Abstract
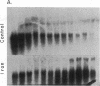
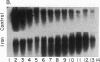
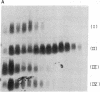
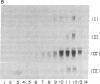
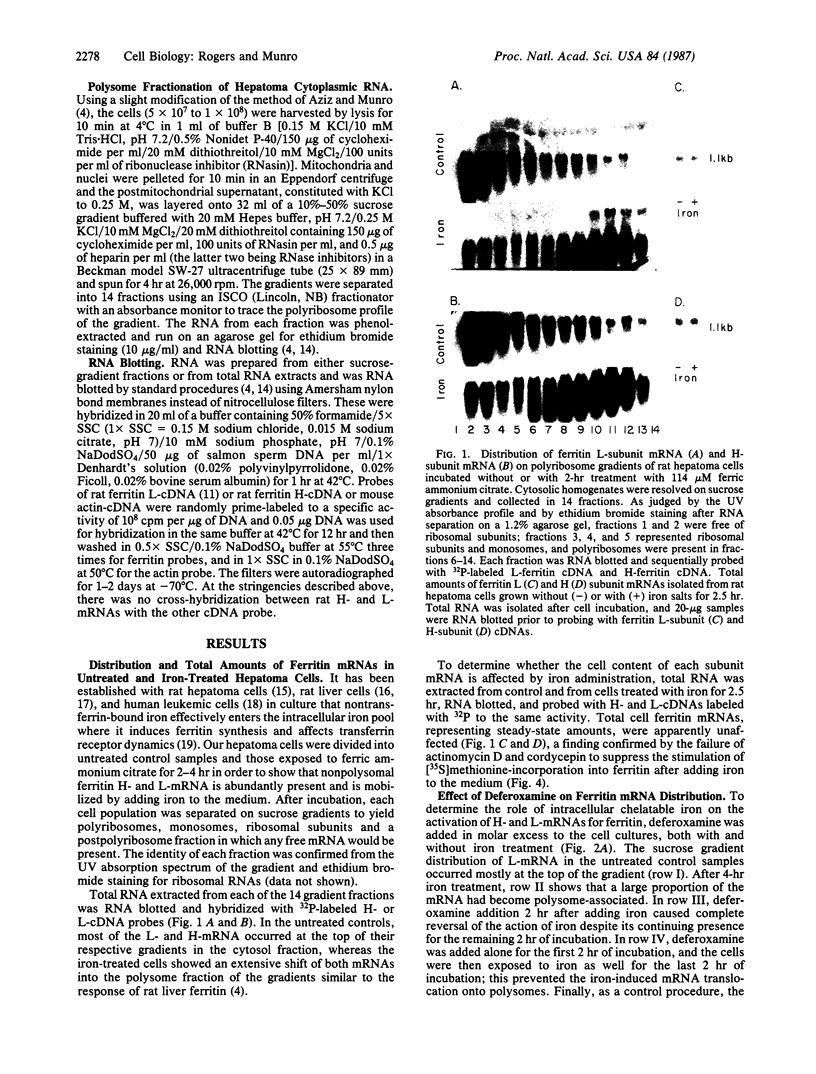
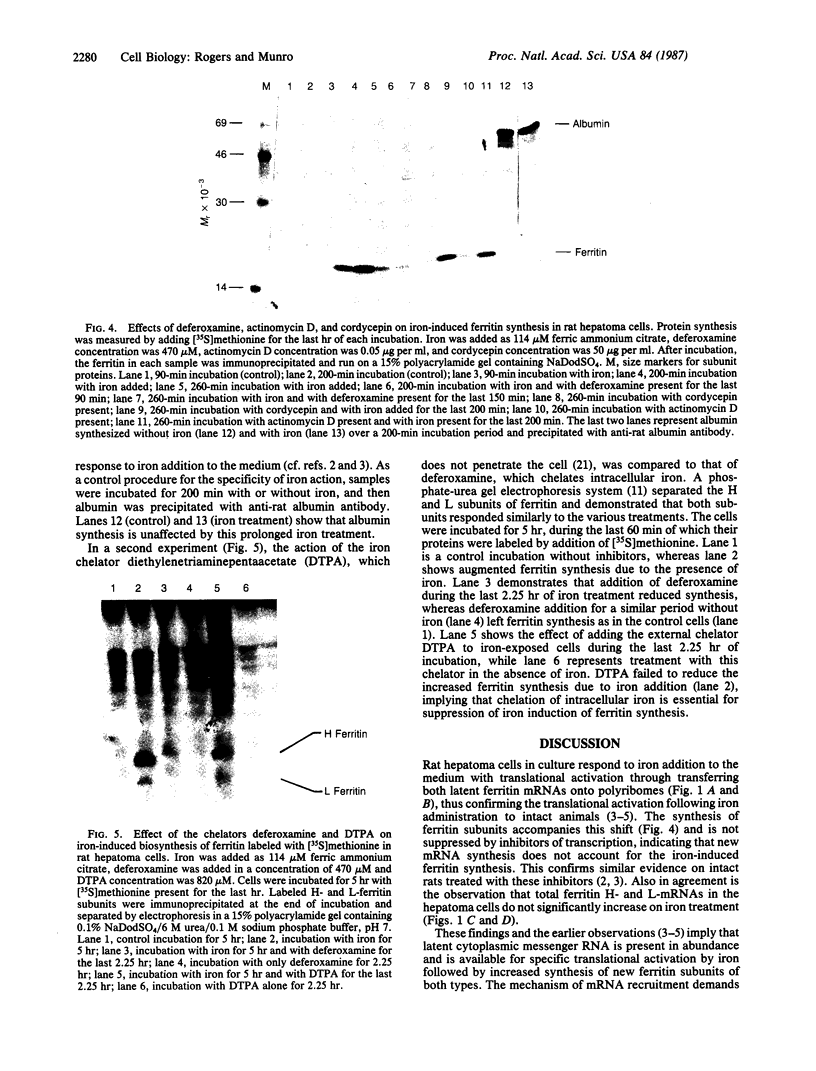
Acute administration of iron to rats has been previously shown to induce liver ferritin synthesis by increasing the translation of inactive cytoplasmic ferritin mRNAs for both heavy (H) and light (L) subunits by mobilizing them onto polyribosomes. In this report rat hepatoma cells in culture are used to explore the relationship of this response to intracellular iron levels. After adding iron as ferric ammonium citrate to the medium, latent ferritin H- and L-mRNAs were extensively transferred to polyribosomes, accompanied by increased uptake of [35S]methionine into ferritin protein. Because total cellular levels of L- and H-mRNA were not significantly changed by exposure to iron, the increased ferritin mRNAs on polyribosomes most probably come from an inactive cytoplasmic pool, consistent with the inability of actinomycin-D and of cordycepin to inhibit iron-induced ferritin synthesis. When deferoxamine mesylate, an intracellular iron chelator, was added after the addition of iron to the medium, ferritin mRNA on the polyribosomes was reduced, while the free messenger pool increased, and ferritin synthesis diminished. In contrast, the extracellular iron chelator diethylenetriaminepentaacetic acid failed to inhibit the induction of ferritin protein synthesis. Addition of iron in the form of hemin also caused translocation of mRNA to polyribosomes, a response that could be similarly quenched by deferoxamine. Because hemin does not release chelatable iron extracellularly, we conclude that the level of chelatable iron within the cell has a regulatory role in ferritin synthesis through redistribution of the messenger RNAs between the free mRNA pool and the polyribosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley S. S., Wolfe L. C., Bridges K. R. Iron metabolism in K562 erythroleukemic cells. J Biol Chem. 1985 Jun 10;260(11):6811–6815. [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Bridges K. R., Hoffman K. E. The effects of ascorbic acid on the intracellular metabolism of iron and ferritin. J Biol Chem. 1986 Oct 25;261(30):14273–14277. [PubMed] [Google Scholar]
- Brown A. J., Leibold E. A., Munro H. N. Isolation of cDNA clones for the light subunit of rat liver ferritin: evidence that the light subunit is encoded by a multigene family. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1265–1269. doi: 10.1073/pnas.80.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DRYSDALE J. W., MUNRO H. N. FAILURE OF ACTINOMYCIN D TO PREVENT INDUCTION OF LIVER APOFERRITIN AFTER IRON ADMINISTRATION. Biochim Biophys Acta. 1965 May 11;103:185–188. doi: 10.1016/0005-2787(65)90554-x. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Theil E. C., Kaufman R. E., Dickey L. F. Multiple red cell ferritin mRNAs, which code for an abundant protein in the embryonic cell type, analyzed by cDNA sequence and by primer extension of the 5'-untranslated regions. J Biol Chem. 1986 Jan 15;261(2):949–955. [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Goto Y., Paterson M., Listowsky I. Iron uptake and regulation of ferritin synthesis by hepatoma cells in hormone-supplemented serum-free media. J Biol Chem. 1983 Apr 25;258(8):5248–5255. [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattia E., Josic D., Ashwell G., Klausner R., van Renswoude J. Regulation of intracellular iron distribution in K562 human erythroleukemia cells. J Biol Chem. 1986 Apr 5;261(10):4587–4593. [PubMed] [Google Scholar]
- Rittling S. R., Woodworth R. C. The synthesis and turnover of ferritin in rat L-6 cells. Rates and response to iron, actinomycin D, and desferrioxamine. J Biol Chem. 1984 May 10;259(9):5561–5566. [PubMed] [Google Scholar]
- Rosenthal E. T., Tansey T. R., Ruderman J. V. Sequence-specific adenylations and deadenylations accompany changes in the translation of maternal messenger RNA after fertilization of Spisula oocytes. J Mol Biol. 1983 May 25;166(3):309–327. doi: 10.1016/s0022-2836(83)80087-4. [DOI] [PubMed] [Google Scholar]
- Rouault T., Rao K., Harford J., Mattia E., Klausner R. D. Hemin, chelatable iron, and the regulation of transferrin receptor biosynthesis. J Biol Chem. 1985 Nov 25;260(27):14862–14866. [PubMed] [Google Scholar]
- Rudolph N. S., Ohlsson-Wilhelm B. M., Leary J. F., Rowley P. T. Regulation of K562 cell transferrin receptors by exogenous iron. J Cell Physiol. 1985 Mar;122(3):441–450. doi: 10.1002/jcp.1041220315. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeux M., Testa U., Louache F., Thomopoulos P., Rochant H., Breton-Gorius J. The role of iron in the growth of human leukemic cell lines. J Cell Physiol. 1984 Oct;121(1):251–256. doi: 10.1002/jcp.1041210131. [DOI] [PubMed] [Google Scholar]
- Trinder D., Morgan E., Baker E. The mechanisms of iron uptake by fetal rat hepatocytes in culture. Hepatology. 1986 Sep-Oct;6(5):852–858. doi: 10.1002/hep.1840060508. [DOI] [PubMed] [Google Scholar]
- Wright T. L., Brissot P., Ma W. L., Weisiger R. A. Characterization of non-transferrin-bound iron clearance by rat liver. J Biol Chem. 1986 Aug 15;261(23):10909–10914. [PubMed] [Google Scholar]
- Wynshaw-Boris A., Lugo T. G., Short J. M., Fournier R. E., Hanson R. W. Identification of a cAMP regulatory region in the gene for rat cytosolic phosphoenolpyruvate carboxykinase (GTP). Use of chimeric genes transfected into hepatoma cells. J Biol Chem. 1984 Oct 10;259(19):12161–12169. [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Nuclear RNA is spliced in the absence of poly(A) addition. Cell. 1981 Oct;26(1 Pt 1):39–46. doi: 10.1016/0092-8674(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]