Abstract
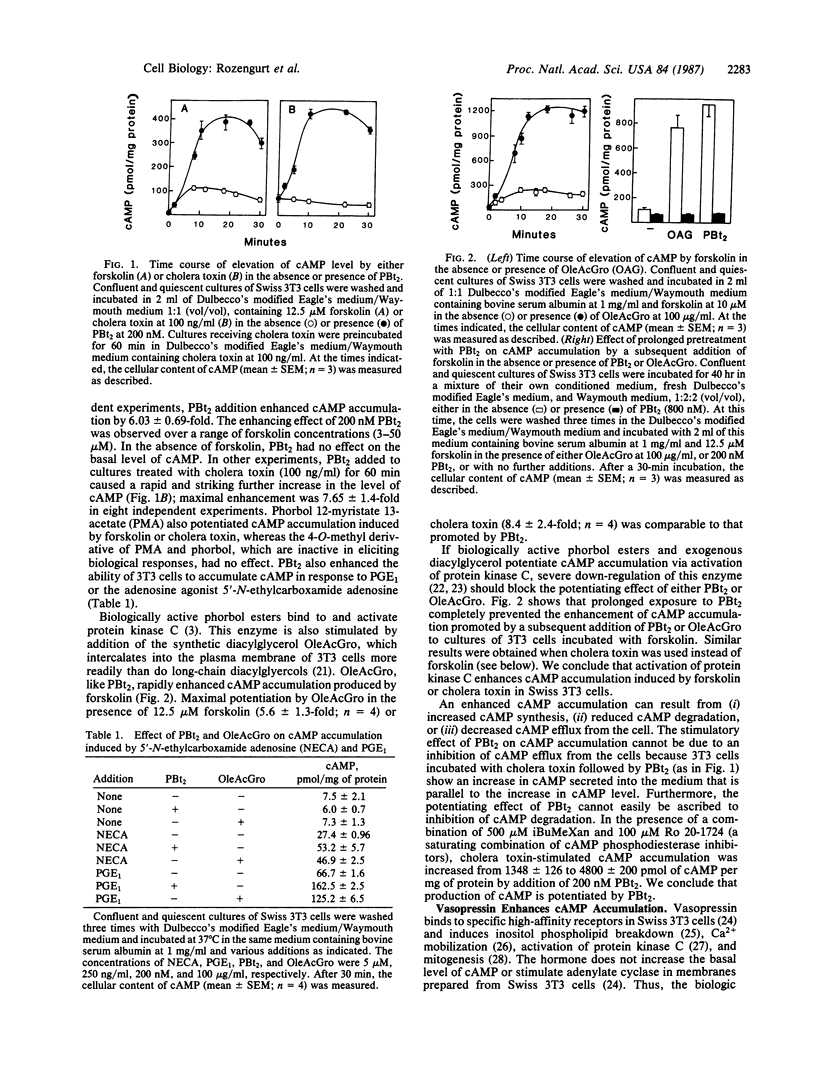
Addition of phorbol 12,13-dibutyrate (PBt2) in the presence of forskolin or cholera toxin caused marked (6- to 8-fold) and rapid accumulation of cAMP in Swiss 3T3 cells. The effect of PBt2 is mediated by protein kinase C because the synthetic diacylglycerol 1-oleoyl-2 acetylglycerol substitutes for PBt2 in enhancing cAMP accumulation and because the enhancing effect of either PBt2 or 1-oleoyl-2-acetylglycerol was prevented by down-regulation of protein kinase C. Vasopressin, which activates protein kinase C but does not directly affect adenylate cyclase in 3T3 cells, also enhanced cAMP accumulation in cells treated with cholera toxin or forskolin. This effect was abolished by down-regulation of protein kinase C. Treatment with pertussis toxin blocked the enhancing effect of PBt2 in a concentration- and time-dependent manner. Pertussis toxin neither prevented protein kinase C activation by PBt2 nor other biologic responses elicited by PBt2. The results presented here suggest an unusual function for a pertussis toxin substrate--namely, coupling protein kinase C activation to cAMP production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Bankowski K., Manning M., Haldar J., Sawyer W. H. Design of potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1978 Sep;21(9):850–853. doi: 10.1021/jm00207a002. [DOI] [PubMed] [Google Scholar]
- Bell J. D., Buxton I. L., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Putative effect of C kinase on alpha s-GTP-catalytic subunit interaction. J Biol Chem. 1985 Mar 10;260(5):2625–2628. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Brostrom M. A., Brostrom C. O., Brotman L. A., Lee C., Wolff D. J., Geller H. M. Alterations of glial tumor cell Ca2+ metabolism and Ca2+-dependent cAMP accumulation by phorbol myristate acetate. J Biol Chem. 1982 Jun 25;257(12):6758–6765. [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Vasopressin induces selective desensitization of its mitogenic response in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1924–1928. doi: 10.1073/pnas.80.7.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Halvorsen S. W., Nathanson N. M. Ontogenesis of physiological responsiveness and guanine nucleotide sensitivity of cardiac muscarinic receptors during chick embryonic development. Biochemistry. 1984 Nov 20;23(24):5813–5821. doi: 10.1021/bi00319a021. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Bauer S., Watanabe Y. Modulation of adenylate cyclase of human platelets by phorbol ester. Impairment of the hormone-sensitive inhibitory pathway. Eur J Biochem. 1985 Sep 2;151(2):425–430. doi: 10.1111/j.1432-1033.1985.tb09119.x. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986 Jun 25;261(18):8182–8191. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza S. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Phorbol esters and diacylglycerol inhibit vasopressin-induced increases in cytoplasmic-free Ca2+ and 45Ca2+ efflux in Swiss 3T3 cells. Exp Cell Res. 1986 Jun;164(2):536–545. doi: 10.1016/0014-4827(86)90051-0. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Schneider J. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. II. Changes in Na+ and Ca2+ fluxes, Na+/K+ pump activity, and intracellular pH. J Cell Biol. 1986 Jun;102(6):2223–2233. doi: 10.1083/jcb.102.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambi P., Peters J. R., Sibley D. R., Lefkowitz R. J. Desensitization of the turkey erythrocyte beta-adrenergic receptor in a cell-free system. Evidence that multiple protein kinases can phosphorylate and desensitize the receptor. J Biol Chem. 1985 Feb 25;260(4):2165–2171. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Vasopressin rapidly stimulates protein kinase C in quiescent Swiss 3T3 cells. J Cell Physiol. 1986 Oct;129(1):124–130. doi: 10.1002/jcp.1041290117. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Uzumaki H., Yamamoto S., Goto H., Kato R. Potentiation of prostaglandin E1-stimulated cAMP formation by 12-O-tetradecanoylphorbol-13-acetate in BALB/c mouse 3T3 cells. Biochem Pharmacol. 1986 Mar 1;35(5):835–838. doi: 10.1016/0006-2952(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Vara F., Schneider J. A., Rozengurt E. Ionic responses rapidly elicited by activation of protein kinase C in quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2384–2388. doi: 10.1073/pnas.82.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Modulation of the epidermal growth factor receptor by mitogenic ligands: effects of bombesin and role of protein kinase C. Cancer Surv. 1985;4(4):729–765. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]




