Abstract
Beneficial effects of estrogen therapy on cognitive performance diminish with age and time following the loss of ovarian function. This has led to the ‘Window of Opportunity’ hypothesis, which states that estrogen therapy must be administered within a limited period of time following menopause in order to be effective. Effects of estrogen therapy on cognitive performance are due, at least in part, to effects on cholinergic afferents innervating the hippocampus and cortex, and it has been suggested that the loss of estrogen effect with age and time following menopause is due to a substantial reduction in the function of these projections. The mechanisms that underlie the effects are not clear. GPR30 is a novel G-protein coupled estrogen receptor that is expressed in brain and other tissues. Our recent studies show that GPR30 is expressed in areas of the brain important for spatial learning, memory, and attention. In addition, GPR30 in expressed by the vast majority of cholinergic neurons in the basal forebrain, and appears to be an important regulator of basal forebrain cholinergic function. We hypothesize that GPR30 plays an important role in mediating direct effects of estradiol on basal forebrain cholinergic neurons, with corresponding effects on cognitive performance. Hence, GPR30 may be an important target for developing new therapies that can enhance or restore estrogen effects on cognitive performance in older women. Here we briefly review the cholinergic hypothesis and summarize our findings to date showing effects of a GPR30 agonist and antagonist on basal forebrain cholinergic function and cognitive performance.
Estrogen Effects on Cognition
Animal studies show that estrogens have many beneficial effects on the brain, including reducing neuronal loss following cardiac arrest and stroke (Suzuki et al., 2006), increasing neuronal connectivity (Spencer et al., 2008), improving cognitive performance (Daniel, 2006), and preventing or slowing age-related cognitive decline (Frick, 2009; Gibbs and Gabor, 2003). Young adult ovariectomized rats and mice treated with estradiol perform better on learning and memory tasks such as T-maze and radial arm maze tasks (Daniel et al., 1997; Gibbs, 1999), place and object recognition tasks (Fernandez et al., 2008; Frye et al., 2007; Gresack and Frick, 2006), and contextual or cued fear conditioning (Jasnow et al., 2006), than ovariectomized controls. These effects are selective and can vary depending on the type of memory tested (Davis et al., 2005; Fader et al., 1999; Galea et al., 2001; Korol and Kolo, 2002), level of hormone (Wide et al., 2004), and prior stress (Wood and Shors, 1998). Human studies likewise show beneficial effects of estrogens on specific cognitive tasks in younger surgically menopausal or perimenopausal women, particularly in the realm of verbal memory and executive functioning (Sherwin and Henry, 2008). Several studies have reported estrogen-mediated increases in cerebral blood flow (Greene, 2000; Resnick and Maki, 2001), and reductions in hippocampal and cortical atrophy associated with aging and Alzheimer’s disease (AD) (Erickson et al., 2007; Hu et al., 2006), as well as significant reductions in memory decline (Resnick et al., 1997) and in the risk of developing Alzheimer’s disease (AD)-related dementia (Henderson, 2009). A recent study also reported that women who experience an early menopause are at significantly greater risk for age-related cognitive decline and dementia, and that this risk is mitigated by early estrogen therapy (Shuster et al., 2010).
Despite these encouraging findings, recent randomized trials suggest far fewer benefits when estrogen therapy is initiated in older women. In fact, several large trials have reported either no beneficial effect or increased harm for women receiving hormone therapy (HT) in old age (Resnick et al., 2006; Shumaker et al., 2003; Shumaker et al., 2004). The Women’s Health Initiative Memory Study (WHIMS), one of the largest prospective randomized trials conducted to date, found that older women (mean age 69 years at enrollment) treated daily with a combination of conjugated equine estrogens and medroxyprogesterone acetate were at significantly greater risk for stroke and dementia than placebo-treated controls (Shumaker et al., 2004). Likewise, attempts to use estrogen therapy to reduce AD-related cognitive impairment in women have been largely unsuccessful (Henderson et al., 2000; Mulnard et al., 2000; Mulnard et al., 2004; Wang et al., 2000). Taken together, these studies have led to the ‘Window of Opportunity’ or ‘Critical Period’ hypothesis, which posits that estrogen therapy must be initiated at or near the time of menopause in order to positively affect brain aging and cognition. This is supported by recent animal studies which show that the beneficial effects of estrogens on cognitive performance in rats and mice diminish with age and time following loss of ovarian function when treatment is delayed (Daniel et al., 2006; Gibbs et al., 2009; Markowska and Savonenko, 2002; Talboom et al., 2008).
The Cholinergic Hypothesis
The reasons for the loss of estrogen effect with age and time following menopause have yet to be explained. Cholinergic inputs to the hippocampus and cortex are known to play an important role in mediating effects of estrogens on cognitive performance (reviewed in (Gibbs, 2010)). Therefore, it is possible that deficits in cholinergic function that occur with age and time following menopause are responsible, at least in part, for the loss of estrogen effect.
Cholinergic neurons in the medial septum (MS), diagonal band of Broca (DBB), and nucleus basalis magnocellularis (NBM) are the primary source of cholinergic inputs to the hippocampus and cerebral cortex (Gritti et al., 1997; Mesulam, 1996; Woolf, 1991). These cholinergic projections are important for learning, memory, and attention (Baxter and Chiba, 1999; Everitt and Robbins, 1997). Early human studies demonstrate that anticholinergic drugs produce cognitive impairments in young healthy adults similar to impairments seen in patients with dementia (Deutsch, 1971; Drachman and Leavitt, 1974), while cholinergic enhancing drugs improve performance in older patients (Drachman, 1977). Cholinergic lesions in the MS or NBM as well as use of selective muscarinic antagonists impair learning and memory (Everitt and Robbins, 1997). Moreover, cholinergic neurons in the basal forebrain (BFCNs) undergo degenerative changes during aging that correlate with progressive memory deficits (Bartus et al., 1982; Baxter and Chiba, 1999). Loss of BFCNs is a hallmark of Alzheimer’s disease, and has been shown to play a role in the cognitive deficits associated with the dementia (Lanari et al., 2006; Linstow and Platt, 1999; Schliebs and Arendt, 2006; Smith et al., 1999).
Estradiol’s ability to influence the functionality of the cholinergic system may contribute to the timing of the Window of Opportunity hypothesis. Following ovariectomy, 17β-estradiol (E2) treatment can significantly enhance basal forebrain cholinergic function in females. E2 treatment increases choline acetyltransferase (ChAT) activity (Luine, 1985), ChAT mRNA and protein (Gibbs, 1997; Gibbs, 2000; Singh et al., 1994), high-affinity choline uptake (O'Malley et al., 1987; Tinkler et al., 2004), and potassium-stimulated acetylcholine release in the hippocampus (Gabor et al., 2003; Gibbs et al., 1997). E2 treatment also can increase the density of cholinergic fibers innervating specific regions of frontal cortex (Gibbs et al., 1997; Tinkler et al., 2004). These effects are relevant to effects on cognitive performance. For example, E2 treatment can attenuate the amnestic effects of scopolamine, a muscarinic acetylcholine receptor blocker in rats (Fader et al., 1998; Fader et al., 1999; Gibbs, 1999). A similar effect has been reported in humans (Dumas et al., 2006; Dumas et al., 2008). The effects of E2 on NMDA receptor binding and dendritic spine density in region CA1 of the hippocampus also appear to be mediated via cholinergic inputs (Daniel and Dohanich, 2001; Lam and Leranth, 2003). In addition, selectively destroying cholinergic projections to the hippocampus (Gibbs, 2002; Gibbs, 2007) or blocking M2 muscarinic receptors in the hippocampus (Daniel et al., 2005) prevents estradiol effects on spatial learning. This demonstrates that the cholinergic inputs are essential for mediating beneficial effects of estradiol on certain tasks. Notably, recent studies suggest that the effects of E2 on ChAT expression and cholinergic function are altered by age and time following ovariectomy (Bohacek et al., 2008; Gibbs, 1998; Gibbs, 2003; Gibbs et al., 2009; McMillan et al., 1996), which may account for the loss of beneficial effects of estrogen therapy on cognition in older women and in women with AD. We propose that a better understanding of the mechanisms that underlie the effects of estrogens on BFCNs will lead to the development of more effective therapies for preventing and treating cognitive decline associated with aging and with AD.
Mechanisms of Estrogen Effects on Cholinergic Function
The mechanism(s) by which estradiol increases the functionality of BFCNs is unclear. Two nuclear estrogen receptors (ERs) have been identified, ERα and ERβ (Toran-Allerand, 2004). These nuclear receptors regulate gene transcription but are also capable of activating second messenger signaling pathways such as mitogen-activated protein kinases (MAPK), calcium/calmodulin-dependent protein kinases (CamKII), and cAMP response element-binding proteins (CREB) (Manavathi and Kumar, 2006; McEwen, 2002). Approximately 30% of cholinergic neurons in the MS express ERα, but not ERβ (Shughrue et al., 2000). Estradiol can stimulate CREB activation in BFCNs in adult mice, and this effect was blocked by inhibition of MEK1/2 and by treatment with ICI182,780 (a classical ERα and β antagonist). These effects were observed in ERβ knockout mice but not in ERα knockout mice, suggesting that estradiol may act through ERα to activate MAPK signaling and CREB phosphorylation in a subset of the cholinergic neurons (Szego et al., 2006).
Recently, a novel membrane-associated estrogen receptor GPR30 was identified (Moriarty et al., 2006; Prossnitz et al., 2007). GPR30 is a seven-transmembrane-spanning G-protein coupled receptor that is localized to both intracellular and plasma membranes (Funakoshi et al., 2006), and promotes rapid estrogen signaling in a variety of cell types including breast cancer cells, uterine epithelial cells, keratinocytes, thymus, and select cell lines (Albanito et al., 2007; Filardo and Thomas, 2005; Thomas et al., 2005; Vivacqua et al., 2006). Radioligand assays have found that GPR30 shows specific, high affinity binding to estradiol and related estrogens. In SKBR3 cells, GPR30 confers high affinity (Kd: 2.7 nM), saturable, low capacity (Bmax: 114 pM) estrogen binding (Thomas et al., 2005). In Cos-7 cells, transfection with GPR30 confers E2 binding with a Ki of 6.6 nM, and a Kd similar to that calculated for E2 binding in SKBR3 cells (Revankar, 2005). Heterologous expression studies and RNAi or antisense knock-down experiments confirm that binding is associated specifically with GPR30, and does not require classical estrogen receptors. Competitive binding assays show that steroid binding to GPR30 is specific for E2 and for certain phytoestrogens, whereas other steroid hormones (e.g., 17α-estradiol, progesterone, cortisol, and testosterone) have little binding at concentrations up to 10 µM. In contrast, the antiestrogens ICI182,780 and tamoxifen compete effectively, with relative binding affinities approximately 10% that of E2 (Thomas et al., 2005). Functional studies using breast cancer cells lacking classical estrogen receptors found that GPR30 mediates upregulation of c-fos proto-oncogene by E2, as well as by other estrogen-like compounds such as the phytoestrogens genistein and quercetin (Maggiolini et al., 2004). Using Ishikawa cells (which express ERα) and human endometrial cancer cells (HEC1A cells; which do not express functional ERα), Vivacqua et al. (Vivacqua et al., 2006) demonstrated that E2 and the major antiestrogenic metabolite of tamoxifen (4-hydroxytamoxifen) up-regulate c-fos expression specifically through interaction with GPR30 and that this effect involves activation of epidermal growth factor receptor.
Both Northern blot and Western blot studies have confirmed that GPR30 is expressed in a variety of tissues, including the central nervous system. In the brain, GPR30 immunoreactivity has been detected in many regions including the hippocampus, hypothalamus, and midbrain (Brailoiu et al., 2007). GPR30 also is expressed in spinal cord and in dorsal root ganglion neurons, and G-1, a selective GPR30 agonist (Bologa et al., 2006), can depolarize spinal cord neurons as well as stimulate protein kinase Cξ-dependent hyperalgesia in rats (Dun et al., 2009; Kuhn et al., 2008). The role that GPR30 plays in mediating estrogen effects in brain is still largely unclear. One recent study found that G-1 reduces 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus, analogous to E2 (Xu et al., 2009). Other studies suggest that GPR30 is involved in estrogen-mediated regulation of GnRH neurons in non-human primates (Noel et al., 2009; Terasawa et al., 2009). Another study reports that G-1 significantly reduced freezing behavior in a mouse model of depression (analogous to E2) and that this effect was blocked by G-15 (a selective GPR30 antagonist) (Dennis et al., 2009). Recently we have investigated the role of GPR30 in mediating estrogen effects on cholinergic neurons in the basal forebrain, and have generated evidence that GPR30 is an important regulator of BFCN function with corresponding effects on cognitive performance.
GPR30 is Expressed by Cholinergic Neurons in the Basal Forebrain
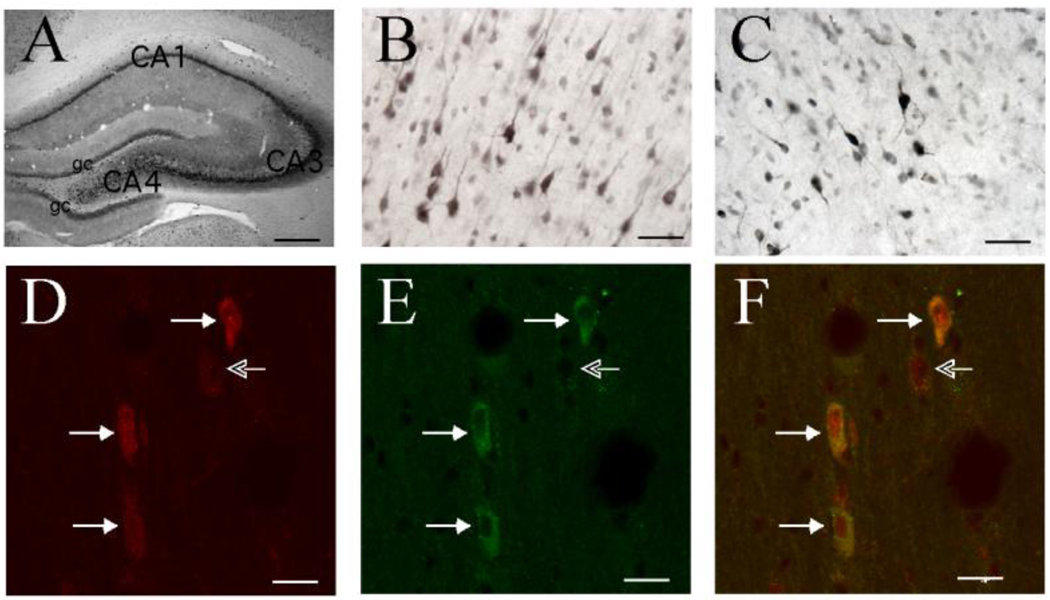
Our initial studies evaluated GPR30 expression in the rat forebrain and tested whether GPR30 is expressed by specific cholinergic cell groups. GPR30-like immunoreactivity was detected in many regions of the forebrain including hippocampal pyramidal cells, pyramidal neurons in frontal cortex, cells in the hypothalamus, as well as regions containing basal forebrain cholinergic cell groups (Hammond et al., 2010) (Figure 1). Using RT-PCR, we also verified that GPR30 mRNA is detectable in each of these regions, with the highest levels detected in the hippocampus and frontal cortex (Hammond et al., 2010). ChAT is the enzyme responsible for the production of acetylcholine (ACh) and is a specific marker for cholinergic neurons. Using fluorescence immunohistochemistry, we evaluated the co-localization of GPR30 with cholinergic neurons in the MS, DBB, NBM and striatum. For comparison, we also evaluated co-localization with parvalbumin (Parv)-containing cells in these same regions. Parv is a calcium binding protein that is expressed by GABAergic neurons in the basal forebrain (Freund, 1989; Kiss et al., 1990). Results showed that the vast majority of ChAT+ cells in each region of the basal forebrain contained GPR30-like immunoreactivity (IR) (Hammond et al., 2010) (Figure 1). In the MS and STR, nearly all ChAT+ cells contained GPR30-like-IR (95% and 99%). In the VDB, HDB, and NBM, (62%, 68%, and 81%) of cholinergic cells also contained GPR30-like-IR. In contrast, the majority of parvalbumin (GABAergic) neurons in the same regions of the brain were GPR30 negative (Hammond et al., 2010). Only 19%, 35%, 42%, and 0.4% of parvalbumin cells in the MS, VDB, HDB, and STR contained GPR30-IR. These data demonstrate that GPR30 is differentially expressed by cell types in the basal forebrain, and that GPR30 is ideally positioned to play a significant role in mediating direct effects of E2 on BFCNs.
Figure 1.
Immunostaining showing GPR30-IR in forebrain regions and illustrating co-localization of GPR30-IR with ChAT in adult female Sprague-Dawley rats. Panels A and B show GPR30-IR staining in the hippocampus, and in pyramidal cells in layer III of frontal cortex. Panel C shows GPR30-IR staining in the medial septum. Panels D and E are confocal images showing co-localization of GPR30 (panel D-labeled with Cy3) and ChAT (panel E-labeled with Alexa-488) in the medial septum. The merged image is shown in panel F. Solid arrows point to double-labeled cells. Open arrow points to a weak GPR30-positive cell that is negative for ChAT. Scale bar = 400 µm in A, 50 µm in B–C, and 20 µm in D–F. Portions adapted from (Hammond et al., 2010).
Activation and Inhibition of GPR30 Affects Cholinergic Function
To determine whether GPR30 influences basal forebrain cholinergic function, we evaluated whether treating rats with G-1 or G-15 significantly affects ACh release in the hippocampus. In one experiment, ovariectomized (Ovx) rats were treated with G-1, E2, or vehicle using a miniosmotic pump implanted subcutaneously. These pumps deliver continuously at a rate of 12 µL/day, and rats were treated with a dose of 5 µg/day G-1 or E2. Rats also received a guide cannula positioned immediately over the right hippocampus. After one week of treatment, a 3 mm concentric microdialysis probe was lowered into the hippocampus, and in vivo microdialysis was performed to measure both basal and potassium-stimulated ACh release. E2 did not affect basal ACh release, but significantly increased potassium-stimulated release (Table 1) consistent with previous reports (Gabor et al., 2003; Gibbs et al., 2004; Marriott and Korol, 2003). G-1 produced essentially the same effect, i.e., G-1 did not affect basal ACh release, but significantly increased potassium-stimulated release similar to E2 (Hammond et al., 2010) (Table 1). This suggests that activation of GPR30 enhances ACh release in the hippocampus similar to E2.
Table 1.
Effects of Estradiol (E2) and G-1 on ACh Release in the Hippocampus
| Group | Basal ACh Release (pmol/ 20 microliters) |
Potassium- Stimulated ACh Release (Percentage Change) |
|---|---|---|
| Vehicle | 0.38 ± 0.02 | 135.4 ± 6.8% |
| E2 | 0.46 ± 0.03 | 289.8 ± 28.9%* |
| G-1 | 0.34 ± 0.03 | 290.7 ± 21.2%* |
Basal Ach levels represent baseline amounts of Ach collected 90–150 minutes following probe insertion. Potassium-stimulated Ach levels represent the percentage change between amount of Ach in basal levels and amount of Ach collected during perfusion of the probe with high potassium added to the artificial cerebrospinal fluid.
p<0.05 relative to OVX vehicle. Adapted from (Hammond et al., 2010).
In a separate experiment, gonadally intact rats were treated with G-15 or vehicle and then tested for effects on ACh release. Preliminary results indicate G-15 at a dose of 10 µg/day has no effect on basal ACh release, but significantly decreases ACh release in response to elevated potassium (not shown). The profile of release suggests a depletion of ACh stores during the period of enhanced neural activity, consistent with a reduction in ACh synthesis. These preliminary results are consistent with the hypothesis that GPR30 is an important regulator of basal forebrain cholinergic function, and that effects of E2 on basal forebrain cholinergic function are mediated, at least in part, via GPR30.
Effects of G-1 and G-15 on Spatial Learning
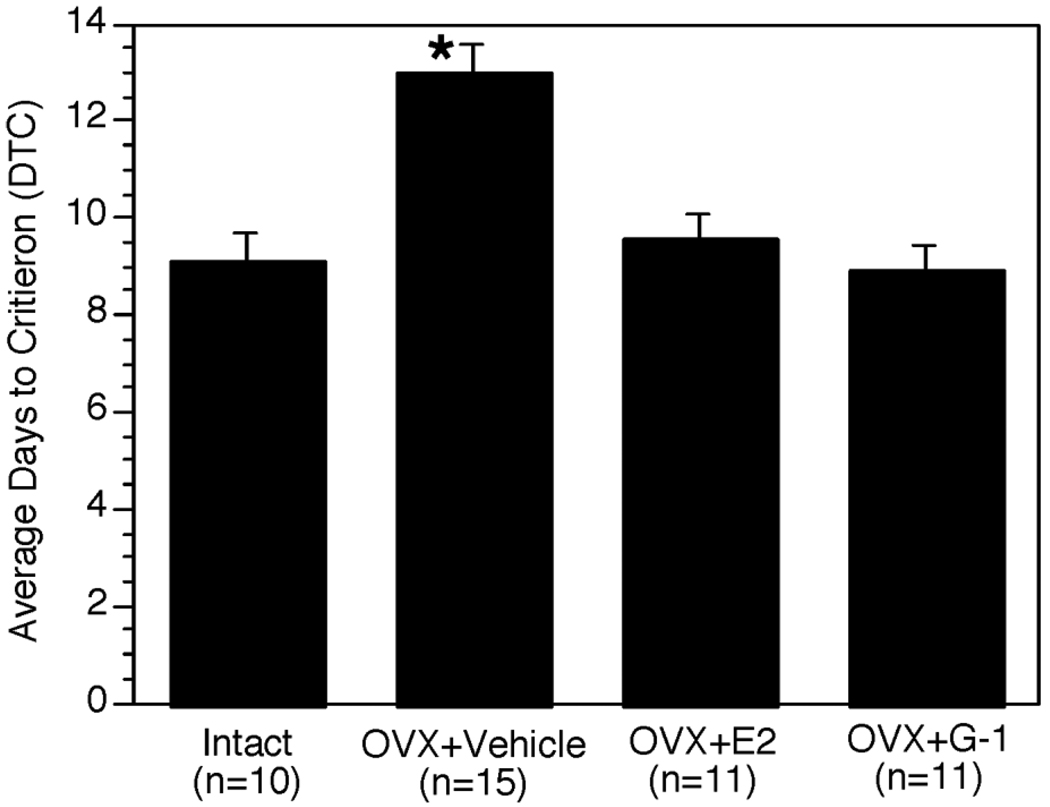
E2 enhances acquisition of a delayed matching-to-position T-maze task by Ovx rats, and cholinergic inputs to the hippocampus are critical for this effect (Gibbs, 2002; Gibbs, 2007). To test whether the effects of GPR30 on cholinergic function are relevant to cognitive performance, we evaluated the effects of G-1 and G-15 on DMP acquisition in comparison with the effects of ovariectomy and E2 replacement. Ovx rats were treated with G-1, E2, or vehicle via miniosmotic pump as described above, and compared with vehicle-treated gonadally intact controls. G-1 and E2 were delivered at a dose of 5 µg/day for the duration of the experiment. After one week of treatment, rats were trained on the DMP task as previously described (Gibbs, 2007), and the number of days required to reach criterion was compared. Ovariectomy significantly reduced the rate of acquisition as demonstrated by a significant increase in the number of days required to reach criterion compared with gonadally intact controls. This effect was reversed by chronic treatment with either E2 or G-1 (Hammond et al., 2009) (Fig 2). In a separate experiment gonadally intact rats were treated with G-15 at a dose of 5 or 10 µg/day and then compared with vehicle-treated controls. Preliminary results suggest G-15 dose-dependently reduces the rate of acquisition on the DMP task, and that a dose of 10 µg/day has an effect comparable to ovariectomy (G-15-treated rats took and average of 13 days to reach criterion compared with 10 days for gonadally intact controls). These effects are consistent with the effects on ACh release, and suggest that the effects of Ovx and E2 on DMP acquisition are mediated via GPR30 and the regulation of BFCN function. Future studies will focus on identifying the specific mechanisms of GPR30-mediated regulation of BFCN function, showing that G-15 specifically blocks the effects of estradiol, and on demonstrating whether GPR30 is both necessary and sufficient to mediate the effects of E2 on cognitive performance.
Figure 2.
Bar graph illustrating the effects of ovariectomy (OVX), estradiol, and G-1 treatment on rate of DMP acquisition in adult female Sprague-Dawley rats treated systemically with 10 µg/day of treatment. Bars indicate mean number of days to reach criterion (DTC) ± s.e.m. OVX vehicle significantly differs from all over groups *p<0.05. Adapted from (Hammond et al., 2009).
Summary & Conclusions
Human and animal studies support the ‘Window of Opportunity’ hypothesis and show that the effects of estrogen therapy on cognitive performance are due, at least in part, to effects on cholinergic inputs to the hippocampus and cortex. We hypothesize that the loss of estrogen effect with age and time following menopause is due to a reduction in the function of these projections. Our recent findings support the hypothesis that GPR30 plays an important role in mediating the effects of estradiol on the function of cholinergic inputs to the hippocampus and cortex, with corresponding effects on cognitive performance. Consequently, treatment with a GPR30 agonist may be a viable strategy for preventing or reversing the loss of estrogen effect with age and time following menopause.
Other mechanisms also may contribute to the loss of estrogen effect following menopause. For example, decreases in estrogen receptor expression in the hippocampus or cortex have been described (Mehra et al., 2005; Wilson et al., 2002) and could contribute to reductions in the effects of estrogen therapy. Data from our laboratory suggest that reductions in estrogen receptor expression also may occur in response to loss of cholinergic function. Specifically, preliminary data suggest that septal cholinergic lesions can lead to significant reductions in ERα and ERβ mRNA in the hippocampus (Hammond et al., 2010 abstract). This may be another mechanism by which cholinergic impairment associated with age and time following menopause can contribute to the Window of Opportunity. Whether activation of GPR30 can reverse this effect currently is being evaluated.
Based on our data and on evidence supporting the cholinergic hypothesis, we also propose that enhancing cholinergic activity, whether indirectly via a GPR30 agonist or AChE inhibitors, or directly via selective AChR agonists, could be an effective means of reopening the window of opportunity and restoring beneficial effects of estrogen therapy on cognitive performance in older women. This is supported by one recent study showing that donepezil, a cholinesterase inhibitor used in treating Alzheimer’s disease, was effective at restoring effects of estradiol on DMP acquisition in aged rats (Gibbs et al., 2009). We have observed similar effects of donepezil + estradiol in young rats with cholinergic lesions, as well as the ability of galanthamine (another cholinesterase inhibitor used in treating Alzheimer’s disease) to enhance effects of estradiol on DMP acquisition in aged rats (unpublished observations). These findings are consistent with a report by Schneider et al. (Schneider et al., 1996) that postmenopausal women with AD and who also received estrogen therapy responded better to tacrine (a cholinesterase inhibitor formerly used to treat AD) than women not on estrogen therapy. Hence, combining estrogen therapy with selective cholinergic enhancers may be a particularly effective strategy for re-opening the window of opportunity in older women (discussed in (Gibbs, 2010)). Sustained estrogen therapy, however, is not without significant risks and side effects, particularly in older women (Grady et al., 1995; Grady et al., 2000; Ross et al., 2000). Selective GPR30 agonists (such as G-1) may offer added benefits by avoiding such adverse effects. Whether G-1 is effective at enhancing cholinergic function and cognitive performance in aged rats and is similarly affected by co-administration of cholinesterase inhibitors needs to be evaluated. We hypothesize that such strategies will prove beneficial, particularly in older women who are beginning to show signs of mild cognitive impairment or early Alzheimer’s disease. More studies are needed to address these questions.
Acknowledgments
I acknowledge Douglas Nelson and Amanda Chipman, whose technical assistance contributed to this work. I also acknowledge the National Institutes of Health and the National Science Foundation, which have funded much of the work described in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174:788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Drachman DA. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977;27:783–790. doi: 10.1212/wnl.27.8.783. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, Oprea TI, Dun NJ. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res. 2009;87:1610–1619. doi: 10.1002/jnr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28:179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen Improves Performance of Reinforced T-Maze Alternation and Prevents the Amnestic Effects of Scopolamine Administered Systemically or Intrahippocampally. Neurobiol. Learning and Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacology Biochemistry and Behavior. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res. 2003;962:244–247. doi: 10.1016/s0006-8993(02)04053-2. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997;749:143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;810:294. doi: 10.1016/s0006-8993(98)00945-7. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of ageing and long-term hormone replacement on cholinergic neurones in the medial septum and nucleus basalis magnocellularis of ovariectomized rats. J Neuroendocrinol. 2003;15:477–485. doi: 10.1046/j.1365-2826.2003.01012.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocrine Reviews. 2010;31 doi: 10.1210/er.2009-0036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–313. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, Vittinghoff E, Hulley S. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–696. doi: 10.7326/0003-4819-132-9-200005020-00002. [DOI] [PubMed] [Google Scholar]
- Greene RA. Estrogen and cerebral blood flow: a mechanism to explain the impact of estrogen on the incidence and treatment of Alzheimer's disease. Int J Fertil Womens Med. 2000;45:253–257. [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogens, episodic memory, and Alzheimer's disease: a critical update. Semin Reprod Med. 2009;27:283–293. doi: 10.1055/s-0029-1216281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Yue Y, Zuo PP, Jin ZY, Feng F, You H, Li ML, Ge QS. Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J. 2006;21:214–218. [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Freund TF. Distribution of septohippocampal neurons containing parvalbumin or choline acetyltransferase in the rat brain. J Comp Neurol. 1990;298:362–372. doi: 10.1002/cne.902980308. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hip {Rhodes, 1997 #13} pocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer's disease. Mech Ageing Dev. 2006;127:158–165. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Linstow Ev, Platt B. Biochemical dysfunction and memory loss: the case of Alzheimer's dementia. Cell. Mol. Life Sci. 1999;55:601–616. doi: 10.1007/s000180050318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–322. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J Neurosci. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. The systems-level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer's disease. Prog Brain Res. 1996;109:285–297. doi: 10.1016/s0079-6123(08)62112-3. [DOI] [PubMed] [Google Scholar]
- Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer's Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Corrada MM, Kawas CH. Estrogen replacement therapy, Alzheimer's disease, and mild cognitive impairment. Curr Neurol Neurosci Rep. 2004;4:368–373. doi: 10.1007/s11910-004-0083-8. [DOI] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley CA, Hautamaki RD, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987;403:389–392. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007:265–266. doi: 10.1016/j.mce.2006.12.010. 138-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann N Y Acad Sci. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Climino DanielF, Sklar LaryyA, Arterburn JeffreyB, Prossnitz EricR. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer's disease. J Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Farlow MR, Henderson VW, Pogoda JM. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer's disease. Neurology. 1996;46:1580–1584. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol. 2009;21:316–321. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]