Abstract
Objective
To examine changes in brachial artery conductance (BAC) during reactive hyperemia in women with polycystic ovary syndrome (PCOS) compared to controls.
Study Design
This is a pilot case-control study performed at a single academic medical center. Changes in BAC during reactive hyperemia were evaluated in 31 women with PCOS and 11 healthy control women. Fasting glucose, insulin, lipids and androgen levels were also determined. A mixed-effects model was used to compare the PCOS curve to the control curve for change in BAC from baseline during reactive hyperemia.
Results
Body mass index (BMI) and testosterone levels were significantly increased in the PCOS group compared to controls (P < 0.05). In addition, the PCOS group had higher total and LDL cholesterol levels (P = 0.05 and 0.09, respectively). Change in BAC from baseline during reactive hyperemia was significantly increased in the PCOS group compared to controls even after adjusting for age, BMI and LDL cholesterol levels (P < 0.0001). There were no significant differences between the two groups in age, blood pressure, or fasting glucose or insulin levels.
Conclusions
Brachial artery conductance during reactive hyperemia is significantly increased in women with PCOS compared to controls and may be a novel early indicator of increased cardiovascular risk in women with PCOS.
Keywords: polycystic ovary syndrome, androgen excess, brachial artery, conductance, cardiovascular risk
Introduction
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder that affects up to 10% of reproductive-age women (1). PCOS is characterized by oligoanovulation and hyperandrogenism. This disorder is associated with multiple independent cardiometabolic risk factors, including central obesity, insulin resistance, dyslipidemia, subclinical atherosclerosis, and inflammation (2–7). These cardiovascular risk factors can manifest themselves in the earliest stages of endothelial dysfunction, a precursor to atherosclerosis that may be present years before structural atherosclerotic disease becomes clinically evident (8). Several studies have demonstrated endothelial dysfunction in young, otherwise healthy, women with PCOS (9–11). This suggests that women with PCOS are at increased risk for early onset cardiovascular disease. However, the evidence for a cause-effect relationship between PCOS and cardiovascular disease remains limited (12). Therefore, early vascular dysfunction needs to be better characterized in women with PCOS. These studies will improve our understanding of the nature and implications of cardiovascular risk in women with PCOS.
Vascular dysfunction can be non-invasively characterized by the assessment of brachial artery responses to reactive hyperemia (13). Reactive hyperemia is the transient increase in blood flow that occurs following relief from a brief period of arterial occlusion. Mediated by neurogenic, myogenic, and endothelial factors in resistance vessels, reactive hyperemia has been recognized as an important research tool for assessing vascular function (14, 15). A number of factors that typify the reactive hyperemic response have been linked to atherosclerosis and cardiovascular risk (16). For example, decreased brachial artery reactive hyperemic blood flow has been shown to independently predict all-cause mortality in patients with end-stage renal disease (17).
Several studies have reported that reactive hyperemic brachial artery flow-mediated dilation (FMD), the percent change in brachial artery diameter during reactive hyperemia, is reduced in women with PCOS compared to controls (9–11). During reactive hyperemia, the change in brachial artery diameter is preceded by, and may be dependent on, a change in brachial artery conductance (BAC), an index of limb vasodilator capacity that takes into account differences in blood pressure (16). However, BAC during reactive hyperemia has not been characterized in women with PCOS.
Women with PCOS who have altered vascular reactivity may be at increased risk for early cardiovascular disease. Abnormal changes in reactive hyperemic brachial artery diameter have been reported in women with PCOS, but it is not known whether there are also alterations in BAC during reactive hyperemia. The objective of this study was to examine changes in BAC during reactive hyperemia in women with PCOS compared to controls.
Materials and Methods
We studied 31 women with PCOS and 11 healthy control women, recruited through the Obstetrics and Gynecology and Endocrinology clinics of Penn State Hershey Medical Center. PCOS was defined using the 1990 National Institutes of Health criteria: 1) spontaneous intermenstrual periods of ≥ 45 days or ≤ 8 periods per year, and 2) serum total testosterone >50 ng/dL or unbound testosterone >10 ng/dL, while off of oral contraceptives. Complete evaluation for secondary causes included measurements of thyroid stimulating hormone (TSH), prolactin, dehydroepiandrosterone sulfate (DHEAS) and 17-hydroxyprogesterone. Control subjects were women with regular menses and no signs of androgen excess. All participants were off of metformin for 3 months prior to screening and enrollment. Patients on oral cyclic progestins underwent a one-month washout period. Only one woman with PCOS was on an oral contraceptive. None of the control women were on oral contraceptives. None of the subjects were on lipid-lowering or antihypertensive medications, none were smokers and none had hypertension, diabetes or known cardiovascular disease. None of the subjects were pregnant. The research protocol was approved by the Institutional Review Board at the Penn State Hershey Medical Center. Written informed consent was obtained from all participants.
Participants presented in a 12-hour fasting state, having abstained from caffeine and chocolate for at least 24 hours. After excluding pregnancy, participants completed a medical history questionnaire and underwent measurements of height, weight, waist circumference, and Ferriman-Gallwey hirsutism score (18). Fasting blood was obtained to measure hormonal and metabolic parameters. A pelvic ultrasound was performed using a 6.5 MHz probe of an ATL 400 machine to characterize ovarian size and morphology (19). When a control had a dominant follicle, the ovary with the dominant follicle was excluded and the other ovary was used to determine the ovarian volume.
To minimize the effects of mood and emotion, participants were studied in a calm environment and when they were not under any acute stress. Participants were placed in a supine position in a quiet, dimly lit room. Heart rate (HR) and rhythm and blood pressure were continuously recorded. A large pneumatic cuff was placed on the upper part of the experimental arm above the antecubital fossa. A second cuff was placed around the wrist of the same arm to exclude any potential effects of hand blood flow on BAC. The brachial artery was imaged by using an ATL Doppler ultrasound probe (5–12MHz linear array scanhead, HDI 5000, Advanced Technology Laboratories, Bothell, WA). Mean blood flow velocity (MBV) and brachial artery diameter (BAD) were recorded at baseline and used to calculate baseline conductance (MBV/MAP) and flow ((π)(BAD/2)2(MBV)(60)).
Prior to inducing reactive hyperemia, a priming procedure was performed. Both cuffs were inflated to suprasystolic pressure for one to two minutes and then released. This priming conditions the blood vessels to vasodilate and generates more reproducible results. After the priming, there was a 10-minute period of rest so that the blood vessels returned to baseline. Then the wrist cuff was re-inflated. After a minute, the arm cuff was re-inflated for 10 minutes and then released. Upon release of the arm cuff, we continuously measured MBV and intermittently measured BAD by Doppler ultrasound for up to 5 minutes. We then calculated BAC at specific time points: at peak MBV and 15, 30, 45, 60, 75, 90, 105, 120, 150, and 180 seconds after release of the arm cuff. We used one sonographer who was experienced in the assessment of brachial artery reactive hyperemia. The sonographer was not blinded.
All blood samples were analyzed in either the General Clinical Research Center or Core Endocrine Laboratory at the Penn State Hershey Medical Center using validated assays. Plasma glucose was measured by a glucose oxidase method (YSI 2300 Stat Plus, Yellow Springs, Ohio). Insulin was measured using a double antibody/polyethylene glycol radioimmunoassay (RIA) that utilizes 125I-labeled human insulin and human insulin antiserum obtained from Linco Research (St. Charles, Missouri). Total testosterone was measured with a solid-phase RIA which utilizes testosterone-specific antibodies that are immobilized to the wall of polypropylene tubes and 125I-labeled testosterone. Free and weakly bound testosterone was determined using a 3H-testosterone exchange equilibrium assay. Total and HDL cholesterol were measured spectrophotometrically using a cholesterol esterase method while triglycerides were measured enzymatically on an Olympus AU5400 chemistry analyzer. LDL-cholesterol was calculated using the Friedewald equation (20).
As this was a pilot study, we did not have previous reactive hyperemia data for women with PCOS so the sample size was based on standardized effect size. We calculated that a sample size of 40 subjects (20 PCOS and 20 controls) would provide at least 85% power to detect a standardized effect size of 1.0 using a two-sided, two-sample t-test with a significance level of 5%. This sample size estimate incorporated an anticipated 5% subject dropout. The software package nQuery Advisor 5.0 (Statistical Solutions Ltd.) was utilized to perform the sample size estimate.
To account for potential confounding, all analyses comparing the PCOS group to the control group were adjusted for age, BMI and LDL-cholesterol by including these variables as continuous covariates in the statistical models. Analysis of covariance (ANCOVA) model was used to compare the clinical characteristics and metabolic profiles of the two groups. At each time point during reactive hyperemia, the change in BAC from baseline was compared between PCOS and controls, using a mixed-effects model to account for the correlation inherent among repeated measurements in a single subject (21). Linear and quadratic contrasts were constructed from the mixed-effects model to compare the PCOS curve to the control curve for change in BAC from baseline. Residual diagnostics were used to assure modeling assumptions, such as normality, were met. For completeness we also explored nonparametric analysis of the data, but the inference did not change from those obtained from the parametric methods (e.g., ANCOVA model). No corrections were made for multiple comparisons because analyses were exploratory in nature. We considered a p-value < 0.05 to be significant for the primary factor of interest, i.e., group status (PCOS versus control). All hypothesis tests were two-sided and all analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC).
Results
For the PCOS group, we screened 50 women, and 15 were excluded due to labs, 2 were lost to follow-up, 1 chose to participate in another study and 1 went on metformin. Women in the control group were selected from a pool of control women who had participated in our genetics study. We selected the controls based on their BMI in an attempt to obtain a similar BMI distribution as the PCOS group. We enrolled relatively more PCOS women than controls because many controls did not have a high enough BMI to match the more obese PCOS women.
The reproductive and metabolic characteristics of the PCOS and control groups are shown in Table 1. The PCOS group had a significantly greater body mass index (BMI) but a similar age compared to the control group. After adjusting for age and BMI, Total and LDL cholesterol appeared to be higher in the PCOS group compared to the control group (P = 0.05 and 0.09, respectively). As expected, testosterone levels and the Ferriman-Gallwey scores were significantly higher in the PCOS group compared to controls (P < 0.01 for all). There were no significant differences in blood pressure, fasting glucose or insulin between the two groups. Polycystic ovary morphology, defined as the presence of 12 or more antral follicles in either ovary, was present in 26 PCOS women and one control (19). Ovarian volume was > 10 cm3 in only 36% of controls.
Table 1.
Reproductive and metabolic characteristics of the PCOS and control groups.
| PCOS (n=31) |
Controls (n=11) |
P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 30.5 ± 5.5 | 32.1 ± 7.5 | 0.45 |
| Body mass index (kg/m2) | 36.8 ± 10.6 | 28.6 ± 5.5 | 0.02 |
| Waist-hip ratio | 0.87 ± 0.06 | 0.82 ± 0.05 | 0.39* |
| Reproductive characteristics | |||
| Testosterone (ng/dl) | 77.8 ± 37.3 | 30.9 ± 9.3 | <0.01* |
| Free and Weakly bound Testosterone (ng/dl) | 21.2 ± 9.7 | 7.5 ± 3.4 | <0.01* |
| Ferriman-Gallwey score | 14.6 ± 6.5 | 3.4 ± 4.7 | <0.01* |
| Mean ovarian volume (cm3) | 17.9 ± 11.6 | 7.1 ± 4.2 | 0.02* |
| Prevalence of polycystic ovary morphology† | 26 (93%) | 1 (9%) | <0.01* |
| Metabolic characteristics | |||
| Systolic blood pressure (mmHg) | 116.7 ± 14.0 | 114.8 ± 9.5 | 0.75* |
| Diastolic blood pressure (mmHg) | 67.1 ± 11.0 | 65.7 ± 7.3 | 0.32* |
| Total cholesterol (mg/dl) | 195.5 ± 35.2 | 172.5 ± 37.3 | 0.05** |
| LDL Cholesterol (mg/dl) | 122.7 ± 28.4 | 104.0 ± 32.1 | 0.09** |
| HDL Cholesterol (mg/dl) | 45.7 ± 10.9 | 48.5 ± 9.1 | 0.92* |
| Triglycerides (mg/dl) | 136.0 ± 67.2 | 99.6 ± 50.1 | 0.24* |
| Fasting glucose (mg/dl) | 86.8 ± 8.6 | 86.0 ± 6.5 | 0.44* |
| Fasting insulin (uU/ml) | 19.6 ± 15.2 | 14.0 ± 5.3 | 0.69* |
| Fasting glucose-Insulin ratio | 6.6 ± 3.9 | 7.0 ± 2.7 | 0.67* |
Data are presented as mean ± SD, unadjusted;
ANCOVA, adjusted for age, BMI, and LDL cholesterol;
ANCOVA, only adjusted for age and BMI.
Polycystic ovary morphology is defined as the presence of ≥ 12 follicles measuring 2–9 mm in either ovary. N (%) and Fisher’s Exact test are reported.
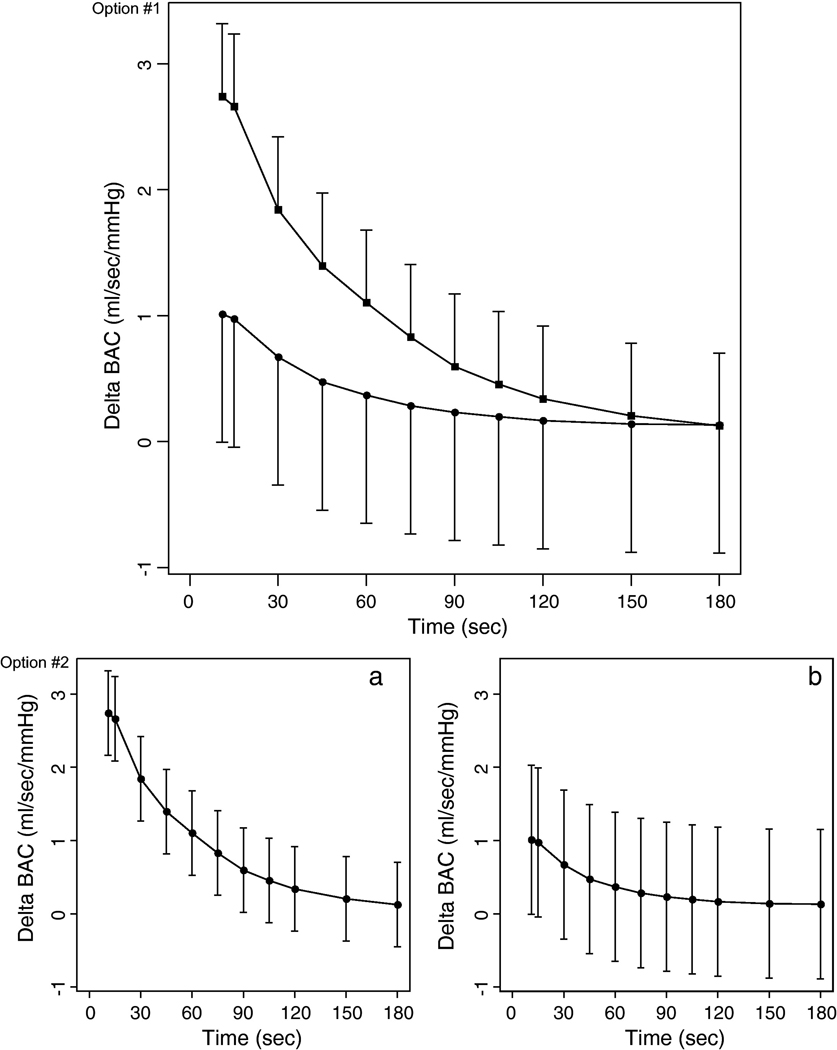
Change in BAC from baseline during reactive hyperemia was significantly higher in the PCOS group compared to controls, even after adjusting for age, BMI and LDL-cholesterol (P < 0.0001) (Fig. 1). Baseline flow, baseline BAD and baseline and peak MBV were similar in the two groups.
Figure 1.
Mean change (two standard errors above the mean change for women with PCOS; two standard errors below the mean change for healthy control women) in brachial artery conductance from baseline during reactive hyperemia in women with PCOS (■) and healthy control women (●), adjusted for age, BMI and LDL-cholesterol.
Comment
This study demonstrates that change in BAC from baseline during reactive hyperemia is significantly higher in women with PCOS compared to controls, after adjusting for age, BMI, and LDL-cholesterol. To the best of our knowledge, this is the first study to characterize changes in BAC during reactive hyperemia in women with PCOS.
Previous studies have reported alterations in brachial artery diameter during reactive hyperemia in women with PCOS, but these studies did not evaluate conductance (9–11, 22, 23). Changes in conductance may be more important because they precede and may ultimately determine changes in brachial artery diameter following reactive hyperemia (16). We noted significant differences in BAC despite similar MBV in PCOS and controls. Thus, increased BAC during reactive hyperemia may be an early indicator of vascular dysfunction among otherwise healthy women with PCOS, even in the setting of preserved brachial artery blood flow velocity.
Whether vascular dysfunction in PCOS is primarily due to insulin resistance, dyslipidemia, obesity, hyperandrogenism, or a combination of these factors is currently a subject of controversy in the literature (24, 25). Blood pressure, glucose and insulin levels were similar in our PCOS and control groups and do not explain the increased BAC observed in the PCOS group. Although dyslipidemia and obesity may contribute to greater change in BAC from baseline, the increase in BAC in women with PCOS remained significant even after adjusting for LDL-cholesterol and BMI. After adjusting for age, BMI and LDL-cholesterol, we observed that androgen levels remained significantly elevated while BAC was significantly increased in our PCOS group compared to controls. This suggests that hyperandrogenism may contribute to the increased BAC observed in women with PCOS. Even though we accounted for major potential confounders, it is still possible that some unknown factor may have affected our measurement of BAC.
A potential limitation of our study is that our PCOS sample may not be representative of the general PCOS population. Many women with PCOS have insulin resistance, however the glucose and insulin levels between our PCOS group and controls were comparable (3, 26). In addition, our control group may not be representative of the general healthy population as 36% of controls had increased ovarian volume. Since polycystic ovaries can result from increased ovarian sympathetic nerve activity (27), and increased sympathetic tone adversely affects blood flow (28), heightened sympathetic tone could have adversely affected BAC in the controls. Even if the measurement of BAC in the control group was affected by the presence of increased ovarian volume in some of the controls, this would be expected to decrease any differences between the two groups so our conclusion that BAC is increased in PCOS would still be valid. Furthermore, isolated polycystic ovaries have been demonstrated in normal healthy women and are only rarely associated with subtle metabolic abnormalities (29–31). Another limitation is that we only measured BAC in one randomly selected arm and there may be differences within an individual depending on the arm used (32).
Measuring BAC in additional healthy participants at various ages would have allowed us to further evaluate whether age influences BAC. We recognize this as a potential weakness. However, we do not have the resources to study additional controls. We sufficiently adjusted for age in all our analyses and the literature does not support age as a significant cardiovascular factor in women less than 50 years old (33–35). Therefore, we believe our finding that BAC is increased in women with PCOS is novel and adds to the existing literature (9–11, 22, 23).
In conclusion, we have demonstrated a statistically significant increase in the change in BAC from baseline during reactive hyperemia in women with PCOS compared to controls. Our findings could have important public health implications as PCOS is a prevalent disorder, affecting up to 10% of reproductive-age women. Changes in BAC from baseline during reactive hyperemia can be assessed noninvasively and could prove useful for characterizing early vascular dysfunction in young women with PCOS. Early identification and reversal of increased BAC during reactive hyperemia could be an important target for therapeutic interventions aimed at improving cardiovascular health in women with PCOS. Future research should confirm these findings in a larger study population, determine whether the degree of increase in BAC reflects the severity of the vascular dysfunction, and clarify up to what age BAC may be effectively applied in the cardiovascular risk assessment of women with PCOS. Although BAC has the potential to become a useful clinical tool for the diagnosis of early vascular dysfunction in women who have PCOS, at this time it would be premature to apply BAC to patients in the clinical setting. Until further study, BAC should only be applied in the research setting for the investigation of vascular dysfunction.
Acknowledgments
This study would not have been possible without Barbara Scheetz, B.S., the study coordinator, and the personnel of the General Clinical Research Center (GCRC) and the Core Endocrine Laboratory at the Penn State Hershey Medical Center.
This study was supported by PHS K24 HD01476 (R.S.L.), a GCRC grant MO1 RR 10732 and construction grant C06 RR016499 to Pennsylvania State University. The project described was also supported by Grant Number K 12HD055882, "Career Development Program in Women's Health Research at Penn State," from the National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raja-Khan N, Legro RS. Diagnosis and management of polycystic ovary syndrome. J Clin Outcomes Manage. 2005;12:218–227. [Google Scholar]
- 2.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi M. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 3.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 5.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 6.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–5716. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo M, Berneis K, Carmina E, Rini GB. How should we manage atherogenic dyslipidemia in women with polycystic ovary syndrome? Am J Obstet Gynecol. 2008;198(28):e1–e5. doi: 10.1016/j.ajog.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 9.Kravariti M, Naka KK, Kalantaridou SN, et al. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- 10.Orio F, Jr., Palomba S, Cascella T, et al. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:4588–4593. doi: 10.1210/jc.2003-031867. [DOI] [PubMed] [Google Scholar]
- 11.Tarkun I, Arslan BC, Canturk Z, Turemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592–5596. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 12.Carmina E. Cardiovascular risk and events in polycystic ovary syndrome. Climacteric. 2009;12 Suppl 1:22–25. doi: 10.1080/13697130903003842. [DOI] [PubMed] [Google Scholar]
- 13.Korkmaz H, Onalan O. Evaluation of endothelial dysfunction: flow-mediated dilation. Endothelium. 2008;15:157–163. doi: 10.1080/10623320802228872. [DOI] [PubMed] [Google Scholar]
- 14.Dakak N, Husain S, Mulcahy D, et al. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension. 1998;32:9–15. doi: 10.1161/01.hyp.32.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Tousoulis D, Antoniades C, Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non-invasive techniques. Heart. 2005;91:553–558. doi: 10.1136/hrt.2003.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 17.London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 2004;65:700–704. doi: 10.1111/j.1523-1755.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 18.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–830. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 19.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, New Jersey: John Wiley & Sons Inc.; 2004. [Google Scholar]
- 22.Bickerton AS, Clark N, Meeking D, et al. Cardiovascular risk in women with polycystic ovarian syndrome (PCOS) J Clin Pathol. 2005;58:151–154. doi: 10.1136/jcp.2003.015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagre A, Lekakis J, Mihas C, et al. Association of dehydroepiandrosterone-sulfate with endothelial function in young women with polycystic ovary syndrome. Eur J Endocrinol. 2006;154:883–890. doi: 10.1530/eje.1.02153. [DOI] [PubMed] [Google Scholar]
- 24.Dokras A, Jagasia DH, Maifeld M, Sinkey CA, VanVoorhis BJ, Haynes WG. Obesity and insulin resistance but not hyperandrogenism mediates vascular dysfunction in women with polycystic ovary syndrome. Fertil Steril. 2006;86:1702–1709. doi: 10.1016/j.fertnstert.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 25.Romualdi D, Costantini B, Selvaggi L, et al. Metformin improves endothelial function in normoinsulinemic PCOS patients: a new prospective. Hum Reprod. 2008;23:2127–2133. doi: 10.1093/humrep/den230. [DOI] [PubMed] [Google Scholar]
- 26.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–3242. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 27.Lara HE, Dorfman M, Venegas M, et al. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: Studies on norepinephrine release. Microsc Res Tech. 2002;59:495–502. doi: 10.1002/jemt.10229. [DOI] [PubMed] [Google Scholar]
- 28.Sinoway LI, Wilson JS, Zelis R, et al. Sympathetic tone affects human limb vascular resistance during a maximal metabolic stimulus. Am J Physiol. 1988;255:H937–H946. doi: 10.1152/ajpheart.1988.255.4.H937. [DOI] [PubMed] [Google Scholar]
- 29.Carmina E, Wong L, Chang L, et al. Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod. 1997;12:905–909. doi: 10.1093/humrep/12.5.905. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone EB, Rosen MP, Neril R, et al. The Polycystic Ovary Post-Rotterdam: A Common, Age-Dependent Finding in Ovulatory Women without Metabolic Significance. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0202. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries--a common finding in normal women. Lancet. 1988;1:870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- 32.Clark CE, Campbell JL, Powell RJ, Thompson JF. The inter-arm blood pressure difference and peripheral vascular disease: cross-sectional study. Fam Pract. 2007;24:420–426. doi: 10.1093/fampra/cmm035. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 34.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 35.Virdis A, Ghiadoni L, Giannarelli C, Taddei S. Endothelial dysfunction and vascular disease in later life. Maturitas. 67:20–24. doi: 10.1016/j.maturitas.2010.04.006. [DOI] [PubMed] [Google Scholar]