immunological response elicited by the same AMA1 vaccine differs depending on the population immunized. While Malian children showed similar levels of antibody as Malian adults on Day 42 as judged by ELISA, U.S. adults had significantly lower antibody levels after vaccination than either of these two populations. In terms of the functionality of the antibodies as judged by in vitro GIA and antigen-reversal GIA, Malian children with higher growth-inhibitory activity on Day 0 were similar to Malian adults (i.e., AMA1 vaccination did not induce higher activity on Day 42 and the activity was not reversed by pre-incubation with AMA1 protein); in contrast Malian children with lower activity on Day 0 were similar to U.S. adults (i.e., AMA1 vaccination could induce higher activity on Day 42, and the activity was reversible). This likely reflects varying degrees of previous malaria exposure in these 2–3 year old Malian children.
There are several factors which are considered to alter the host immune response to vaccination, such as ethnicity (genetic background), age, nutrition status and environmental infections [16-19]. In the case of malarial vaccines, to the best of our knowledge, this is the first study which directly compares the immune responses elicited by the same malaria vaccine administered according to the same regimen in different populations. While many papers have been published for malaria vaccine trials, it is not straightforward to compare the results since different adjuvants or different immunization schedules and/or different measurements (i.e., expressed the levels of antibodies either in μg/ml or ELISA units/ml) were used in these studies. However, one may partially compare the responses by combining results from multiple publications. For example, in contrast to our AMA1/Alhydrogel vaccination, when an AMA1-3D7 vaccine adjuvanted with AS02A was administered at 0, 1 and 2 months, the vaccine induced approximately 200 μg/ml of anti-AMA1 antibody in both U.S. adults [12] and in Malian children [13] at 1 month after the third immunization. When RTS,S, which is the most advanced malaria vaccine candidate, adjuvanted with AS02A was administered at 0 and 1 month, the vaccine induced approximately 20–30 μg/ml of antibody at 2–4 weeks after the second immunization regardless of whether the recipients were U.S. adults [23], Kenyan adults [24] or Gambian adults [25]. On the other hand, other RTS,S/AS02A studies showed different levels of antibodies in different populations: when Kenyan adults received the vaccine at 0, 1 and 2 months, the antibody levels reached ~20 ELISA units/ml at 1 month after the third immunization [26], but the level was almost 10 times higher in the Mozambiquean children [27, 28]. Not only immune responses, but also clinical responses induced by a vaccine might be different in different populations. For example, a human vaccine trial with thrombospondin-related adhesion protein induced partial protection after sporozoite challenge in a malaria naïve population [29], but not in malaria exposed populations [30, 31]. In this study, we tried to compare immunological responses in the three populations under as similar vaccination conditions as possible. Ethnicity in the adult trial (5 of them were Bamanan and the other 7 were Sarakole) was not the same as the pediatric trial (more than 90% of them were Malinke), however none of these ethnic groups are known to be resistant to malaria. In addition, the blood collections were at slightly different time during the malaria transmission season. These factors may also affect the differences in humoral responses seen in this study. However, from a practical point of view, it is very difficult to control all of the factors in multiple human trials for a vaccine during the whole development path. Taken together, immune responses (and/or clinical protection) induced by vaccination in a non-target population (such as malaria-naïve US or malaria exposed adults) appear to be imperfect predictors of responses in malaria exposed African children.
In this study, we did not observe different patterns of IgG subclasses in the three populations studied (i.e., IgG1 is the dominant subclass), and this pattern is consistent with reports from epidemiologic studies [32-34]. These results indicate that an AMA1 protein by itself may strongly skew the IgG subclass toward IgG1 production. Because of the limited quantity of blood available, especially from the Mali pediatric trial, we did not investigate difference in cross-reactivity against multiple allelic forms of AMA1 or in cellular immunity in the three populations in this study. Further studies could be conducted to compare the cross-reactivity and/or the cellular immunity in different populations with a more immunogenic vaccine formulation.
There were significant correlations between anti-AMA1 antibody levels and % inhibition in GIA on Day 0 in Malian children (Figure 5A) and in Malian adults [5] when the sample size was reasonably large (i.e., n=54, instead of only n=12 used in this study). However, growth-inhibitory activity on Day 0, likely induced by prior exposure to malaria, was not readily reversed by pre-incubation with AMA1 protein ([5] and Figure 6A). Our other study [21] also showed that the fraction of IgGs which did not bind to an AMA1-affinity column showed similar growth-inhibitory activity to the original IgGs purified from Malian adult sera. Taken together, we conclude that natural malaria infections induce growth-inhibitory antibodies in these populations and that antibodies directed to antigens other than AMA1 also cause growth-inhibitory activity in vitro. High antibody levels to AMA1 antigen may be a marker for high levels of antibodies to other plasmodial antigens which also induce growth-inhibitory activity.
We have shown that a high level of growth-inhibitory activity before the malaria transmission season is significantly associated with a reduction of subsequent malaria risk in a previous longitudinal study in Malian children aged 2 to 10 years [35]. Another study has demonstrated that time to first infection is significantly associated with the level of growth-inhibitory activity when controlled for age [36], but other studies have not given similar results [37, 38]. In addition to conflicting results relating to growth-inhibitory activity and clinical protection, data on the relationship between inhibitory activity and age are inconsistent. Our current results show that growth-inhibitory activity is significantly higher in Malian adults than in Malian children, and is similar to our previous epidemiologic study in Mali [35]. However, Dent et al reported that Kenyan children showed higher activity than adults [36], while studies conducted in Senegal [38] and Gambia [39] showed no association between age and inhibitory activity. Another study conducted in Kenya showed no association with age when the GIA was performed with 3D7 strain parasites, and a negative correlation with W2mef parasites (i.e., the adults showed lower inhibition than the children) [40]. The discrepancy among the studies may be explained at least in part by varying prevalence of malaria at the study sites and the way the assay is performed (e.g., using serum versus purified IgG, strain of parasites, etc). Different endpoints relating to clinical protection may also in part explain the discrepancy. Indeed, there was no significant correlation when time to first episode or overall malaria incidence were used as clinical endpoints in our previous study [35]. Standardization and/or harmonization of the in vitro assay and clinical endpoints among different laboratories would be desirable.
In the current study, there was no significant correlation (p=0.594) between the growth-inhibitory activity on Day 42 and subsequent malaria risk in AMA1-vaccinated Malian children. One of the possible explanations for the lack of correlation in this study is that the growth-inhibitory activity elicited by the AMA1/Alhydrogel vaccine might be too weak. In the previous longitudinal study [35], 40% inhibition was calculated as the optimal cutoff for predicting risk of subsequent clinical malaria. However, in this Mali pediatric Phase 2 trial, the proportion of children with more than 40% inhibition only increased from 7 to 20 % after vaccination. An Aotus monkey challenge model with an AMA1 vaccine demonstrated that only monkeys with more than 70% inhibition at 1:10 serum dilution were protected against a virulent P. falciparum challenge [41]. These results suggest that an AMA1 vaccine may need to induce much higher immune responses in the vaccinees to demonstrate protection in the field. Another possibility is that a proportion of anti-malarial antibodies, which interferes with biological activity of anti-AMA1 antibodies, may modulate this putative mechanism of action in the target populations with previous malaria exposure. Our previous study suggests this hypothesis [21]. Further studies are required to determine which assays are likely to predict clinical protection. However, this study indicates that immune responses in non-target populations (i.e., malaria naïve adults or malaria exposed adults) are not predictive of those in target populations (i.e., children and infants who live in a malaria endemic area) who have uneven degrees of previous malaria exposure.
Acknowledgments
We are very grateful to all volunteers who participated in the clinical trials. We also thank John Treanor, Amagana Dolo, Dapa Diallo, Gregory Mullen and Allan Saul for their contributions to the human trials. This study was supported by the intramural program of the National Institute of Allergy and Infectious Diseases/NIH and the GIA Reference Center was supported by the PATH/Malaria Vaccine Initiative.
Abbreviations
- AMA1
Apical Membrane Antigen 1
- GIA
Growth Inhibition Assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, et al. Estimating the global clinical burden of Plasmodium falciparum Malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- [3].Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008;24:74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [4].Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dicko A, Diemert DJ, Sagara I, Sogoba M, Niambele MB, Assadou MH, et al. Impact of a Plasmodium falciparum AMA1 Vaccine on Antibody Responses in Adult Malians. PLoS ONE. 2007;2:e1045. doi: 10.1371/journal.pone.0001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008;3:e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sagara I, Ellis RD, Dicko A, Niambele MB, Kamate B, Guindo O, et al. A randomized and controlled Phase 1 study of the safety and immunogenicity of the AMA1-C1/Alhydrogel((R))+CPG 7909 vaccine for Plasmodium falciparum malaria in semi-immune Malian adults. Vaccine. 2009;27:7292–8. doi: 10.1016/j.vaccine.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dicko A, Sagara I, Ellis RD, Miura K, Guindo O, Kamate B, et al. Phase 1 Study of a Combination AMA1 Blood Stage Malaria Vaccine in Malian Children. PLoS ONE. 2008;3:e1563. doi: 10.1371/journal.pone.0001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Polhemus ME, Magill AJ, Cummings JF, Kester KE, Ockenhouse CF, Lanar DE, et al. Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2.1, adjuvanted with AS02A, in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2007;25:4203–12. doi: 10.1016/j.vaccine.2007.03.012. [DOI] [PubMed] [Google Scholar]
- [10].Thera MA, Doumbo OK, Coulibaly D, Diallo DA, Kone AK, Guindo AB, et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS ONE. 2008;3:e1465. doi: 10.1371/journal.pone.0001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE. 2008;3:e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Kone AK, Guindo AB, et al. Safety and Immunogenicity of an AMA1 Malaria Vaccine in Malian Children: Results of a Phase 1 Randomized Controlled Trial. PLoS ONE. 2010;5:e9041. doi: 10.1371/journal.pone.0009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sagara I, Dicko A, Ellis RD, Fay MP, Diawara SI, Assadou MH, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27:3090–8. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thera MA, Doumbo OK, D.Coulibaly, Laurens MB, Kone A, A.Guindo, et al. Randomized, controlled, phase 2b clinical trial to evaluate the safery, immunogenicity and efficacy of WRAIR's AMA-1 malaria vaccine (FMP2.1) adjuvanted in GSK biologicals'AS02A vs. Rabies vaccine in 1–6 year old children in Bandiagara, Mali. Program and abstracts of the American society of tropical medicine and hygiene 58th annual meeting. 2009:1074. [Google Scholar]
- [16].Poland GA, Jacobson RM, Colbourne SA, Thampy AM, Lipsky JJ, Wollan PC, et al. Measles antibody seroprevalence rates among immunized Inuit, Innu and Caucasian subjects. Vaccine. 1999;17:1525–31. doi: 10.1016/s0264-410x(98)00362-4. [DOI] [PubMed] [Google Scholar]
- [17].Rager-Zisman B, Bazarsky E, Skibin A, Tam G, Chamney S, Belmaker I, et al. Differential immune responses to primary measles-mumps-rubella vaccination in Israeli children. Clin Diagn Lab Immunol. 2004;11:913–8. doi: 10.1128/CDLI.11.5.913-918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281:1520–7. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- [19].Kimman TG, Vandebriel RJ, Hoebee B. Genetic variation in the response to vaccination. Community Genet. 2007;10:201–17. doi: 10.1159/000106559. [DOI] [PubMed] [Google Scholar]
- [20].Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miura K, Zhou H, Moretz SE, Diouf A, Thera MA, Dolo A, et al. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J Immunol. 2008;181:8776–83. doi: 10.4049/jimmunol.181.12.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miura K, Zhou H, Diouf A, Moretz SE, Fay MP, Miller LH, et al. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin Vac Immunol. 2009;16:963–8. doi: 10.1128/CVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, Double-Blind, Phase 2a Trial of Falciparum Malaria Vaccines RTS,S/AS01B and RTS,S/AS02A in Malaria-Naive Adults: Safety, Efficacy, and Immunologic Associates of Protection. J Infect Dis. 2009;200:337–46. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- [24].Stoute JA, Heppner DG, Jr., Mason CJ, Siangla J, Opollo MO, Kester KE, et al. Phase 1 safety and immunogenicity trial of malaria vaccine RTS,S/AS02A in adults in a hyperendemic region of western Kenya. Am J Trop Med Hyg. 2006;75:166–70. [PubMed] [Google Scholar]
- [25].Bojang K, Milligan P, Pinder M, Doherty T, Leach A, Ofori-Anyinam O, et al. Five year safety and immunogenicity of GlaxoSmithKline's candidate malaria vaccine RTS,S/AS02 following administration to semi-immune adult men living in a malaria-endemic region of The Gambia. Hum Vaccin. 2009;5:242–7. doi: 10.4161/hv.5.4.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS ONE. 2009;4:e6465. doi: 10.1371/journal.pone.0006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- [28].Macete EV, Sacarlal J, Aponte JJ, Leach A, Navia MM, Milman J, et al. Evaluation of two formulations of adjuvanted RTS, S malaria vaccine in children aged 3 to 5 years living in a malaria-endemic region of Mozambique: a Phase I/IIb randomized double-blind bridging trial. Trials. 2007;8:11. doi: 10.1186/1745-6215-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–35. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- [30].Moorthy VS, Imoukhuede EB, Milligan P, Bojang K, Keating S, Kaye P, et al. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 2004;1:e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bejon P, Mwacharo J, Kai O, Mwangi T, Milligan P, Todryk S, et al. A Phase 2b Randomised Trial of the Candidate Malaria Vaccines FP9 ME-TRAP and MVA ME-TRAP among Children in Kenya. PLoS Clin Trials. 2006;1:e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin g subclass switching in malaria. Infect Immun. 2006;74:257–64. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nebie I, Diarra A, Ouedraogo A, Soulama I, Bougouma EC, Tiono AB, et al. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun. 2008;76:759–66. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Crompton PD, Miura K, Traore B, Kayentao K, Ongoiba A, Weiss G, et al. In vitro growth-inhibitory activity and malaria risk in a cohort study in mali. Infect Immun. 2010;78:737–45. doi: 10.1128/IAI.00960-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dent AE, Bergmann-Leitner ES, Wilson DW, Tisch DJ, Kimmel R, Vulule J, et al. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS ONE. 2008;3:e3557. doi: 10.1371/journal.pone.0003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- [38].Perraut R, Marrama L, Diouf B, Sokhna C, Tall A, Nabeth P, et al. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J Infect Dis. 2005;191:264–71. doi: 10.1086/426398. [DOI] [PubMed] [Google Scholar]
- [39].Corran PH, O'Donnell RA, Todd J, Uthaipibull C, Holder AA, Crabb BS, et al. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infect Immun. 2004;72:6185–9. doi: 10.1128/IAI.72.10.6185-6189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McCallum FJ, Persson KE, Mugyenyi CK, Fowkes FJ, Simpson JA, Richards JS, et al. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS ONE. 2008;3:e3571. doi: 10.1371/journal.pone.0003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dutta S, Sullivan JS, Grady KK, Haynes JD, Komisar J, Batchelor AH, et al. High antibody titer against apical membrane antigen-1 is required to protect against malaria in the Aotus model. PLoS ONE. 2009;4:e8138. doi: 10.1371/journal.pone.0008138. [DOI] [PMC free article] [PubMed] [Google Scholar]