Abstract
The lack of suitable biomarkers of oxidative stress is a common problem for antioxidant intervention studies in humans. We evaluated the efficacy of vitamin C supplementation in decreasing biomarkers of lipid peroxidation in nonsmokers and in cigarette smokers, a commonly studied, free-living human model of chronic oxidative stress. Participants received ascorbic acid (500 mg twice per day) or placebos for 17 d in a double blind, placebo-controlled, randomized crossover design study. The urinary biomarkers assessed and reported herein are derived from 4-hydroperoxy-2-nonenal (HPNE) and include the mercapturic acid (MA) conjugates of 4-hydroxy-2(E)-nonenal (HNE); 1,4-dihydroxy-2(E)-nonene (DHN); and 4-oxo-2(E)-nonenol (ONO). Vitamin C supplementation decreased the urinary concentrations of both ONO-MA (p=0.0013) and HNE-MA (p=0.0213) by ~30%; however, neither cigarette smoking nor sex affected these biomarkers. In contrast, vitamin C supplementation decreased urinary concentrations of DHN-MA (3-way interaction p=0.0304) in nonsmoking men compared with nonsmoking women (p<0.05), as well as in nonsmoking men compared with smoking men (p<0.05). Vitamin C supplementation also decreased (p=0.0092) urinary total of metabolites by ~20%. Thus, HPNE metabolites can be reduced favorably in response to improved plasma ascorbic acid concentrations, an effect due to ascorbic acid antioxidant function.
Keywords: vitamin C, lipid peroxidation, 4-hydroxy-2-nonenal, 4-oxo-2-nonen-1-ol, mass spectrometry, oxidative stress, F2-isoprostanes
Introduction
Oxidative stress plays a pivotal role in the pathogenesis of various chronic diseases including atherosclerosis, Alzheimer disease, diabetes, and nonalcoholic fatty liver disease [1-3]. The notion that oxidative stress is a causative factor in the etiology of chronic disease has provided the rationale for dietary antioxidant interventions. Several large-scale, double-blind, placebo-controlled trials with the antioxidant vitamins C or E, alone or in combination, have shown disappointing effects on clinical endpoints [4-6]. Consequently, these findings have raised the question whether the dose and/or administration frequency of these antioxidants were effective to mitigate damage from reactive oxygen species (ROS) under conditions of oxidative stress [4].
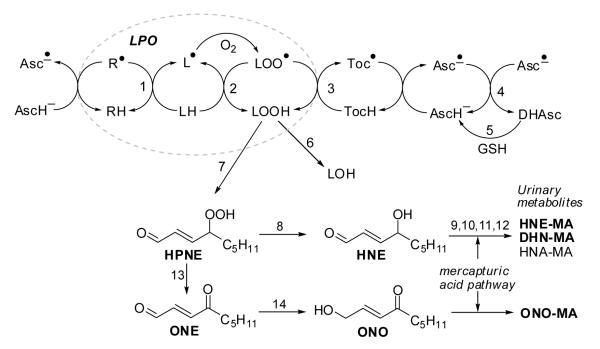
One of the challenges in assessing antioxidant efficacy in vivo is to identify validated endpoints of oxidative stress. Numerous markers are derived from polyunsaturated fatty acids because they are vulnerable targets of ROS and form ‘stable’ end-products from unstable lipid hydroperoxides (LOOHs): malondialdehyde [7], conjugated dienes [8], isoprostanes [9, 10], alkanes [8, 11], and (reactive) aldehydes [12, 13]. LOOHs spontaneously degrade into cytotoxic and genotoxic lipid peroxidation (LPO) products, especially 2-alkenals. 4-Hydroxy-2(E)-nonenal (HNE) and 4-oxo-2(E)-nonenal (ONE) are two of the most well-studied LPO products [14] (Figure 1). Both are derived from 4-hydroperoxy-2-nonenal (HPNE) [14, 15] and are toxic because they can covalently modify proteins and DNA through Michael-type additions and Schiff base formation [16]. Indeed, findings from the past several decades indicate that HNE levels are associated with the severity of Alzheimer disease and cancer [1, 17].
Fig. 1. Lipid peroxidation (LPO) and fate of LPO products.
The LPO chain reaction can be initiated by many radical species (indicated by R•) and converts LH into LOO•, which attacks another LH generating L• (paths 1 and 2, dotted oval). Ascorbic acid may scavenge the initiating radical species R• and reduce the tocopheroxyl radical, generating the ascorbyl radical, which can be reduced by glutathione dependent enzymes.
Key to reaction steps: 1, initiating event; 2, radical propagation reaction; 3, termination of the radical reaction by tocopherol (TocH); 4, dismutation of ascorbyl radicals (Asc•−); 5, reduction of dehydroascorbate (DHAsc) by GSH-dependent dehydroascorbate reductase; 6, GSH peroxidase (GPx); 7, further oxygenation and non-enzymatic cleavage of carbon-carbon bonds; 8, reduction. Steps 9-12 represent the mercapturic acid pathway to form mercapturic acid (= N-acetyl cysteine) conjugates: 9, glutathione-S-transferase (GST); 10, γ-glutamyl transferase; 11, cysteinyl glycinase; 12, N-acetyl transferase. HNE-GSH/MA metabolites can be converted into DHN-MA and HNA-MA. Step 13, dehydration; 14, aldo-keto reductase (AKR).
Humans have several metabolic pathways to detoxify 2-alkenals. Phase I enzymes catalyze the conversion of HNE into 4-hydroxy-2(E)-nonenoic acid (HNA) and 1,4-dihydroxy-2(E)-nonene (DHN) while ONE is converted into 4-oxo-2(E)-nonenoic acid (ONA) and 4-oxo-2(E)-nonenol (ONO). Phase II enzymes, particularly glutathione-S-transferases (GSTs) catalyze the conjugation of HNE, ONE, and ONO with glutathione (GSH). The resulting GSH conjugates themselves may be substrates for Phase I enzymes, forming, e.g., HNA-GSH and DHN-GSH from HNE-GSH. Enzymes in the mercapturic acid pathway convert GSH conjugates into the corresponding N-acetylcysteine (mercapturic acid) conjugates, which are the metabolites excreted in the urine (Figure 1) [18-21].
Prior studies from our group have shown that phase I and II metabolites of HPNE are elevated in rats following administration of CCl4, a well-established model of acute oxidative stress [18]. We also demonstrated that urinary HPNE metabolites decrease following smoking cessation in humans [22]. Thus, urinary HPNE metabolites are responsive to changes in oxidative stress levels. In the present study, we hypothesized that vitamin C supplementation would decrease urinary HPNE metabolites in cigarette smokers. We specifically examined these urinary metabolites in smokers because smoking is an accessible free-living model of chronic oxidative stress in humans [23, 24].
Materials and methods
Materials
HNE and ONO as well as their mercapturic acid (MA) and N-(acetyl-d3)-L-cysteine (MAd3) conjugates were prepared and chemically characterized as described previously [18, 25]. DHN-MA and DHN-MAd3 were prepared by NaBH4-reduction of HNE-MA and HNE-MAd3 [18]. HPLC-grade solvents were obtained from Honeywell Burdick and Jackson, Muskegon, MI (0.1% formic acid in water) and from EMD Chemicals, San Diego, CA (acetonitrile). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Subjects and Study Design
The Institutional Review Board for the Protection of Human Subjects at Oregon State University approved the study protocol. Men and women were recruited from the University area and were enrolled on the basis of age (18-35 y), non-nutritional supplement user for the past 6-mo, stable exercise patterns (<7 h/week), cigarette smoker (>10 cigarettes/d) or nonsmoker. The complete details of the investigation and additional biochemical endpoints have been reported previously [26, 27].
This study was conducted as a double-blind, placebo-controlled, randomized crossover study with the primary aim to determine the effect of vitamin C supplementation on vitamin E kinetics in smokers and nonsmokers [26]. Participants received ascorbic acid (500 mg twice per day) or a placebo (70.5% calcium phosphate dibasic, 29.5% cellulose, 0.5% magnesium stearate) for 17 d; all supplements were kindly provided by Pharmavite, LLC (Northridge, CA). Participants were instructed by a registered dietitian (RSB) to follow a diet low in ascorbic acid during the intervention periods. On Day 14, participants ingested approximately 50 mg each of an equimolar mixture of d6-α- and d2-γ-tocopheryl acetates. Blood was collected at regular intervals for 72 h and urine was collected in 8 h intervals from 0 to 24 h. After a 3-month washout period, participants received the alternate treatment and repeated the study as described above. Plasma ascorbic acid concentrations were measured from each fasting sample obtained during the 72 h study and averages of these plasma concentrations are reported herein.
Extraction of urinary HPNE metabolites and LC-MS/MS Analysis of HPNE metabolites
Urine samples were prepared as described previously [25]. Briefly, an aliquot of urine (0.2 ml) was acidified to pH 3 with 20 μl of 1 N HCl, then deuterium-labeled (d3)-internal standards (0.5 nmol DHN-MAd3, 0.1 nmol HNE-MAd3, and 0.1 nmol ONO-MAd3) were added. The samples were extracted twice with ethyl acetate (700 μl), and the extracts were combined and evaporated under nitrogen. The residues were then reconstituted in 100 μl of 1:4 acetonitrile:water containing 0.1 % formic acid and analyzed using a liquid chromatography/mass spectrometry system (LC-MS).
The LC system consisted of a Shimadzu Prominence HPLC with two LC-20AD pumps, a DQU-20A5 degasser, a SIL-HTc autosampler and post-column switching valves (Shimadzu, Columbia, MD). Separation was achieved on a 250 × 2 mm Synergi Max RP C12 column (Phenomenex, Torrance, CA) by gradient elution using aqueous 0.1 % (v/v) formic acid as solvent A and acetonitrile containing 0.1 % (v/v) formic acid as solvent B at a flow rate of 0.2 ml/min. The linear solvent gradient proceeded from 20 to 50% B in 10 min, from 50 to 90% B over the next 2 min, held constant at 90% B for 7 min, returned to 20% B over 1 min, and equilibrated at 20% B for 5 min.
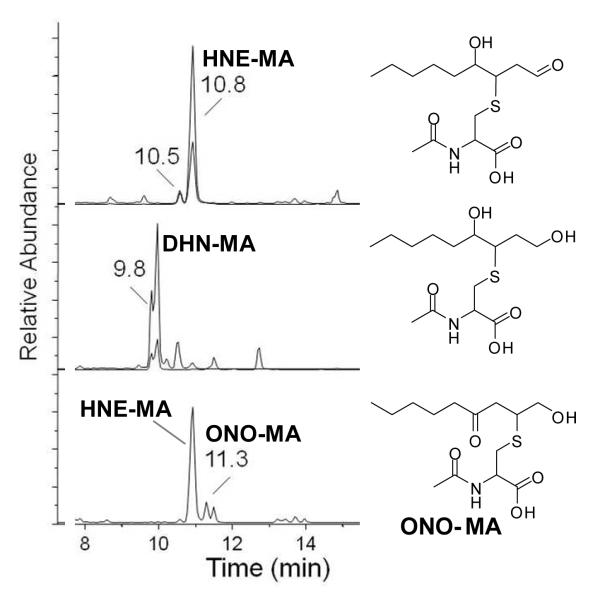
The mass spectrometer was an Applied Biosystems MDS Sciex hybrid triple quadrupole/linear ion trap instrument (4000 QTrap) equipped with a TurboV electrospray source (Concord, Canada). Detection was achieved by selected reaction monitoring (SRM) in the negative ion mode with the collision energy set at 25 eV. The following SRM transitions were used for quantitative purposes (Figure 2) with qualifying SRMs listed in parentheses:
HNE-MA, tR 10.5 and 10.8 min, m/z 318 →189 (m/z 318→171);
HNE-MA-d3, tR 10.5 and 10.8 min, m/z 321→189 (m/z 321→171); ONO-MA, tR 11.3 min, m/z 318→162;
ONO-MA-d3, tR 11.3 min, m/z 321→165;
DHN-MA, tR 9.8 min, m/z 320→191 (m/z 320→143);
DHN-MA-d3, tR 9.8 min, m/z 323→191 (m/z 323→143)
Fig. 2. LC-MS/MS-SRM chromatogram of a human urine extract.
Chromatographic peaks: Top: HNE-MA, tR 10.5 and 10.8 min, m/z 318 →189 (m/z 318→171); Middle: DHN-MA, tR 9.8 min, m/z 320→191 (m/z 320→143); Bottom: HNE-MA, tR 10.5 and 10.8 min, m/z 318→162 (qualifying transition) and ONO-MA, tR 11.3 min, m/z 318→162. The structures from top to bottom are HNE-MA, DHN-MA, and ONO-MA.
Calibration curves were constructed from standard solutions of HNE-MA, DHN-MA, and ONO-MA in 1:4 acetontrile:water (both with 0.1 % formic acid) spiked with HNE-MAd3 (10 μl of a 10 μM solution), DHN-MAd3 (5 μl of a 100 μM solution), and ONO-MAd3 (10 μl of a 10 μM solution) using materials we described previously [18, 25]. Analyte concentrations were calculated from peak area ratios of the analyte/internal standard using Analyst 1.4.1 (Applied Biosystems). HPNE metabolite concentrations, normalized to urinary creatinine, were measured in aliquots from each of the three (0-8, 8-16 and 16-24 h) collections. Urinary creatinine was measured spectrophotometrically using a kit (#500701; Cayman Chemical Co., Ann Arbor, MI). No apparent effects of urinary interval (P>0.05) were observed. Therefore, the average was calculated from the three intervals to yield the urinary concentration per g creatinine for the 24 h period for each individual.
Statistical analysis
Data are expressed as means ± SD. Repeated measures MANOVA was performed using JMP Statistical Discovery Software (5.0.1a, SAS Institute, Cary, NC) to evaluate effects attributed to smoking status, sex, and with-in subject vitamin C supplementation effects and time effects from urine samples collected in three 8 h intervals over 24 h (0-8, 8-16, and 16-24 h). Results were considered to be statistically significant at p < 0.05. Urinary metabolite data from the different time points were not significantly different. Therefore, concentrations from the three 8 h intervals were averaged and mean values were used to examine whether vitamin C supplementation reduces urinary metabolite concentrations. For the analysis of repeated measures (multiple samples from the same subject), the natural logarithm of the dependent variables (urine concentration for placebo or vitamin C supplementation) was used in the MANOVA analysis to minimize the correlation between the mean and variance of the data. When the effects of either cigarettes or sex were not statistically significant, then matched pairs analysis was used.
Results
Participant characteristics and plasma vitamin C
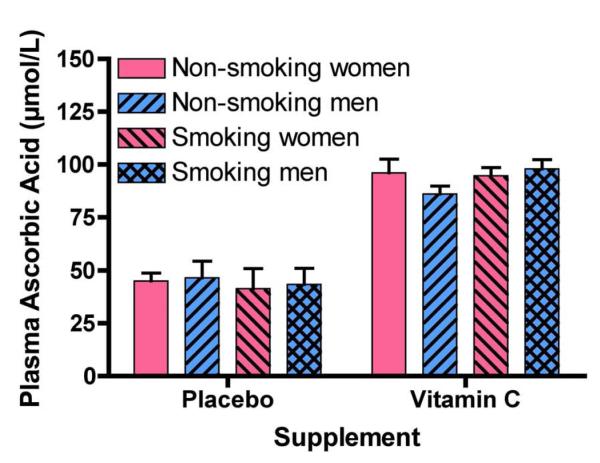
Urinary HPNE metabolites were measured in samples collected throughout a study examining vitamin C supplementation on vitamin E pharmacokinetics. Complete details of this study have been published elsewhere [26]. In brief, vitamin C supplementation ameliorated the faster rate of vitamin E disappearance in smokers, but had no effect on nonsmokers’ vitamin E kinetics [26]. No significant differences (p > 0.05) were observed between nonsmokers and smokers with respect to age or body mass index (BMI) (Table 1). However, concentrations of urinary cotinine, a marker of nicotine metabolism, were greater in smokers (>10 cigarettes/d). Vitamin C supplementation increased (p <0.0001) ascorbic acid concentrations [26] to a similar extent in smokers and nonsmokers regardless of sex (Figure 3). Plasma ascorbic acid during the placebo trial also did not differ between smokers and nonsmokers and was ~50% lower in comparison to the supplement trial. Thus, smokers and nonsmokers had essentially identical plasma ascorbic acid concentrations in the respective study phases.
Table 1.
Study participant characteristics
| Parameter | Nonsmokers (n = 12) |
Smokers (n = 10) |
|---|---|---|
| Sex | 6 men | 6 men |
| 6 women | 4 women | |
| Age (years) | 23.1 ± 2.5 | 21.1 ± 1.7 |
| BMI (kg/m2) | 24.7 ± 2.8 | 23.2 ± 3.9 |
| Cigarettes smoked (averaged over study) | 0 | 10.6 ± 3.7 |
| Cotinine (μg/ml; averaged over study) | 0.027 ± 0.013 | 2.6 ± 1.6 |
These and additional details have been published previously [26].
Fig. 3. Plasma ascorbic acid concentrations in nonsmoking and smoking men and women following supplementation with vitamin C or placebos.
During this double-blind, placebo-controlled, randomized crossover study, participants received 17-day treatments with ascorbic acid (500 mg twice per day) or with placebo. On Day 14, participants’ blood was collected at various intervals for 72 h and plasma ascorbic acid concentrations were measured in each of the samples taken; averages of these concentrations are reported herein. Vitamin C supplementation increased ascorbic acid plasma concentrations (mean ± SD, p <0.0001, n=22), but the concentrations were not different between smokers and nonsmokers, or between men and women. These data have been published previously [26].
Vitamin C supplementation decreases urinary HPNE metabolites
The present investigation aimed to evaluate novel biomarkers of LPO in cryogenically archived samples from our prior work. HPNE metabolite concentrations were measured in urine samples following 17 d of supplementation with placebo and vitamin C for each subject (crossover study design). Thus, each participant served as their own control thereby allowing for within-subject assessment of vitamin C supplementation on HPNE metabolite levels compared to placebo treatment.
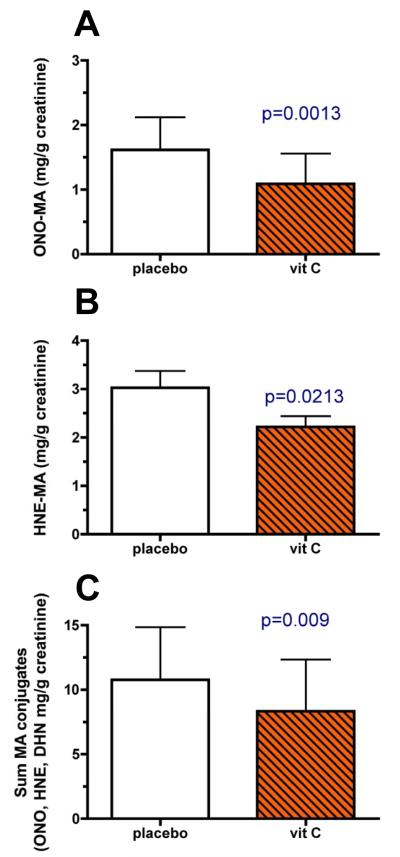
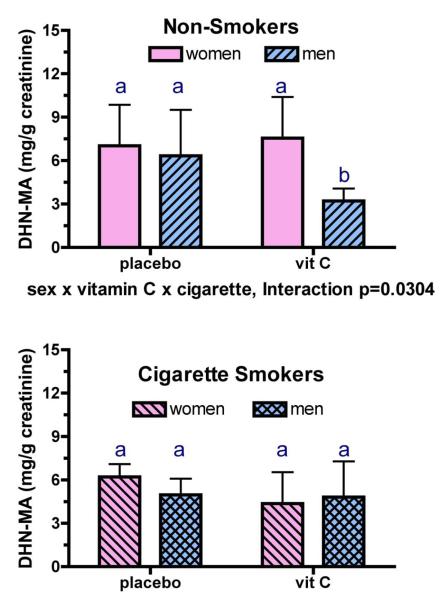
Vitamin C supplementation decreased urinary ONO-MA (p=0.0013); however, neither cigarette smoking nor sex affected this parameter (Figure 4A). Similarly, vitamin C supplementation decreased urinary HNE-MA concentrations (p=0.0213; Figure 4B), while smoking and sex had no effect on its urinary levels. In contrast, a 3-way interactive effect for vitamin C supplementation, sex, and smoking was observed for urinary DHN-MA concentrations (vitamin C effect p = 0.0165, 3-way interaction p=0.0304) indicating that smokers and nonsmokers as well as men and women responded differently to vitamin C supplementation. As with the other LPO metabolites, there was a main effect of vitamin C supplementation on urinary DHN-MA (p=0.0165). Post-hoc analysis indicated that vitamin C supplementation decreased urinary DHN-MA (p<0.05) in nonsmoking men compared with nonsmoking women as well as in male nonsmokers compared with male smokers (Figure 5). Lastly, when the urinary metabolites were summed for each individual, vitamin C decreased total metabolite concentrations (Figure 4C; p=0.0092).
Fig. 4. Modulation of urinary levels of HPNE metabolites (mean ± SD) by vitamin C supplements.
Urine was collected from all subjects shown in Figure 3. Vitamin C supplementation decreased urinary levels of ONO-MA (p=0.0013, panel A), HNE-MA (p=0.0213, panel B), and the total of ONO-MA, HNE-MA and DHN-MA (p=0.0092, panel C).
Fig. 5. Modulation of urinary DHN-MA metabolite levels (mean ± SD) by vitamin C supplements in nonsmoking men and women (top panel) and in smoking men and women (bottom panel).
Vitamin C supplementation had a different effect on urinary DHN-MA depending upon the sex and cigarette smoking habits of the participants (3-way interaction p=0.0304). As with the other metabolites, there was an overall effect of vitamin C supplementation on urinary DHN-MA (p=0.0165). Additionally, vitamin C supplementation decreased urinary DHN-MA (p<0.05) in non-smoking men compared with nonsmoking women, and also in non-smoking men compared with smoking men. Bars within a graph bearing the same letter are not different from each other.
We also performed regression analysis to better define the interrelations between concentrations urinary HPNE metabolites in the presence and absence of vitamin C supplementation (Table 2). Each HPNE metabolite was highly correlated (p <0.02 to <0.0001) with each other during placebo trial (r = 0.51-0.83) and to a greater extent during the vitamin C intervention trial (r = 0.70-0.90)
Table 2.
Correlations (p-value) of urinary HPNE metabolites during each of the supplementation trials.
| Supplement | |||
|---|---|---|---|
| Pairwise-correlations between: |
Placebo | Vitamin C | |
| ONO-MA | DHN-MA | 0.51 (p=0.015) |
0.70 (p=0.0003) |
| ONO-MA | HNE-MA | 0.57 (p=0.006) |
0.75 (p=0.0000) |
| HNE-MA | DHN-MA | 0.83 (p=0.0000) |
0.90 (p=0.0000) |
Discussion
The findings of this study demonstrate for the first time that vitamin C supplementation dramatically reduces urinary concentrations of HPNE metabolites in a cohort of healthy nonsmokers and smokers. Urinary levels of ONO-MA and HNE-MA, independent of sex or smoking status, are reduced by ~30% following vitamin C supplementation (Figure 4). Similarly, vitamin C supplementation decreased the total of HPNE metabolites by ~20%. These findings can be explained by the antioxidant effects of vitamin C, causing a decrease in LOOH production by preventing the radical initiation reaction (Figure 1, Step 1) through scavenging of oxygen radicals. In addition, vitamin C promotes the tocopherol-dependent radical termination reaction (Figure 1, Step 3), as demonstrated by measurements of deuterium-labeled vitamin E kinetics in these subjects [26]. Both effects favorably decrease the pool of lipid hydroperoxy radicals that would otherwise perpetuate the radical propagation reaction (Figure 1, Step 2). The net result is decreased formation of HPNE, HNE and ONO.
Further to a direct antioxidant effect of ascorbate, prolonged vitamin C supplementation may elevate the expression/activity of glutathione peroxidase (GPx) as demonstrated in rats [28, 29]. Increased GPx activity would favor the reduction of LOOHs into hydroxylipids (LOHs, step 6 in Fig. 1) at the expense of HPNE production, because the hydroperoxy group in LOOHs induces non-enzymatic cleavage of carbon–carbon bonds to form HPNE [15, 30]. Membrane-derived LOOHs, including fatty acid hydroperoxides, are substrates for GPx-1 and GPx-4 [31, 32]. Therefore, ascorbate-induced expression of GPx would lead to reduced production of HPNE. Unfortunately, direct testing of this hypothesis by measuring liver glutathione or GPx activities is not possible when humans, not rats, are subjects.
The decrease in urinary concentrations of ONO-MA and HNE-MA by vitamin C supplementation sheds light on the question whether HPNE metabolites are biomarkers of oxidative stress or biomarkers of the physiological response to oxidative stress. If the predominant effect of vitamin C supplementation was enhancing phase I and phase II metabolism, preserving cellular levels of GSH, and promoting cellular excretion of GSH metabolites as we observed previously in cultured cells treated with HNE [33], we would expect higher concentrations of HPNE metabolites following vitamin C supplementation. We observed the opposite effect, that is, an overall decrease in the levels of urinary HPNE metabolites, thus supporting the notion that vitamin C supplementation reduces oxidative stress and LPO.
If the urinary HPNE metabolite levels reflect oxidative stress status, why are the metabolite levels not elevated in the smoking subjects? Although the present study does not provide a definitive answer to this question, there are several aspects to consider. First, greater accumulation of oxygen radicals, either from the smoke itself [24] or indirectly from activated inflammatory cells, does not necessarily translate into increased oxidative stress because of cellular compensatory mechanisms that induce the expression of antioxidant enzymes [34, 35]. Although prior studies demonstrate that smoking decreases plasma ascorbic acid due to its increased utilization [36], we observed no difference in plasma ascorbic acid concentrations between smokers and nonsmokers regardless of intervention study phase. This may indicate that ascorbic acid utilization did not increase due to adequate defense against greater ROS in the smokers, who were all confirmed to be otherwise healthy young adults on the basis of their health history and normal serum clinical chemistries [26]. Consistent with this notion, this specific cohort of smokers had plasma F2-isoprostane concentrations that did not differ from those of the nonsmokers [26]. Second, increased production of LOOH and HPNE does not necessarily translate into increased levels of HPNE metabolites from the mercapturic acid pathway. Smoking increases the expression of phase I enzymes, such as aldo-keto reductase 1 B10 (AKR1B10) [37-39] and aldehyde dehydrogenase (ALDH) [40], which would increase the production of DHN and HNA, respectively, since HNE is a known substrate of both AKR1B10 [41] and ALDH [42, 43]. Because free DHN cannot form conjugates with GSH and HNA is a poor substrate for GST enzymes, increased expression of phase I enzymes decreases HNE as a substrate pool for GST. Thus, it is possible that smokers had greater production of DHN and HNA that was not paralleled by greater production of phase II metabolites of HNE. Clearly, complete interpretations of our findings are limited since we did not measure both free and conjugated DHN and HNA. Lastly, smoking is known induce GPx enzymes [44] that are responsible for converting LOOHs into LOHs [45], an action that would be expected to blunt the increase in HPNE production.
The three urinary HPNE metabolites, ONO-MA, HNE-MA and DHN-MA, were significantly correlated with each other during the placebo phase as well as the vitamin C supplementation phase of the study (Table 2). In addition, the relationships during the vitamin C supplementation phase were stronger (r = 0.70-0.90) than those from the placebo phase (r = 0.51-0.82). DHN-MA and HNE-MA correlated better with each other than ONO-MA with each of the two other HPNE metabolites, which is a reflection of DHN-MA being a direct metabolite of HNE-MA. HPNE [14, 15] is the common precursor to HNE and ONO, the AKR metabolite of ONE [41]. ONO differs from HNE in that the formation of ONO from HPNE does not involve reduction of the γ-hydroperoxy group. Assuming that reduction of the γ-hydroperoxy group is mediated by vitamin C-inducible GPx, vitamin C supplementation would differentially affect HNE-MA and ONO-MA levels consistent with our observation that ONO-MA levels were reduced (33%) to a greater extent (p=0.027) than HNE-MA (27%).
The subjects we examined for urinary HPNE metabolites in this study were previously analyzed for plasma and urinary F2-isoprostane levels [26, 27], allowing a comparison between F2-isoprostanes and HPNE metabolites as endpoints in this vitamin C supplementation study. Vitamin C supplementation did not change the urinary concentrations of any of the three F2-isoprostanes quantified (i.e. 8-iso-PGF2α, 8-iso-15(R)-PGF2α and PGF2α; p > 0.05) [27] nor did vitamin C supplementation change plasma levels of F2-isoprostanes [26]. Like the γ-hydroxy group in HNE [15], the side-chain hydroxy groups in F2-isoprostanes originate from hydroperoxy groups [46], most likely by GPx-mediated reduction as reported for the related hydroperoxy eicosatetraenoic acids [32, 47]. Thus, vitamin C-induced GPx expression would enhance F2-isoprostane production from hydroperoxy precursors, thereby blunting the antioxidant activity of vitamin C. The different response of urinary F2-isoprostane and ONO-MA levels to vitamin C supplementation implies that HPNE metabolites, especially ONO-MA, are more sensitive to the antioxidant effects of vitamin C than are F2-isoprostanes.
Previous studies using an animal model of acute oxidative stress [18] and smoking cessation as a model of oxidative stress relief [22] have established the value of HPNE metabolites as biomarkers of oxidative stress. Our present findings provide evidence that HPNE metabolite levels can be reduced favorably in response to improved plasma vitamin C concentrations. Although additional work is needed to fully validate these metabolites as biomarkers of oxidative stress, they show promise as a potentially more sensitive index of oxidative stress compared to F2-isoprostanes. Indeed, our prior work indicated no change in plasma or urinary F2-isoprostanes following vitamin C supplementation whereas the present work in the same participant population demonstrates that short-term vitamin C administration decreases HPNE metabolites by 20-30%. Clearly, additional study is needed to further characterize the responsiveness of these metabolites to dose-dependent changes in oxidant exposure as well as to assess their role in the etiology of chronic disease.
Acknowledgments
This study was supported by NIH grants R01HL081721 (JFS), R01DK059576 (MGT), R01DK067930 (MGT), and S10RR022589 (JFS). The authors acknowledge the use of the Mass Spectrometry Facility of the Environmental Health Sciences Center at Oregon State University (NIH grant P30ES000210).
Abbreviations
- AKR
aldo-keto reductase
- ALDH
aldehyde dehydrogenase
- BMI
body mass index
- DHN
1,4-dihydroxy-2(E)-nonene
- GPx
glutathione peroxidase
- GSH
glutathione
- GST
glutathione-S-transferase
- HNA
4-hydroxy-2(E)-nonenoic acid
- HNE
4-hydroxy-2(E)-nonenal
- HPLC
high performance liquid chromatography
- HPNE
4-hydroperoxy-2-nonenal
- LC
liquid chromatography
- LOH
hydroxylipid
- LOOH
lipid hydroperoxide
- LPO
lipid peroxidation
- MA
mercapturic acid
- MAd3
N-(acetyl-d3)-L-cysteine
- MS
mass spectrometry
- ONA
4-oxo-2(E)-nonenoic acid
- ONE
4-oxo-2(E)-nonenal
- ONO
4-oxo-2(E)-nonenol
- ROS
reactive oxygen species
- SRM
selected reaction monitoring
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Butterfield DA, Yatin SM, Varadarajan S, Koppal T. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer’s disease. Methods Enzymol. 1999;309:746–768. doi: 10.1016/s0076-6879(99)09050-3. [DOI] [PubMed] [Google Scholar]
- [2].Suzuki D, Miyata T, Saotome N, Horie K, Inagi R, Yasuda Y, Uchida K, Izuhara Y, Yagame M, Sakai H, Kurokawa K. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J. Am. Soc. Nephrol. 1999;10:822–832. doi: 10.1681/ASN.V104822. [DOI] [PubMed] [Google Scholar]
- [3].Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am. J. Clin. Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- [4].Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- [5].Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetiere P, Hercberg S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler. Thromb. Vasc. Biol. 2004;24:1485–1491. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]
- [6].Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004;7:407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- [7].Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin. Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- [8].Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- [9].Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. U. S. A. (2nd.) 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Awad JA, Morrow JD, Takahashi K, Roberts LJ. Identification of non-cyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J. Biol. Chem. (2nd.) 1993;268:4161–4169. [PubMed] [Google Scholar]
- [11].Kazui M, Andreoni KA, Norris EJ, Klein AS, Burdick JF, Beattie C, Sehnert SS, Bell WR, Bulkley GB, Risby TH. Breath ethane: a specific indicator of free-radical-mediated lipid peroxidation following reperfusion of the ischemic liver. Free Radic. Biol. Med. 1992;13:509–515. doi: 10.1016/0891-5849(92)90145-7. [DOI] [PubMed] [Google Scholar]
- [12].Blasig IE, Grune T, Schonheit K, Rohde E, Jakstadt M, Haseloff RF, Siems WG. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am. J. Physiol. 1995;269:H14–22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- [13].Meagher EA, FitzGerald GA. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic. Biol. Med. 2000;28:1745–1750. doi: 10.1016/s0891-5849(00)00232-x. [DOI] [PubMed] [Google Scholar]
- [14].Lee SH, Blair IA. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- [15].Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- [16].Blair IA. Lipid hydroperoxide-mediated DNA damage. Exp. Gerontol. 2001;36:1473–1481. doi: 10.1016/s0531-5565(01)00133-4. [DOI] [PubMed] [Google Scholar]
- [17].Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat. Res. 2005;591:34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [18].Kuiper HC, Miranda CL, Sowell JD, Stevens JF. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J. Biol. Chem. 2008;283:17131–17138. doi: 10.1074/jbc.M802797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alary J, Bravais F, Cravedi JP, Debrauwer L, Rao D, Bories G. Mercapturic acid conjugates as urinary end metabolites of the lipid peroxidation product 4-hydroxy-2-nonenal in the rat. Chem. Res. Toxicol. 1995;8:34–39. doi: 10.1021/tx00043a004. [DOI] [PubMed] [Google Scholar]
- [20].Alary J, Debrauwer L, Fernandez Y, Cravedi JP, Rao D, Bories G. 1,4-Dihydroxynonene mercapturic acid, the major end metabolite of exogenous 4-hydroxy-2-nonenal, is a physiological component of rat and human urine. Chem. Re.s Toxicol. 1998;11:130–135. doi: 10.1021/tx970139w. [DOI] [PubMed] [Google Scholar]
- [21].Alary J, Gueraud F, Cravedi JP. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Mol. Aspects Med. 2003;24:177–187. doi: 10.1016/s0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- [22].Kuiper HC, Langsdorf BL, Miranda CL, Joss J, Jubert C, Mata JE, Stevens JF. Quantitation of mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites in a smoking cessation study. Free Radic. Biol. Med. 2010;48:65–72. doi: 10.1016/j.freeradbiomed.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bruno RS, Ramakrishnan R, Montine TJ, Bray TM, Traber MG. α-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am. J. Clin. Nutr. 2005;81:95–103. doi: 10.1093/ajcn/81.1.95. [DOI] [PubMed] [Google Scholar]
- [24].Church DF, Pryor WA. The oxidative stress placed on the lung by cigarette smoke. In: Crystal RG, West JB, editors. The Lung. Raven Press; New York: 1991. pp. 1975–1979. [Google Scholar]
- [25].Kuiper HC, Stevens JF. LC-MS/MS quantification of mercapturic acid conjugates of lipid peroxidation products as markers of oxidative stress. Curr. Protocols Tox. 2010;45:17.14.11–17.14.16. doi: 10.1002/0471140856.tx1714s45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006;40:689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- [27].Taylor AW, Bruno RS, Traber MG. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 2008;43:925–936. doi: 10.1007/s11745-008-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nishi EE, Oliveira-Sales EB, Bergamaschi CT, Oliveira TG, Boim MA, Campos RR. Chronic antioxidant treatment improves arterial renovascular hypertension and oxidative stress markers in the kidney in Wistar rats. Am. J. Hypertens. 2010;23:473–480. doi: 10.1038/ajh.2010.11. [DOI] [PubMed] [Google Scholar]
- [29].Sadi G, Guray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: effect of antioxidants. Mol. Cell Biochem. 2009;327:127–134. doi: 10.1007/s11010-009-0050-4. [DOI] [PubMed] [Google Scholar]
- [30].Schneider C, Boeglin WE, Yin H, Ste DF, Hachey DL, Porter NA, Brash AR. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids. 2005;40:1155–1162. doi: 10.1007/s11745-005-1480-3. [DOI] [PubMed] [Google Scholar]
- [31].Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 1996;271:4653–4658. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- [32].Mates JM, Perez-Gomez C, de Castro I. Nunez. Antioxidant enzymes and human diseases. Clin. Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- [33].Miranda CL, Reed RL, Kuiper HC, Alber S, Stevens JF. Ascorbic acid promotes detoxification and elimination of 4-hydroxy-2(E)-nonenal in human monocytic THP-1 cells. Chem. Res. Toxicol. 2009;22:863–874. doi: 10.1021/tx900042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rohrdanz E, Schmuck G, Ohler S, Tran-Thi QH, Kahl R. Changes in antioxidant enzyme expression in response to hydrogen peroxide in rat astroglial cells. Arch. Toxicol. 2001;75:150–158. doi: 10.1007/s002040000206. [DOI] [PubMed] [Google Scholar]
- [35].Yatin SM, Aksenova M, Aksenov M, Markesbery WR, Aulick T, Butterfield DA. Temporal relations among amyloid beta-peptide-induced free-radical oxidative stress, neuronal toxicity, and neuronal defensive responses. J. Mol. Neurosci. 1998;11:183–197. doi: 10.1385/JMN:11:3:183. [DOI] [PubMed] [Google Scholar]
- [36].Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000;71:530–536. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- [37].Wang R, Wang G, Ricard MJ, Ferris B, Strulovici-Barel Y, Salit J, Hackett NR, Gudas LJ, Crystal RG. Smoking-induced Up-regulation of AKR1B10 Expression in the Airway Epithelium of Healthy Individuals. Chest. doi: 10.1378/chest.09-2634. epub date 2010/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fukumoto S, Yamauchi N, Moriguchi H, Hippo Y, Watanabe A, Shibahara J, Taniguchi H, Ishikawa S, Ito H, Yamamoto S, Iwanari H, Hironaka M, Ishikawa Y, Niki T, Sohara Y, Kodama T, Nishimura M, Fukayama M, Dosaka-Akita H, Aburatani H. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers’ non-small cell lung carcinomas. Clin. Cancer Res. 2005;11:1776–1785. doi: 10.1158/1078-0432.CCR-04-1238. [DOI] [PubMed] [Google Scholar]
- [39].Nagaraj NS, Beckers S, Mensah JK, Waigel S, Vigneswaran N, Zacharias W. Cigarette smoke condensate induces cytochromes P450 and aldo-keto reductases in oral cancer cells. Toxicol. Lett. 2006;165:182–194. doi: 10.1016/j.toxlet.2006.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer. 2008;59:340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- [41].Martin HJ, Maser E. Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. Chem. Biol. Interact. 2009;178:145–150. doi: 10.1016/j.cbi.2008.10.021. [DOI] [PubMed] [Google Scholar]
- [42].Srivastava S, Chandra A, Wang LF, Seifert WE, Jr., DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brichac J, Ho KK, Honzatko A, Wang R, Lu X, Weiner H, Picklo MJ., Sr. Enantioselective oxidation of trans-4-hydroxy-2-nonenal is aldehyde dehydrogenase isozyme and Mg2+ dependent. Chem. Res. Toxicol. 2007;20:887–895. doi: 10.1021/tx7000509. [DOI] [PubMed] [Google Scholar]
- [44].Gilks CB, Price K, Wright JL, Churg A. Antioxidant gene expression in rat lung after exposure to cigarette smoke. Am. J. Pathol. 1998;152:269–278. [PMC free article] [PubMed] [Google Scholar]
- [45].Toppo S, Flohe L, Ursini F, Vanin S, Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim. Biophys. Acta. 2009;1790:1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- [46].Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Imai H, Narashima K, Arai M, Sakamoto H, Chiba N, Nakagawa Y. Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. J. Biol. Chem. 1998;273:1990–1997. doi: 10.1074/jbc.273.4.1990. [DOI] [PubMed] [Google Scholar]