Abstract
Functional noradrenergic transmission requires the coordinate expression of enzymes involved in norepinephrine (NE) synthesis, as well as the norepinephrine transporter (NET) which removes NE from the synapse. Inflammatory cytokines acting through gp130 can suppress the noradrenergic phenotype in sympathetic neurons. This occurs in a subset of sympathetic neurons during development and also occurs in adult neurons after injury. For example, cytokines suppress noradrenergic function in sympathetic neurons after axotomy and during heart failure. The molecular basis for suppression of noradrenergic genes is not well understood, but previous studies implicated a reduction of Phox2a in cytokine suppression of dopamine beta hydroxylase. We used sympathetic neurons and neuroblastoma cells to investigate the role of Phox2a in cytokine suppression of NET transcription. Chromatin immunoprecipitation experiments revealed that Phox2a did not bind the NET promoter, and overexpression of Phox2a did not prevent cytokine suppression of NET transcription. Hand2 and Gata3 are transcription factors that induce noradrenergic genes during development and are present in mature sympathetic neurons. Both Hand2 and Gata3 were decreased by cytokines in sympathetic neurons and neuroblastoma cells. Overexpression of either Hand2 or Gata3 was sufficient to rescue NET transcription following suppression by cytokines. We examined expression of these genes following axotomy to determine if their expression was altered following nerve injury. NET and Hand2 mRNAs decreased significantly in sympathetic neurons 48 hours after axotomy, but Gata3 mRNA was unchanged. These data suggest that cytokines can inhibit NET expression through downregulation of Hand2 or Gata3 in cultured sympathetic neurons, but axotomy in adult animals selectively suppresses Hand2 expression.
Keywords: Sympathetic neurons, Cytokines, Axotomy, Phox2a, Hand2, Gata3
1. Introduction
Functional noradrenergic transmission requires the synthesis, secretion, and reuptake of the neurotransmitter norepinephrine (NE). Dopamine-β-hydroxylase (DBH), the final enzyme in the NE biosynthetic pathway, and the NE transporter (NET), which terminates NE neurotransmission by reuptake of NE from the synaptic cleft, are unique to noradrenergic neurons. Their expression is coordinately regulated during development by several transcription factors including Insm1, Gata3, Hand2, Phox2a, and Phox2b. Regulation of noradrenergic properties in mature neurons is less clearly understood, but Hand2 is required for maintenance of noradrenergic genes.
Although the majority of sympathetic neurons are noradrenergic, there are two well-characterized scenarios when the noradrenergic phenotype is suppressed and replaced by a cholinergic or peptidergic phenotype. First, a small sub-set of sympathetic neurons becomes cholinergic during development, using acetylcholine as their primary neurotransmitter. In addition, adult sympathetic neurons whose axons have been damaged decrease expression of noradrenergic genes and production of NE. This can occur acutely due to axotomy, or it can develop over a longer period of time after damage to the target tissue, such as the switch to a cholinergic phenotype in cardiac sympathetic neurons during the development of heart failure. Sympathetic dysfunction in heart failure includes a loss of NE uptake, which contributes to the cardiac pathology. Suppression of noradrenergic function in both of these cases is caused by inflammatory cytokines that act through the gp130 receptor.
Cytokines suppress noradrenergic genes in cultured sympathetic neurons, providing a model system to study the mechanisms underlying this phenomenon. Cytokines also suppress the homeodomain transcription factor Phox2a in cultured sympathetic neurons. Phox2a disappears from sympathetic neurons that become cholinergic during development, suggesting that it might be involved in maintenance of the noradrenergic phenotype. Two other transcription factors that have been linked to maintenance of noradrenergic identity are Gata3 and Hand2. We have used neuroblastoma cell lines and sympathetic neurons to investigate which transcription factors mediate cytokine suppression of noradrenergic genes. We paid special attention to the NET gene and promoter, since very little is known about what regulates this important gene.
2. Materials & Methods
2.1. Materials
SK-N-BE(2)M17 human neuroblastoma cells and pNET4000(i)Luc luciferase promoter construct were generous gifts from Dr. Kwang-Soo Kim (McLean Hospital/Harvard Medical School, Waltham, MA). This construct includes the entire regulatory domain required to drive noradrenergic-cell specific expression of NET. The Hand2 expression construct was a generous gift from Dr. Peter Cserjesi (Tulane University, New Orleans, LA). The 4.5TH-fLuc promoter construct was a generous gift from Dr. Dona Chikaraishi (Duke University Medical Center, Durham, NC), and includes the promoter region sufficient to drive tissue-specific expression of TH. The Arix/Phox2a expression construct was a generous gift from Dr. Elaine Lewis (NIH). The Gata3 expression construct was purchased from Addgene (Cambridge, MA; deposited to Addgene by Gokhan S. Hotamisligil, Harvard University). The pcDNA 3.1 expression vector and cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum was purchased from ATCC (Manassas, VA). CNTF was from PeproTech (Rocky Hills, NJ) and UO126 was from Cell Signaling Technology (Beverly, MA). Nerve growth factor (NGF) was purchased from Austral Biologicals (San Ramon, CA) and from BD Biosciences (Bedford, MA). Dispase was purchased from Boehringer Mannheim (Indianapolis, IN). Collagenase type II was purchased from Worthington Biochemicals (Freehold, NJ). Nitrocellulose membranes were purchased from Schleicher & Schuell (Dassel, Germany). Protease inhibitor cocktail tablets were purchased from Roche Applied Science (Indianapolis, IN). Phosphatase inhibitor cocktails, poly-L-lysine and phenylmethanesulfonyl fluoride (PMSF) were from Sigma Chemical Co. (St. Louis, MO), and Maxi Prep Kits were from Qiagen (Valencia, CA). SuperSignal Dura was purchased from Thermo Scientific (Rockford, IL). 4x sample buffer and 20x reducing agent were from Bio-Rad (Hercules, CA). The pRL-null renilla luciferase construct and the Dual-Luciferase Reporter Assay system were purchased from Promega (Madison, WI). The ChIP-IT Express Enzymatic kits were from Active Motif (Carlsbad, CA).
2.2. Antibodies
Rabbit anti-actin (A2066) was obtained from Sigma Chemical Co. (St. Louis, MO), rabbit anti-tyrosine hydroxylase (AB152) from Millipore (Temecula, CA), rabbit anti-Hand2 (H-110), rabbit anti-c-fos (H-125), goat anti-Phox2b (H-20), and rabbit anti-CREB (C-21) were from Santa Cruz Biotechnologies (Santa Cruz, CA). Rabbit anti-ERK1/2 (#9102) was from Cell Signaling Technologies (Danvers, MA). Goat anti-GATA3 (AF2605) was from R&D Systems (Minneapolis, MN). The Phox2a antibody was a generous gift from Dr Jean-Francois Brunet (Ecole Normale Superieure, CNRS, Paris, France). Anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP) and anti-goat secondary conjugated to HRP were from Santa Cruz Biotechnologies (Santa Cruz, CA).
2.3 Animals
Pregnant Sprague-Dawley rats were purchased from Charles River (Cambridge, MA). C57Bl/6 mice were purchased from The Jackson Laboratories West (Sacramento, CA). gp130DBH-Cre/lox mice were generated as previously described. All mice were kept on a 12h:12h-light dark cycle with ad libitum access to food and water.
2.4. Cell Culture
Sympathetic neurons were dissociated from the superior cervical ganglia of newborn rat pups as described. Neurons were plated onto plates coated with poly-L-lysine and mouse type IV collagen. Cultures were maintained at 37°C in 5% CO2. Neurons were cultured in C2 media (DMEM/F12 1:1, BSA 0.5mg/ml, L-glutamate 1.4mM, selenium 30nM, transferrin 10μg/ml, insulin 10μg/ml) supplemented with 100 units/ml penicillin G, 100 μg/ml streptomycin sulfate, 50ng/ml NGF, and 3% fetal bovine serum. Neurons were cultured with the anti-mitotic agent AraC (1μM) for 2 days to deplete non-neuronal cells prior to treatment with CNTF. Half the media was changed every 2 days, including new CNTF.
The human neuroblastoma SK-N-BE(2)M17 cells and SH-SY5Y cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin G, and 100μg/mL streptomycin sulfate and maintained in 5% CO2.
2.5. Chromatin Immunoprecipitation Assays (ChIPs)
For ChIP experiments, CNTF (100ng/ml) and UO126 (20 μM) were used for the indicated times. ChIP assays were performed with the aid of ChIP-IT Express Enzymatic kits. Briefly, for both M17 and SH5Y cells, two 10cm plates of confluent cells were used for each condition. Cells were fixed with a formaldehyde solution for 5 minutes (M17) or 8 minutes (SH5Y). Cells were washed with 1X PBS and fixation is stopped using a Glycine solution for 5 minutes. Cells were scraped, lysed, gently dounced with a homogenizer on ice for 10 strokes to aid in nuclei release. Nuclei were isolated and an enzymatic cocktail was used to shear the chromatin for 15 minutes (M17) or 10 minutes (SH5Y). Sheared chromatin was immunoprecipitated overnight at 4°C with the appropriate antibody (see antibody section) or negative IgG. Immunoprecipitates were isolated using magnetic beads, chromatin was eluted, cross-links were reversed in a thermocycler at 95°C for 15 minutes, and Proteinase K was used to digest the protein. DNA was used in PCR reactions using the following parameters: denaturing at 94° for 3 minutes followed by 36 cycles of 94°C for 20 seconds, 59°C for 30 seconds, 72°C for 30 seconds. The following primer sets were used: DBH Forward-CCGCCTGTCTACTTCAACTCC, DBH Reverse- TGTACATGAAGGCTGCCTCC, TH Forward- CACACGGCCTGGAATCTTCTGG, TH Reverse- TCAAGAGAGCACACAGGGAGGG, NET Forward- ATCAGAGGCCTGGTGTCCTTGG, NET Reverse- TGGAGCCTGTTAGCGCTAATGG. Predicted amplicon sizes are: DBH- 283 base pairs, TH- 171 base pairs, NET- 161 base pairs.
2.6. Real-time PCR
Real-time PCR was performed as described previously. Cultured neurons were harvested using the Cells-to-cDNA II kit from Ambion/ABI (Foster City, CA) and reverse transcribed. RNA was isolated from individual ganglia using the Ambion RNAqueous micro kit. For PCR amplification, 4μl of reverse transcriptase reactions were used in a total volume of 20μl. Each sample was assayed in duplicate. Real-time PCR was performed using the 7500 Real-Time PCR System from ABI, and fluorescence measurements were taken from the exponential phase of amplification for all standards and samples. TaqMan® Universal PCR Master Mix was used with pre-validated TaqMan primers to GAPDH, TH, NET, Hand2, GATA3, and Phox2a from ABI. Standard curves were generated for each primer set from known amounts of sympathetic neuron RNA (100ng-0.8ng), and each gene of interest was normalized to GAPDH mRNA from the same sample.
2.7. Transient Transfection and Reporter Assays
DNA used for transfection was purified using the Qiagen Maxiprep kit. M17 cells were plated at a density of 1.0 × 105 cells per well in 6-well plates and immediately treated with 100ng/mL CNTF. 48 hours after plating, media were removed and cells were transfected using the CaPO4 method. Cells were transfected with 1μg of pNET4000(i)Luc or 1μg of 4.5TH-fLuc and 1μg of Hand2 expression construct, 1μg Arix/Phox2a expression construct, 1μg Gata3 expression construct, or 1μg empty pcDNA3 vector to bring the total amount of DNA to 2μg. 100ng pRL-null was used as a control for transfection efficiency. After a 4 hour incubation with DNA, cells were shocked with 15% glycerol/PBS, washed with PBS, and placed back into culture media containing CNTF. Firefly luciferase activity from promoter luciferase constructs and Renilla luciferase activity from the pRL-null internal control were assayed 48 hours after transfection using the Dual-Luciferase Reporter Assay system. Firefly luciferase values were normalized to Renilla luciferase values.
For inhibitor experiments, M17 cells were plated at a density of 1.0 × 105 cells per well in 6-well plates and allowed to adhere overnight before being treated with 100ng/mL CNTF and 10μM UO126. Transfections were performed 48 hours later as described above and cells were placed back in culture media containing CNTF and UO126. Cells were lysed 48 hours after transfection in RIPA buffer (50mM Tris, 150mM NaCl, 1mM EDTA, 5μg/mL aprotinin, 5μg/mL leupeptin, 1% Triton x-100, 1% sodium deoxycholate, 0.1% SDS, pH 7.4) with phosphatase inhibitor cocktail I (1:1000), phosphatase inhibitor cocktail II (1:1000), phenylmethanesulfonyl fluoride (1:1000), and 25x protease inhibitor cocktail to a total volume of 200μL.
2.8. Western Blot Analysis
SK-N-BE(2)M17 cells- Transfections and cell lysis were performed as described previously. Samples were sonicated for 5 seconds on ice to reduce viscosity. Samples were diluted in sample buffer (4x sample buffer and 20x reducing agent) to a total volume of 60μL before heating at 95°C for 10 minutes and separating on 4–12% Bis-Tris acrylamide gels. Membranes were blocked in 5% nonfat dry milk in TBST (100mM NaCl, 10mM Tris, and 0.1% Tween 20, pH 7.4) and incubated at 4°C overnight in antibodies against β-Actin, Hand2, or Gata3 diluted 1:1000 in TBST with 5% dry milk. After washing in TBST, membranes were incubated for 1 hour at room temperature with appropriate secondary antibody diluted 1:10,000 in TBST with 5% dry milk. Membranes were washed again in TBST and immunoreactive bands were visualized by chemiluminescence. Band intensity was recorded using a −40°C charge-coupled device camera and quantified using Labworks software (UVP, Upland, CA). Band intensities obtained for Hand2 and Gata3 were normalized to β-Actin to control for differences in cell number between the wells.
Sympathetic neurons- Neurons were placed on ice and lysed in RIPA buffer (1% Triton-X 100, 1% sodium deoxycholate, 0.2% sodium dodecyl sulfate, 125mM NaCl, 50mM Tris pH 8.0, 10% glycerol, 1mM EDTA, 25mM β-glycerolphosphate, 25mM NaF, 1mM sodium orthovanadate, 10μg/ml aprotinin, 10μg/ml leupeptin, 1mM phenylmethylsulfonyl fluoride, 1x complete protease inhibitor cocktail-EDTA free). Samples were sonicated for 30 seconds in a bath sonicator. Proteins were size fractionated on denaturing gels and transferred to nitrocellulose membranes for blotting. Membranes were blocked in 5% nonfat dry milk in TBST and incubated at 4°C overnight in antibodies against TH, ERK1/2, β-Actin, Phox2a, and Phox2b diluted 1:1000 in TBST with 5% dry milk. After washing in TBST, membranes were incubated for 1 hour at room temperature with appropriate secondary antibody diluted 1:10,000 in TBST with 5% dry milk. Membranes were washed again in TBST and immunoreactive bands were visualized by chemiluminescence. Bands were captured by a UVP camera and analyzed using Labworks Software.
2.9. Axotomy
Six week old mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and neurons in one SCG were axotomized by unilateral transection of the major postganglionic nerves of the ganglion, the external and internal carotid nerves. Surgical procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Forty eight hours after surgery both ganglia were removed into RNAlater and mRNA levels were measured as described above.
2.10. Statistics
Statistical analyses were carried out using GraphPad Prism 5.0. Student’s t-test was used for a single comparison between 2 groups. One way analysis of variance with the Dunnett post hoc test used to compare multiple treatments to the control. One way analysis of variance with a Newman-Keuls post hoc test was used to compare multiple groups to one another.
3. Results
3.1. CNTF suppresses noradrenergic genes and Phox2a
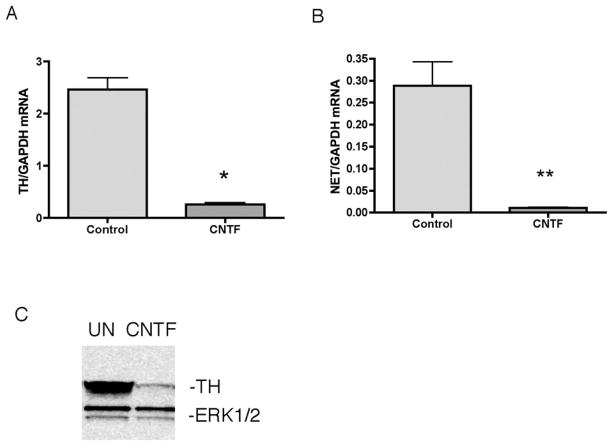
Gp130 cytokines, such as ciliary neurotrophic factor (CNTF), suppress noradrenergic genes in sympathetic neurons. In order to investigate the mechanisms of this suppression we first confirmed that CNTF decreased TH and NET mRNA in sympathetic neurons (Fig 1AB). Western blot analysis revealed significantly reduced TH protein levels 3 days after CNTF treatment, consistent with previous studies (Fig 1C).
Fig 1.
Cytokines suppress noradrenergic genes. (A) Sympathetic neurons were treated for 6 days with CNTF. Real-time PCR analysis of TH (A) and NET (B) mRNA normalized to GAPDH. (C) A representative western blot showing TH (top panel) and ERK1/2 (bottom panel). Data are mean values ± SEM of triplicate values. Each experiment was repeated at least 3 times. *p<0.05. **p<0.01.
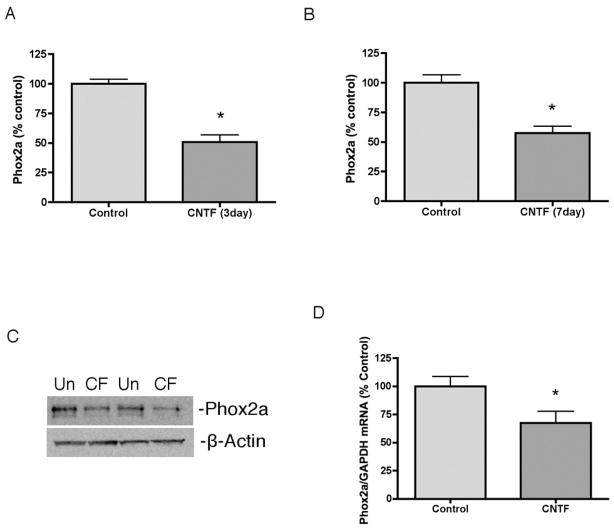
Phox2a has been implicated in regulation of noradrenergic genes during development. Phox2a expression is suppressed in sympathetic neurons that acquire a cholinergic phenotype, and the timing is consistent with a role for Phox2a in regulating the loss of noradrenergic gene expression. Cytokines suppress Phox2a in cultured sympathetic neurons and we confirmed that 3 and 7 days of CNTF treatment decreased Phox2a levels compared to untreated controls (Fig 2A–C). Phox2a mRNA was also significantly reduced following CNTF treatment (Fig 2D).
Fig 2.
Cytokines suppress expression of Phox2a. (A,B) Sympathetic neurons were treated with vehicle (control) or CNTF for 3 (A) or 7 (B) days. Phox2a was quantified by western blot. (C) A representative western blot of sympathetic neurons treated with vehicle (Un) or CNTF (CF) for 7 days. Two samples from each condition are shown, each blotted for Phox2a (top panel) and β-Actin (bottom panel). (D) Phox2a mRNA normalized to GAPDH mRNA in sympathetic neurons treated with vehicle or CNTF for 6 days. Data are mean values ± SEM (n=3). *p<0.05.
3.2. Phox2a does not bind the NET promoter
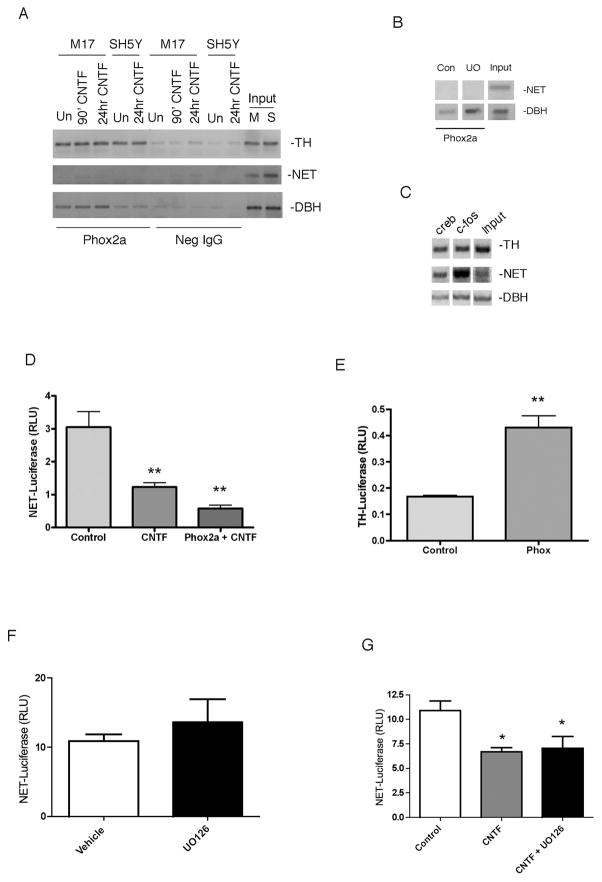
Using chromatin immunoprecipitation (ChIP), we tested whether Phox2a was bound to promoters of noradrenergic genes. We tested two neuroblastoma cell lines that express noradrenergic genes, and Phox2a bound the DBH and TH promoters in both M17 and SH5Y cells. Short-term (90 min and 24 hr) CNTF stimulation, which activates ERK1/2, , did not alter this interaction (Fig 3A). Phox2a exhibited no binding to the NET promoter in either cell line under any conditions. The immunoprecipitation pulled down more Phox2a bound to the DBH promoter in the M17 cell line than in the SH5Y line, but no differences between cell type were observed with the TH promoter. Inputs for all primer sets were positive for both cell lines (Fig 3A). Phox2a binding to the DBH promoter is stimulated by inhibition of the ERK1/2 pathway. We tested whether pharmacological inhibition of ERK1/2 might promote Phox2a binding to the NET promoter in a fashion comparable to DBH. As expected, we observed an increase in binding of Phox2a to the DBH promoter with the MEK inhibitor UO126 compared to vehicle treated cells (Fig 3B). However, inhibition of ERK1/2 activation did not promote binding of Phox2a to the NET promoter. Both CREB and c-fos bound all 3 promoters (Fig 3C). Although there is a Phox2a/b binding site in the NET promoter, we could not detect any Phox2a bound to the promoter, in contrast to robust Phox2a binding to the TH and DBH promoters.
Fig 3.
Phox2a does not regulate transcription of the NET gene. (A) M17 and SH5Y cells were untreated or treated with CNTF and ChIP analysis was performed with a Phox2a antibody or a negative IgG. PCR reactions were done with primers specific for TH, NET, and DBH. Positive signals from input DNA are shown from both M17 cells (M) and SH5Y cells (S). (B) ChIP analysis was performed with M17 cells that were treated with DMSO (Con) or UO126 (UO) for 90 minutes and PCR reactions were done with primers specific for NET and DBH. (C) M17 cells were subjected to ChIP analysis using antibodies against CREB or c-fos as a positive control. Positive signals from input DNA are shown. PCR reactions were performed with primers specific for TH, NET and DBH, and all three promoters were pulled down with CREB and c-fos antibodies. (D) Cells were treated with control medium (Control) or CNTF (CNTF) for 2 days prior to transfection with luciferase reporter constructs (NET-Luc+pRLNull) and a control vector or Phox2a (Phox2a + CNTF). Cells were maintained for an additional two days in vehicle or CNTF prior to analysis of luciferase. Data are a ratio of firefly luciferase/renilla luciferase. There was no significant difference between CNTF treated cells and CNTF+Phox2a. (E) Cells were treated with vehicle for 2 days prior to transfection with luciferase reporter constructs (TH-fLuc+pRLNull) and a control vector or Phox2a (Phox2a). Cells were maintained for an additional two days prior to analysis of luciferase. Data are a ratio of firefly luciferase/renilla luciferase. (F) Cells were treated with DMSO (Vehicle) or UO126 for 2 days prior to transfection with NET-Luc and pRL-Null. Cells were maintained in vehicle or UO126 for two more days prior to analysis of luciferase. Data is shown as a ratio of firefly luciferase/renilla luciferase. (G) Cells were treated with DMSO (all conditions), CNTF, or CNTF + UO126 for 2 days prior to transfection and then maintained in drug treatments for two days after transfection. Data are a ratio of firefly luciferase/renilla luciferase. Data are mean values ± SEM of triplicate values. Each experiment was repeated at least 3 times. *p<0.05, **p<0.01 vs control.
Although we did not observe Phox2a binding to the NET promoter using ChIP, Phox2a may still regulate NET transcription indirectly via protein-protein interactions with other transcription factors. We used a luciferase reporter assay in M17 cells to test the role of Phox2a in cytokine regulation of NET transcription. The NET-Luciferase reporter construct used included the 4Kb of upstream sequence required to drive noradrenergic-cell specific transcription of NET during development. CNTF treatment decreased NET-Luciferase transcription significantly. Addition of Phox2a did not alter NET transcription significantly (Fig 3D), although there was a trend toward decreased (not increased) transcription. The same Phox2a construct stimulated a significant increase in TH transcription, confirming expression and activity of Phox2a (Fig 3E). Blockade of ERK1/2 activation increased Phox2a-dependent basal transcription of the DBH promoter, but had no effect on basal transcription of NET-Luciferase (Fig 3F). In addition, inhibiting ERK1/2 activation did not prevent CNTF-dependent decrease in NET transcription (Fig 3G). Thus, it appears that Phox2a does not regulate the NET promoter.
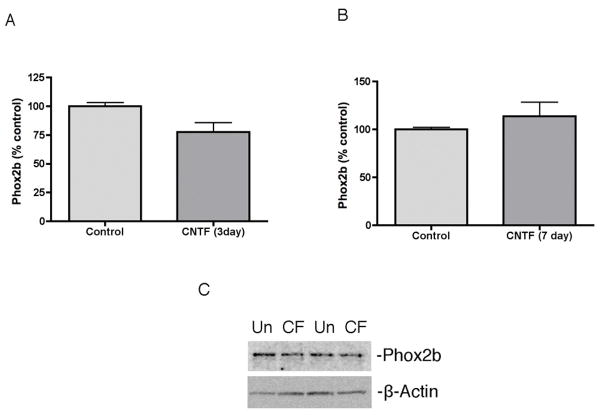
3.3. CNTF does not alter expression of Phox2b
Since our results suggest Phox2a does not mediate cytokine suppression of NET, we analyzed other candidate transcription factors. Phox2b has an identical DNA binding domain and can compensate for the absence of Phox2a during sympathetic neuron development to stimulate expression of noradrenergic genes. We analyzed Phox2b protein levels after CNTF treatment and saw no significant change in Phox2b in cultured sympathetic neurons (Fig 4).
Fig 4.
Cytokines do not suppress Phox2b. (A, B) Phox2b protein was quantified in control and CNTF treated sympathetic neurons. (C) A representative western blot showing Phox2b (top panel) and β-Actin (bottom panel) in untreated (Un) and treated with CNTF (CF) for 3 days. Data are mean values ± SEM (n=3).
3.4. Hand2 and Gata3 are regulated by CNTF in cultured neurons and rescue NET transcription
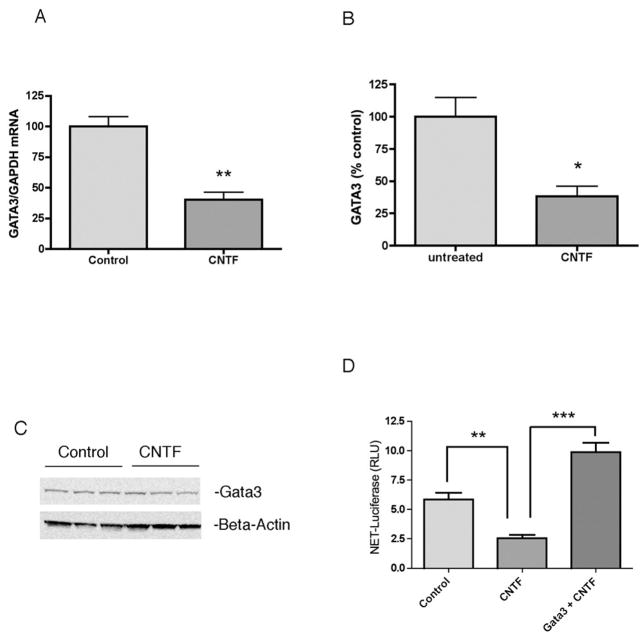
Hand2 and Gata3 are involved in the development of the noradrenergic phenotype. To determine if these transcription factors were regulated by cytokines, we treated dissociated sympathetic neurons with CNTF for 6 days and analyzed mRNA levels using RT-PCR. Both Gata3 (Fig. 5) and Hand2 (Fig. 6) mRNA were significantly reduced by CNTF treatment. Due to a lack of good antibodies for rodent Gata3 and Hand2, we analyzed protein levels of Hand2 and Gata3 in the M17 human neuroblastoma cells. After 4 days of CNTF, there was significantly less Gata3 (Fig. 5) and Hand2 (Fig. 6) protein when compared to untreated control cells. Using a luciferase reporter assay, we tested whether the CNTF-stimulated reduction of NET transcription could be rescued by overexpression of either Hand2 or Gata3. Both Gata3 (Fig 5D) and Hand2 (Fig 6D) rescued NET luciferase expression in cytokine-treated M17 cells. These data support a role for both Hand2 and Gata3, and not Phox2a/2b, in cytokine suppression of NET expression.
Fig 5.
Gata3 is suppressed by CNTF and rescues NET transcription. CNTF suppresses Gata3 mRNA in sympathetic neurons (A) and decreases Gata3 protein (B) in M17 cells. Gata3 levels were normalized to β-Actin levels and shown as a percent of control (mean values ± SEM, n=3). (C) A representative western blot showing Gata3 (top panel) and β-Actin (bottom) protein levels. (D) M17 cells were treated for 2 days with vehicle (Control) or CNTF. Cells were then transfected with NET-Luc+pRL-Null to monitor transcription, together with a control vector or Gata3 expression plasmid (Gata3 + CNTF) and maintained for two more days in CNTF or control medium. Data are a ratio of firefly luciferase/renilla luciferase. Data are mean values ± SEM of triplicate values. Each experiment was repeated at least 3 times. *p<0.05, **p<0.01, ***p<0.001.
Fig 6.
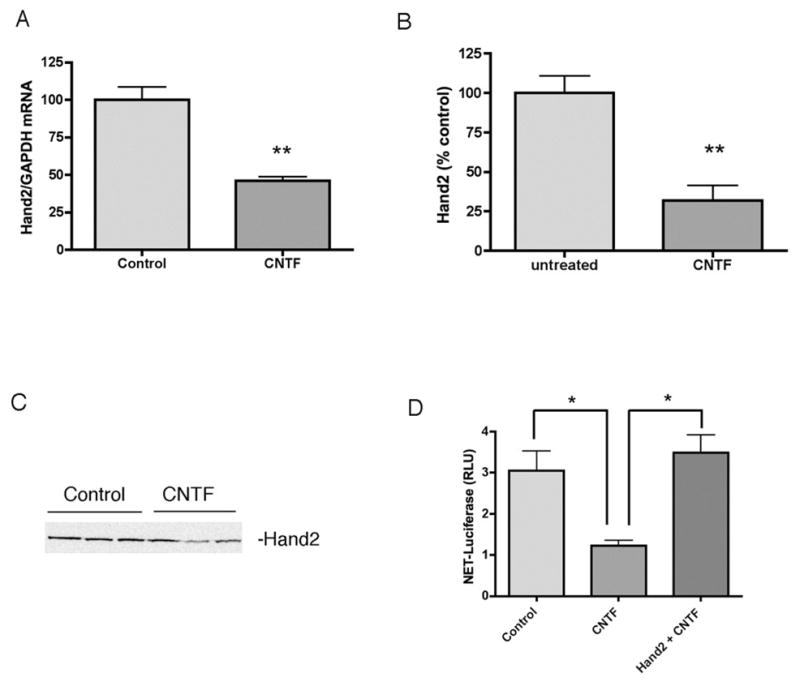
Hand2 is suppressed by CNTF and rescues NET transcription. CNTF suppresses Hand2 mRNA in sympathetic neurons (A) and decreases Hand2 protein (B) in M17 cells. Hand2 levels were quantified in the same cells analyzed for Gata3, and Hand2 protein was normalized to the β-Actin shown in Fig 5C. Data shown are percent of control (mean values ± SEM, n=3). (C) A representative western blot showing Hand2 (top panel). (D) M17 cells were treated for 2 days with vehicle (Control) or CNTF. Cells were then transfected with NET-Luc+pRL-Null to monitor transcription, together with a control vector or Hand2 expression plasmid (Hand2 + CNTF) and maintained for two more days in CNTF or control medium. Data are a ratio of firefly luciferase/renilla luciferase. Data are mean values ± SEM of triplicate values. Each experiment was repeated at least 3 times. *p<0.05, **p<0.01.
3.5 Axotomy downregulates expression of Hand2 but not Gata3
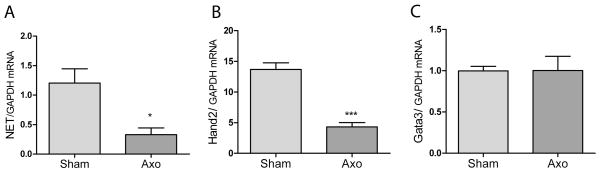
Culture studies suggested that cytokines suppress NET transcription through suppression of Gata3 and/or Hand2. To examine these transcription factors in vivo we carried out axotomy experiments with the SCG. Axon transection leads to the loss of TH expression and NE synthesis in sympathetic neurons due in large part to the actions of inflammatory cytokines. We asked if NET expression, which had not previously been examined in this injury paradigm, was decreased after axotomy. We found that 48 hours after injury NET mRNA levels were less than a third of sham control levels (Fig. 7). If cytokine suppression of Hand2 or Gata3 played a role in the loss of NET transcription, then their expression should also be decreased significantly. Hand2 mRNA levels were approximately 30% of sham control 48 hours after axotomy, but Gata3 levels were unchanged (Fig. 7). This suggests that Hand2, but not Gata3, plays a role in decreasing noradrenergic genes following axotomy.
Fig. 7.
Axotomy regulates NET and Hand2, but not Gata3. NET (A), Hand2 (B), and Gata3 (C) mRNAs were quantified in SCG 48 hrs after axotomy. The contralateral SCG from the same animal served as a control. NET and Hand2 mRNA were decreased significantly after axotomy, but Gata3 mRNA was unchanged. Data are mean values ± SEM (n=4), *p<0.05, ***p<0.001.
4. Discussion
4.1. Phox2a/2b
Phox2a was once proposed to be a master regulator of the noradrenergic phenotype because it was expressed in all neurons that express DBH. Further evidence strengthening this hypothesis showed DBH expression was dependent on Phox2a. However, Phox2b has an identical DNA-binding domain and can also transactivate noradrenergic genes in vitro. Several studies carried out with the proximal DBH promoter implicated Phox2a and/or Phox2b in regulating DBH transcription. However, generation of mice lacking Phox2a or Phox2b, and more recent reciprocal replacement experiments, revealed that Phox2b plays the more important role in sympathetic neuron differentiation and expression of noradrenergic genes. The differentiation of neural crest cells into noradrenergic sympathetic neurons is complex and requires a network of transcription factors including Sox 4&11, Insm1, MASH1/Ascl1, Phox2a/b, Hand2, and Gata3.
We looked at Phox2a initially because expression of Phox2a is suppressed in vivo when noradrenergic sympathetic neurons are converted to a cholinergic phenotype. Decreased transcription of the DBH gene is tied to the loss of a transcriptional activator rather than the induction of a suppressor, and adding back Phox2a restores DBH expression in cytokine treated cells, and Phox2a binds to the DBH promoter. However, we did not detect Phox2a binding to the NET promoter, and Phox2a did not rescue cytokine suppression of NET transcription (Fig 3D). Furthermore, Phox2b levels were unchanged in cytokine-treated sympathetic neurons, suggesting the lack of Phox2 DNA binding cannot explain the loss of NET transcription. There is only one homeodomain binding site in the NET promoter, in contrast to multiple sites in the TH and DBH promoters, so we expected a weak signal at best with Phox2a binding. However, we did not detect Phox2a bound to the NET promoter even after inhibition of ERK1/2 activity, which increases Phox2a binding to the DBH promoter. We did detect CREB and c-fos binding to the NET promoter, confirming that the ChIP assay and the primers for the NET promoter were working.
4.2. Hand2
Hand2 is critical for development of the heart, the enteric nervous system, and sympathetic ganglia, in addition to its role as a key transcription factor for noradrenergic specific genes like DBH and TH. More recent studies have implicated Hand2 in the acquisition and maintenance of the noradrenergic phenotype. Our data are consistent with this model and suggest that cytokines suppress noradrenergic function, and NET in particular, through downregulation of Hand2. ChIP analysis shows that Hand2 binds the NET promoter, and our luciferase data indicate that Hand2 stimulates NET transcription. Cytokines decrease Hand2 mRNA in sympathetic neurons and Hand2 protein in M17 cells, and Hand2 overexpression was able to rescue cytokine-suppressed NET transcription. Importantly, in vivo studies revealed the coordinate loss of Hand2 and NET mRNAs in sympathetic ganglia after axotomy, while Gata3 was unchanged. This is the first evidence in an adult nerve injury model that Hand2 is suppressed and might play a role in the downregulation of noradrenergic genes.
Hand2 may interact directly with the NET promoter since the hNET sequence contains E- boxes, which are Hand2 binding sites. However, indirect interactions may also be involved since Hand2 can increase DBH transcription without directly binding DNA. For example, cAMP stimulates NET transcription. Our data show CREB binds the NET promoter, but Hand2 can also drive CRE-dependent transcription through interactions with CREB binding protein (CBP). The mechanisms underlying Hand2-dependent NET transcription will need further study and might involve interactions with multiple transcription factors.
4.3. Gata3
Gata3 plays a role in development of the ear, heart, T cells, and sympathoadrenal cell types. Gata3 is also required for the survival of developing sympathetic neurons and adult sympathetic neurons, but it is difficult to separate its role in survival from a role in differentiation. Our data indicate that Gata3 is still present in fully differentiated neonatal neurons, and is suppressed by treatment with cytokines. Restoring Gata3 by transfection was sufficient to rescue transcription of a NET promoter construct. This suggests Gata3 affects differentiation without affecting cell survival. It is unclear at this point if Gata3 binds directly to the NET promoter, or if it interacts with other DNA binding proteins. At least six putative Gata3 binding sites [WGATAR W=A/T, R=A/G] are present in 5Kb of the human NET promoter sequence (Genbank AF061198.1), but Gata3 can also interact with other transcription factors to regulate noradrenergic genes.
During development Hand2 and Gata3 are part of a network of transcription factors that can regulate each other in addition to having direct effects on noradrenergic genes. Since over-expression of Gata3 and Hand2 both rescued NET transcription, but only Hand2 was downregulated in vivo following axotomy, we wondered if Gata3 was upstream of Hand2 in this context. We were unable to detect an increase in Hand2 protein after overexpression of Gata3 in neuroblastoma cells. However, we cannot conclude that Gata3 did not stimulate Hand2 expression because we were unable to control for transfection efficiency with the western blot procedure.
4.4. Concluding remarks
The differentiation of sympathetic neurons during development requires the coordinate expression of specific transcription factors to regulate noradrenergic genes. We asked if any of these transcription factors are involved in the suppression of these noradrenergic genes by gp130 cytokines. We focused on NET since little is known about regulation of NET and it is one of the genes specific to the noradrenergic phenotype.
Our data identified several differences in transcription factor binding and stimulation of the NET promoter compared to the synthetic enzymes TH and DBH. Although we have examined NET transcription indirectly through NET-luciferase, the 4Kb promoter used in our experiments is sufficient to drive noradrenergic-specific expression of NET in vivo, and encompasses a polymorphism site linked to attention-deficit hyperactivity disorder. Therefore, we believe that regulation of this construct accurately reflects regulation of the NET gene in vivo. Similarly, the TH-luciferase construct used here contains the full promoter region necessary for tissue-specific expression. Inflammatory cytokines acting through gp130 decreased Hand2, Gata3, and Phox2a in sympathetic neurons, but NET transcription was only regulated by Hand2 and Gata3. Our new data in an adult injury model, together with recent studies by Schmidt and co-workers, implicate Hand2 as a key injury-regulated transcription factor that may control the suppression of noradrenergic function. An interesting new study has identified Satb2 as a cytokine-induced transcription factor that stimulates cholinergic function and suppresses NET expression in sympathetic neurons. It’s not known if Satb2 is increased in sympathetic neurons after axotomy. If Satb2 is elevated after injury in vivo, it might act together with decreased expression of Hand2 to suppress NET transcription. However, our data indicate that restoring Hand2 is sufficient to bring NET transcription back to control levels. Understanding the factors that regulate NET expression after injury is important. The loss of NE uptake in the face of increased NE release is a key factor of heart failure, and sympathetic dysfunction occurs in other pathologies that include inflammation, such as metabolic syndrome. Thus, these culture and axotomy studies lay the groundwork for further in vivo studies investigating the regulation of NET and noradrenergic transmission in other pathological conditions.
Acknowledgments
This work was supported by RO1 HL068231 (BAH) and R01 NS17512 (REZ). We would like to thank Dr. Kwang-Soo Kim, Dr. Peter Cserjesi, Dr. Dona Chikaraishi, Dr. Elaine Lewis, and Dr. Gokhan Hotamisligil for reagents.
Abbreviations
- NE
Norepinephrine
- NET
Norepinephrine Transporter
- DBH
Dopamine-β-hydroxylase
- TH
Tyrosine Hydroxylase
- CNTF
Ciliary Neurotrophic Factor
- ChIP
Chromatin Immunoprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Browne D, Lewis EJ. Paired-like homeodomain proteins Phox2a/Arix and Phox2b/NBPhox have similar genetic organization and independently regulate dopamine beta-hydroxylase gene transcription. DNA Cell Biol. 2000;199:539–54. doi: 10.1089/104454900439773. [DOI] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Apostolova G, Dechant G. Development of neurotransmitter phenotypes in sympathetic neurons. Auton Neurosci. 2009;1511:30–8. doi: 10.1016/j.autneu.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Apostolova G, Loy B, Dorn R, Dechant G. The sympathetic neurotransmitter switch depends on the nuclear matrix protein satb2. J Neurosci. 2010;3048:16356–64. doi: 10.1523/JNEUROSCI.3502-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev. 2002;124:435–40. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Buss SJ, Backs J, Kreusser MM, Hardt SE, Maser-Gluth C, Katus HA, Haass M. Spironolactone preserves cardiac norepinephrine reuptake in salt-sensitive Dahl rats. Endocrinology. 2006;1475:2526–34. doi: 10.1210/en.2005-1167. [DOI] [PubMed] [Google Scholar]
- Coppola E, Pattyn A, Guthrie SC, Goridis C, Studer M. Reciprocal gene replacements reveal unique functions for Phox2 genes during neural differentiation. Embo J. 2005;2424:4392–403. doi: 10.1038/sj.emboj.7600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxakis E, Howard L, Rohrer H, Davies AM. HAND transcription factors are required for neonatal sympathetic neuron survival. EMBO Rep. 2008;910:1041–7. doi: 10.1038/embor.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziennis S, Habecker BA. Cytokine suppression of dopamine-beta-hydroxylase by extracellular signal-regulated kinase-dependent and -independent pathways. J Biol Chem. 2003;27818:15897–904. doi: 10.1074/jbc.M212480200. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Habecker BA. Ciliary neurotrophic factor suppresses Phox2a in sympathetic neurons. Neuroreport. 2004;151:33–6. doi: 10.1097/00001756-200401190-00008. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;939:1667–76. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;1021:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Faron-Gorecka A, Rogoz Z, Bruss M, Caron MG, Dziedzicka-Wasylewska M, Bonisch H. Norepinephrine transporter knockout-induced up-regulation of brain alpha2A/C-adrenergic receptors. J Neurochem. 2006;964:1111–20. doi: 10.1111/j.1471-4159.2005.03598.x. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;37:531–41. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Gritman KR, Willison BD, Van Winkle DM. Myocardial infarction stimulates galanin expression in cardiac sympathetic neurons. Neuropeptides. 2005;392:89–95. doi: 10.1016/j.npep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol. 2009;696:392–400. doi: 10.1002/dneu.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E, Patterson PH. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, Firulli AB, Pittman DL, Howard MJ. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev Biol. 2008;3192:179–91. doi: 10.1016/j.ydbio.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Sarkar AA, Giovannucci DR, Clouthier DE, Abe M, Howard MJ. Expression of Hand2 is sufficient for neurogenesis and cell type-specific gene expression in the enteric nervous system. Dev Dyn. 2007;2361:93–105. doi: 10.1002/dvdy.20989. [DOI] [PubMed] [Google Scholar]
- Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;1254:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- Holler KL, Hendershot TJ, Troy SE, Vincentz JW, Firulli AB, Howard MJ. Targeted deletion of Hand2 in cardiac neural crest-derived cells influences cardiac gene expression and outflow tract development. Dev Biol. 2010;3411:291–304. doi: 10.1016/j.ydbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Choi HJ, Hong S, Huh Y, Chae H, Kim KS. Transcription factor GATA-3 regulates the transcriptional activity of dopamine beta-hydroxylase by interacting with Sp1 and AP4. Neurochem Res. 2008;339:1821–31. doi: 10.1007/s11064-008-9639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Huh Y, Chae H, Hong S, Lardaro T, Kim KS. GATA-3 regulates the transcriptional activity of tyrosine hydroxylase by interacting with CREB. J Neurochem. 2006;983:773–81. doi: 10.1111/j.1471-4159.2006.03924.x. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, Engel JD. GATA-3 is required for early T lineage progenitor development. J Exp Med. 2009;20613:2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;2772:271–86. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;12718:4073–81. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- Hsieh MM, Lupas G, Rychlik J, Dziennis S, Habecker BA, Lewis EJ. ERK1/2 is a negative regulator of homeodomain protein Arix/Phox2a. J Neurochem. 2005;946:1719–27. doi: 10.1111/j.1471-4159.2005.03333.x. [DOI] [PubMed] [Google Scholar]
- Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;241:131–41. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi-Ueda H, Ogawa S, Fukuda K. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;1202:408–21. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Hahn MK, Joung Y, Anderson SL, Steele AH, Mazei-Robinson MS, Gizer I, Teicher MH, Cohen BM, Robertson D, Waldman ID, Blakely RD, Kim KS. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci U S A. 2006;10350:19164–9. doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Hwang DY, Park JJ, Kim KS. A proximal promoter domain containing a homeodomain-binding core motif interacts with multiple transcription factors, including HoxA5 and Phox2 proteins, and critically regulates cell type-specific transcription of the human norepinephrine transporter gene. J Neurosci. 2002;227:2579–89. doi: 10.1523/JNEUROSCI.22-07-02579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kim HS, Cubells JF, Kim KS. A previously undescribed intron and extensive 5′ upstream sequence, but not Phox2a-mediated transactivation, are necessary for high level cell type-specific expression of the human norepinephrine transporter gene. J Biol Chem. 1999;27410:6507–18. doi: 10.1074/jbc.274.10.6507. [DOI] [PubMed] [Google Scholar]
- Kim HS, Seo H, Yang C, Brunet JF, Kim KS. Noradrenergic-specific transcription of the dopamine beta-hydroxylase gene requires synergy of multiple cis-acting elements including at least two Phox2a-binding sites. J Neurosci. 1998;1820:8247–60. doi: 10.1523/JNEUROSCI.18-20-08247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Yang C, Kim KS. The cell-specific silencer region of the human dopamine beta-hydroxylase gene contains several negative regulatory elements. J Neurochem. 1998;711:41–50. doi: 10.1046/j.1471-4159.1998.71010041.x. [DOI] [PubMed] [Google Scholar]
- Kreusser MM, Buss SJ, Krebs J, Kinscherf R, Metz J, Katus HA, Haass M, Backs J. Differential expression of cardiac neurotrophic factors and sympathetic nerve ending abnormalities within the failing heart. J Mol Cell Cardiol. 2008;442:380–7. doi: 10.1016/j.yjmcc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Landis SC. Target regulation of neurotransmitter phenotype. Trends Neurosci. 1990;138:344–50. doi: 10.1016/0166-2236(90)90147-3. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;863:774–83. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- Lillevali K, Haugas M, Matilainen T, Pussinen C, Karis A, Salminen M. Gata3 is required for early morphogenesis and Fgf10 expression during otic development. Mech Dev. 2006;1236:415–29. doi: 10.1016/j.mod.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Lillevali K, Matilainen T, Karis A, Salminen M. Partially overlapping expression of Gata2 and Gata3 during inner ear development. Dev Dyn. 2004;2314:775–81. doi: 10.1002/dvdy.20185. [DOI] [PubMed] [Google Scholar]
- Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;252:209–12. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- Lo L, Morin X, Brunet JF, Anderson DJ. Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron. 1999;224:693–705. doi: 10.1016/s0896-6273(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;1254:609–20. doi: 10.1242/dev.125.4.609. [DOI] [PubMed] [Google Scholar]
- Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;13320:4015–24. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Maves L, Tyler A, Moens CB, Tapscott SJ. Pbx acts with Hand2 in early myocardial differentiation. Dev Biol. 2009;3332:409–18. doi: 10.1016/j.ydbio.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Wiedemann P, Okladnova O, Bruss M, Staab T, Stober G, Riederer P, Bonisch H, Lesch KP. Cloning and functional characterization of the human norepinephrine transporter gene promoter. J Neural Transm. 1998;10510–12:1341–50. doi: 10.1007/s007020050136. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim KC, Engel JD. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;13319:3871–81. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;3071:114–26. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;183:411–23. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;12420:4065–75. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;3996734:366–70. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, Rosenthal A, Taga T, Paoni NF, Wood WI. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;27018:10915–22. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- Potzner MR, Tsarovina K, Binder E, Penzo-Mendez A, Lefebvre V, Rohrer H, Wegner M, Sock E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;1375:775–84. doi: 10.1242/dev.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raid R, Krinka D, Bakhoff L, Abdelwahid E, Jokinen E, Karner M, Malva M, Meier R, Pelliniemi LJ, Ploom M, Sizarov A, Pooga M, Karis A. Lack of Gata3 results in conotruncal heart anomalies in mouse. Mech Dev. 2009;1261–2:80–9. doi: 10.1016/j.mod.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Rao MS, Landis SC. Characterization of a target-derived neuronal cholinergic differentiation factor. Neuron. 1990;56:899–910. doi: 10.1016/0896-6273(90)90350-o. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;116:1175–85. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- Rychlik JL, Gerbasi V, Lewis EJ. The interaction between dHAND and Arix at the dopamine beta-hydroxylase promoter region is independent of direct dHAND binding to DNA. J Biol Chem. 2003;27849:49652–60. doi: 10.1074/jbc.M308577200. [DOI] [PubMed] [Google Scholar]
- Rychlik JL, Hsieh M, Eiden LE, Lewis EJ. Phox2 and dHAND transcription factors select shared and unique target genes in the noradrenergic cell type. J Mol Neurosci. 2005;273:281–92. doi: 10.1385/JMN:27:3:281. [DOI] [PubMed] [Google Scholar]
- Saadat S, Sendtner M, Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J Cell Biol. 1989;1085:1807–16. doi: 10.1083/jcb.108.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AA, Howard MJ. Perspectives on integration of cell extrinsic and cell intrinsic pathways of signaling required for differentiation of noradrenergic sympathetic ganglion neurons. Auto Neurosci. 2006;126–127:225–231. doi: 10.1016/j.autneu.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Schimmel JJ, Crews L, Roffler-Tarlov S, Chikaraishi DM. 4.5 kb of the rat tyrosine hydroxylase 5′ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Brain Res Mol Brain Res. 1999;741–2:1–14. doi: 10.1016/s0169-328x(99)00234-x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Lin S, Pape M, Ernsberger U, Stanke M, Kobayashi K, Howard MJ, Rohrer H. The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Dev Biol. 2009;3292:191–200. doi: 10.1016/j.ydbio.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotzinger RJ, Landis SC. Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature. 1988;3356191:637–9. doi: 10.1038/335637a0. [DOI] [PubMed] [Google Scholar]
- Schotzinger RJ, Landis SC. Acquisition of cholinergic and peptidergic properties by sympathetic innervation of rat sweat glands requires interaction with normal target. Neuron. 1990;51:91–100. doi: 10.1016/0896-6273(90)90037-g. [DOI] [PubMed] [Google Scholar]
- Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;1331:141–50. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996;674:1751–60. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur J Neurosci. 1996;810:2213–20. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Tsarovina K, Pattyn A, Stubbusch J, Muller F, van der Wees J, Schneider C, Brunet JF, Rohrer H. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;13119:4775–86. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- Tsarovina K, Reiff T, Stubbusch J, Kurek D, Grosveld FG, Parlato R, Schutz G, Rohrer H. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J Neurosci. 2010;3032:10833–43. doi: 10.1523/JNEUROSCI.0175-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarche I, Tissier-Seta JP, Hirsch MR, Martinez S, Goridis C, Brunet JF. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development. 1993;1193:881–96. doi: 10.1242/dev.119.3.881. [DOI] [PubMed] [Google Scholar]
- Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;1353:473–81. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- Xu H, Firulli AB, Zhang X, Howard MJ. HAND2 synergistically enhances transcription of dopamine-beta-hydroxylase in the presence of Phox2a. Dev Biol. 2003;2621:183–93. doi: 10.1016/s0012-1606(03)00361-0. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann MJ, Patterson PH. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989;2464936:1412–6. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- Yang C, Kim HS, Seo H, Kim CH, Brunet JF, Kim KS. Paired-like homeodomain proteins, Phox2a and Phox2b, are responsible for noradrenergic cell-specific transcription of the dopamine beta-hydroxylase gene. J Neurochem. 1998;715:1813–26. doi: 10.1046/j.1471-4159.1998.71051813.x. [DOI] [PubMed] [Google Scholar]