Summary
We determined the temporal dynamics of cambial activity and xylem cell differentiation of Scots pine (Pinus sylvestris L.) within a dry inner Alpine valley (750 m asl, Tyrol, Austria), where radial growth is strongly limited by drought in spring. Repeated micro-sampling of the developing tree ring of mature trees was carried out during 2 contrasting years at two study plots that differ in soil water availability (xeric and dry-mesic site).
In 2007, when air temperature at the beginning of the growing season in April exceeded the long-term mean by 6.4 °C, cambial cell division started in early April at both study plots. A delayed onset of cambial activity of c. 2 wk was found in 2008, when average climate conditions prevailed in spring, indicating that resumption of cambial cell division after winter dormancy is temperature-controlled. Cambial cell division consistently ended about the end of June/early July in both study years. Radial enlargement of tracheids started almost 3 wk earlier in 2007 compared with 2008 at both study plots. At the xeric site, the maximum rate of tracheid production in 2007 and 2008 was reached in early and mid-May, respectively, and c. 2 wk later, at the dry-mesic site. Since in both study years, more favorable growing conditions (i.e., an increase in soil water content) were recorded during summer, we suggest a strong sink competition for carbohydrates to mycorrhizal root and shoot growth. Wood formation stopped c. 4 wk earlier at the xeric compared with the dry-mesic site in both years, indicating a strong influence of drought stress on cell differentiation. This is supported by radial widths of earlywood cells, which were found to be significantly narrower at the xeric than at the dry-mesic site (P < 0.05).
Repeated cellular analyses during the two growing seasons revealed that, although spatial variability in the dynamics and duration of cell differentiation processes in Pinus sylvestris exposed to drought is strongly influenced by water availability, the onset of cambial activity and cell differentiation is controlled by temperature.
Keywords: Cambium, dry inner Alpine valley, intra-annual growth, Scots pine, tracheid production, xylogenesis
Introduction
Several tree ring studies conducted in the dry inner Alpine valleys have shown that precipitation in spring limits radial growth of trees (e.g., Kienast et al. 1987, Oberhuber et al. 1998, Rigling et al. 2002, Jolly et al. 2005) and severe drought during the growing season results in long-lasting growth reductions and increased tree mortality (Oberhuber 2001, Rebetez and Dobbertin 2004, Bigler et al. 2006, Waldboth and Oberhuber 2009). On the other hand, the record-breaking heat wave in summer 2003 (Beniston 2004) was found to have a minor impact on the growth of drought-exposed coniferous forest trees in an inner Alpine environment (Pichler and Oberhuber 2007). Dendroecological studies provide information on climate-growth relationships and about the year-to-year variability of radial growth, but not about variability of crucial phenological stages—i.e., onset, maximum rate, and ending. Radial stem growth is a complex process and involves cell division in the cambial zone, followed by cell enlargement, secondary wall thickening, and lignification. During the period of cambial activity and tracheid differentiation, the trees and their wood cells are influenced by environmental signals, which are directly incorporated in the developing tree ring (Plomion et al. 2001, Frankenstein et al. 2005). In recent years, fine-scaled histological analyses of xylem cell production and development during the growing season have become more frequent, particularly in conifers of cold climates (e.g., Deslauriers et al. 2003, Schmitt et al. 2004, Rossi et al. 2006b, 2008, Heinrichs et al. 2007). Because of intra-annual differences in weather, some variability in the onset of cambial activity was found, with temperature in spring being a key factor in inducing cambial reactivation after winter dormancy (e.g., Rossi et al. 2007, Deslauriers et al. 2008, Seo et al. 2008, Gruber et al. 2009a). Several authors also reported positive effects of localized stem heating on reactivation of the cambium in evergreen conifers (e.g., Savidge and Wareing 1981, Oribe et al. 2001, Gričar et al. 2006), which led to the hypothesis that stem temperature is a limiting factor in the onset of cambial activity. The maximum growth rate of conifers at high altitudes and latitudes was found to occur close to the summer solstice, suggesting photoperiodic control of xylogenesis to allow tracheid differentiation to be completed before winter (Rossi et al. 2006c). On the other hand, there is still a lack of description of cellular phenology of annual ring formation in Scots pine (Pinus sylvestris L.), which forms widespread forest ecosystems in the lower montane region within dry inner Alpine valleys in the central Austrian and Swiss Alps (Ellenberg 1988). Studies on the seasonal dynamics of cambial growth and xylem cell differentiation are required to better understand the effect of climate variables on xylem development and to determine the period of cambial activity and wood formation at xeric sites (Eilmann et al. 2006, Pichler and Oberhuber 2007).
The present study focuses on the timing and dynamics of cambial activity and xylem cell differentiation in Pinus sylvestris exposed to soil dryness in the lower montane region of the Eastern Alps (Austria) during 2007 and 2008. Whereas climate in 2007 was characterized by exceptionally warm and dry conditions at the beginning of the growing season in April, at the same time in 2008, cool-moist conditions corresponding to long-term average prevailed. Because it has been found that trees within the study area respond quite differently to identical climatic conditions, depending on the interaction of soil condition and topographic features on water availability (cf. Oberhuber and Kofler 2000, Oberhuber 2001), and there is evidence that trees at xeric sites are better adapted to water deficits than those at mesic sites (Orwig and Abrams 1997, Martín-Benito et al. 2008), wood formation dynamics was monitored at two sites differing in soil moisture availability (xeric vs. dry-moist) by repeatedly taking small punched cores of the outermost tree rings (micro-cores) during short time intervals. This technique has been successfully applied to follow seasonal formation of xylem (e.g., Loris 1981, Deslauriers et al. 2003, Rossi et al. 2006b, Mäkinen et al. 2008, Gruber et al. 2009a). We hypothesized that (i) cambial resumption in spring is controlled by temperature, and (ii) water availability throughout the growing season determines the temporal dynamics of xylem cell development.
Materials and methods
Study area
The study site (for the geographical location, see Oberhuber et al. 1998) is part of a postglacial rock-slide area situated in the montane belt (c. 750 m asl) within the inner Alpine dry valley of the Inn River (Tyrol, Austria, 47° 14′ 00″ N, 10° 50′ 20″ E) and has a relatively continental climate with mean annual precipitation and temperature of 715 mm and 7.3 °C, respectively (long-term mean during 1911-2007 at Ötz, 812 m asl, 5 km from the study area). The widespread plant community in the study area is a Spring Heath-Pine wood (Erico-Pinetum typicum, Ellenberg 1988). Human impact in this area was generally restricted to sporadic gathering of firewood and livestock grazing.
Two sites that differ in water availability were selected: a most xeric open south-facing stand growing on shallow stony soil and a less xeric site with deeper soil and higher stand density in a hollow (partly facing north). On the xeric site, pioneer vegetation prevails in the ground flora, whereas crowberry (Vaccinium vitis-idaea L.) and a thick moss layer dominate the understory in the hollow, which indicates slightly moist conditions at the latter site. All measurements were carried out at dominant trees to reduce the influence of competition on radial growth. Whereas mean age and stem diameter of trees, which on an average amounted to 155 yr and 27 cm, respectively, were statistically not significantly different, dominant trees were twice as high and annual increments were c. 30% wider at the dry-mesic compared with those at the xeric site (Table 1).
Table 1.
Site description and characteristics of Pinus sylvestris trees selected for micro-core sampling at the xeric and dry-mesic study plots (n = five trees/site, SD = standard deviation, RW = ring width). Statistically significant differences of mean values between sites are indicated by different letters (P ≤ 0.05; Student's t test).
| Site | Aspect | Slope (°) |
Stand height (m) |
Canopy coverage (%) |
Soil type | Humus type | Soil depth (cm) |
Tree age1 (yr) mean ± SD |
Stem diameter2 (cm) mean ± SD |
RW3 (μm) mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Xeric | SW | 40 | 4-5 | 33 | Syrosem | Xeromoder | 0-10 | 148 ± 25a | 24.8 ± 5.4a | 352 ± 140a |
| Dry-mesic | N | <10 | 10 | 66 | Protorendzina | Raw humus | 20-30 | 163 ± 27a | 30.0 ± 4.9a | 503 ± 120b |
Cambial age at sampling height
Mean tree diameter measured at 1m stem height
Mean values for the period 2002–2006
Within the study area, shallow soils, predominantly of the protorendzina type—i.e. rendzic and lithic leptosols according to the FAO classification system (FAO 1998)—are developed; they consist of unconsolidated, coarse-textured materials with low waterholding capacity. Distinct soil horizons are hardly ever developed and are restricted to small-scale areas within deep hollows.
Xylem sampling and determination of wood formation
Seasonal wood formation dynamics was monitored during the growing seasons of 2007 and 2008 by taking small punched cores from five trees/site of the outermost tree rings (micro-cores) with a diameter and length of 2.5 mm and c. 2 cm, respectively (Rossi et al. 2006a). To determine the variability in intra-annual wood formation between trees at each plot (xeric and dry-mesic site), individual trees were randomly selected. Based on previous dendroclimatological studies carried out within the study area (Oberhuber et al. 1998, Oberhuber and Kofler 2000), xylem formation was expected to start in April. Therefore, micro-cores were taken at all study plots from March to October in weekly to 10-day intervals to include the whole dynamics of xylem formation. Shorter time intervals were chosen at the beginning and end of the growing season to determine the onset and end of cambial activity and xylem differentiation more precisely.
Because c. 20 samples were taken throughout the growing season from each selected tree, micro-cores were sampled from different trees at both study plots in 2007 and 2008, to avoid the effects of wounding on wood formation dynamics. Samples were taken on the slope-parallel side of the stem following a spiral trajectory up the stem starting at c. 1 m stem height. A distance of c. 2 cm in tangential and longitudinal direction was kept to avoid lateral influence of wound reactions on adjacent sampling positions.
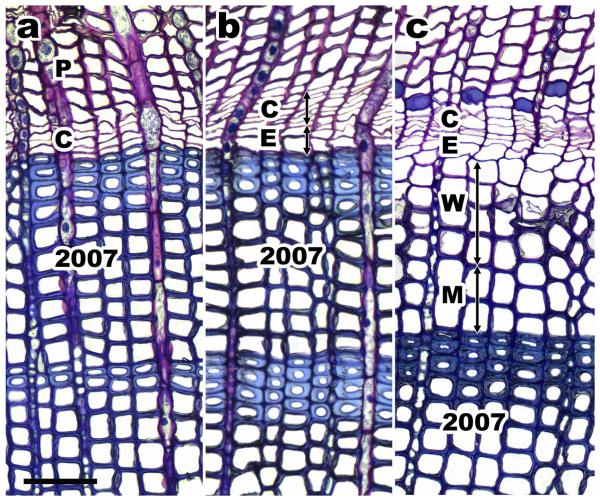
Immediately after extraction, cores were placed in a solution of 70% ethanol, propionic acid, and 40% formaldehyde (mixing ratio: 90/5/5), subsequently embedded in glycolmethacrylate (Technovit 7100) and polymerized after adding an accelerator. Transverse sections of c. 12 μm were cut with a rotary microtome, stained with a water solution of 0.05 % cresyl fast violet and observed under a light microscope with polarized light to differentiate the development of xylem cells—i.e. the discrimination between tracheids in enlarging and cell-wall thickening phase (Antonova and Stasova 1993, Deslauriers et al. 2003, Rossi et al. 2006b). The number of cambial cells (i.e., fusiform cells lacking radial enlargement), radial-enlarging cells, cells undergoing secondary wall thickening and lignification, and mature xylem cells were counted on all sampled cores in three radial rows. Cells in the cambial zone had thin cell walls and small radial diameters (Figure 1). Cells in radial enlargement were larger than cambial cells and observations under polarized light discriminated between enlarging and birefringent wall-thickening tracheids (Figure 1b). During secondary wall thickening, the walls of cells changed from light violet (unlignified secondary cell walls) to blue (lignified cell walls) and tracheids were considered mature when cell walls were completely blue (Figure 1c; cf. Rossi et al. 2006b).
Figure 1.
Transverse sections of Pinus sylvestris at 100X magnification from March to May 2008. a cambial zone (C) during dormancy in early March; P = phloem. b cambial zone after onset of cell division in late April; E = tracheids in radial enlargement. c developing xylem in late May; W = wall-thickening tracheids, M = mature tracheids. Scale bar = 100 μm.
In accordance with recent studies (e.g., Deslauriers et al. 2008, Thibeault-Martel et al. 2008, Gruber et al. 2009a, Rossi et al. 2009b), we defined onset and end of cambial activity on the basis of number of cells in the cambial zone. These studies also clearly distinguished between cambial activity and enlargement of tracheids. Hence, onset and end of cambial activity was defined when the standard deviation of the number of cambial cells did not cross the number of dormant cells in the cambial zone, whereas xylem cell differentiation was considered to have begun and to be complete when one horizontal row of cells was detected in the enlarging phase and cell wall thickening and lignification were completed, respectively. At the end of the growing season, when cambial activity had already stopped, single partly expanded cells in some samples were detected. However, these cells were not counted because they differed from fusiform cambial cells and could also not be assigned to mature latewood due to missing cell wall thickening and lignification. Total xylem cell number was determined by adding the number of cells in radial enlargement, cell wall thickening, and the number of mature xylem cells (Deslauriers et al. 2003, Rossi et al. 2006c). Values—i.e. the number of cells in different zones of five cores (trees) per date and for each site—were averaged.
Radial cell width (RCW) of earlywood xylem was calculated as: RCW earlywood = (ring width − latewood width)/(n xylem cells − n latewood cells). RCW of latewood xylem was determined by dividing latewood width by number of latewood cells. The earlywood-latewood boundary was defined as two times double the wall thickness equal to or greater than the width of the lumen (Denne 1988).
The time of bud break in the upper crown was recorded at the same trees selected for micro-coring. Five additional trees per site were included to account for the large within-tree variability observed. Bud break was assessed weekly and defined as readily identifiable swelling of buds. At this time, bud scales were still covering the new needles.
Standardization of cell number and fitting of xylem growth
Because cell number varies within the tree circumference and hence among different samples, standardization is required (Rossi et al. 2003). The total cell numbers of the previous three tree rings were recorded in every sample and used for a cell number correction for each tree. Cell number in each j sample (i.e., micro-cores taken throughout the 2007 and 2008 growing seasons) and by each i phase (i.e., enlarging, wall-thickening, and mature tracheids) was corrected as follows:
where
ncij = corrected cell number,
nij = counted cell number,
nm= mean cell number of previous ring of all j samples, and
ns = cell number of previous ring for each j sample.
Short-term variation in total number of tracheids (sum of enlarging, wall-thickening and mature cells) were modelled with a Gompertz function using the nonlinear regression procedure included in the Origin software package (OriginLab Corporation, Northampton, MA, USA). The Gompertz equation proved its versatility in describing growth-limiting processes and assessing cell dynamics of tree-ring growth (e.g., Zeide 1993, Deslauriers and Morin 2005, Rossi et al. 2003, 2006c).
Microclimate records
During the study period, daily precipitation and air temperature were collected automatically at 2 m height (ONSET, Pocasset, MA, USA) at the xeric site. Since the dry-mesic plot was located at the same elevation and within less than 200 m in linear distance, climate records from this plot were regarded as representative of the whole study area. Long-term records (LTM) of total monthly precipitation and mean monthly temperatures since 1911 were available from a nearby meteorological station (Ötz, 812 m asl, 5 km from the study area).
Soil moisture dynamics (volumetric water content) and soil temperature in the upper 5–10 cm of the soil layer were continuously monitored. Moisture sensors are based on a capacitive method (Cyclobios, proprietary development at University of Innsbruck, Austria). Due to small-scale variability of soil structure and soil depth, records of three soil moisture and temperature sensors placed at each plot were averaged. Measuring intervals for all sensors were 30 min. Mean daily air and soil temperature and soil water content (vol %) were calculated by averaging all measurements (48 values/day).
Environmental variables during growing seasons 2007 and 2008
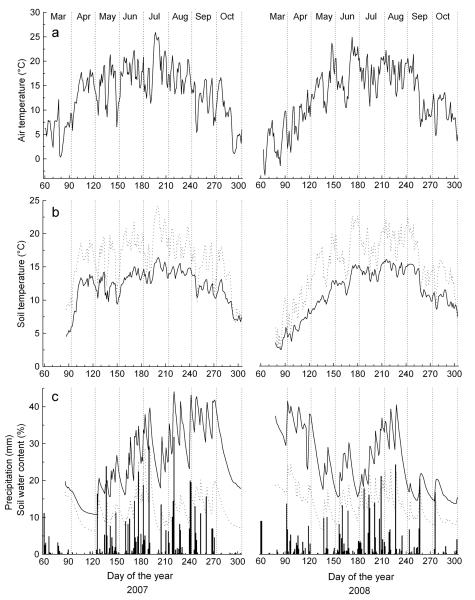
Climate in 2007 was characterized by exceptionally mild temperatures in spring (Figure 2, Table 2). An almost continuous drought period was recorded from 20 March to 6 May 2007, when total monthly precipitation in April reached <2 mm (LTM: 39 mm) and mean monthly air temperature was 13.9 °C, i.e. 6.4 °C above LTM (Table 2). Air temperature in May and during summer (June–August) 2007 exceeded LTM by 2.7 and 2 °C, respectively (Table 2). In contrast to 2007, climate at the beginning of the growing season in 2008 was cool and wet, whereby in April, mean monthly air temperature of 7.6 °C corresponded to LTM (7.5 ± 1.7 °C) and precipitation exceeded LTM by almost 50% (Figure 2, Table 2). While mean daily air temperatures recorded in summer 2008 corresponded to temperature records of 2007, total summer precipitation was c. 25 % lower than LTM (Table 2). During the drought period lasting from late March through early May 2007, soil water content dropped to 10.8 and 6.3 vol% at the dry-mesic and at the xeric site, respectively (Figure 2). Starting with rainfall events in May 2007, soil moisture at the dry-mesic site reached 20 and 30 vol% in May and June, respectively. In contrast to 2007, frequent rainfall events in spring 2008 caused high soil moisture at the start of the growing season in April and May. Throughout both growing seasons studied, records of mean soil water content were predominantly c. 10–15% lower at the xeric than at the dry-mesic site (Figure 2).
Figure 2.
Climate variables recorded during the growing seasons of 2007 and 2008 within the study area. a Mean daily air temperature b Mean daily soil temperature. c Daily precipitation sum (bars) and soil water content. In b-c study sites are denoted by dotted and solid lines for the xeric and dry-mesic site, respectively.
Table 2.
Monthly and summer (June–August) mean daily air temperature and precipitation sum during 2007 and 2008 growing seasons recorded within the study area. Long-term mean values (LTM, 1911–2006) of air temperature and precipitation were available from a nearby meteorological station (Oetz, 5 km from the study area). Mean values ± standard deviation are shown.
| Air temperature (°C) | Precipitation (mm) | |||||
|---|---|---|---|---|---|---|
| LTM | 2007 | 2008 | LTM | 2007 | 2008 | |
| April | 7.5 ± 1.7 | 13.9 ± 3.4 | 7.6 ± 3.2 | 39 ± 19 | 2 | 58 |
| May | 12.0 ± 1.8 | 14.7 ± 3.8 | 15.1 ± 3.7 | 64 ± 26 | 86 | 28 |
| Summer | 15.8 ± 1.0 | 17.8 ± 3.3 | 17.7 ± 3.2 | 316 ± 65 | 308 | 236 |
Results
Dynamics of tree-ring growth
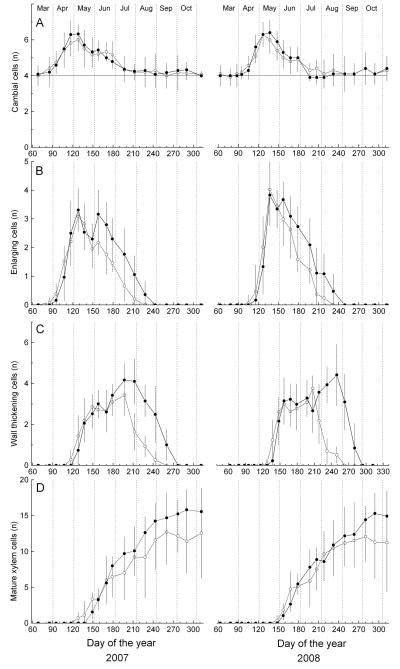
The dormant cambium consisted of c. four cells, when there was no cambial activity from July through March (Figure 3). In early April 2007, the number of cells in the cambial zone rapidly increased to about 6, whereby maximum values were reached at both plots in late April through early May (Figure 3). Annual dynamics of cambial activity in 2007 were quite similar at the xeric and dry-mesic sites. A delayed onset and maximum of c. 2 wk was found in 2008. Again, the number of cambial cells synchronously increased and decreased at the xeric and dry-mesic sites. The low numbers of cambial cells and variability between trees precluded a precise determination and a differentiation between sites. However, it can be deduced that cambial activity within the study area lasted from early/mid-April to about end of June/early July—i.e., cambial cells in 2007 and 2008 divided throughout c. 90 and c. 75 d, respectively (Figure 3). At both study plots, bud break in the upper crown occurred by the end of March (85 d) in 2007 and 2 wk later (99 d) in 2008, i.e., about 1 wk before the start of cambial activity and cell enlargement was detected (see Figures 3 and 4).
Figure 3.
Number of cells a in the cambial zone, b in radial enlargement, c in secondary wall thickening and lignification, and d mature xylem cells during 2007 and 2008 at study plots. Bars represent standard deviations and horizontal thin lines in a indicate the number of dormant cambial cells. Study sites are denoted by open and filled circles for the xeric and dry-mesic site, respectively.
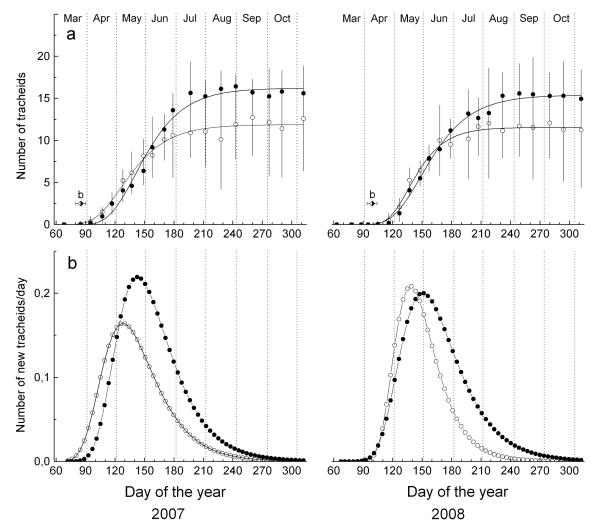
Figure 4.
a Dynamics of xylem growth (including enlarging, wall-thickening, and mature xylem cells) modelled by applying the Gompertz function in 2007 and 2008 at study plots. Time of bud break is indicated by symbol b. b Daily xylem growth rates calculated on the basis of modelled growth. Symbols as in Figure 3.
Delayed bell-shaped curves (enlarging and wall-thickening cells) and a growing S-shaped curve (mature cells) characterize the dynamics of cell differentiation (Figure 3). In 2007, higher numbers of enlarging cells than in 2008 were recorded early in the growing season in April at both study plots. Mean onset of radial enlargement at the xeric site in 2007 and 2008 occurred on 12 and 30 April (102 and 120 d of the year, respectively; Table 3). At the dry-mesic site, the start of radial cell enlargement was delayed in 2007 and 2008 by c. 1 wk and in both years, the number of enlarging cells decreased more rapidly in early June at the xeric compared with the dry-mesic site. Corresponding to cambial activity and number of cells in the enlargement phase, first tracheids were undergoing wall thickening around 27 April and 17 May in 2007 and 2008, respectively. Wall thickening and lignification in 2007 and 2008 were completed about 4–5 wk earlier at the xeric site than at the dry-mesic site (early September and early October, respectively, Table 3). Whereas in 2007, first mature tracheids were already detected on 8 May (128 d) and 29 May (149 d) at the xeric and dry-mesic site, respectively, in 2008, maturation of some tracheids was completed around 28 May (148 d) at the xeric site and around 6 June (157 d) at the dry-mesic site (Figure 3). Hence, mature xylem cells were found about 3 wk (2007) and 10 days (2008) earlier at the xeric compared with the dry-mesic site, respectively. In 2007 and 2008, mean duration of wood formation, including all developmental phases from cell enlargement to the end of wall thickening and lignification, lasted for about 137 and 161 days at the xeric and dry-mesic site, respectively (Table 3). Hence, growing season length was reduced by about 3 wk at the xeric compared with the dry-mesic site.
Table 3.
Onset, end, and overall duration of wood formation in Pinus sylvestris and number of mature xylem and latewood cells (LW) in 2007 and 2008 at study plots (n = five trees/site). Timing of wood formation is given in days of the year (mean values ± standard deviation). Statistically significant differences of mean values between sites and years are indicated by different letters (P ≤ 0.05; Student's t test).
| Onset | End | Duration (days) | Xylem cells (n) | LW cells (n) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | |
| Xeric | 102 ± 9a | 120 ± 9b | 243 ± 9a | 253 ± 11a | 141 ± 8a | 133 ± 9a | 13 ± 6a | 11 ± 7a | 4 ± 2a | 5 ± 4a |
| Dry-mesic | 110 ± 10a | 128 ± 7b | 271 ± 9b | 287 ± 7b | 162 ± 12b | 159 ± 11b | 16 ± 2a | 16 ± 3a | 5 ± 3a | 6 ± 2a |
Increase in total number of tracheids (including cells in enlarging and wall thickening phase and mature cells) during the growing seasons of 2007 and 2008 are depicted in Figure 4. The total number of new tracheids produced was higher at the dry-mesic site but was statistically not significantly different from the cell number determined at the xeric site (Table 3). Based on modelled xylem cell number increase by applying the Gompertz function, the maximum cell production rate (tracheids d−1) at the xeric site occurred around 126 and 136 d of the year in 2007 and 2008, respectively, and was c. 25% lower in 2007 compared with 2008 (Figure 4, Table 4). At the dry-mesic site, the maximum cell production rate was reached about 2 wk later compared with the xeric site in both study years. Thereafter, daily xylem growth rates decreased, although in both years, an increase in soil moisture content was recorded later on (Figure 2, Table 2). A low cell production rate was found during both study years, which on average amounted to 1.2 and 1.4 cells within 14 days during the whole increment period at the xeric and dry-mesic site, respectively (cf. Table 3). At the most, two to three cells within 14 days were developed in mid-May through early June, when daily growth rates reached maximum values (Figure 4). Total ring width and radial width of earlywood cells in 2007 and 2008 were significantly wider at the dry-mesic than at the xeric site (Table 5). Although there is a tendency for a wider latewood zone at the dry-mesic site, latewood width and radial width of latewood cells were not significantly different between sites in both study years, except for the width of latewood cells in 2008.
Table 4.
Parameters of the Gompertz function for xylem cell dynamics in 2007 and 2008 (see Figure 4) at study plots and R2 of the model (A = upper asymptote, Ip = inflection point, κ = rate of change parameter, mean values ± standard deviation).
| Site | Year | A (n cells) |
Ip (day of the year) |
κ | R2 |
|---|---|---|---|---|---|
| Xeric | 2007 | 12 ± 0.2 | 126 ± 1.8 | 0.038 ± 0.004 | 0.987 |
| 2008 | 12 ± 0.2 | 136 ± 1.3 | 0.049 ± 0.004 | 0.993 | |
| Dry-mesic | 2007 | 16 ± 0.3 | 141 ± 1.8 | 0.036 ± 0.003 | 0.990 |
| 2008 | 15 ± 0.2 | 148 ± 1.3 | 0.035 ± 0.002 | 0.995 |
Table 5.
Ring width, latewood width, and radial cell width (RCW) of earlywood (EW) and latewood (LW) cells in 2007 and 2008 at study plots (n = five trees/site, mean values ± standard deviation). Statistically significant differences of mean values between sites are indicated by different letters (P < 0.05; Student's t test).
| Site | Ring width (μm) | LW width (μm) | RCW EW (μm) | RCW LW (μm) | ||||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | |
| Xeric | 304 ± 137a | 279 ± 171a | 59 ± 25a | 71 ± 62a | 27 ± 4a | 30 ± 5a | 16 ± 3a | 15 ± la |
| Dry-mesic | 438 ± 72b | 487 ± 87b | 76 ± 14a | 107 ± 39a | 34 ± 3b | 38 ± 3b | 18 ± 3a | 18 ± 3b |
Discussion
Impact of temperature on onset of cambial activity
Timing of bud break, onset of cambial activity, and cell differentiation processes differed by almost 3 wk between 2007 and 2008 at both study plots, suggesting marked effects of early spring temperature on early processes of xylogenesis. Oribe et al. (2001) and Gričar et al. (2006) reported that heating experiments carried out on stems of evergreen conifers during the quiescent stage were able to induce reactivation of the cambium. Their findings demonstrated that cambium activity is highly responsive to temperature. Similarly, at several timberline sites, warm spring temperatures were found to result in earlier onset of cambial activity, which increased the duration of wood formation by several weeks (Rossi et al. 2007, Deslauriers et al. 2008, Gruber et al. 2009a). The influence of temperature on wood formation in early spring can also be deduced from the earlier onset of cell differentiation processes during both growing seasons at the xeric compared with the dry-mesic site. Based on our findings, a temporal shift in tree ring-climate relationships in response to climate change-induced warmer spring temperatures in recent decades (e.g., Menzel et al. 2006) is conceivable but needs further dendroclimatological investigations.
Site-specific differences in the temporal dynamics of wood formation in spring might be caused by earlier soil warming under open sparse canopy at the south-facing xeric site compared with shaded conditions prevailing in the hollow. Because cold soils inhibit root activity and water uptake (for a review, see Pregitzer et al. 2000), a delay in cell turgor increase and hence, enlargement of newly formed tracheids in the stem, is reasonable to assume. Furthermore, several authors reported that root-zone temperatures exert direct influences on aboveground metabolism (e.g., Hellmers et al. 1970, DeLucia 1986, Day et al. 1989, Gruber et al. 2009b) and Lopushinsky and Kaufmann (1984) and Vapaavuori et al. (1992) found that low soil temperature in spring can delay the establishment of planted conifer seedlings by impairing shoot growth. However, although there are several lines of evidence on the impact of root-zone temperature on aboveground stem growth, we cannot exclude the possibility that differences in stem temperature influenced the onset of cell differentiation processes in our study.
Impact of drought on xylem cell development
Wood formation during 2007 and 2008 stopped c. 4 wk earlier at the drought-prone xeric site than at the dry-mesic site, which correspondingly resulted in shorter duration of cell differentiation processes (cell enlargement, wall thickening) and narrower ring width. In 2003, when extraordinary hot and dry conditions prevailed during the growing season (Beniston 2004, Rebetez et al. 2006), an early cessation of cambial activity in conifers was also found within the study area (Pichler and Oberhuber 2007) and in the southeastern Alps (Levanič et al. 2009). Similarly, results of Rigling et al. (2003) and Thabeet et al. (2009) indicate that duration of wood formation in Pinus sylvestris is adversely affected by high water deficits in summer. Additionally, limited water supply in summer might impair bud development (Kozlowski et al. 1991), which. in the following year. impacts tree growth by reduction of leaf area and auxin-mediated stimulation of cambial cell division (Larson 1994, Uggla et al. 1998). That annual increments in Pinus sylvestris are influenced by previous-year drought conditions was shown in several dendroecological studies (e.g., Oberhuber et al. 1998, Rigling et al. 2002, Friedrichs et al. 2009). Therefore, we explain the missing significant increase in ring width in 2008 compared with 2007 by predisposition through the buds developed under drought stress in 2007.
In comparison with timberline sites, where tree growth is regarded to be restricted by low temperature throughout the growing season (Körner 1998, cf. Rossi et al. 2007), the total number of tracheids produced per year was quite low within the study area—e.g., 16 tracheids were developed in 2007 at the dry-mesic site compared to c. 60 tracheids recorded during the same growing season in mature Pinus cembra at timberline (c. 1950 m a.s.l.) on Mt. Patscherkofel, located c. 50 km in linear distance of the study area (Gruber et al. 2009a). These results point to the importance of tree water status on stem radial growth, which has frequently been shown to limit radial growth within dry inner Alpine valleys (e.g., Kienast et al. 1987, Oberhuber et al. 1998, Rigling et al. 2002, Eilmann et al. 2006, Zweifel et al. 2006). The inherent low soil water content within the study area and the observed abrupt fluctuations following precipitation events are caused by low waterholding capacity of the shallow, stony soils. Besides limited water availability, however, tree growth might also be restricted within the study area due to inherent low nutrient content and retarded soil development of dolomite parent material (Krapfenbauer 1969), which is supported by almost twice as wide annual increments of Pinus sylvestris trees of comparable age reported from Valais, an inner Alpine dry valley in Switzerland (Rigling et al. 2002). The overall low cell production rate within the study area, which amounted to two–three cells/14 days at the most at the dry-mesic site, also precluded the determination of the relationship between xylogenesis and climate variables (cf. Gruber et al. 2009a).
Lower soil water availability during the growing season at the xeric compared with the dry-mesic site caused significantly reduced radial cell width in earlywood (for 2007 and 2008) and latewood tracheids (for 2008 only). This inhibition of cell expansion of tracheids with the loss of cell turgor during drought is a well-known phenomenon (e.g., Zahner 1968, Kramer 1983, Sheriff and Whitehead 1984, Abe and Nakai 1999). Recently, Rossi et al. (2009b) reported a reduction of up to 50% in lumen area and xylem cell diameter in Abies balsamea seedlings exposed to drought. Because the radial number of developed xylem cells in earlywood and latewood was not significantly different between contrasting sites, our results also indicate that larger annual radial increments at the dry-mesic site compared with those at the xeric site were primarily caused by an increase in radial cell diameter. However, several authors reported a decline in cambial cell division during drought (e.g., Dünisch and Bauch 1994b, Abe and Nakai 1999, Savidge 2000), which indicates that missing significant differences in number of tracheids between sites might also be caused by small-scale variability in soil water availability at both sites, rather than insensitivity of cambial activity to water deficit.
Timing of maximum tracheid production
Several authors (Rossi et al. 2006c, Heinrichs et al. 2007, Gruber et al. 2009c) reported that the maximum daily growth rate of conifers from cold environments, i.e., at the alpine treeline and in boreal forests, peaks around summer solstice. Rossi et al. (2006c) hypothesized that, in these environments, photoperiodic growth constraint is an adaptation allowing tracheid differentiation, particularly latewood cell wall formation and lignification, to be completed before winter. In our study, however, the timing of maximum cell production in 2007 and 2008 already peaked c. 4–6 wk before summer solstice and before the occurrence of more favorable growing conditions—i.e., an increase in precipitation and soil water content during summer. Additionally, the early start of the growing season in 2007 did not extend the duration of wood formation compared with 2008, when time period of xylogenesis was shifted by 2–3 wk. Hence, we suggest that, instead of photoperiodic regulation of maximum growth rate, a pronounced internal competition in carbon allocation between aboveground (terminal and radial shoot growth, bud development, foliage growth) and belowground organs (mycorrhiza-associated root system) exists. Litton et al. (2007) stated that, in forests, carbon partitioning to aboveground and belowground sinks are most sensitive to resources and environment, whereby aboveground production is low and belowground production is high at low resource availability. Therefore, we regard the early decrease in tracheid production in late spring as an adaptation to cope with extreme environmental conditions prevailing within the study area. Low precipitation and recurring drought periods in spring, combined with limited waterholding capacity and nutrient deficiency of shallow stony soils, might cause elevated carbohydrate requirements belowground to ensure adequate water and nutrient supply for shoot growth. That the root system generally is a strong sink for carbohydrates is supported by the results of Brunner et al. (2009), who reported a lack of increase in fine root biomass in Pinus sylvestris dominating in a comparable dry inner Alpine environment in response to irrigation treatment.
Conclusion
Despite an extended drought period in spring, cambial activity commenced earlier in 2007 compared with 2008, when cool-moist conditions prevailed at the beginning of the growing season. This suggests that temperature, rather than water availability, controls cambial reactivation in drought-exposed Pinus sylvestris. On the other hand, spatial variability in the dynamics and duration of wood formation and radial widths of earlywood cells indicate a strong influence of drought stress on cell differentiation processes. Hence, this study demonstrates the plasticity of tree-ring formation in Pinus sylvestris in response to temperature and water availability as a result of modifying the onset of cambial activity and duration of xylem cell differentiation, respectively. A better understanding of the dynamics of growth ring development in Pinus sylvestris will also enable improvement of climate-growth models used to reconstruct precipitation variability within inner Alpine dry valleys based on tree ring widths (Oberhuber and Kofler 2002, cf. Wilson et al. 2005).
Acknowledgments
This work was supported by the Austrian Science Fund (FWF Project No. P19563-B16 “Dynamics of cambial activity and wood formation of Scots pine (Pinus sylvestris L.) exposed to soil dryness.”. Special thanks go to I. Swidrak for technical support. We also thank the anonymous reviewers for their valuable suggestions and comments to improve the manuscript. We greatly acknowledge Hydrographischer Dienst, Innsbruck, for providing us the climate data.
References
- Abe H, Nakai T. Effect of the water status within a tree on tracheid morphogenesis in Cryptomeria japonica D. Don. Trees. 1999;14:124–129. [Google Scholar]
- Antonova GF, Stasova VV. Effects of environmental factors on wood formation in Scots pine stems. Trees. 1993;7:214–219. [Google Scholar]
- Beniston M. The 2003 heat wave in Europe: A shape of things to come? An analysis based on Swiss climatological data and model simulations. Geophys. Res. Lett. 2004;31:2022–2026. [Google Scholar]
- Bigler C, Bräker OU, Bugmann H, Dobbertin M, Rigling A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems. 2006;9:330–343. [Google Scholar]
- Brunner I, Pannatier EG, Frey B, Rigling A, Landolt W, Zimmermann S, Dobbertin M. Morphological and physiological responses of Scots pine fine roots to water supply in a dry climatic region in Switzerland. Tree Physiol. 2009 doi: 10.1093/treephys/tpn046. doi: 10.1093/treephys/tpm046. [DOI] [PubMed] [Google Scholar]
- Day TA, DeLucia EH, Smith WK. Influence of cold soil and snowcover on photosynthesis and leaf conductance in two Rocky Mountain conifers. Oecologia. 1989;80:546–552. doi: 10.1007/BF00380080. [DOI] [PubMed] [Google Scholar]
- DeLucia EH. Effect of low root temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engelmann spruce (Picea engelmannii Parry ex Engelm.) seedlings. Tree Physiol. 1986;2:143–154. doi: 10.1093/treephys/2.1-2-3.143. [DOI] [PubMed] [Google Scholar]
- Denne MP. Definition of latewood according to Mork (1928) IAWA Bull. 1988;10:59–62. [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Can. J. For. Res. 2003;33:190–200. [Google Scholar]
- Deslauriers A, Morin H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees. 2005;19:402–408. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T, Saracino A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008;28:863–871. doi: 10.1093/treephys/28.6.863. [DOI] [PubMed] [Google Scholar]
- Dünisch O, Bauch J. Influence of soil substrate and drought on wood formation of spruce (Picea abies L. Karst.) under controlled conditions. Holzforschung. 1994;48:447–457. [Google Scholar]
- Eilmann B, Weber P, Rigling A, Eckstein D. Growth reactions of Pinus sylvestris L. and Quercus pubescens Willd. to drought years at a xeric site in Valais, Switzerland. Dendrochronologia. 2006;23:121–132. [Google Scholar]
- Ellenberg H. Vegetation ecology of Central Europe. Cambridge University Press; UK, Cambridge: 1988. p. 731. [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 1998. [Google Scholar]
- Frankenstein C, Eckstein D, Schmitt U. The onset of cambium activity – a matter of agreement? Dendrochronologia. 2005;23:57–62. [Google Scholar]
- Friedrichs DA, Trouet V, Büntgen U, Frank DC, Esper J, Neuwirth B, Löffler J. Species-specific climate sensitivity of tree growth in Central-West Germany. Trees. 2009;23:729–739. doi: 10.1093/treephys/tpn003. [DOI] [PubMed] [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Primož O. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci. Techn. 2006;41(6):463–475. [Google Scholar]
- Gruber A, Baumgartner D, Zimmermann J, Oberhuber W. Temporal dynamic of wood formation in Pinus cembra along the alpine treeline ecotone and the effect of climate variables. Trees. 2009a;23:623–635. doi: 10.1007/s00468-008-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Wieser G, Oberhuber W. Effects of simulated soil temperature on stem diameter increment of Pinus cembra at the alpine timberline: a new approach based on root zone roofing. Eur. J. For. Res. 2009b doi: 10.1007/s10342-009-0305-3. doi:10.1007/s10342-009-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Zimmermann J, Wieser G, Oberhuber W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 2009c;66:503. doi: 10.1051/forest/2009038. doi:10.1051/forest/2009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs DK, Tardif JC, Bergeron Y. Xylem production in six tree species growing on an island in the boreal forest region of western Quebec, Canada. Can. J. Bot. 2007;85:518–525. [Google Scholar]
- Hellmers H, Genthe MK, Ronco F. Temperature affects growth and development of Engelmann spruce. For. Sci. 1970;16:447–452. [Google Scholar]
- Jolly WM, Dobbertin M, Zimmermann NE, Reichstein M. Divergent vegetation growth response to the 2003 heat wave in the Swiss Alps. Geophys. Res. Lett. 2005;32:L18409. doi:10.1029/2005GL023252. [Google Scholar]
- Kienast F, Schweingruber FH, Bräker OU, Schär E. Tree-ring studies on conifers along ecological gradients and the potential of single-year analyses. Can. J. For. Res. 1987;17:683–696. [Google Scholar]
- Körner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Kozlowski TT, Kramer PJ, Pallardy SG. The physiological ecology of woody plants. Academic Press; San Diego: 1991. p. 657. [Google Scholar]
- Kramer PJ. Water relations of plants. Academic Press; USA, New York: 1983. p. 489. [Google Scholar]
- Krapfenbauer A. Böden auf Dolomit und Serpentin in ihrer Auswirkung auf die Waldernährung. Cbl. Ges. Forstw. 1969;86:189–219. [Google Scholar]
- Larson PR. The vascular cambium: development and structure. Springer; Berlin: 1994. p. 740. [Google Scholar]
- Levanič T, Gričar J, Gagen M, Jalkanen R, Loader NJ, McCarroll D, Oven P, Robertson I. The climatic sensitivity of Norway spruce [Picea abies (L.) Karst.] in the southeastern European Alps. Trees. 2009;23:169–180. [Google Scholar]
- Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Glob. Ch. Biol. 2007;13:2089–2109. [Google Scholar]
- Lopushinsky W, Kaufmann MR. Effects of cold soil on water relations and spring growth of Douglas-fir seedlings. For. Sci. 1984;30:628–634. [Google Scholar]
- Loris K. Dickenwachstum von Zirbe, Fichte und Lärche an der alpinen Waldgrenze/Patscherkofel. Mitt. Forstl. Bundesvers. Wien. 1981;142:417–441. [Google Scholar]
- Mäkinen H, Seo J-W, Nöjd P, Schmitt U, Jalkanen R. Seasonal dynamics of wood formation: a comparison between pinning, microcoring and dendrometer measurements. Eur. J. For. Res. 2008;127:235–245. [Google Scholar]
- Martín-Benito D, Cherubini P, Río M, Canellas I. Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees. 2008;22:363–373. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavska O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Mage F, Mestre A, Nordli Ø, Penuelas J, Pirinen P, Remisova V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski F-E, Zach S, Zust A. European phenological response to climate change matches the warming pattern. Glob. Ch. Biol. 2006;12(10):1969–1976. [Google Scholar]
- Oberhuber W, Stumböck M, Kofler W. Climate-tree-growth relationships of Scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees. 1998;13:19–27. [Google Scholar]
- Oberhuber W, Kofler W. Topographic influences on radial growth of Scots pine (Pinus sylvestris L.) at small spatial scales. Plant Ecol. 2000;146:229–238. [Google Scholar]
- Oberhuber W. The role of climate in the mortality of Scots pine (Pinus sylvestris L.) exposed to soil dryness. Dendrochronologia. 2001;19(1):45–55. [Google Scholar]
- Oberhuber W, Kofler W. Dendroclimatological spring rainfall reconstruction for an inner Alpine dry valley. Theor. Appl. Clim. 2002;71:97–106. [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Orwig DA, Abrams MD. Variation in radial growth responses to drought among species, site, and canopy strata. Trees. 1997;11:474–484. [Google Scholar]
- Pichler P, Oberhuber W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manage. 2007;242:688–699. [Google Scholar]
- Plomion C, Leprovost G, Stokes A. Wood formation in trees. Plant Physiol. 2001;127:1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Pregitzer KS, King JS, Burton AJ, Brown SE. Responses of tree fine roots to temperature. New Phytol. 2000;147:105–115. [Google Scholar]
- Rebetez M, Dobbertin M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theor. Appl. Clim. 2004;79:1–9. [Google Scholar]
- Rebetez M, Mayer H, Dupont O, Schindler D, Gartner K, Kropp JP, Menzel A. Heat and drought 2003 in Europe: a climate synthesis. Ann. For. Sci. 2006;63:569–577. [Google Scholar]
- Rigling A, Bräker OU, Schneiter G, Schweingruber FH. Intra-annual tree-ring parameters indicating differences in drought stress of Pinus sylvestris forests within the Erico-Pinion in the Valais (Switzerland) Plant Ecol. 2002;163:105–121. [Google Scholar]
- Rigling A, Brühlhart H, Bräker OU, Forster T, Schweingruber FH. Effects of irrigation on diameter growth and vertical resin duct production in Pinus sylvestris L. on dry sites in the central Alps, Switzerland. For. Ecol. Manage. 2003;175:285–296. [Google Scholar]
- Rossi S, Deslauriers A, Morin H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 2003;21:33–39. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 2006a;27:89–97. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA J. 2006b;27:383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006c;170(2):301–310. doi: 10.1111/j.1469-8137.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carraro V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia. 2007;152(1):1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Gričar J, Seo J-W, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008;17:696–707. [Google Scholar]
- Rossi S, Rathgeber CBK, Deslauriers A. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann. For. Sci. 2009a;66:206. doi: 10.1051/forest/2008088. [Google Scholar]
- Rossi S, Simard S, Rathgeber CBK, Deslauriers A, Zan CD. Effects of a 20-day-long dry period on cambial and apical meristem growth in Abies balsamea seedlings. Trees. 2009b;23:85–93. [Google Scholar]
- Savidge RA. Intrinsic regulation of cambial growth. J. Plant Growth Regul. 2000;20:52–77. [Google Scholar]
- Savidge RA, Wareing PF. Plant growth regulators and the differentiation of vascular elements. In: Barnett JR, editor. Xylem Cell Development. Castle House Publications; Tunbridge Wells, U.K.: 1981. pp. 192–235. [Google Scholar]
- Schmitt U, Jalkanen R, Eckstein D. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn. 2004;38(2):167–178. [Google Scholar]
- Seo J-W, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiol. 2008;28:105–112. doi: 10.1093/treephys/28.1.105. [DOI] [PubMed] [Google Scholar]
- Sheriff DW, Whitehead D. Photosynthesis and wood structure in Pinus radiata D. Don during dehydration and immediately after rewatering. Plant Cell Env. 1984;7:53–62. [Google Scholar]
- Thabeet A, Vennetier M, Gadbin-Henry C, Denelle N, Roux M, Caraglio Y, Vila B. Response of Pinus sylvestris L. to recent climatic events in the French Mediterranean region. Trees. 2009;23:843–853. [Google Scholar]
- Thibeault-Martel M, Krause C, Morin H, Rossi S. Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Ann. Bot. 2008;102:667–674. doi: 10.1093/aob/mcn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EJ, Sundberg B. Indole-3-acetic acid controls cambial growth in Scots Pine by positional signaling. Plant Physiol. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapaavuori EM, Rikala R, Ryyppö A. Effects of root temperature on growth and photosynthesis of conifer seedlings during shoot elongation. Tree Physiol. 1992;10:217–230. doi: 10.1093/treephys/10.3.217. [DOI] [PubMed] [Google Scholar]
- Waldboth M, Oberhuber W. Synergistic effect of drought and chestnut blight (Cryphonectria parasitica) on growth decline of European chestnut (Castanea sativa) For. Path. 2009;39:43–55. [Google Scholar]
- Wilson RJS, Luckman BH, Esper J. A 500 year dendroclimatic reconstruction of spring-summer precipitation form the lower Bavarian forest region, Germany. Int. J. Climatol. 2005;25:611–630. [Google Scholar]
- Zahner R. Water deficits and growth of trees. In: Kozlowski TT, editor. Water deficits and plant growth. II. Academic Press; New York: 1968. pp. 191–254. [Google Scholar]
- Zeide B. Analysis of growth equations. For. Sci. 1993;39:594–616. [Google Scholar]
- Zweifel R, Zimmermann L, Zeugin F, Newberry DM. Intra-annual radial growth and water relations of trees: implications towards a growth mechanism. J. Exp. Bot. 2006;57(6):1445–1459. doi: 10.1093/jxb/erj125. [DOI] [PubMed] [Google Scholar]