Abstract
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS). MS is thought to be T-cell-mediated, with prior research predominantly focusing on CD4+ T-cells. There is a high prevalence of CNS-specific CD8+ T-cell responses in MS patients and healthy subjects. However, the role of neuroantigen-specific CD8+ T-cells in MS is poorly understood, with the prevalent notion that these may represent pathogenic T-cells. We show here that healthy subjects and MS patients demonstrate similar magnitudes of CD8+ and CD4+ T-cell responses to various antigenic stimuli. Interestingly, CD8+ T-cells specific for CNS autoantigens, but not those specific for control foreign antigens, exhibit immune regulatory ability, suppressing proliferation of CD4+CD25- T-cells when stimulated by their cognate antigen. While CD8+ T-cell-mediated immune suppression is similar between healthy subjects and clinically quiescent treatment-naïve MS patients, it is significantly deficient during acute exacerbation of MS. Of note, the recovery of neuroantigen-specific CD8+ T-cell suppression correlates with disease recovery post-relapse. These studies reveal a novel immune suppressor function for neuroantigen-specific CD8+ T-cells that is clinically relevant in the maintenance of peripheral tolerance and the intrinsic regulation of MS immune pathology.
Keywords: multiple sclerosis, CD8, Treg, immune regulation, exacerbation, suppressor
1. Introduction
Multiple sclerosis (MS) is the most common disabling neurological disease of young people, typically presenting as a relapsing-remitting form (RRMS). MS is thought to be immune-mediated and is characterized by temporally and spatially separated central nervous system (CNS) lesions that may be accompanied by acute exacerbation of clinical symptoms, which remit over time with limited accumulating disability. The immune dysregulation that underlies the pathology of MS and its clinical exacerbations remains poorly understood. Much of our understanding of the immunology of MS derives from work in the murine model, experimental autoimmune encephalomyelitis (EAE). The vast majority of studies in MS and EAE have focused on the role of CD4+ T-cells as mediators and regulators of disease. The preponderant belief is that MS and EAE are mediated through CNS-specific CD4+ Th1/Th17 responses and regulated by CD4+ regulatory T-cells [1]. However, considerable evidence points to an important role for CD8+ T-cells in the pathogenesis and/or regulation of MS and EAE [2-18]. CNS lesions show a predominance of CD8+ T-cells with oligoclonal expansion [4], indicating an active role at the site of pathology. While it is thought that these cells represent a key pathogenic element of MS lesions, neither the antigenic specificity of these cells nor their role has been elucidated. MS patients show a high prevalence of CNS-specific CD8+ T-cell responses in their circulation [3]. These cells appear to have a mixed functional phenotype in that they express cytotoxic/inflammatory as well as regulatory effector molecules [3]. Again, the intuitive function attributed to these responses is that of pathogenesis. However, their role has not been adequately investigated. Moreover, healthy subjects also harbor such responses, raising the possibility that they may not be purely pathogenic.
Antigen-specific immune regulation has high therapeutic potential. Global defects in immune regulatory T-cell (Treg) function have been demonstrated in a wide variety of human immune-mediated diseases [14, 19-27]. In most cases, antigenic specificity of the regulatory population is poorly defined and this would be key in the ability to expand and utilize such populations. In the current study, we investigated the role of neuroantigen-specific CD8+ T-cells in MS and discovered an unexpected, novel and clinically relevant immune regulatory role for autoantigen-specific T-cells. This role has both biologic and therapeutic implications.
2. Methods and Materials
2.1. Subject characteristics
MS patients were recruited and gave written informed consent at the UT Southwestern Clinical Center for Multiple Sclerosis. Table 1 summarizes patient characteristics. 11 treatment-naïve adult clinically definite RRMS patients (McDonald criteria) with quiescent disease were recruited. Exclusion criteria included pregnancy, HIV positivity, active cancer, other autoimmune, immunosuppressive, neurodegenerative conditions, clinical relapse or corticosteroid treatment within last 3 months and any history of disease-modifying immunomodulatory therapy. In addition, 9 treatment-naïve MS patients were recruited during an active acute clinical episode/relapse. 15 healthy subjects were recruited as controls (HC). All studies were approved by the UT Southwestern IRB according to Declaration of Helsinki principles.
Table 1. Summary of Patient Characteristics.
| Healthy Controls (HC) | RRMS: Quiescent (MS) | MS: Acute Exacerbation | MS: Exacerbation Follow-up | |
|---|---|---|---|---|
| Number of Subjects | 15 | 11 | 9 | 4 |
| Average age, y (Range) | 44 (21-65) | 40 (23-56) | 45 (31-65) | 44 (35-53) |
| Sex (M/F) | 5/10 | 2/9 | 3/6 | 2/2 |
| Days from Last Relapse [Mean (Range)] | N/A | 599 (90-2920) | 8 (2-50) | 81 (31-118) |
2.2. Cell preparation and bead sorting
PBMC were isolated from whole blood using Ficoll Hypaque (GE Healthcare Biosciences, Pittsburgh, PA) density gradient. Purified CD8+ T-cells were isolated using CD8+ Microbeads positive selection kit (Miltenyi Biotec, Auburn, CA) and AutoMacs separation, according to the manufacturer's instructions. CD8+ enriched populations were >95% CD8+ and <0.1% CD4+ by flow cytometric analysis. “Untouched” CD4+ T-cells were isolated using CD4 negative selection kits (Miltenyi Biotech). CD25+ T-cells were depleted from the purified CD4+ using CD25 Microbeads (Miltenyi Biotec). CD4+CD25- enriched populations were >98% CD4+, <1% CD25+, and <0.1% CD8+ by flow cytometric analysis. CD4+CD25+ enriched populations were >98% CD4+ and <0.1% CD8+. CD25 expression ranged from 40.5-73.8%. The CD4+ and CD8+ T-cell-depleted PBMC population was irradiated with 3000 rads before being used as antigen-presenting cells (APC).
2.3. CFSE staining
To detect proliferative responses upon antigenic challenge, cells were stained with carboxyfluorescien diacetate succinimidyl ester (CFSE) (Invitrogen Molecular Probes, Eugene, OR), as described previously [3, 28]. Briefly, cells are suspended at 1 × 106 cells/mL and incubated for 7 min at 37°C with 0.25 μM CFSE (Invitrogen), then washed twice with media containing 5% human serum.
2.4. CMTPX staining
Cell Tracker Red CMTPX (Invitrogen Molecular Probes) was used to stain putative regulatory cells. CD8+ and CD4+CD25+ suppressor cells, or CD4+CD25-negative control cells were marked with CMTPX, as described previously [15]. Briefly, cells were suspended at 1 × 106 cell/mL and incubated 15 min at 37°C with 700 nM CMTPX, then washed twice with media containing 5% human serum. The longer-wavelength CMTPX exhibits bright red fluorescence that is easily distinguished from that of green fluorescent probes, such as CFSE.
2.5. Flow cytometry-based suppression assay cultures
1 × 106 CFSE-stained CD4+CD25- T-cells were used as responders in a 1 ml culture. 1 × 106 CD4- and CD8-depleted PBMC were irradiated with 3000 rads and used as APC. In replicate cultures, varying ratios of CMTPX-stained suppressors were added and cultured with various antigenic stimuli for 7 days in complete RPMI 1640 media containing 5% human serum, 100U/mL Penicillin, 100 μg/mL Streptomycin, and 0.92 mg/mL L-glutamine. Cells were washed and stained for flow cytometry, as described below.
2.6. Antigenic Stimulation
Pools of 15-mer peptides, overlapping by 10, spanning entire neuroantigenic proteins were used, as described previously [3]. These were used at 1 μg/ml final concentration for each peptide and covered myelin basic protein (MBP), proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG), myelin associated glycoprotein (MAG), oligodendrocyte myelin glycoprotein (OMGP) and αβ-crystallin (CRAB). In addition, whole bovine MBP (wbMBP) was also used at 20 μg/ml. For control foreign antigens, we utilized pools of known CD4 and CD8 epitopes of CMV (5 and 14 peptides, respectively) as well as whole cytomegalovirus (CMV) (Microbix Biosystems, Ontario, Canada) and tetanus toxoid (TT) (Accurate Chemical & Scientific Corp, Westbury, NY). 1 μg/mL anti-CD3 monoclonal antibody (OKT3) was used for mitogenic stimulation.
2.7. T-cell line generation
We generated neuroantigen- and control antigen-specific CD8+ and CD4+ T-cell lines by bead-sorting CD8+ (or CD4+) T-cells after 1 week of in vitro PBMC stimulation, followed by repeated antigen-specific expansion with autologous APC. CD8+ T-cell lines were maintained with 25 IU/mL IL-2 (Peprotech, Rocky Hill, NJ), 10 ng/mL IL-7 (Peprotech), 1 ng/mL IL-12 (Peprotech), and 1 ng/mL IL-15 (Peprotech), as previously described [29, 30].
2.8. Flow cytometric antibody staining
On day 7 of in vitro stimulation, cells were washed with 0.1% (w/v) sodium azide/phosphate-buffered saline (Mediatech Cellgro). Cells were stained with anti-CD3-PE (BD Biosciences, San Jose, CA), anti-CD4-PECy5.5 (Invitrogen), anti-CD8-Pacific Blue (BD Biosciences), and anti-CD25-APC (BD Biosciences), then resuspended in 1% paraformaldehyde (Electron Microscopy Sciences, Hatfiled, PA). Flow cytometric data were acquired on a 4-Laser, 17-color LSRII using FACSDiva software (Becton Dickinson). CFSE was detected in the FITC channel and CMTPX in the PE-Texas red channel on the LSR.
2.9. Data Analysis
Linear uncompensated data was transferred as FCS 3.0 files and analysed after compensation and transformation using FlowJo version 8.4.1 (TreeStar, Ashland, OR). Using Flowjo software (Treestar), putative Treg (CD4+CD25+, CD8+, and CD4+CD25- as a negative control) were CMTPX(high) and were gated out from flow cytometric analysis of CFSE-stained cells. Similar PKH-26 (Sigma-Aldrich, St. Louis, MO)-labeling techniques have been utilized for the purpose of excluding Treg from proliferative quantitation of CD4+ responder T-cells [31]. T-cell activation and proliferation was quantified by the percentage of CD25(high) and CFSE(low) events among gated CD4+ (or CD8+) T-cells. Cut-offs for positive populations were determined by using either fluorescence minus one (FMO) staining for polychromatic flow cytometry, no stimulus background CFSE staining, or isotype control staining, as appropriate [32]. A “positive” T-cell response to antigen was defined as having (1) a response index (RI) greater than or equal to 1.5 and (2) a %CD25+CFSElow response of the antigen-stimulated cells at least 1% greater than the %CD25+CFSElow response of the cells in the no antigen tube. Response index (RI) was the stimulated cells' %CD25+CFSElow divided by 100-%CD25+CFSElow divided by the unstimulated cells' %CD25+CFSElow divided by 100-%CD25+CFSElow. If these criteria were unmet, absence of T-cell response was indicated. For suppression assays, % response was calculated by normalizing the ‘responder only’ proliferation to 100%. %Suppression was 100 minus %response. CMTPX(high)CD8+ cells were analyzed for CD25+ expression. Stimulation index of the CD8 response was defined as the percentage of CD25+ cells with antigenic stimulus divided by percentage with no antigen.
2.10. 3H-thymidine based assays
Assays were performed in triplicate in 96-well plates using antigen-specific T-cell lines. 1×105 CD4+ line cells were cultured with 1×105 irradiated autologous PBMC in a total volume of 200 μl/well, with or without indicated antigens. 1×105 CD8+ line cells were added to the cultures as suppressors. The cultures were pulsed with 3H-thymidine on day 3 and harvested after 20 hours to measure proliferation in CPM, as previously described [3, 28]. ΔCPM was calculated by subtracting background proliferation in the absence of antigen.
2.11. Statistical analyses
Statistical tests were performed using Prism 5 (Graphpad Software, La Jolla, CA). Correlation regression and t tests were used to compute a two-tailed P value assuming a 95% confidence interval. P values >0.05 were not significant with “ns” notated where applied in figures. Likewise P values 0.01 to 0.05, 0.001 to 0.01, and <0.001 were significant with “*”, “**”, and “***” notated respectively. R squared values were computed from non-transformed raw data with the use of non-linear regression, assuming a semi-log X line model (days since start of last relapse is plotted on a logarithmic X axis).
3. Results
3.1. CD8+ T-cells specific for CNS autoantigens, but not those specific for control foreign antigens, suppress CD4+ T-cell proliferation
Most prior studies comparing CNS-specific T-cell responses between MS patients and healthy subjects have employed proliferation assays using bulk PBMC. Using CFSE-based flow cytometric proliferation assays, we have shown high prevalence of CD4+ and CD8+ T-cell responses to neuroantigens in both healthy subjects and MS patients, with some functional differences [3]. In the current study, we performed CFSE assays using magnetically purified CD4+CD25- and CD8+ cells. We observed that, similar to bulk PBMC, purified populations of CD4+CD25- and CD8+ T-cells from treatment-naïve MS patients (MS) and healthy control subjects (HC) showed similar responses to neuroantigens, foreign antigens and mitogenic (anti-CD3) stimulation (Fig. 1). Fig. 1A shows examples of CD4 proliferation from representative HC and MS subjects, whereas Fig. 1B shows cumulative data from 15 HC and 11 MS, representing 50 and 37 detectable CNS-specific CD4 responses and 25 and 13 CD8 responses, respectively. We also performed parallel assays using bulk PBMC versus purified CD4+CD25- T-cells [i.e., in the absence of CD8+ T-cells and CD4+CD25+ regulatory T-cells], predominantly using HC PBMC. We observed that depletion of CD8+ T-cells and CD25+ cells, resulted in a significant increase in CD4+ T-cell responses to neuroantigens, but not to control foreign antigens like CMV or TT (Fig. 1C). This suggested that, in addition to CD4+CD25+ T cells, CNS-specific CD8+ T-cells may potentially possess immune suppressive ability.
Figure 1. Multiple sclerosis patients and healthy control subjects share similar T-cell responses. CFSE-based proliferation assays were performed on purified CD4+CD25- or CD8+ T-cells from 15 HC and 11 MS patients.
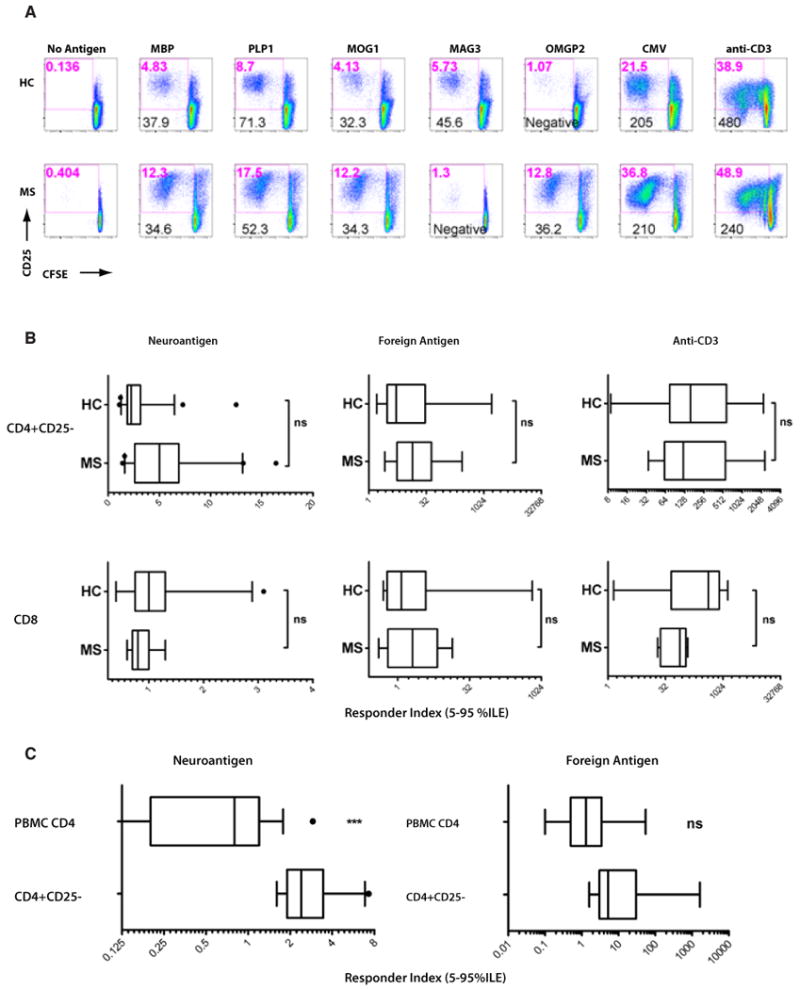
(A) Representative responses from CD4+ T-cells from a single HC (top row) and single MS patient (bottom row) are shown, with CFSE on X-axis and CD25 on the Y-axis. Various stimuli are indicated above each column. The numbers in red toward the top of each dotplot indicate the %CD25+/CFSE(low) (activated/proliferating) cells, representing the response. Numbers in black toward the bottom represent the response index (RI), calculated based on background proliferation in the absence of any stimulus. “Negative” represents lack of a response, based on criteria described in the methods. (B) Cumulative results from 15 HC and 11 MS patients (9 neuroantigenic responders) are shown as RI for both CD4 responses (top row) and CD8 responses (bottom row), stimulated with neuroantigens, foreign antigens or anti-CD3 (as indicated). These results represent 85 and 60 positive assays with neuroantigens performed on HC and MS, respectively. (C) From 9 HC, CFSE-based proliferation assays were performed on both bulk PBMC as well as sorted CD4+CD25- T-cells. Cumulative results from gated CD4 responses from each condition are shown as RI. *** indicates significant elevation of neuroantigen-specific responses (p<0.001), whereas foreign antigen-specific responses were not significantly different (ns).
To test this hypothesis, we adapted a sensitive flow cytometry-based suppression assay [31] to measure suppressive ability of autologous CD8+ T-cells (Fig. 2A). This assay measured the proliferation and activation of CFSE-stained CD4+CD25- responder T-cells. Putative suppressor cells were stained with a tracker dye, CMTPX [15], allowing their exclusion from the analysis. CMTPX-stained CD4+CD25+ (positive control), CD8+ or CD4+CD25- (negative control) T-cells were added in increasing numbers and their effect on responder proliferation was quantified, by normalizing to the RI of CD4+CD25- T-cells (treated as 100% proliferation or 0% suppression). Representative dotplots of anti-CD3-stimulated assays are shown in Fig. 2A, with cumulative %proliferation shown in Fig. 2B and %suppression from a single responder to suppressor ratio in Fig. 2C. Using anti-CD3 stimulation, we observed consistent suppressive activity in the CD4+CD25+ and CD8+ populations, while CMTPX-stained CD4+CD25- T-cells did not significantly dampen pan-stimulated CD4+CD25- T-cells (negative control). Interestingly, non-fractionated CD8+ T-cells showed greater suppressive capacity than CD4+CD25+ T-cells, a fraction known to contain regulatory T-cells.
Figure 2. Anti-CD3-stimulated and neuroantigen-specific CD8+ T-cells suppress CD4+ T-cells.
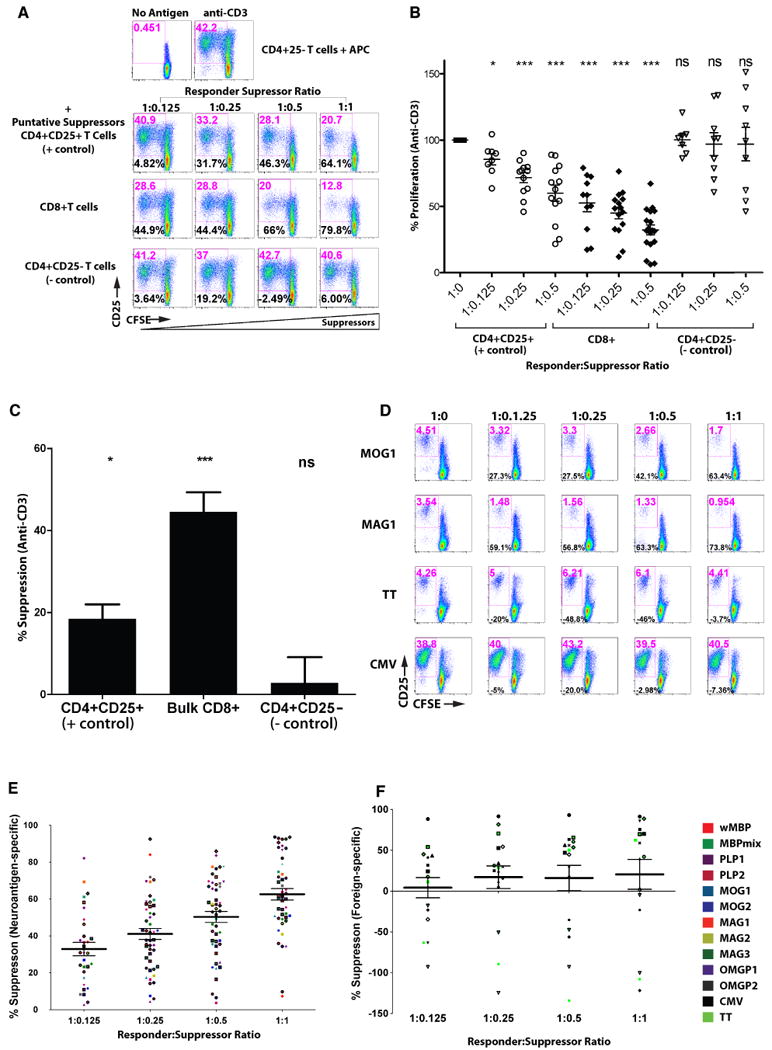
(A) CFSE-stained healthy ex vivo purified CD4+CD25- T-cells were used as responders in anti-CD3-stimulated suppression assays. Dotplots from a single representative experiment demonstrate CFSE on the X-axis and CD25 expression on Y-axis. Indicated in red at the top of each dot plot is the gated percentage of CD25+/CFSE-low cells (activated and proliferating), representing the “response”. Indicated in black in the lower left is the calculated %suppression, based on normalizing to the anti-CD3-mediated response in the absence of suppressors (top row). Indicated to the left of the bottom three rows are the CMTPX-stained cell populations used as suppressors at the indicated ratios over each column. The results are representative of 15 flow-based suppression assays from 15 healthy controls. (B) Cumulative results from suppression assays from 15 healthy controls are displayed as percent proliferative response normalized to the response without suppressors (defined as 100%), indicated as 1:0. Open circles represent the response in the presence of increasing numbers of CD4+CD25+ T-cells (positive controls), closed diamonds for bulk CD8+ T-cells and open triangles for CD4+CD25- T-cells (negative controls). (C) Results from Panel B are represented as %suppression at a single responder: suppressor ratio (1:0.25). (D) Representative dotplots from a single subject demonstrate CD8-mediated suppression assays in the presence of neuroantigens (MOG1, MAG1) and foreign antigen (TT, CMV). The left column represents CD4+CD25- responders only, where positive responses were selected to evaluate suppression. The right three columns contain increasing numbers of CMTPX-stained CD8+ T-cells, with %proliferation and %suppression indicated. (E, F) Cumulative results are shown from 67 suppression assays. Data points represent neuroantigen- (E) and foreign antigen- (F) specific %suppression. Each of 15 subjects is indicated by a different shape. Neuroantigen or foreign antigen used in the suppression assay is indicated by the color legend at right (for some proteins, multiple pools were made to limit the number of peptides in each pool, as described previously [3]).
We then sought to evaluate the suppressive ability of CD8+ T-cells in cultures stimulated with specific antigens by conducting suppression assays using a panel of CNS and control antigens. Positive CD4 T-cell responses to specific antigens were selected and suppression was quantified. Fig. 2D shows representative responses from one HC, where the addition of increasing numbers of CD8+ T-cells suppressed the proliferation and activation of neuroantigen-stimulated responses, in contrast to foreign-antigen-stimulated responses. Figs. 2E and 2F show cumulative data from 15 HC, demonstrating consistent dose-dependent suppression in neuroantigen-stimulated cultures (2E), contrasting with lack of consistent suppression in response to foreign antigens (2F), which in many cases led to enhanced proliferation of the responders [denoted as “negative suppression”]. This suggested that neuroantigen-specific CD8+ T-cells obtained ex vivo possessed immune suppressive ability, whereas foreign antigen-specific ones did not show consistent suppression.
3.2. CNS-specific CD8+ T-cells require stimulation with cognate antigen for suppressive activity
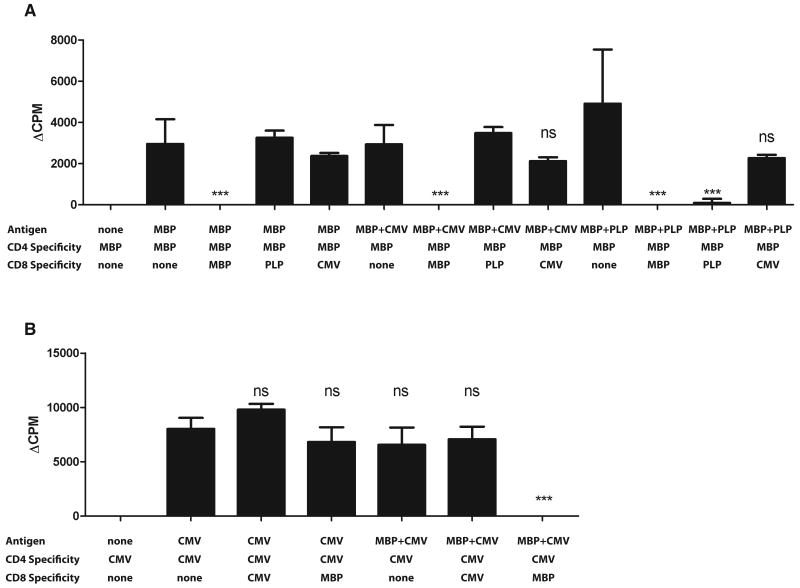
In the experiments above, the antigens added to the bulk culture presumably stimulated both the CD4+ T-cells and CD8+ T-cells. Thus, it was possible only to test the effect of neuroantigen-stimulated CD8+ T-cells on CD4+ T-cells stimulated by the same antigenic peptides. To ascertain that these results were based on cognate antigen-specific recognition, we generated over thirty-four CD4+ and CD8+ T-cell lines, using PBMC from 8 HC. Specificity was confirmed by 3H-thymidine uptake, showing reactivity to the intended antigen but not to other CNS or foreign antigens (Suppl. Fig. 1). Using these lines, we performed autologous 3H-thymidine-based suppression assays, culturing CNS-or foreign antigen-specific CD4+ T-cells alone or in the presence of CNS- or foreign antigen-specific CD8+ T-cells in various combinations. These cultures contained APC with antigens that would stimulate just the CD4+ T-cells or both CD4+ and CD8+ T-cells (Fig. 3). Fig. 3A shows a single MBP-specific CD4+ T-cell line, cultured with autologous MBP-, PLP- or CMV-specific CD8+ T-cells. When cultured in the absence of any CD8+ T-cells, the MBP-specific CD4+ T-cells showed a similar proliferative response to stimulation by MBP, MBP+CMV or MBP+PLP. The addition of MBP-specific CD8+ T-cells to the MBP-stimulated cultures resulted in robust suppression of the response. Importantly, the addition of PLP- or CMV-specific CD8+ T-cells did not affect cultures stimulated only by MBP. However, when PLP antigen was added, the PLP-specific CD8+ T-cells suppressed MBP-specific CD4 proliferation. Most interestingly, this was not true of CMV-specific CD8+ T-cells. In cultures containing CMV peptides and CMV-specific CD8+ T-cells, no significant suppressive effect was exerted. Fig. 3B demonstrates an example of a CMV-specific CD4+ T-cell line, in combination with autologous CMV-specific or MBP-specific CD8+ T-cells. Again, in contrast to CMV-specific CD8+ T-cells, MBP-specific CD8+ T-cells had a suppressive effect in the presence of their cognate antigen. Thus, similar to bulk cultures (Fig. 2), antigen-specific lines confirmed that neuroantigen-specific CD8+ T-cells had robust immune suppressive properties compared to foreign antigen-specific CD8+ T-cells and required the presence of cognate antigen.
Figure 3. Activated neuroantigen-specific CD8+ T-cells suppress CD4+ T-cells.
Responder CD4+ T-cell lines were cultured with APC and indicated antigens in the presence or absence of the indicated CD8+ T-cell lines. 3H-Thymidine-based proliferation assays were performed. Panel A shows ΔCPM (background subtracted) from a single MBP-specific CD4+ line and Panel B shows a CMV-specific CD4+ line. The results are representative of 8 independent assays, each repeated twice, with lines obtained from 8 different HC.
3.3. CNS-specific suppressive ability is significantly diminished during acute exacerbation of MS and recovers during remission
We then postulated that neuroantigen-specific CD8+ T-cell suppressive ability may be relevant in MS and may influence the clinical disease course. Several studies by others and us have demonstrated a global deficit in regulatory CD4+CD25+ [23-25] or CD8+ T-cell function in MS [14, 26, 27]. To test the possibility that CNS-specific suppressive ability has a bearing on MS clinical presentation, we compared flow-based suppression assays on PBMC from 15 HC, 11 treatment-naive RRMS patients (quiescent MS) and 9 treatment-naïve MS patients during an acute exacerbation (Fig. 4). CD8+ T-cells from HC and quiescent MS patients showed similar neuroantigen-specific suppressive ability (Figs. 4A-4B). Interestingly, CD8+ T-cells obtained during an acute clinical episode showed significantly lower neuroantigen-specific suppressor ability, whether viewed as suppression stimulated by independent multiple antigens (Fig. 4B) or as a mean neuroantigen-specific suppression per subject (Suppl. Fig. 2). This corroborated with a global CD8 suppressor deficit, demonstrated in anti-CD3-stimulated suppressor assays (Fig. 4D), whereas none of the patients showed significant foreign antigen-specific CD8+ suppressor ability (Fig. 4C). To address whether the lack of suppression could be explained by major changes in T-cell subsets, we first evaluated CD4:CD8 ratios across various cohorts an found no significant differences between any of the cohorts, especially between quiescent MS (1.75 ± 0.69) vs. acute exacerbation (2.12 ± 0.72). We further evaluated whether there may be an absence of CNS-specific CD8 reactivity in the peripheral blood during acute exacerbation or enhanced activation or proliferation of CNS-specific CD8 cells in the suppression assays. Using CMTPX as a cell tracker, we were able to specifically evaluate CD8 T cell activation. While CMTPX is not optimal for use as a proliferation dye, we could evaluate total CD25 expression on the CMTPX-stained CD8 cells in these cultures, indicating their overall activation/proliferation status. We found that, albeit slightly diminished, CNS-specific CD8+ T-cell reactivity was detectable even during acute exacerbation (Suppl. Fig. 3), suggesting that these responses may be functionally different rather than simply quantitatively suppressed.
Figure 4. Neuroantigen-specific suppressive ability is deficient during acute MS exacerbation.
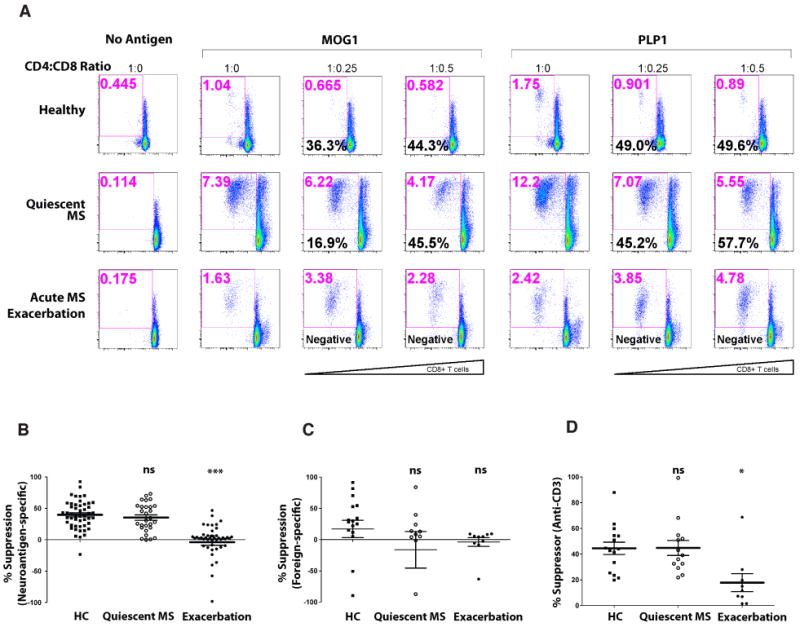
Ex vivo-purified, CFSE-stained CD4+CD25- T-cells from HC, quiescent MS patients or MS patients suffering from an acute exacerbation were used as responders in autologous suppression assays. Panel A shows CFSE vs. CD25 dotplots from representative subjects responding to two neuroantigens (MOG-pool 1 and PLP-pool 1) in the absence of suppressor cells (1:0) or with CD8+ T-cells added at indicated ratios. Red numbers at the top of each dotplot represent proliferative response, whereas the black numbers represent the calculated %suppression. This is representative 15 HC, 11 quiescent MS patients (9 responders) and 9 acute MS exacerbation patients (6 responders), equivalent to 50, 47, and 37 flow-based suppression assays, respectively. Panels B, C and D show cumulative %suppression data at the 1:0.25 responder:suppressor ratio from assays containing neuroantigens, foreign antigens or anti-CD3, as indicated.
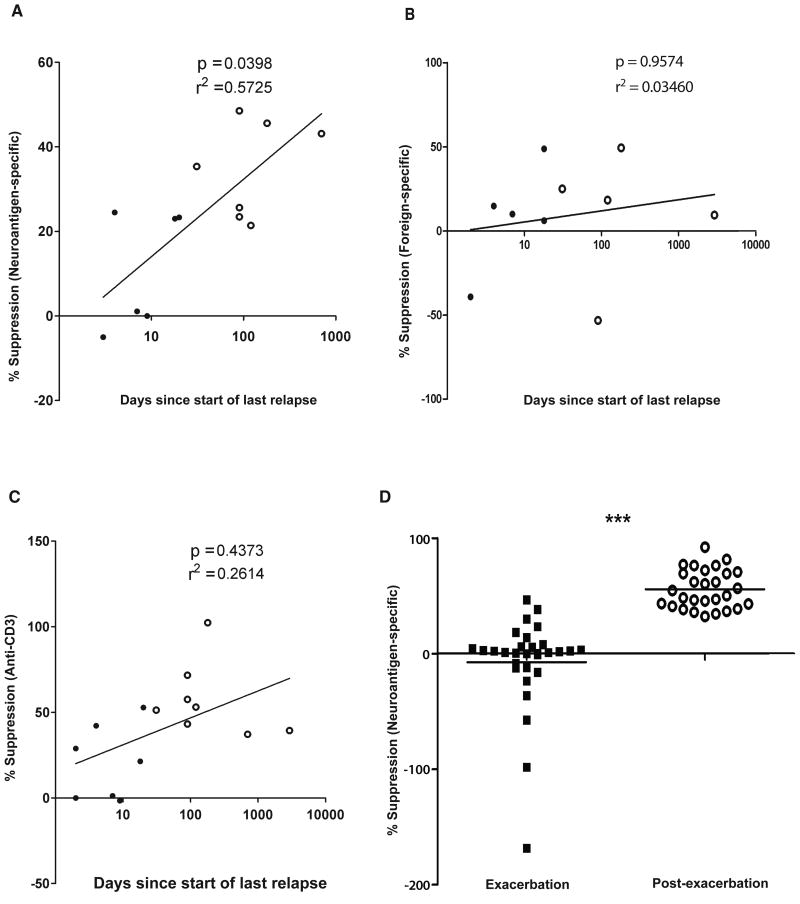
To gauge the clinical relevance of these findings further, we asked whether there was any correlation between CD8 suppressor ability and the distance from an acute clinical episode. We found that the duration from the latest clinical episode correlated significantly with CNS-specific CD8 suppression, but not with foreign-specific or global (anti-CD3-mediated) suppression (Fig. 5A-C). In contrast to foreign-specific and anti-CD3-induced CD8 suppression, most of the neuroantigen-specific CD8+ suppressive function, plotted versus time since last relapse, could be explained by the regression line demonstrated in Fig. 5A. This suggested that correction of the CNS-specific CD8 suppressor deficit would correlate with recovery from an acute relapse. To test this hypothesis prospectively, we re-evaluated a subset of the subjects longitudinally, after their disease had become clinically quiescent either with or without immunomodulatory therapy. We observed a robust and significant recovery of the CNS-specific CD8 suppressive ability, whether viewed as suppression against multiple neuroantigens (Fig. 5D) or as mean suppression per subject (Suppl. Fig. 4A). Again, foreign-specific CD8 suppression showed no changes over time, whereas there was some recovery of anti-CD3-based suppression (Suppl. Fig. 4B-D).
Figure 5. Neuroantigen-specific CD8+ T-cell suppressive-ability correlates with days since last relapse and recovery.
(A, B, C) Dots represent average CD8+ T-cell suppressive ability of individual MS patients in the presence of neuroantigens (A), foreign antigens (B) or anti-CD3 (C). Closed and open circles are acute MS exacerbation and quiescent MS patients, respectively. R squared values are shown for nonlinear regression assuming a semi-log X line model. P values are shown for correlation analysis. (D) Dots represent neuroantigen-specific suppression assays performed longitudinally during exacerbation and after a quiescent clinical state as reached. Closed squares and open circles represent patients who averaged 12 and 81 days since start of last relapse, respectively.
4. Discussion
To our knowledge, these studies are the first evidence that human autoreactive CNS-specific CD8+ T-cells play an immune regulatory role, in contrast to foreign-antigen-specific effectors. Moreover, our studies show a clear clinical relevance for this regulatory role, in that suppressive activity is greatly diminished during relapses of MS and recovered as the patients enter remission. Classically, CNS-targeted, MHC Class I-restricted CD8+ T-cells are thought to have a pathogenic role in disease, with reports demonstrating in vitro cytotoxic killing of oligodendrocytes [8, 9]. However, our studies identify an unexpected and novel immune regulatory role in both HC and quiescent MS patients, corroborating studies in EAE, where CNS-specific CD8+ T-cells inhibited disease, whereas control antigen-specific ones did not [15]. Sporadic, acute exacerbations are characteristic of the relapsing-remitting form of MS. While MS suppressor cell dysfunction has been recognized for decades, the role of CNS-specific CD8+ T-cells remains elusive in the context of accumulating disability, axon trans-section, and gliosis which are characteristic of secondary progressive MS [33, 34]. It appears that CNS-specific regulatory ability is directly or indirectly involved in the mechanism of MS clinical phase changes. It still remains unclear whether underlying pathology of chronic progressive MS exhibit similar deficient suppressor CD8+ T-cell activity [25].
We are only beginning to understand the role of autoreactive, regulatory (“autoregulatory”) T-cells in autoimmune disease [35]. There has been some evidence that autoantigen-specific CD8+ T-cells may have immune regulatory properties in diabetes models [36, 37]. Thus, chronic stimulation of CD8+ T-cells with low TCR avidity may induce regulatory function [37], perhaps explaining the therapeutic generation of antigen-specific, cytotoxic immune suppressor CD8+ T-cells following chronic copolymer-based therapy of MS [14, 28]. This may also explain the difference between the roles of foreign-antigen-specific CD8+ T-cells vs. autoreactive ones that tend to bear lower avidity TCR, presumably following thymic deletion of higher avidity responders. In contrast to Qa1/HLA-E-restricted suppressor CD8+ T-cells that recognize immune cell-derived peptides, autoregulatory CD8+ T-cells are stimulated by the same tissue antigens that are targets of destructive effector cells, thereby creating an autoregulatory tolerance loop.
The assay system utilized in our studies took advantage a proliferation dye (CFSE), a cellular tracking dye (CMTPX) and overlapping antigenic peptide pools to monitor neuroantigen-specific CD8+ T-cell suppressive ability. This assay has excellent sensitivity and specificity for detecting functional antigen-specific suppressive ability, by allowing the exclusion of suppressor populations from the analysis. Moreover, it allows an unbiased characterization of T-cell suppressive ability without limited range of HLA haplotype or epitopes. Finally, the assay also enables separate concurrent characterization of CD4+ and CD8+ responses within the same culture. Thus, this unique approach allowed us to discover and quantify this novel autoregulatory function of CNS-specific CD8+ T-cells.
This novel concept also unveils a potential strategy for immune therapy. While using autoreactive CD8+ T-cells as therapy may seem unorthodox, this is principally similar to generating autoantigen-reactive CD4+CD25+FOXP3+ Tregs for adoptive immunotherapy. Other forms of autoreactive CD4+ Tregs (Tr1, Th3) have also shown promise in animal models. CD8+ T-cells, representing an underappreciated arm of peripheral immune tolerance, afford an attractive form of adoptive immunotherapy, especially in the context of clinical relapses. In that regard, we have shown recently that CNS-specific CD8+ T-cells can inhibit ongoing EAE [15], dependent on cytotoxic and immune modulatory mechanisms. The phenotypic characteristics of regulatory CD8+ T-cells are not definite and, depending on the model, may range from a CD28(-) [38-44], γδ+ [45], CD25+ [46], CD122+ [16], CD103+ [47, 48], PD-1+ [49] or FOXP3+ [50-53], among others [54]. In which context neuroantigen-specific CD8+ T-cells regulate, and how, is still unclear. Our preliminary studies reveal autoregulatory CNS-specific suppressor activity in multiple such subsets, with the common features being cytokine- and contact-dependent processes (including cytotoxicity) and an absolute requirement for HLA-Class I (data not shown). Detailed dissection of the characteristics and mechanisms of these cells will be an important pursuit to develop a therapeutic approach.
To summarize, our studies demonstrate a novel, clinically relevant role for neuroantigen-specific CD8+ T-cells, revealing a potential pathway of intrinsic immune regulation that may have implications for the therapy of human MS and other immune-mediated disorders.
Supplementary Material
Acknowledgments
These studies were supported, in part, by grant awards (to NJK) from the NIH and National MS Society, including the Harry Weaver Neuroscience Scholar Award. The authors are indebted to all the patients who participated in this study. We also thank Thomas Abraham, Stephanie Taylor, Megan Quigg and Parul Chaudhary for help with patient recruitment and Drs. Michael Racke, Todd Eagar and Larry Anderson for insightful discussions and experimental support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–9. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Arbour N, Holz A, Sipe JC, Naniche D, Romine JS, Zyroff J, et al. A new approach for evaluating antigen-specific T cell responses to myelin antigens during the course of multiple sclerosis. J Neuroimmunol. 2003;137:197–209. doi: 10.1016/s0165-5728(03)00080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–31. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 4.Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–76. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobsen M, Cepok S, Quak E, Happel M, Gaber R, Ziegler A, et al. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–50. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- 7.Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, et al. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101:2428–33. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–9. [PubMed] [Google Scholar]
- 9.Niland B, Banki K, Biddison WE, Perl A. CD8+ T cell-mediated HLA-A*0201-restricted cytotoxicity to transaldolase peptide 168-176 in patients with multiple sclerosis. J Immunol. 2005;175:8365–78. doi: 10.4049/jimmunol.175.12.8365. [DOI] [PubMed] [Google Scholar]
- 10.Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004;5:469–71. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- 11.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–21. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–42. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–29. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 15.York NR, Mendoza JP, Ortega SB, Benagh A, Tyler AF, Firan M, et al. Immune regulatory CNS-reactive CD8+T cells in experimental autoimmune encephalomyelitis. J Autoimmun. 2010;35:33–44. doi: 10.1016/j.jaut.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–32. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 17.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 18.Chen ML, Yan BS, Kozoriz D, Weiner HL. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol. 2009;39:3423–35. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang X, Zheng P, Liu Y. FoxP3: a genetic link between immunodeficiency and autoimmune diseases. Autoimmun Rev. 2006;5:399–402. doi: 10.1016/j.autrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Bonelli M, Smolen JS, Scheinecker C. Treg and lupus. Ann Rheum Dis. 2010;69 1:i65–6. doi: 10.1136/ard.2009.117135. [DOI] [PubMed] [Google Scholar]
- 21.Brusko T, Atkinson M. Treg in type 1 diabetes. Cell Biochem Biophys. 2007;48:165–75. doi: 10.1007/s12013-007-0018-5. [DOI] [PubMed] [Google Scholar]
- 22.Jaeckel E, Kretschmer K, Apostolou I, von Boehmer H. Instruction of Treg commitment in peripheral T cells is suited to reverse autoimmunity. Semin Immunol. 2006;18:89–92. doi: 10.1016/j.smim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas J, Hug A, Viehover A, Fritzsching B, Falk CS, Filser A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 25.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antel J, Bania M, Noronha A, Neely S. Defective suppressor cell function mediated by T8+ cell lines from patients with progressive multiple sclerosis. J Immunol. 1986;137:3436–9. [PubMed] [Google Scholar]
- 27.Antel J, Brown M, Nicholas MK, Blain M, Noronha A, Reder A. Activated suppressor cell function in multiple sclerosis--clinical correlations. J Neuroimmunol. 1988;17:323–30. doi: 10.1016/0165-5728(88)90123-3. [DOI] [PubMed] [Google Scholar]
- 28.Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, et al. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–9. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yssel H, Spits H. Generation and maintenance of cloned human T cell lines. Curr Protoc Immunol. 2002;Chapter 7(Unit 7 19) doi: 10.1002/0471142735.im0719s47. [DOI] [PubMed] [Google Scholar]
- 30.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029–34. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol. 2006;7:681–5. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 33.Bach MA, Phan-Dinh-Tuy F, Tournier E, Chatenoud L, Bach JF, Martin C, et al. Deficit of suppressor T cells in active multiple sclerosis. Lancet. 1980;2:1221–3. doi: 10.1016/s0140-6736(80)92480-0. [DOI] [PubMed] [Google Scholar]
- 34.Antel JP, Bania MB, Reder A, Cashman N. Activated suppressor cell dysfunction in progressive multiple sclerosis. J Immunol. 1986;137:137–41. [PubMed] [Google Scholar]
- 35.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849–58. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 36.James EA, Kwok WW. CD8+ suppressor-mediated regulation of human CD4+ T cell responses to glutamic acid decarboxylase 65. Eur J Immunol. 2007;37:78–86. doi: 10.1002/eji.200636383. [DOI] [PubMed] [Google Scholar]
- 37.Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–80. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Colovai AI, Mirza M, Vlad G, Wang S, Ho E, Cortesini R, et al. Regulatory CD8+CD28- T cells in heart transplant recipients. Hum Immunol. 2003;64:31–7. doi: 10.1016/s0198-8859(02)00742-5. [DOI] [PubMed] [Google Scholar]
- 39.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–34. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 40.Koide J, Engleman EG. Differences in surface phenotype and mechanism of action between alloantigen-specific CD8+ cytotoxic and suppressor T cell clones. J Immunol. 1990;144:32–40. [PubMed] [Google Scholar]
- 41.Lin YX, Wang LL, Yan LN, Cai P, Li B, Wen TF, et al. Analysis of CD8+CD28- T-suppressor cells in living donor liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2009;8:241–6. [PubMed] [Google Scholar]
- 42.Sindhi R, Manavalan JS, Magill A, Suciu-Foca N, Zeevi A. Reduced immunosuppression in pediatric liver-intestine transplant recipients with CD8+CD28- T-suppressor cells. Hum Immunol. 2005;66:252–7. doi: 10.1016/j.humimm.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Suciu-Foca N, Manavalan JS, Scotto L, Kim-Schulze S, Galluzzo S, Naiyer AJ, et al. Molecular characterization of allospecific T suppressor and tolerogenic dendritic cells: review. Int Immunopharmacol. 2005;5:7–11. doi: 10.1016/j.intimp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Trzonkowski P, Zilvetti M, Chapman S, Wieckiewicz J, Sutherland A, Friend P, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8:338–47. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- 45.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38:640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Yu Y, Li G, Pu L, Zhang F, Zheng S, et al. CD8(+)CD103(+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9:546–8. doi: 10.1016/j.intimp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 49.Izawa A, Yamaura K, Albin MJ, Jurewicz M, Tanaka K, Clarkson MR, et al. A novel alloantigen-specific CD8+PD1+ regulatory T cell induced by ICOS-B7h blockade in vivo. J Immunol. 2007;179:786–96. doi: 10.4049/jimmunol.179.2.786. [DOI] [PubMed] [Google Scholar]
- 50.Bienvenu B, Martin B, Auffray C, Cordier C, Becourt C, Lucas B. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005;175:246–53. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 51.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–58. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 52.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, et al. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–68. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 53.Meloni F, Cascina A, Paschetto E, Marone Bianco A, Morosini M, Pellegrini C, et al. Monocyte chemoattractant protein-1 levels in bronchoalveolar lavage fluid of lung-transplanted patients treated with tacrolimus as rescue treatment for refractory acute rejection. Transplant Proc. 2003;35:1523–6. doi: 10.1016/s0041-1345(03)00476-7. [DOI] [PubMed] [Google Scholar]
- 54.Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol. 2008;69:760–70. doi: 10.1016/j.humimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.