Abstract
Biological differences may underlie individual differences in impulsive behavior, such as choice for a smaller, more immediate reinforcer over a larger, more delayed reinforcer. Repeated exposure to drugs of abuse may have differing effects on such behavior. To evaluate acute and repeated effects of nicotine on impulsive choice, two strains of rats that have been shown to differ in impulsive choice were tested in a delay-discounting paradigm. Eight Lewis and eight Fischer 344 rats were allowed to choose between one food pellet delivered immediately and three food pellets delivered after a delay. The delay systematically increased in blocks of trials within each session, and the delay value at which choice for the two alternatives was equal (i.e., the indifference point) was interpolated. Effects of nicotine (0.1 – 1.0 mg/kg, s.c.) on percent choice and indifference points were determined during the acute-testing phase and during the re-determination of effects of each dose following at least 30 sessions of repeated 1.0 mg/kg nicotine exposure. Lewis rats had shorter indifference points (i.e., made fewer larger reinforcer choices) than the Fischer 344 rats. Acute nicotine administration increased mean larger-reinforcer choices at the 0.3 mg/kg dose in the Lewis rats and at the 1.0 mg/kg dose in the Fischer 344 rats. After repeated exposure to nicotine, indifference points returned to near baseline (pre-drug) levels for both strains. Strain differences were observed in rates of delay discounting and nicotine may decrease impulsive choice acutely, but this effect does not appear to be long-lasting.
Keywords: choice, delay discounting, Fischer 344, impulsivity, Lewis, nicotine, rat, self-control, temporal discounting
Introduction
Increased impulsive choice correlates with various psychiatric and behavioral disorders, such as attention-deficit/hyperactivity disorder (ADHD), gambling, aggression, suicide, and substance abuse. The correlation of increased impulsivity and substance abuse has been shown across drugs of various classes including opioids (Kirby et al. 1999; Madden et al. 1997), alcohol (Vuchinich and Simpson 1998; Petry 2001), cocaine/crack (Coffey et al. 2003; Kirby and Petry 2004), methamphetamine (Hoffman et al. 2006), and nicotine (Bickel et al. 1999; Mitchell 1999; 2004; Reynolds et al. 2004). It is not clear, however, what underlies this correlation between increased impulsive choice and substance abuse. Do individuals make more impulsive choices because of their history with substance use/abuse, does a history of making impulsive choices lead to substance abuse, or do other variables determine both types of behavior? There is growing evidence that increased impulsive choice precedes substance abuse, perhaps increasing susceptibility for the immediate reinforcement that drugs of abuse often provide. Some evidence of this may be found in the literature with human (e.g., Ernst et al. 2003; Dom et al. 2006) and non-human subjects (Logue et al. 1998; Perry et al. 2005). If this is the case, various factors such as differences in behavioral history, neurochemistry, or genetics, could be examined to shed additional light on the issue of what determines increased impulsive choice, subsequent drug taking, and drug effects. Within the framework of animal models, such variables can be systematically evaluated.
A frequently employed operational definition of “impulsivity” states that it is the choice for a smaller, more immediate reinforcer to the exclusion of a larger, more delayed reinforcer. “Self-control” is the converse choice. Increased impulsive choice, like that seen in substance abusers relative to non-abusing control subjects, may occur because the value of the delayed reinforcer (i.e., its effectiveness in maintaining behavior) decreases to a greater extent as the delay to its presentation increases. The delay-discounting hypothesis (Mazur 1987) and related findings (e.g., Green et al. 1994; Myerson and Green 1995; Evenden and Ryan 1996) support this behavioral outcome. When impulsive choices are made to the exclusion of self-controlled choices, overall reinforcement is reduced, making this a type of maladaptive behavior.
Since nicotine is a widely abused drug and greater delay discounting has been reported in cigarette smokers than non-smokers or ex-smokers (Bickel et al. 1999; Reynolds et al. 2004), effects of nicotine on impulsive choice warrant further study. Furthermore, since drug effects on choice may be influenced by individual differences, (e.g., neurochemistry, genetics) it is reasonable to explore this possibility in different strains of rats.
Lewis and Fischer 344 rat strains differ genetically, neurochemically, and behaviorally in ways that might be meaningful for studies of impulsive choice. Low levels of dopamine (DA) and serotonin (5-HT) have been implicated in increased impulsive choice or delay discounting (Wogar et al. 1993; Cherek et al. 1997; Asberg 1998; Cardinal et al. 2000; 2001; Lesch and Merschdorf 2000; Mobini et al. 2000; Crean et al. 2002; Winstanley et al. 2003; 2005; 2006). Decreased levels of DA and 5-HT have been observed in various brain regions of Lewis rats when compared to Fischer 344 rats (Burnet et al. 1996; Selim and Bradberry 1996; Flores et al. 1998; Lindley et al. 1999). Additionally, previous research has shown that Lewis rats make more impulsive choices than Fischer 344 rats in a delay-discounting task (Anderson and Woolverton 2005). Lewis rats are also more likely to self-administer various drugs of abuse, including cocaine, morphine, codeine, ethanol and nicotine than Fischer 344 rats (Li and Lumeng 1984; Suzuki et al. 1988a,b; Kosten et al. 1997; Martin et al. 1999, 2003; Brower et al. 2002). In addition, the development of a conditioned place preference following nicotine administration is more likely for Lewis rats than Fischer 344 rats (Horan et al. 1997; Philibin et al. 2005) and a conditioned place avoidance following nicotine withdrawal precipitated by mecamylamine, a nicotine antagonist, is also more likely for Lewis rats (Suzuki et al. 1999). Thus, because of established neurochemical and behavioral differences, these two strains are well suited for the study of effects of nicotine on delay discounting.
In animal models, variations of delay-discounting procedures have been used, but all procedures have common elements. Generally, the subject chooses between two reinforcers (usually food) differing in magnitude, with the smaller one delivered immediately and the larger one delivered either after an adjusting delay or a fixed delay that increases across trials in an experimental session. Indifference points (delay values where choice for either alternative is 50%) may be determined or interpolated, and comparisons may be made between groups of subjects or after administration of various doses of drugs (or other experimental variations).
In an adjusting-delay procedure (e.g., Dallery and Locey 2005), Long-Evans rats engaged in increased impulsive responding following acute and repeated (chronic) nicotine administration, but choice eventually returned to baseline levels following drug termination. These findings are interesting because if nicotine increases 5-HT and DA levels (e.g., Summers and Giacobini 1995; Singer et al. 2004), then decreases, rather than increases, in impulsive choice might be expected, as is seen with other stimulant-type drugs (e.g., Richards et al. 1999; Wade et al. 2000; Winstanley et al. 2005). To further explore effects of nicotine on impulsive choice in a different behavioral model and with different rat strains, the present experiment employed a variation of a procedure first reported by Evenden and Ryan (1996) in which the delay to three food pellets was increased within each experimental session in a fixed sequence. Choice was assessed between that option and immediate delivery of one food pellet. Effects of acute and repeated nicotine administration, followed by termination of drug administration, on large reinforcer choice and indifference points were then determined for the two rat strains, Lewis and Fischer 344. It was predicted that nicotine, because of its effects on 5-HT and DA, would decrease impulsive choice and that the Lewis rats would be more sensitive to these effects.
Methods
Subjects
Eight experimentally naïve, male Lewis rats and eight experimentally naïve, male Fischer 344 rats (Hilltop Lab Animals, Inc., Scottdale, PA) weighing approximately 300 g served as subjects. Subjects were housed individually with controlled environmental conditions (temperature, 24°C; 12-h reverse light/dark cycle) and continuous access to water. In addition, subjects were fed approximately 15 g of food immediately following the experimental session, and body weights were maintained or increased slightly throughout the duration of the experiment. Thus, the subjects were food-restricted for approximately 22 h preceding the experimental sessions, in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals. All procedures with animals were approved by West Virginia University’s Animal Care and Use Committee.
Apparatus
Experimental sessions were conducted in eight identical rat operant-conditioning chambers (Med Associates, St. Albans, VT). The working area inside each chamber measured 30.5 cm × 24.1 cm × 21.0 cm and had a grid floor. In each chamber, two response levers (each 4.8 cm × 1.9 cm) were mounted on one wall (11.5 cm apart, center to center, 8 cm above the floor) and a minimal downward force of 0.30 N was required to activate each lever. Between the two levers there was a receptacle in which 45-mg food pellets could be dispensed. Each chamber was illuminated by a single 28-V white light located on the wall opposite the two levers and by a white light (28-V, 2.5 cm in diameter) located above each lever. Extraneous noise was diminished by enclosing each chamber in a melamine sound-attenuating box and by operating a ventilation fan mounted on the outside of each box. Data collection and experimental events were controlled by a computer with MedPC-IV software and interfaces (Med Associates, St. Albans, VT) located in an adjacent room.
Procedure
After initial lever-press training, subjects were exposed to a discrete-trials choice procedure in which they chose between one food pellet delivered immediately following one lever press, and three delayed food pellets after pressing the opposite lever. Lever assignments were counterbalanced across subjects. This method was first described by Evenden and Ryan (1996) for use in studying impulsive choice in rats. Sessions consisted of five blocks of eight trials, which began after a 15-min pre-session blackout (fan ventilation on, but no illumination). The blackout allowed any administered drug to become physiologically active before behavior was assessed.
Trials were of two types, forced-choice and free-choice, and started every 100 s. The first two trials in each block were forced-choice trials that provided exposure to both contingencies before allowing a choice between them. In the first forced-choice trial of each block, one of the two lever lights, randomly determined, was illuminated and the outcome associated with that lever was available for lever pressing on a fixed-ratio (FR) 1 schedule. For the second forced-choice trial in the block, the other outcome was available as a consequence of pressing the other lever (FR 1). For example, for one outcome, the lever press resulted in an immediate delivery of one food pellet. After the variable intertrial interval (ITI) had elapsed, the houselight and the light above the other lever were illuminated and a lever press on that lever was followed by three food pellets delivered after a delay. The ITI duration was equal to 100 s minus the sum of the latency to respond and any programmed delay, and thus varied across blocks of trials and trials in each block.
After presentation of both outcomes at the beginning of a block during forced-choice trials, the next six trials in each block were free-choice trials. During free-choice trials, the houselight and lights above both levers were illuminated and the subject was allowed to choose one alternative (FR 1). The free-choice trial contingencies remained the same as those programmed during the forced-choice trials in that block. After completion of the six free-choice trials within a block, the delay to the larger reinforcer was increased and presented during the subsequent forced-choice trial. In the first block of trials, the delay to the larger reinforcer was 0 s. This value was then increased within each session in the following order: 0, 1, 2, 4, 6 s. This delay sequence was in effect until the rats were reliably responding throughout the sessions and the number of larger-reinforcer choices during the free-choice trials in the 0-s delay condition was 80% or greater for three consecutive sessions. Using that same protocol, the delays were then increased to 0, 2, 4, 8, 16 s followed by 0, 5, 10, 20, 40 s and ending with the terminal values of 0, 10, 20, 40, 60 s across the five blocks.
Sessions ended following 40 total (10 forced- and 30 free-choice) trials. If a subject failed to respond during the first 30 s of each trial, an omission was recorded and a 70-s ITI began. Regardless of a left response, a right response, or an omission, trials began every 100 s. Experimental sessions were conducted five days per week (Monday-Friday). Training conditions (each delay series) were in effect for at least five sessions and until responding was stable. Stable responding was defined as 80% or greater choice (at least 5 of 6 trials) for the larger reinforcer under equal delay (0-s) conditions and less than 20% variation between the number of larger reinforcer choices in each block, with no increasing or decreasing shifts of the delay-discounting curve across the last three sessions of a condition. Due to few larger-reinforcer choices in the condition with the 60-s terminal delay value, the final series was reduced to the one ending with a 40-s delay value. The terminal (baseline) condition (0, 5, 10, 20, 40-s delay series) was in effect for at least 10 sessions and until responding was stable. Mean percent choice for the larger reinforcer in each of the five blocks and interpolated indifference points were the primary dependent variables for both strains.
Once a week (Wednesdays), all delays for the larger reinforcer were set to 0 s for the entire session (a probe/control session). Since both outcomes (1 or 3 food pellets) were delivered immediately following a lever press, this enabled periodic assessment of discrimination and preference for the larger reinforcer. If fewer than 4 of the 6 responses during the free-choice trials occurred on the lever associated with the larger reinforcer in any block of the session, then a 0-s probe session was conducted during the following (consecutive) session until the criterion was met. Once the criterion was met, the increasing-delay (baseline) sessions were reinstated. These 0-s probe sessions were omitted during acute and repeated drug exposure (see below).
Drug Treatment
During acute drug administration, subcutaneous (s.c.) injections were given on Tuesdays and Fridays of each week. Data from Mondays and Thursdays served as non-drug control data. All injections were given immediately before the subject was placed in the chamber and the 15-min blackout commenced. Prior to any drug exposure, saline was administered in at least two sessions to ensure no behavioral disruption due to injection procedures. Then, nicotine was tested at doses of 1.0, 0.3, and 0.1 mg/kg, in a descending order, at least twice. Before repeated exposure to nicotine, 0-s probe sessions were reinstated to verify that subjects were discriminating the two reinforcer magnitude differences and choosing the larger one on at least 80% of the trials.
During repeated (chronic) drug administration, 1.0 mg/kg nicotine was administered prior to each experimental session for at least 30 sessions and until no increasing or decreasing trends in larger-reinforcer choice were observed. Effects of each dose previously tested (saline, 0.1, 0.3 mg/kg) were redetermined at least twice on Tuesdays and Fridays, with the repeated 1.0 mg/kg dose administered before all other sessions. Following assessment of effects of repeated exposure to nicotine, all injections were terminated (withdrawal of nicotine administration) for ten sessions. No 0-s probe sessions were conducted during repeated drug administration or the withdrawal phase.
Drugs
Each dose of nicotine was delivered in a saline vehicle at a concentration of 1.0 mg/ml and delivered in a volume of 1.0 ml/kg. Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO).
Data Analysis
Percent choice for the larger reinforcer as a function of delay to the larger reinforcer was the primary dependent variable. Reporting data in this way is consistent with other published studies (cf. Anderson and Woolverton 2005; Cardinal et al. 2000; 2001; Evenden and Ryan 1996; 1999) and thus allows for comparisons among studies that have used similar procedures. Repeated-measures ANOVAs were used to examine differences between the groups during baseline conditions and during drug administration. Follow-up tests (e.g., one-way ANOVAs and paired-samples t-tests) were employed as appropriate. For all analyses, statistical significance was defined as p < 0.05.
Additionally, indifference points (50% choice for each alternative) were interpolated for individual subjects. Interpolation of mean indifference points was done by fitting a logistic equation by non-linear regression using Microsoft Excel Solver. The logistic equation was selected because of history with it in our lab, where better fits to the data have been obtained over other models. Group means with standard error of the mean (SEM) are reported. Indifference points suggest the delay at which the value of the larger reinforcer is equal to that of the smaller, immediate reinforcer. Higher indifference points indicate more larger-reinforcer (self-controlled) choices. T-tests were conducted to examine differences in the mean indifference points between nicotine doses and the strains.
During assessment of baseline choice, one Fischer 344 rat died, so its data have been omitted from all analyses. During assessment of repeated nicotine effects one Lewis rat and one additional Fischer 344 rat died and their data have been omitted from the later analyses, i.e., chronic drug effects and withdrawal.
Results
Percent choice for the larger reinforcer alternative is presented for each block of trials (i.e., each delay) in Table 1. In the control (0-s) block, the Fischer 344 rats emitted a greater percentage of lever presses (M = 98.4%, range = 96.2–100%) on the alternative associated with the larger reinforcer than the Lewis rats (M = 91.9%, range = 87.8–98.8%). This difference between the strains was statistically significant [t(13) = −4.14, p < 0.01], but choice for both strains was above the 80% criterion value.
Table 1.
Mean percent larger-reinforcer choice and mean interpolated indifference points (in seconds) for Lewis and Fischer 344 rats
| 0 s | 5 s | 10 s | 20 s | 40 s | Ind.Pt. (s) | |
|---|---|---|---|---|---|---|
| LEWIS | ||||||
| Control | 91.9 (1.37) | 26.4 (3.82) | 5.1 (2.00) | 2.6 (1.25) | 0.3 (0.22) | 3.6 (0.22) |
| Acute | ||||||
| Saline [4.1; 3–6] | 93.3 (2.48) | 32.3 (4.20) | 8.5 (2.84) | 3.8 (1.38) | 2.0 (0.97) | 4.1 (0.33) |
| 0.1 [2.3; 2–3] | 86.0 (3.63) | 33.6 (9.53) | 5.3 (2.24) | 2.1 (1.39) | 1.1 (1.06) | 3.7 (0.63) |
| 0.3 [2.6; 2–3] | 95.1 (2.67) | 46.5 (5.09) | 7.7 (2.28) | 1.8 (1.19) | 1.8 (1.19) | 5.0 (0.23) |
| 1.0 [3.5; 3–4] | 92.7 (2.46) | 38.4 (3.80) | 8.7 (1.42) | 4.6 (1.59) | 1.3 (0.83) | 4.4 (0.19) |
| Post-Chronic | ||||||
| Saline [2.7; 2–3] | 90.4 (2.91) | 42.1 (7.36) | 4.1 (1.62) | 0.8 (0.81) | 0.0 (0.00) | 4.3 (0.49) |
| 0.1 [3.6; 3–4] | 83.2 (5.73) | 34.6 (5.42) | 2.4 (2.39) | 0.8 (0.81) | 2.0 (1.32) | 3.4 (0.52) |
| 0.3 [3.3; 3–4] | 84.7 (4.39) | 25.1 (7.90) | 7.8 (2.73) | 2.0 (1.35) | 0.0 (0.00) | 3.2 (0.63) |
| 1.0 [9.3; 8–12] | 94.2 (0.75) | 25.1 (4.69) | 3.8 (1.56) | 1.3 (0.68) | 0.9 (0.59) | 3.6 (0.29) |
| Withdrawal | 84.7 (3.86) | 20.8 (3.82) | 4.1 (0.88) | 2.2 (0.87) | 1.2 (0.61) | 2.8 (0.37) |
| FISCHER 344 | ||||||
| Control | 98.4 (0.62) | 36.6 (4.68) | 4.7 (1.30) | 2.5 (0.78) | 1.5 (0.45) | 4.4 (0.25) |
| Acute | ||||||
| Saline [4.7; 4–6] | 97.9 (1.04) | 47.5 (5.82) | 6.9 (1.73) | 3.1 (1.40) | 1.5 (0.73) | 5.0 (0.38) |
| 0.1 [2.7; 2–3] | 96.0 (2.01) | 37.7 (6.48) | 4.0 (2.01) | 0.0 (0.0) | 0.0 (0.00) | 4.5 (0.41) |
| 0.3 [2.6; 2–4] | 95.8 (1.61) | 45.2 (9.09) | 8.9 (5.79) | 1.8 (1.27) | 1.2 (1.21) | 5.2 (0.77) |
| 1.0 [2.7; 2–4] | 95.3 (3.04) | 65.0 (8.62) | 25.9 (9.70) | 14.2 (5.79) | 6.3 (2.23) | 8.3 (1.50) |
| Post-Chronic | ||||||
| Saline [3.2; 2–6] | 94.4 (3.53) | 35.6 (5.09) | 3.8 (2.02) | 2.3 (1.51) | 0.0 (0.00) | 4.3 (0.27) |
| 0.1 [3.4; 2–6] | 92.6 (5.83) | 34.1 (8.87) | 5.3 (3.87) | 2.7 (1.40) | 0.5 (0.47) | 4.1 (0.79) |
| 0.3 [3.4; 2–6] | 87.5 (6.95) | 37.1 (11.06) | 12.8 (5.16) | 6.3 (3.20) | 2.4 (1.85) | 4.2 (1.15) |
| 1.0 [10.0; 9–12] | 88.9 (4.95) | 41.2 (9.58) | 16.1 (4.88) | 6.4 (1.45) | 6.3 (1.69) | 4.6 (1.02) |
| Withdrawal | 93.3 (3.01) | 38.8 (6.60) | 8.1 (2.94) | 2.7 (1.42) | 2.3 (0.73) | 4.5 (0.55) |
Data are shown for all experimental conditions (control, acute dose determinations, dose determinations after repeated drug exposure, and withdrawal). For all values, SEM is given in parentheses. Nicotine doses are mg/kg, with the mean number of dose determinations and range in parentheses.
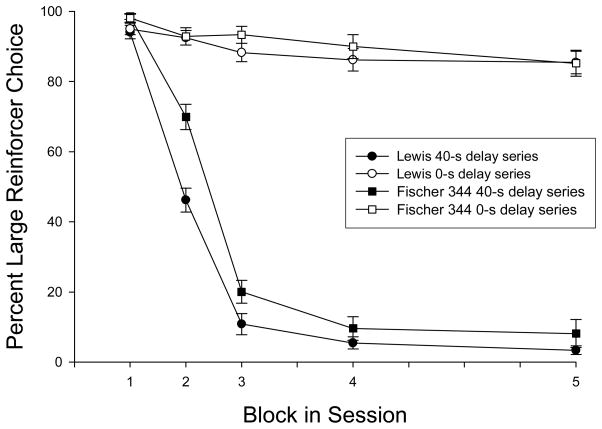
Baseline training took a mean of 150.4 (± 2.06) sessions for the Lewis rats and a mean of 160.3 (±0.57) sessions for the Fischer 344 rats. This difference was statistically significant [t(13) = −4.36, p < 0.01]. Within those baseline training sessions, the number of 0-s probe sessions (all delays 0 s) were 33.3 (±2.05) for the Lewis rats and 25.0 (±0.53) sessions for the Fischer 344 rats. This difference was statistically significant [t(13) = 3.65, p < 0.01]. Thus, the Lewis rats had fewer baseline training sessions while requiring more 0-s probe sessions and they, on average, chose the larger reinforcer less often in the 0-s (control) block during training than the Fischer 344 rats. Despite these strain differences, both groups met or exceeded stability criteria. Figure 1 shows the mean percent larger-reinforcer choice during the last five baseline sessions and the last five presentations of the 0-s delay probe sessions for Lewis and Fischer 344 rats. Choice for the larger reinforcer was nearly exclusive during the 0-s delay probe sessions, but decreased as a function of increasing delays during baseline sessions. This shows that, before any drug testing commenced, both strains of rats were sensitive to the differing reinforcer amounts and delays.
Figure 1.
Mean percent large reinforcer choice by Lewis and Fischer 344 rats, as a function of reinforcer delay in each of the five blocks during a session. Data are presented from the last five sessions of the baseline condition (the 0–5–10–20–40-s delay series is represented by the block number, respectively; filled symbols) and the last five probe sessions (the 0–0–0–0–0-s delay series is also represented by the respective block number; open symbols). Data are mean values ± SEM.
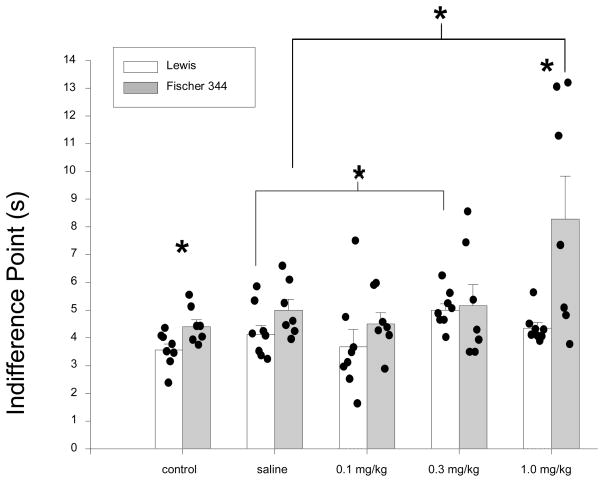
Under control (non-drug) conditions, the Lewis rats emitted fewer large reinforcer choices than the Fischer 344 rats (see Table 1). A repeated-measures ANOVA revealed statistically significant differences in percent larger-reinforcer choice between the strains [F(1, 13) = 1077.23, p < 0.01]. For all subjects in both strains, however, percent larger-reinforcer choice decreased as the delay to the presentation of the food pellets increased. This is supported by the finding of a significant main effect of delay [F(4,52) = 771.05, p < 0.01)] that was qualified by a significant delay × group interaction [F(4, 52) = 2.63, p < 0.05]. However, in baseline conditions, Fischer 344 rats generally chose the three-pellet alternative more often than the Lewis rats. Similar conclusions were identified using the indifference points as a dependent measure. The mean indifference point for the Lewis rats was 3.58 s (± 0.22) and 4.43 s (± 0.25) for the Fischer 344 rats (see the first set of bars in Figure 2). This difference between the strains was statistically significant [t(13) = −2.59, p <= 0.02].
Figure 2.
Mean indifference points for Lewis and Fischer 344 rats under non-drug (control) conditions and following acute administration of saline or nicotine (0.1, 0.3, 1.0 mg/kg). Data from individual subjects are represented by points overlaid on each bar. Variance is represented by standard error bars around the mean and asterisks indicate statistically significant differences between the strains at the doses tested (control and 1.0 mg/kg) and between saline and nicotine (0.3 mg/kg, Lewis and 1.0 mg/kg, Fischer 344) within each strain (p < 0.05).
When acute nicotine was administered, it was found that, when averaging across delay values and collapsing across subjects, the administration of nicotine altered larger-reinforcer choice. Paired-samples t-tests were used to clarify the significant main effect of dose. Statistically significant differences were observed between saline (M = 29.83± 1.15) and 1.0 mg/kg nicotine (M = 35.16 ± 2.75) [t(13) = −2.17, p < 0.05], 0.1 mg/kg nicotine (M = 27.10 ± 1.41) and 0.3 mg/kg nicotine (M = 30.55 ± 2.75) [t(13) = −2.64, p < 0.05], and 0.1 mg/kg and 1.0 mg/kg [t(13) = −3.15, p < 0.01].
For both strains, one dose of nicotine tested increased mean indifference points relative to acute saline effects (see Figure 2). For the Lewis rats, this dose was 0.3 mg/kg [t(7) = −3.20, p < 0.02] and for the Fischer 344 rats, this dose was 1.0 mg/kg [t(7) −2.32, p < 0.05]. The mean indifference points represent the individual-subject data as 7 of 8 Lewis rats and 5 of 7 Fischer 344 rats had increased indifference points following these doses of nicotine (see Table 2). The effect on indifference points at the highest dose tested (1.0 mg/kg) was statistically significant between the two strains [t(13) = −2.72, p < 0.02], with a greater increase observed for the Fischer 344 rats. Determination of the acute effects of nicotine took a mean of 37.0 (± 1.36) sessions for the Lewis rats and a mean of 39.3 (± 0.29) sessions for the Fischer 344 rats. This difference was not statistically significant.
Table 2.
Individual and mean indifference points (s) and percent change from acute saline conditions at the acute nicotine dose that resulted in a statistically significant difference from saline (0.3 mg/kg for Lewis rats and 1.0 mg/kg for Fischer 344 rats).
| Subject Number | Saline | 0.3 mg/kg | % Change |
|---|---|---|---|
| L1 | 4.1 | 4.8 | 17.1 |
| L2 | 5.2 | 4.6 | −11.5 |
| L3 | 5.8 | 6.2 | 6.9 |
| L4 | 3.4 | 4.6 | 35.3 |
| L5 | 3.3 | 4.0 | 21.2 |
| L6 | 4.1 | 5.2 | 26.8 |
| L7 | 4.0 | 5.5 | 37.5 |
| L8 | 3.1 | 5.0 | 61.3 |
| Mean | 4.1 | 5.0 | 25.4 |
| Subject Number | Saline | 1.0 mg/kg | % Change |
| F1 | 6.6 | 13 | 97.0 |
| F2 | 5.2 | 11.2 | 115.4 |
| F3 | 4.4 | 7.3 | 65.9 |
| F4 | -- | -- | -- |
| F5 | 6.1 | 5.0 | −18.0 |
| F6 | 3.9 | 4.7 | 20.5 |
| F7 | 4.6 | 13.1 | 184.8 |
| F8 | 4.2 | 3.7 | −11.9 |
| Mean | 5.0 | 8.3 | 59.4 |
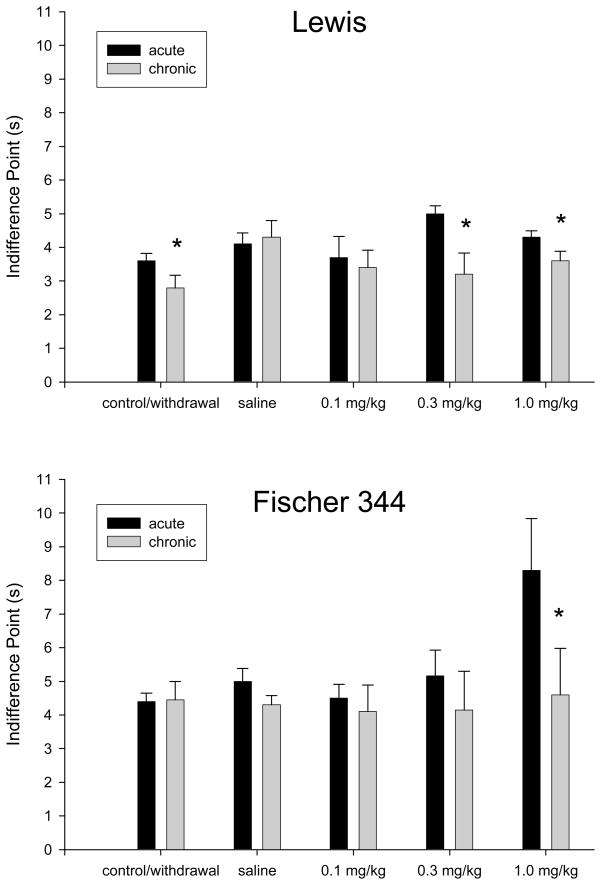
After repeated pre-session nicotine administration (chronic 1.0 mg/kg), mean indifference points for both strains returned to near control (non-drug) levels. These reductions in mean indifference points following repeated 1.0 mg/kg nicotine exposure were statistically significant for both groups (see Figure 3, last set of bars). Mean indifference points between acute and repeated 1.0 mg/kg nicotine for Lewis rats fell from 4.35 s to 3.63 s [t(7) = 2.54, p < 0.05] and for Fischer 344 rats fell from 8.29 s to 4.60 s [t(6) = 3.14, p < 0.02]. Figure 3 shows, for both strains, a comparison of mean indifference points following acute and repeated (chronic) administration of each dose. Exposure to repeated nicotine administration (including determinations of effects of all previous doses) lasted a mean of 66.43 (± 0.90) sessions for the Lewis rats and a mean of 65.83 (± 1.08) sessions for the Fischer 344 rats. This difference was not statistically significant.
Figure 3.
Mean indifference points for Lewis rats (upper panel) and Fischer 344 rats (lower panel) under non-drug (control vs. withdrawal) conditions and following acute and repeated administration of saline or nicotine (0.1, 0.3, 1.0 mg/kg). Values are means (± SEM) and asterisks indicate statistically significant differences from acute means at the same dose (p < 0.05).
A mixed three-way repeated-measures ANOVA with strain as a between-subjects factor and dose and delay as within-subjects factors performed on the chronic-dosing data revealed a significant three-way (dose by delay by strain) interaction [F(16, 176) = 2.57, p < 0.01], a significant dose-by-delay interaction [F(16, 176) = 2.41, p < 0.01], and a significant main effect of delay [F(4, 44) = 402.35, p < 0.01]. To further analyze the significant three-way interaction, repeated-measures ANOVAs were conducted for the Lewis and Fischer 344 rats separately. For the Lewis rats, a significant main effect of delay was found [F(4, 24) = 261.93, p < 0.01]. However, no statistically significant effect of drug dose was found. For the Fischer 344 rats, a significant main effect of delay [F(4, 20) = 155.97, p < 0.01] and dose-by-delay interaction [F(16, 80) = 2.03, p < 0.01] were found. When paired-samples t-tests were conducted comparing the average percent choice between drug conditions, a statistically significant difference was observed between 0.1 mg/kg and 1.0 mg/kg nicotine [t(5)= −3.45, p < 0.05], with the 1.0 mg/kg dose resulting in larger percent larger-reinforcer choice. Thus, for the Lewis rats, there were no statistically significant dose-related differences when collapsing across delay values during chronic drug administration, but for the Fischer 344 rats, larger-reinforcer choice was differentially affected by the lower (0.1 mg/kg) and higher (1.0 mg/kg) doses.
Upon termination of pre-session nicotine exposure (withdrawal on Figure 3), the mean indifference point for the Fischer 344 rats (4.45 s, ± 0.55) approximated that observed under control (non-drug) conditions (4.43 s, ± 0.25), and no statistically significant difference was observed (see Figure 3, first set of bars). The difference between the same points in Lewis rats, however, was statistically significant [t(7) = 2.93, p < 0.02] as the mean indifference point fell from 3.58 s (± 0.22) to 2.81 s (± 0.38). For both groups, the percent choice for the larger reinforcer in the 0-s block was lower than under control conditions, but was still above criterion (see the Table). Throughout the study, either no or few response omissions were observed.
Discussion
For all subjects, choice for the larger reinforcer decreased as the delay to its presentation increased, and delay discounting (cf. Mazur 1987) was thus supported. Strain differences in rate of delay discounting were observed. Lewis rats emitted fewer larger-reinforcer choices (i.e., were more impulsive) than the Fischer 344 rats. This is also indicated by the shorter indifference points observed in the Lewis strain. The finding of increased impulsive choice in Lewis rats, compared to Fischer 344 rats, supports that reported in previous literature (Anderson and Woolverton 2005; Madden et al. 2008).
Lewis rats, on average, required more early training with the 0-s probe sessions. This strain difference may be due to differences in the discrimination of reinforcer amount, which was evaluated during the 0-s probe sessions. With repeated exposures to the 0-s delay probe conditions, however, and by the time of drug testing, both strains were responding above criterion.
In early training when the 0–10–20–40–60-s delay series was used, as based on the work of Evenden and Ryan (1996; 1999), responding was nearly exclusive for the smaller reinforcer during the later blocks, so the delays were reduced to a 0–5–10–20–40-s delay series to minimize a “floor effect”. This might indicate a strain difference between the Lewis and Fischer 344 rats used in the present study and the Sprague-Dawley rats used by Evenden and Ryan (1996, 1999). Additional research could explore differences in other rat strains (e.g., Wilhelm and Mitchell 2009). It is noted that somewhat of a “floor effect” still existed after decreasing the delays and any potential decreases in large reinforcer choice following nicotine administration may be difficult to identify at the longer delays. A similar floor effect was observed with the Lewis rats in the study by Anderson and Woolverton (2005). Interestingly, the Fischer 344 rats in the current study selected the large reinforcer less frequently than the Fischer rats in the Anderson and Woolverton (2005) study. This difference may be accounted for by procedural variations. For example, in the present study, the delay values were systematically increased before being decreased to 40 s. The additional exposure to the procedure in the present study may account for the relatively steep discounting functions obtained. Future work with these strains may adjust delay values such that both decreases and increases in larger-reinforcer choice may be observed. Despite the slight “floor effect” in the present study, the increases in larger-reinforcer choice are still significant. Had drug administration resulted in decreases in larger-reinforcer choice rather than increases, the “floor effect” would be more problematic.
With regard to the baseline differences in choice seen between Lewis and Fischer 344 rats, neurochemistry may be an important factor. Differences in DA and 5-HT systems exist between Lewis and Fischer 344 rats and these systems have been implicated in impulsive choice or delay discounting (see Winstanley et al. 2006 for a review). More specifically, decreased levels of DA and 5-HT have been correlated with increased impulsive behaviors in humans (e.g., Cherek et al. 1997; Asberg 1998; Lesch and Merschdorf 2000) and rats (e.g., Wogar et al. 1993; Cardinal et al. 2000). Lewis rats not only have been shown to make more impulsive choices than Fischer 344 rats, but have also been shown to have hypofunctioning DA and 5-HT systems when compared to Fischer 344 rats. It has been reported that Lewis rats have lower levels of DA in the nucleus accumbens shell (Sziraki et al. 2001) and in the dorsal striatum (Lindley et al. 1999) and lower levels of DA receptors and DA transporters in the striatum and nucleus accumbens (Flores et al. 1998). Lewis rats also have been shown to have lower levels of 5-HT in the nucleus accumbens (Selim and Bradberry 1996) and fewer 5-HT1A binding sites in the hippocampus and frontal cortex (Burnet et al. 1996) than Fischer 344 rats. Whether or not any of these (and other) neurochemical differences underlie the behavioral differences observed in impulsive choice between the two rat strains warrants further study. Although more work in the neurobiological determinants of impulsive behavior needs to be conducted, a foundation has been laid by the existing literature. To further expand on the investigations into roles for DA and 5-HT, use of other rat strains that vary with regard to these systems may prove fruitful.
Lewis rats were more sensitive to the acute effects of nicotine on larger-reinforcer choice, as statistically significant increases were observed at a lower dose than that seen in Fischer 344 rats. Although nicotine increased larger-reinforcer choice at the highest dose administered (1.0 mg/kg) in the Fischer 344 strains, only the Lewis rats showed increases with a lower (0.3 mg/kg) dose. The differences in baseline choice and differential sensitivity to the behavioral effects of nicotine may be attributed to differences in neurochemistry or pharmacokinetics between the two rat strains (cf. Sziraki et al. 2001). This increase in sensitivity to nicotine in Lewis relative to Fischer 344 rats is supported in other literature that includes self-administration (Brower et al. 2002), conditioned place preference (Horan et al. 1997; Philibin et al. 2005), drug discrimination (Philibin et al. 2005), and pharmacokinetic profile (Sziraki et al. 2001). It should be noted that the highest dose of nicotine tested in the Lewis rats (1.0 mg/kg) resulted in increased mean indifference points, relative to those following saline administration, but they did not reach statistical significance as they did with the Fischer 344 rats. Why the increase in larger-reinforcer choice was seen with 0.3 mg/kg, but not with 1.0 mg/kg in the Lewis rats is not clear. It is possible that the effect is bitonic, but higher doses were not tested in the Fischer 344 rats due to concerns of toxicity.
In the present study, although lower doses of nicotine had a greater effect on decreasing impulsive choice of Lewis rats than Fischer 344 rats, there was an overall decrease in impulsive choice in both strains at one of the highest doses tested. This finding of a stimulant-like drug decreasing impulsive choice is consistent with previous findings with other stimulant drugs such as methamphetamine and amphetamine (e.g., Richards et al. 1999; Wade et al. 2000). Drugs that increase DA and 5-HT may, as a result of effects in these neurochemical systems, decrease impulsive behavior. It should be acknowledged, however, that there are variations or exceptions to this finding with nicotine (e.g., Blondel et al. 1999; Dallery and Locey 2005), but differences in operational definitions of “impulsivity” or other procedural variations may account for the discrepant outcomes. For instance, in the study of Blondel et al.(1999), nicotine only increased “impulsive responding” following repeated administration. When given acutely, nicotine improved performance (e.g., decreased “anticipatory responses”) in the five-choice serial reaction time task. At least with this finding, there may be similarities with the present study. However, the operational definition of “impulsivity” used by Blondel et al. (1999) is different than that used in the present study. The effects of repeated administration are somewhat different, but may map onto the return-to-baseline choice observed in both strains. Further work may be aimed at identifying common mechanisms between various procedures and operational definitions (cf. Evenden 1999).
Opposite drug effects in a more similar procedure were presented by Dallery and Locey (2005). In their study, an adjusting-delay procedure was used with Long-Evans rats. This procedure resulted in unstable data in about half of the subjects, so the final drug analyses were based on five of the nine rats. Of the five rats, the mean adjusted delays (where three food pellets were equal in value to the one, immediate food pellet) ranged from 17.9 s to 22.4 s at stability. These delays are much longer than the interpolated indifference points in the present study (Lewis = 3.6 s and Fischer 344 = 4.4 s) and these baseline differences may have affected the effects of nicotine on choice. Perhaps the effects of nicotine are to decrease relatively long indifferences points and increase relatively short ones. The use of different procedures may also impact sensitivity to reinforcement amount or delay, and drugs may have different effects on choice as a result of varying impact on such behavioral mechanisms (cf. Pitts and Febbo 2004). It should also be noted that the choice patterns seen with delays that adjust throughout the session or delays that are presented in a fixed order may be influenced by pharmacokinetic factors. Presenting delays in different orders may reveal such effects.
In addition to the differences in delay presentation between the study of Dallery and Locey (2005) (adjusting delays) and the present one (fixed delays), the former study also included were delay-correlated stimuli. The addition of colored (red or green) lever lights during the delay may have impacted choice, although Dallery and Locey (2005) argue that it would do so equally between the large and small alternatives. In the present study, the loss of lever lights was an immediate consequence of either lever press. It is not clear what impact presenting or removing stimuli has on choice in isolation or in combination with nicotine, although nicotine may enhance the effectiveness of conditioned reinforcers in other situations (Raiff and Dallery 2006).
Furthermore, the use of a different rat strain (Long-Evans) may account for some of the differences between the present study and that of Dallery and Locey (2005). It is possible that the neurochemical differences between these strains may result in differential responses to nicotine administration. Certainly, there is much to be learned about the behavioral and neurochemical variables that determine impulsive choice and the specific conditions under which stimulant drugs like nicotine may decrease or increase impulsive behavior.
With regard to behavioral variables, it is possible that nicotine affected sensitivity to reinforcer delay or to reinforcer magnitude (see Pitts and Febbo, 2004). Another factor that may warrant future investigation is that nicotine increased the probability of perseveration on the lever associated with the larger-reinforcer. It is not clear, however, why nicotine would result in selective perseveration on the lever associated with the larger, but not the smaller, reinforcer. Also, during forced-choice trials, subjects responded on both alternatives, which were counterbalanced across subjects, before choosing between them. The levers were presented randomly during these forced-choice trials, so it does not seem that there was an effect of the subject just staying in front of the last lever presented, because that would have resulted in indifference during the free-choice trials. It would appear then, that there was a selective effect of nicotine to increase larger-reinforcer choice. The mechanisms behind this effect require additional study.
Stimulant-like drugs such as nicotine may work to decrease impulsive choice due to their effects on DA and 5-HT systems. Nicotine is known to increase DA and 5-HT concentrations in various brain regions (e.g., Summers and Giacobini 1995; Singer et al. 2004), and increased delay discounting or impulsive choice is correlated with decreases in DA and 5-HT (see Winstanley et al. 2006 for a review). Better understanding of the relevant mechanisms of action of drugs such as nicotine may aid in drug development for impulse-control disorders. In addition to specifying better the mechanisms of drug actions, as through the use of receptor-specific ligands, it is necessary to evaluate effects of long-term exposure to various drugs. In the present study, the initial effects of nicotine on increasing self-control choice were diminished after repeated exposure to the drug, possibly suggesting the development of tolerance. This is an interesting finding in that the drug’s early effect was to increase overall reinforcement density, but it was not a long-lasting effect. Having exposure to, and experience with, increased reinforcement was not sufficient to produce long-term changes in choice. With repeated exposure to nicotine, the initial effects were diminished in both strains. The Lewis rats, following termination of nicotine administration, made more impulsive choices than before drug exposure. Thus, repeated exposure to drugs of abuse, such as nicotine, may result in changes that lead to increased impulsive choice in some individuals already at risk for such maladaptive behavior. It is important to note that development of tolerance to the effects of nicotineon impulsive choice, although in an opposite direction and in a different procedure with a different rat strain, have also been reported (Dallery and Locey 2005).
The differential effects following nicotine withdrawal may also be due to differences in DA and 5-HT systems (see Suzuki et al. 1999 for a review). In the study of Suzuki et al. (1999), aversion associated with mecamylamine-precipitated nicotine withdrawal (assessed with place conditioning) was greater in the Lewis rats when compared to Fischer 344 rats. The authors concluded that this aversion is heavily impacted by genetic factors and that the Lewis rats have greater sensitivity to physical dependence to nicotine than Fischer 344 rats. It has been shown that nicotine withdrawal decreases D2 binding in the nucleus accumbens and decreases DA in the striatum and nucleus accumbens in Sprague-Dawley rats (Fung et al. 1996). If a similar effect occurs in other strains and Lewis rats have reduced DA and DA receptors in the nucleus accumbens and other areas compared to Fischer 344 rats (see above), then it would be expected that Lewis rats have increased sensitivity to nicotine withdrawal. Such a finding would support the differential effects of nicotine withdrawal in the present study. The increase in impulsive choice observed in Lewis rats following nicotine withdrawal may be accounted for by their lower baseline and post-withdrawal levels of DA and DA receptors, since lower levels of DA correlate with increased impulsive choice.
Another consideration surrounds better characterization of the impact of response-independent versus response-dependent drug administration on impulsive choice (delay discounting). In the present study and others (e.g., Wade et al. 2000; Winstanley et al. 2005), a drug with abuse potential, particularly with stimulant-like properties, decreased delay discounting. This effect is in contrast to the well-established correlation between substance abuse and increased delay discounting. Although there is a correlation between increased impulsive choice (delay discounting) and self-administration of abused drugs such as nicotine, amphetamine, and cocaine (e.g., Perry et al. 2005), there is evidence that experimenter-injected drugs with stimulant-like effects may decrease impulsive choice (e.g., Richards et al. 1999; Wade et al. 2000). Thus, the behavioral processes underlying effects of response-independent and response-dependent drug administration should be further explored.
In summary, Lewis rats had higher rates of delay discounting, i.e., made more impulsive choices, than Fischer 344 rats. This supports earlier work and may suggest that genetic or neurochemical differences play a role in individual differences seen in impulsive behavior. Further work should address how these differences may lead to maladaptive choices, e.g., drug abuse, pathological gambling, suicide. Also, the finding that acute administration of nicotine reduced impulsive choice in both strains of rats, but that the effect was not long lasting, has potential implications for pharmacologic treatment of impulse-control disorders.
Acknowledgments
The authors wish to acknowledge Mirari Elcoro for technical support and for writing the computer program used to conduct the daily experimental sessions. Rebecca Dover is acknowledged for additional technical support in the laboratory. Paul Soto, Ph.D. generously provided the program used to calculate indifference points. West Virginia University and the National Institutes of Health DA-019842 provided financial support of this project.
Funding for this project came from NIH DA-019842 to K.G.A.
References
- 1.Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Annals New York Academy of Science. 1998;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- 3.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 4.Blondel A, Simon H, Sanger DJ, Moser P. The effect of nicotine administration on the performance of drug-naive rats in a five-choice serial reaction time task. Behav Pharmacol. 1999;10:665–673. doi: 10.1097/00008877-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- 6.Burnet PW, Mefford IN, Smith CC, Gold PW, Sternberg EM. Hippocampal 5-HT1A receptor binding site densities, 5-HT1A receptor messenger riboneucleic acid abundance and serotonin levels parallel the activity of the hypothalamo-pituitary-adrenal axis in rats. Behav Brain Res. 1996;73:365–368. doi: 10.1016/0166-4328(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 7.Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signaled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- 8.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 9.Cherek DR, Moeller FG, Dougherty DM, Rhoades H. Studies of violent and nonviolent male parolees: II. Laboratory and psychometric measurements of impulsivity. Biol Psychiatry. 1997;41:523–529. doi: 10.1016/s0006-3223(96)00426-x. [DOI] [PubMed] [Google Scholar]
- 10.Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Crean J, Richards JB, de Wit H. Effects of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav Brain Res. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 12.Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Dom G, D’haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006;101:50–59. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- 14.Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psych. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 16.Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 17.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: VI. The effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- 18.Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptor levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- 19.Fung YK, Schmid MJ, Anderson TM, Lau YS. Effects of nicotine withdrawal on central dopamine systems. Pharmacol Biochem Behav. 1996;53:635–640. doi: 10.1016/0091-3057(95)02063-2. [DOI] [PubMed] [Google Scholar]
- 20.Green L, Fistroe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychon Bull Rev. 1994;1:383–389. doi: 10.3758/BF03213979. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 22.Horan B, Smith M, Gardner EL, Lepore M, Ashby CR. (-)Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–94. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 24.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-abusing controls. J Exp Psych: Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Kosten TA, Miserendino MJD, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- 26.Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Li TK, Lumeng L. Alcohol preference and voluntary intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol: Clin Exp Res. 1984;8:485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindley SE, Bengoechea TG, Wong DL, Schatzberg AF. Strain differences in mesotelencephalic dopaminergic neuronal regulation between Fischer 344 and Lewis rats. Brain Res. 1999;832:152–158. doi: 10.1016/s0006-8993(99)01446-8. [DOI] [PubMed] [Google Scholar]
- 29.Logue SF, Swartz RJ, Wehner JM. Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol: Clin Exp Res. 1998;22:1912–1920. [PubMed] [Google Scholar]
- 30.Madden GJ, Petry NM, Dadger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- 31.Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J Exp Anal Behav. 2008;90:333–44. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- 33.Martin S, Lyupina Y, Crespo JA, Gonzalez B, Garcia-Lecumberri C, Ambrosio E. Genetic differences in NMDA and D1 receptor levels, and operant responding for food and morphine in Lewis and Fischer 344 rats. Brain Res. 2003;973:205–213. doi: 10.1016/s0006-8993(03)02482-x. [DOI] [PubMed] [Google Scholar]
- 34.Mazur JE. An adjusting procedure for studying delayed reinforcement. The effect of delay and intervening events on reinforcement value. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior. Vol. 5. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- 35.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cog Neurosci Rev. 2004;3:261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- 37.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- 38.Myerson J, Green L. Discounting of delayed rewards: models of individual choice. J Exp Anal Behav. 1995;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 40.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 41.Philibin SD, Vann RE, Varvel SA, Covington HE, III, Rosecrans JA, James JR, Robinson SE. Differential behavioral responses to nicotine in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Pitts RC, Febbo SM. Quantitative analyses of methamphetamine’s effects on self-control choices: implications for elucidating behavioral mechanisms of drug action. Behav Proc. 2004;66:213–233. doi: 10.1016/j.beproc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Exp Clin Psychopharmacol. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Proc. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 45.Richards JB, Sabol KE, deWit H. Effects of methamphetamine on the adjusting-amount procedure: a model of impulsive behavior in rats. Psychopharmacology. 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- 46.Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- 47.Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29:1779–1792. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- 48.Summers KL, Giacobini E. Effects of local and repeated systemic administration of (-)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res. 1995;20:753–759. doi: 10.1007/BF01705545. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988a;245:164–170. [PubMed] [Google Scholar]
- 50.Suzuki T, Otani K, Koike Y, Misawa M. Genetic differences in preferences for morphine and codeine in Lewis and Fischer 344 inbred rat strains. Jap J Pharmacol. 1988b;47:425–431. doi: 10.1254/jjp.47.425. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Ise Y, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. Eur J Pharmacol. 1999;369:159–162. doi: 10.1016/s0014-2999(99)00086-2. [DOI] [PubMed] [Google Scholar]
- 52.Sziraki I, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;26:609–617. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- 53.Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;150:90–101. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 54.Wade TR, deWit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- 55.Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- 57.Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacol. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 58.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–243. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]