Abstract
Lesion and transplantation studies in the cockroach, Leucophaea maderae, have located its bilaterally symmetric circadian pacemakers necessary for driving circadian locomotor activity rhythms to the accessory medulla of the optic lobes. The accessory medulla comprises a network of peptidergic neurons, including pigment-dispersing factor (PDF)-expressing presumptive circadian pacemaker cells. At least three of the PDF-expressing neurons directly connect the two accessory medullae, apparently as a circadian coupling pathway. Here, the PDF-expressing circadian coupling pathways were examined for peptide colocalization by tracer experiments and double-label immunohistochemistry with antisera against PDF, FMRFamide, and Asn13-orcokinin. A fourth group of contralaterally projecting medulla neurons was identified, additional to the three known groups. Group one of the contralaterally projecting medulla neurons contained up to four PDF-expressing cells. Of these, three medium-sized PDF-immunoreactive neurons coexpressed FMRFamide and Asn13-orcokinin immunoreactivity. However, the contralaterally projecting largest PDF neuron showed no further peptide colocalization, as was also the case for the other large PDF-expressing medulla cells, allowing the easy identification of this cell group. Although two-thirds of all PDF-expressing medulla neurons coexpressed FMRFamide and orcokinin immunoreactivity in their somata, colocalization of PDF and FMRFamide immunoreactivity was observed in only a few termination sites. Colocalization of PDF and orcokinin immunoreactivity was never observed in any of the terminals or optic commissures. We suggest that circadian pacemaker cells employ axonal peptide sorting to phase-control physiological processes at specific times of the day.
Keywords: Circadian rhythms, Pigment-dispersing hormone, Orcokinin, FMRFamide, Accessory medulla, Cockroach, Leucophaea maderae (Insects)
Introduction
Much research on the structural, functional, and molecular properties of endogenous circadian clocks has been performed in a variety of insect species. Whereas the first circadian pacemaker center controlling behavioral activity patterns was located to the optic lobes of the cockroach Leucophaea maderae, the cellular nature of the clock remained elusive (Nishiitsutsuji-Uwo and Pittendrigh 1968; Roberts 1974; Sokolove 1975; Page 1982). Subsequent lesion and transplantation studies have identified the accessory medulla (AMe, plural AMae; Ehnbom 1948) at the anterior ventromedial border of the medulla as this circadian clock (Stengl and Homberg 1994; Reischig and Stengl 2003a; for a review, see Homberg et al. 2003). The AMe is formed by a set of about 250 associated neurons, most of which can be classified into several groups according to their morphological and immunohistochemical characteristics (Reischig and Stengl 2003b; see also Materials and methods). Among these is a distinct set of about a dozen pigment-dispersing factor (PDF)-expressing medulla neurons (PDFMe, Fig. 1a, b). In Drosophila melanogaster, homologous PDF-expressing neurons (the ventral group of the lateral neurons, LNvs) have been shown to be circadian pacemakers indispensable for maintaining circadian locomotor rhythms under constant conditions (for a review, see Helfrich-Förster 2005). In cockroaches and other insects, the homologous PDFMe are circadian pacemaker cells that control locomotor activity rhythms (Stengl and Homberg 1994; Singaravel et al. 2003; Reischig and Stengl 2003a; Wen and Lee 2008). Furthermore, the neuropeptide PDF as a clock output factor is indispensable for circadian locomotor rhythms as pdf RNAi experiments have demonstrated in the German cockroach Blatella germanica (Lee et al. 2009).
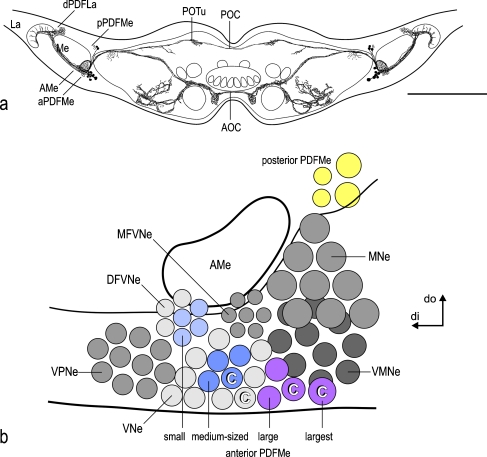
Fig. 1.
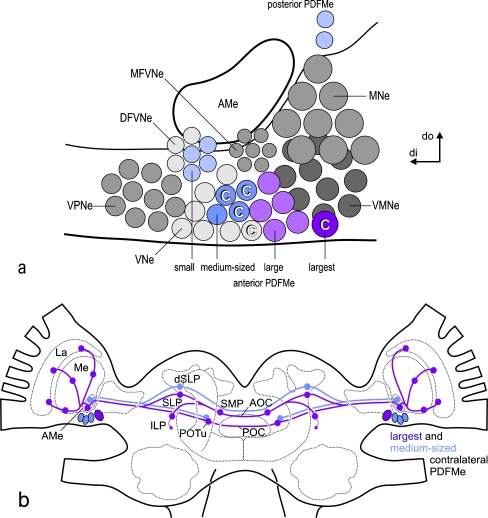
a Horizontal reconstruction of the PDF-expressing neuron system of the cockroach Leucophaea maderae in the supraesophageal ganglion (AMe accessory medulla, aPDFMe anterior group of PDF-expressing neurons, AOC anterior optic commissure, dPDFLa dorsal group of PDF-expressing lamina neurons, La lamina, Me medulla, pPDFMe posterior group of PDF-expressing medulla neurons, POC posterior optic commissure, POTu posterior optic tubercle). Bar 500 μm. b Representation of the neuron groups of the accessory medulla (AMe) including the PDF-expressing medulla neurons (PDFMe, colored) as derived from morphological and immunohistological analyses (DFVNe distal group of frontoventral neurons, MFVNe medial group of frontoventral neurons, MNe medial neurons, VNe ventral neurons, VMNe ventromedial neurons, VPNe ventroposterior neurons). Although the numbers of gray circles do not reflect the actual group sizes, the numbers of colored circles represent the rounded mean numbers of the respective PDFMe neurons according to previous work. Large (purple, plus one largest) and medium-sized (dark blue) PDFMe belong to the VNe, whereas small PDFMe (light blue) appear to belong to the DFVNe neurons. Previous work has suggested that two large and one medium-sized PDFMe together with one unspecified VNe (C) project to the contralateral AMe to provide a mutual pacemaker coupling input. The unspecified VNe is suggested to express either an FMRFamide-related peptide or orcokinin (di distal, do dorsal)
The PDF-expressing circadian pacemaker neurons are assumed to provide circadian timing signals with peptides released from varicosity-studded terminals arborizing in large areas of the optic lobes and central protocerebrum. Moreover, as has been shown in L. maderae and D. melanogaster, fibers of a subgroup of the PDF-expressing neurons of one optic lobe enter the contralateral optic lobe and apparently also the contralateral AMe (Reischig et al. 2004; Helfrich-Förster et al. 2007). These observations suggest that contralaterally projecting PDFMe provide the neuronal pathway for mutual pacemaker coupling as proposed previously by behavioral experiments in L. maderae (Page et al. 1977; Page 1978, 1983a). This hypothesis is further supported by data following PDF injections into the vicinity of the AMe of L. maderae at various Zeitgeber times (ZTs) resulting in a monophasic phase response curve (Petri and Stengl 1997). These experiments have demonstrated that PDF phase delays locomotor activity onset only during the late day. Computer modeling based on experimental observations have predicted that phase delays and phase advances constitute mutual pacemaker coupling (Petri and Stengl 2001). Thus, a search for circadian coupling pathways has been initiated employing dextran tracer injections into one AMe and the subsequent investigation of the contralateral AMe with anti-PDF immunocytochemistry. In a previous study, up to four contralaterally projecting ventral neurons (VNe) at the location of the anterior PDFMe have been labeled by such backfills. Three of these contralateral VNe neurons have been shown to be PDF-immunoreactive (-ir; Reischig et al. 2004). In addition, Hofer and Homberg (2006a) have demonstrated that at least one of these four contralaterally projecting VNe neurons is orcokinin-ir. However, whether orcokinin immunoreactivity is colocalized with PDF immunoreactivity in the group of the four contralaterally projecting VNe remains unknown (Hofer and Homberg 2006b).
Other neuromodulator candidates involved in a bilateral pacemaker coupling pathway are members of the FMRFamide-related peptides (FaRPs). The FaRPs share the RFamide C-terminus and are involved in the regulation of a multitude of physiological activities (Predel 2006; Orchard and Lange 2006). Since studies with antibodies against FMRFamides have revealed the colocalization of PDF with FMRFamide immunoreactivity in up to six PDFMe, FaRPs might be involved in the circadian coupling pathway (Petri et al. 1995). This hypothesis is supported by the finding of FMRFamide immunoreactivity in both the anterior and posterior optic commissures, which connect the two AMae. Finally, injection experiments combined with locomotor activity assays have demonstrated that different FaRPs affect locomotor activity rhythms at distinct circadian times (Soehler et al. 2008). These anatomical and physiological data suggest the involvement of FaRPs in circadian pacemaker coupling of the cockroach L. maderae.
Here, the circadian coupling pathways of L. maderae have been investigated further with neurobiotin backfills from one optic stalk, combined with immunolabeling with anti-PDF together with either anti-FMRFamide or anti-Asn13-orcokinin at higher primary antibody concentrations than those tested previously (Hofer and Homberg 2006a, b). We have found enhanced and more consistent tracer labeling with neurobiotin backfills compared with dextran backfills, thus suggesting that a larger number of neurons is involved in pacemaker coupling than estimated previously. Furthermore, we have demonstrated that neurons that appear to contain at least three different neuropeptides in their somata connect the two AMae with surprisingly sparse colocalization of neuropeptides in their axonal terminals. In addition, further details of the neuroarchitecture of this circadian pacemaker neuropil have been elucidated.
Materials and methods
Animals
Adult male cockroaches (Leucophaea maderae, syn. Rhyparobia maderae) were taken from laboratory colonies. They were reared under a 12:12-h light-dark (LD) photoperiod at about 60% relative humidity and a temperature of 26°C. Animals were fed with dried dog food, potatoes, and water ad libitum. In anatomical descriptions, indications of position (e.g., left, right) are always referred to the animal’s body axis.
Neuron classification
A group of about 250 neurons of which most appear to contribute to the AMe of L. maderae extends frontally, medially, and ventrally to the AMe. According to their size, position, morphological characters, and immunostaining properties, these neurons can be classified into at least six main groups (“morphological groups”; Reischig and Stengl 1996, 2003b; Fig. 1b): the distal and medial frontoventral neurons (DFVNe and MFVNe, respectively), the medial and ventral neurons (MNe and VNe), the ventromedial neurons (VMNe), and the ventroposterior neurons (VPNe). Additionally, neurons anterior to the AMe project into the AMe (Soehler et al. 2008), but these are not included in the scheme.
The PDF-ir medulla neurons (PDFMe) of L. maderae are separated into an anterior and a posterior group (anterior PDFMe and posterior PDFMe, respectively; Fig. 1b). The anterior PDFMe further consist of three subgroups, which can be distinguished by the size and intensity of the anti-PDF immunolabeling (Reischig and Stengl 2003b): the four intensely labeled large anterior PDFMe, the four smaller and generally less intensely labeled medium-sized anterior PDFMe, and the four faintly labeled small anterior PDFMe (all numbers here are approximate). According to the criteria of the six morphological groups, large and medium-sized anterior PDFMe belong to the VNe, whereas the small anterior PDFMe appear to belong to the DFVNe.
Furthermore, three groups of medulla neurons projecting to the contralateral optic lobe have been previously found and have been termed contralaterally projecting medulla neurons (MC I-III), of which at least two (MC I and MC II) definitely project to the contralateral AMe (Reischig et al. 2004). MC I are the contralaterally projecting anterior PDFMe (Fig. 1b) and one non-PDF-ir VNe, whereas MC II is identical with the VMNe. MC III is located in a posterior position similar but not identical to the posterior PDFMe.
Primary antisera
The monoclonal anti-Drosophila-PDF antibody was generated by Dr. Justin Blau (New York University, USA) and purchased from the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa, USA. Mouse B-lymphocytes were raised in cell culture, and pure supernatant was used for immunostaining at a working dilution of 1:5. The anti-β-pigment-dispersing hormone (PDH) antiserum (no. 3B3) was a gift from Dr. Heinrich Dircksen, Stockholm and was raised in rabbits against synthetic Uca pugilator β-PDH (Dircksen et al. 1987). This antiserum has been widely used to detect PDF-expressing neurons in many insect species and has recently been systematically characterized by Honda et al. (2006). Furthermore, a PDF peptide was previously isolated in L. maderae by Hamasaka et al. (2005). The anti-β-PDH antiserum was used at a working dilution of 1:15,000. Double-labeling was performed with the anti-Drosophila-PDF antibody and the anti-β-PDH antiserum to examine the specificity of both antisera. Procedures, buffers, and incubation times were the same as described below. The antibodies were detected with goat anti-mouse Cy3 and goat anti-rabbit Alexa 633. Both antibodies labeled identical structures at the above-mentioned working dilutions (data not shown). The anti-FMRFamide antiserum (no. 671) was a gift from Dr. E. Marder, Massachusetts, USA (Marder et al. 1987) and was raised in rabbits against the C-terminal amino acid sequence FMRFamide. Hence, the antiserum is supposed to detect all members of the large FaRP family. We used the antiserum at a working dilution of 1:4,000. The anti-orcokinin antiserum (provided by Dr. Heinrich Dircksen) was raised in rabbits against the tridecapeptide Asn13-orcokinin of the crayfish Orconectes limosus and was used at a working dilution of 1:1,000. This concentration was considerably higher than that used previously (Hofer and Homberg 2006a, b) in order to ensure the labeling of fine terminals. The specificity of the Asn13-orcokinin antiserum on cockroach brain sections was demonstrated by Hofer et al. (2005), who identified orcokinin-related peptides recognized by the anti-Asn13-orcokinin antiserum in Schistocerca gregaria and L. maderae.
Backfill with neurobiotin
Neurobiotin backfills were accomplished as reported by Reischig and Stengl (2002). At ZT 7-11 (where ZT 0 represents “lights on”), animals were anesthetized with CO2 and fixed facing up in a mounting device, thus enabling a direct view onto the frontal side of the head. The whole operation was performed with the animal under continuous anesthesia produced by a constant flow of CO2. A small window was cut into the head capsule to expose the left optic lobe. The application pipette was filled with 1-2 μl of a solution of 5% neurobiotin in distilled water. The left optic stalk was cut, and the application pipette with a tip opening of 300-400 μm was slipped over the stump of the optic stalk. The pipette was then fixed with modeling clay, and the operation field was closed with Vaseline. The animals were stored overnight in a box at 4°C to allow intracellular transport of the dye.
Immunocytochemistry for backfilled and injected animals
On the day after the operation at ZT 3-6, the brains were removed from the head capsules and fixed for 4 h in 4% paraformaldehyde/7.5% saturated picric acid in sodium phosphate buffer (PB, 0.1 M, pH 7.4) at room temperature. The fixed brains were embedded in gelatin/albumin (4.8% gelatin and 12% ovalbumin in demineralized water) and postfixed overnight in 4% paraformaldehyde in PB in the refrigerator. Then, the brains were sectioned with a vibrating-blade microtome (Leica, Nussloch, Germany) in frontohorizontal slices at a thickness of 50-60 μm. The sections were washed with TRIS-buffered saline (TBS: 0.1 M TRIS-HCl/0.3 M NaCl, pH 7.4) containing 0.1% Triton X-100 (TrX) for 3×10 min and preincubated in TBS with 0.5% TrX and 5% normal goat serum (NGS; DAKO, Hamburg, Germany) for 1 h. Two antisera were applied simultaneously: either monoclonal anti-PDF with anti-FMRFamide or monoclonal anti-PDF with anti-orcokinin (see Primary antisera for dilutions) in TBS containing 0.5% TrX and 1% NGS. The sections were incubated in the antisera cocktails for 3-4 days in the refrigerator and then washed in TBS containing 0.5% TrX 3 (3 x 40 min). Then, they were incubated with a solution containing FITC-conjugated streptavidin (1:100) for detection of neurobiotin, Cy3- conjugated goat anti mouse antiserum (1:300) for detection of anti-PDF, and Alexa 633-conjugated goat anti rabbit antiserum (1:300) for detection of either anti-FMRFamide or anti-orcokinin (all probes from Dianova, Hamburg, Germany). These substances were diluted in TBS containing 0.5% TrX and 1% NGS, and the sections incubated for two hours. The sections were then thoroughly rinsed with PB and cleared with 1:1 Glycerol/P for at least 30 minutes. Finally, the sections were mounted on microscope slides in anatomical order, and coverslipped in 1:1 Glycerol/PB.
Evaluation and visualization
The preparations were examined with a Leica confocal laser scanning microscope TCS SP2 equipped with an acusto-optical beam splitter for separation of excitation and emitted light and with a variable detection filtering system (spectrophotometer) for the arbitrary selection of spectral intervals of emitted light. Most scans were performed with a Leica HC PL apochromat 20×/0.7 dry lens, but a HCX PL apochromat 40×/1.25 oil immersion lens was used for high resolution scans of neuronal fibers. To exclude crosstalk artifacts, all three channels were scanned sequentially, and the detection ranges were separated as far as possible (FITC: excitation with the 488-nm line of an argon laser, detection between 495 and 530 nm; Cy3: excitation with the 543-nm line of a helium/neon laser, detection between 590 and 610 nm; Alexa 633: excitation with the 633-nm line of a helium/neon laser, detection between 680 and 800 nm). All specimens with recognizable backfill labeling (n = 25) were scanned. We scanned every section in which the right AMe, the anterior and posterior PDFMe, and backfilled neurons of the AMe were present (z-distance of single optical sections: 2 μm). In some specimens, the central termination areas of PDF-ir neurons and PDF-ir commissures were additionally scanned with the 40× lens and a z-resolution of 0.5-1 μm. Data evaluation was performed on a graphics computer workstation.
To quantify the results, every soma was identified individually through and between image stacks to prevent counting artifacts resulting from pure counting of neuron profiles. To estimate the average numbers of neurons in certain groups, arithmetic means and standard deviations were calculated. If sections were missing or, more frequently, if the low labeling intensity combined with high background labeling did not allow the unambiguous identification of neurons in groups in which otherwise labeled neurons could always be identified, then the preparations were excluded from the calculation of mean numbers for the respective group.
Results
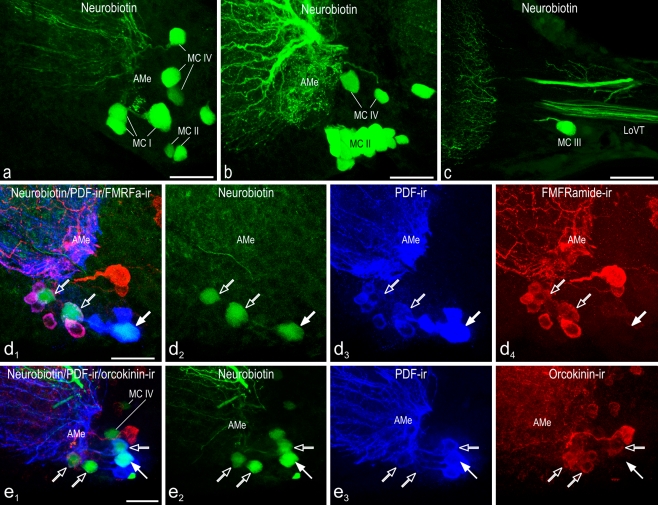
To identify all neurons that couple the two AMae, neurobiotin backfills were performed from one optic stalk. In order to clarify whether, in addition to PDF, the peptides orcokinin and FMRFamide were also involved in the coupling of the two AMae, the backfill experiments were combined with double-label immunohistochemistry. Double-label studies were carried out with antisera either against PDF and FMRFamide (n = 11) or against PDF and orcokinin (n = 14). Labeled neuronal somata of the AMe contralateral to the backfilled side, corresponding fiber projections in the contralateral optic lobe and central brain, and commissural fibers were evaluated with confocal laser scanning microscopy. The neurobiotin backfills generally revealed contrast-enhanced and more reproducible labeling compared with similar experiments employing rhodamine dextran (Reischig and Stengl 2002; Reischig et al. 2004). Moreover, with neurobiotin, larger numbers of backfilled neurons were counted, and a new group of backfilled neurons (MC IV) emerged that was not visible with rhodamine dextran backfills or injections. Contralaterally projecting medium-sized PDFMe could now also be distinguished clearly from large PDFMe, because they were colabeled with both the anti-FMRFamide and anti-orcokinin antisera.
Anti-FMRFamide and anti-orcokinin labeled small and medium-sized PDFMe
Immunocytochemical labeling of AMe neurons of the cockroach with antisera against FMRFamide and orcokinin and colocalization with anti-PDF staining were described previously (Petri et al. 1995; Hofer and Homberg 2006b; Soehler et al. 2008). Our results only partly confirmed these previously published findings. A monoclonal anti-PDF antibody (Cyran et al. 2005) was employed in order to facilitate double-labeling studies. To determine whether this monoclonal antibody, raised in mice against Drosophila-PDF, labeled the same structures as the previously employed anti-Uca-β-PDH antiserum of Dircksen et al. (1987), double-label experiments with both anti-PDF/PDH antisera were employed. The staining pattern obtained with both antisera completely overlapped (data not shown). Within the anterior PDFMe, organization into strongly labeled large PDFMe, weaker labeled medium-sized PDFMe, and the weakest labeled small PDFMe was generally apparent. Compared with the Uca-β-PDH antiserum, the Drosophila anti-PDF antibody gave higher background and weaker specific labeling that impeded the counting of the small anterior PDFMe. Thus, a high concentration (1:5) of antibody solution together with a longer incubation time of at least 2 days was required to achieve satisfactory tissue penetration. In contrast, the difference in labeling intensity between the large and medium-sized anterior PDFMe was enhanced compared with the anti-β-PDH antiserum, thus facilitating discrimination between these two groups (Tables 1, 2).
Table 1.
Mean numbers of anterior and posterior pigment-dispersing factor (PDF)-expressing medulla neurons (PDFMe). Values are given as arithmetic means ± standard deviation; the numbers after the semicolons are the numbers of evaluated animals
| Experimental setup | Large anterior PDFMe | Medium-sized anterior PDFMe | Small anterior PDFMe | Large posterior PDFMe | Small posterior PDFMe |
|---|---|---|---|---|---|
| All experiments (n = 25) | 4.2 ± 1.6; 25 | 3.6 ± 1.0; 24 | 3.9 ± 1.8; 18 | 1.7 ± 1.7; 22 | 2.4 ± 1.3; 22 |
| Anti-PDF + anti-FMRFamide (n = 11) | 0 ± 0; 11 | 3.9 ± 0.9; 10 | 4.4 ± 1.3; 10 | 0.1 ± 0.3; 9a | 1.8 ± 1.5; 9b |
| Anti-PDF + anti-Asn13 -orcokinin (n = 14) | 0 ± 0; 14 | 4.1 ± 1.1; 10 | 2.5 ± 0.6; 4c | 0 ± 0; 13 | 0.1 ± 0.3; 13d |
aOne large posterior PDFMe was FMRFamide-immunoreactive (ir) in only one case
bOne small posterior PDFMe was not FMRFamide-ir in only one case
cThe small number is attributable to difficulties in evaluation of weakly labeled preparations
dOne small posterior PDFMe was orcokinin-ir in only one case
Table 2.
Mean numbers of somata in accessory medulla (AMe) neuron groups labeled with anti-FMRFamide or anti-Asn13-orcokinin (VNe ventral neurons, DFVNe distal group of frontoventral neurons, MNe medial neurons, VPNe ventroposterior neurons, VMNe ventromedial neurons). Values are given as arithmetic means ± standard deviation; the numbers after the semicolons are the number of evaluated animals. Only one AMe per animal was evaluated, since the contralateral optic lobe was cut for backfilling. All animals were evaluated only for the VNe and DFVNe groups
| Immunoreactivity | VNe | DFVNe | MNe | VPNe | VMNe |
|---|---|---|---|---|---|
| FMRFamide-immunoreactive | 10.6 ± 2.2; 11a | 8.9 ± 4.1; 11b | 4.3 ± 0.5; 4 | 2.8 ± 1.0; 4 | 0 ± 0; 4 |
| Orcokinin-immunoreactive | 7.8 ± 1.1; 10a | 10.6 ± 3.7; 10c | 2.4 ± 0.8; 7 | 3.9 ± 1.6; 7 | 3.3 ± 0.5; 7 |
aIncluding the medium-sized PDF, which were all FMRFamide- and orcokinin-immunoreactive
bIncluding the FMRFamide-immunoreactive small PDFMe
cIncluding the orcokinin-immunoreactive small PDFMe
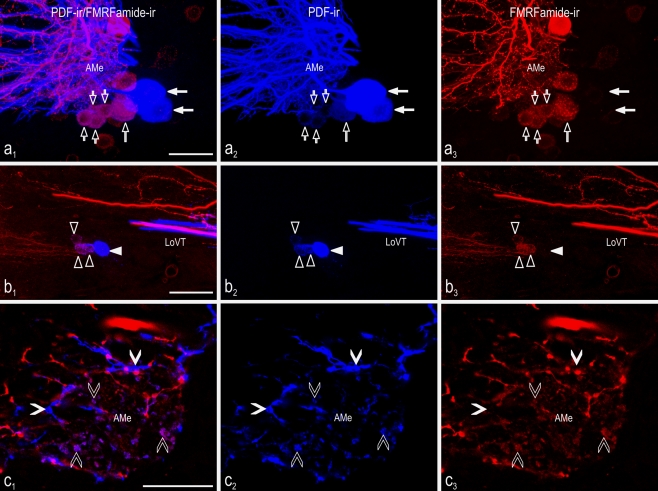
Double-labeling with anti-FMRFamide and anti-Drosophila-PDF revealed colocalized immunoreactivities in subgroups of the PDFMe. All small and all medium-sized anterior PDFMe showed additional FMRFamide immunoreactivity, whereas the large PDFMe never did (Fig. 2a; for numbers of PDFMe, see Table 1). Evaluation of the posterior PDFMe revealed that all small posterior PDFMe were additionally FMRFamide-ir, whereas large posterior PDFMe were not (Fig. 2b, Table 1), except in one case. The cell counts (Table 1) showed that the sum of non-FMRFamide-ir anterior and non-FMRFamide-ir posterior PDFMe was, in most cases, 6 neurons (n = 21 of 25). In cases where no non-FMRFamide-ir PDFMe were found among the posterior PDFMe, six of them were found among the anterior PDFMe (n = 5 of 25). In one preparation, six large posterior PDFMe were present but no large anterior PDFMe. In fiber projections, the colocalization of PDF and FMRFamide immunoreactivity was found only in three areas of the brain, namely in the AMe (Fig. 2c), in a dorsal part of the superior lateral protocerebrum (dSLP, Fig. 3a), and in the posterior optic tubercle (POTu, Fig. 3b). Contrary to previous studies (Reischig and Stengl 1996, 2003a), PDF-ir fibers with varicosities were also identified in the nodular neuropil, in addition to the shell neuropil (including the anterior neuropil) of the AMe. The PDF-ir varicosities of the nodular neuropil exhibited far lower labeling intensity and were generally more irregularly shaped than the strongly labeled PDF-ir varicosities of shell and internodular neuropil (Fig. 2c). Only these weakly labeled nodular PDF-ir projections exhibited additional FMRFamide immunoreactivity. Moreover, the AMe was densely invaded by FMRFamide-ir fibers without additional PDF immunoreactivity. Only PDF/FMRFamide-ir fibers occurred in the dSLP, and no fibers with PDF immunoreactivity alone. Many fibers with FMRFamide immunoreactivity alone were also detected in the dSLP. The origin of these fibers remained unclear. The POTu contained PDF/FMRFamide-ir fibers, fibers that expressed only PDF immunoreactivity, and fibers with only FMRFamide immunoreactivity. As in the dSLP, the origin of the FMRFamide-ir fibers in the POTu remained unclear. As shown previously, FMRFamide immunoreactivity was also present in other neuronal groups of the AMe next to the small and medium-sized PDFMe, namely the MNe, the VPNe, the DFVNe including non-PDF-ir and PDF-ir neurons, and the VNe including non-PDF-ir and PDF-ir neurons. Cell counts generally confirmed previous work (Soehler et al. 2008; Table 2).
Fig. 2.
Colocalization of PDF and FMRFamide immunoreactivity in neurons and neuropil of the accessory medulla (AMe) of the cockroach. Confocal laser scan images were obtained from vibratome sections of the accessory medulla showing PDF-immunoreactive (blue) and FMRFamide-immunoreactive (red) neurons. Left Overlay images. a, b Maximum projections from stacks of optical sections. c Single optical section. a 1-3 Neurons of the small (small open arrowheads), medium-sized (large open arrowhead), and large anterior PDFMe (large filled arrows). b 1-3 Posterior PDFMe comprising small (open triangles) and large (filled triangles) cells. All small, but no large, posterior PDFMe showed colocalized PDF/FMRFamide immunoreactivity (LoVT lobula valley tract). c 1-3 In the AMe, weakly labeled PDF-ir varicosities (open arrowheads) in the noduli showed colocalized PDF/FMRFamide immunoreactivity, whereas large and intensely labeled PDF-ir varicosities (filled arrowheads) in the internodular neuropil did not. Bars 50 μm (a), 25 μm (b), 100 μm (c)
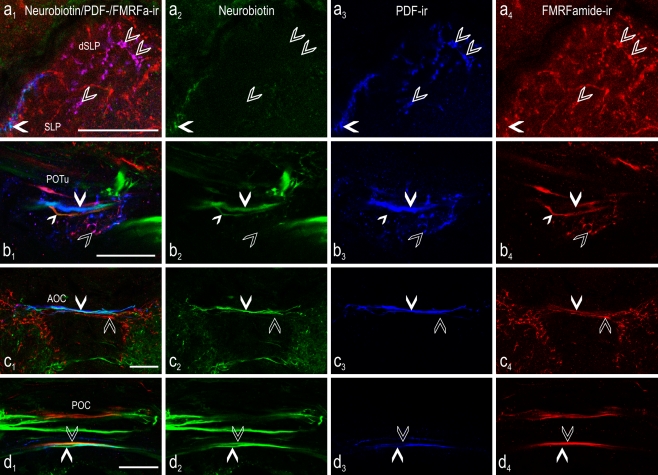
Fig. 3.
Colocalization of PDF (blue) and FMRFamide (red) immunoreactivity in the central brain of the cockroach combined with neurobiotin (green) backfill from a cut optic lobe stump. Confocal laser scan images (single optical sections) of typical projection areas of the PDFMe in the central brain after backfill of neurobiotin from the cut left optic stalk. Left Overlay images. a 1-4 At the dorsal part of the superior lateral protocerebrum (dSLP) contralateral to the backfilled side, fibers were visible with colocalized PDF and FMRFamide immunoreactivity (open arrowheads), but these were not labeled by neurobiotin. At the distal rim of the superior lateral protocerebrum (SLP) fibers were located showing colocalization of neurobiotin backfill and strong PDF immunoreactivity (filled arrowheads) but they did not exhibit FMRFamide immunoreactivity. b 1-4 In the posterior optic tubercle (POTu), fibers were present with colocalized PDF and FMRFamide immunoreactivity (open arrowheads), but these fibers were never labeled by neurobiotin backfills. In contrast, neurobiotin was found in commissural fibers either immunoreactive to anti-PDF (large filled arrowhead) or to anti-FMRFamide (small filled arrowhead) alone. c 1-4 In the anterior optic commissure (AOC), PDF-ir fibers labeled by neurobiotin backfill were frequently visible (filled arrowheads). These were never additionally colabeled by FMRFamide immunoreactivity, and parallel FMRFamide-ir fibers (open arrowheads) were never labeled by neurobiotin backfill. d 1-4 In the posterior optic commissure (POC), many backfilled fibers crossed the midline of the central brain. Backfilled fibers that showed either colocalized neurobiotin and PDF immunoreactivity (filled arrowheads) or neurobiotin and FMRFamide immunoreactivity (open arrowheads) were often found, but triple labeling was never observed. Bars 50 μm
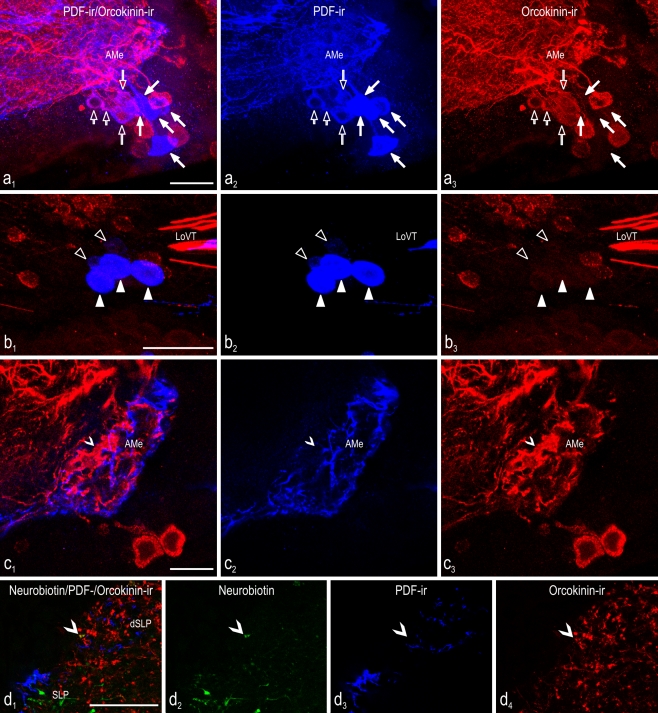
Double-label experiments with anti-Asn13-orcokinin and anti-Drosophila-PDF in part gave results that resembled the anti-FMRFamide labeling (Fig. 4). Generally, after anti-orcokinin labeling, the background staining was relatively high and resulted in ambiguous labeling in some preparations. This mainly affected the DFVNe and VNe in which orcokinin-ir labeling was generally of lower intensity, with the lowest occurring in the DFVNe. Therefore, only preparations with at least three visible orcokinin-ir DFVNe were considered for the counting of orcokinin-ir cells (n = 10 of 14). As in the anti-FMRFamide immunolabeling, the large anterior PDFMe were completely devoid of any additional orcokinin-ir labeling (Fig. 4a; Tables 1, 2). The medium-sized anterior PDFMe were always also orcokinin-ir. In the small PDFMe, this was often, but not always, the case. Within the small posterior PDFMe, one colocalized orcokinin-ir neuron was found only in one preparation. Large posterior PDFMe were never orcokinin-ir (Fig. 4b). With regard to the fiber projections, PDF/orcokinin colocalization was never observed in the AMe (Fig. 4c), the dSLP (Fig. 4d), or the POTu (not shown). However, orcokinin-ir fibers were abundant in these areas. Within the AMe, varicose orcokinin-ir fibers were concentrated in the shell neuropil. Unlike the PDF-ir fibers, most orcokinin-ir fibers were not found in the anterior shell neuropil but rather in the distal shell neuropil, which is the transition zone between AMe and medulla (Fig. 4c3). Moreover, in the core neuropil, orcokinin-ir fibers were present, mainly in the internodular neuropil and in posterior parts of the nodular neuropil. Diffuse orcokinin immunoreactivity was present throughout the nodular neuropil. The orcokinin-ir fiber distribution in other parts of the brain confirmed previous work (Hofer et al. 2005). In addition, orcokinin-ir neurons were found as reported previously in the MNe, the VPNe, and the VMNe (Hofer and Homberg 2006b; for numbers, see Table 2). Within the VNe and the DFVNe, non-PDF-ir and PDF-ir neurons were in part orcokinin-ir (Table 2).
Fig. 4.
Colocalization of PDF (blue) and orcokinin (red) immunoreactivity in the central brain of the cockroach (a–c), plus neurobiotin (green) backfills from the cut optic lobe stump (d). Confocal laser scan images were obtained from vibratome sections of the accessory medulla (AMe; a, c), posterior PDFMe (b), and superior lateral protocerebrum (d). Left Overlay images. a, b Maximum projections from stacks of optical sections. c, d Single optical sections. a 1-4 Small (small open arrows) and medium-sized (large open arrows) anterior PDFMe show colocalized PDF and orcokinin immunoreactivity, whereas large PDFMe (large filled arrows) are only PDF-ir. b 1-3 Of the posterior PDFMe neurons near the lobula valley tract (LoVT), neither the small neurons (open triangles), nor the large neurons (filled triangles) show additional orcokinin immunoreactivity. c 1-3 Most orcokinin-ir fibers in the accessory medulla are not found in the anterior shell neuropil but in the distal shell neuropil between AMe and medulla (arrowhead). Colocalization was not observed in PDF-ir fiber terminals in the AMe. d 1-4 No PDF/orcokinin-ir colocalization is observed in the region of the dorsal superior lateral protocerebrum (dSLP), although orcokinin-ir fibers are abundant. Occasional orcokinin-ir fibers of unknown origin are labeled by neurobiotin backfill (filled arrowheads). Bars 50 μm
Tracer backfills and injections identify neurons with projections in the contralateral AMe
In this study, we evaluated 11 backfills from one cut optic stalk with neurobiotin combined with anti-PDF and anti-FMRFamide antisera and 14 backfills with anti-PDF and anti-orcokinin antisera. We concentrated our studies on the PDFMe. As compared with previous rhodamine-dextran backfills, the neurobiotin labeling showed better signal to noise ratio, and more PDFMe were colabeled (Fig. 5a-c, Table 3). In addition, backfilled neurons appeared in the MNe group of the AMe (Fig. 5a, b); this had not previously been observed. For calculation of the mean numbers of backfilled neurons in a given neuronal group or subgroup, only those preparations were considered that contained at least one labeled neuron per group (see Discussion).
Fig. 5.
Confocal laser scan image projections of accessory medulla neurons backfilled from the contralateral optic lobe. Images are maximum projections from stacks of optical sections (green neurobiotin, blue PDF immunoreactivity, red FMRFamide or orcokinin immunoreactivity). Left in d, e Overlay images. Four groups of neurons of the accessory medulla (AMe) were labeled by neurobiotin backfill from the cut contralateral optic lobe (a-c). Contralaterally projecting medium-sized anterior PDFMe were additionally labeled by FMRFamide (d) or orcokinin (e) immunoreactivity. a Neurobiotin-filled neurons of the contralateral optic lobe projecting medulla cells group 1 (MC I), group 2 (MC II), and the newly discovered group 4 (MC IV). b The contralateral optic lobe projecting medulla cells group 2 (MC II) is the largest of the MC groups and comprises the VMNe group of the AMe. Group 4 (MC IV) neurons are also visible. c One neurobiotin-filled neuron of the group of the contralateral optic lobe projecting medulla cells group 3 (MC III) is immunostained (LoVT lobula valley tract). d 1-4 One large (filled arrows) and two medium-sized (open arrows) anterior PDFMe were backfilled from the optic lobe of the contralateral side. Medium-sized PDFMe could be easily recognized because of colocalized FMRFamide immunoreactivity only being present in these PDFMe. e 1-4 One large (filled arrow) and two medium-sized (open arrows) anterior PDFMe were backfilled from the optic lobe of the contralateral side. All medium-sized PDFMe showed colocalized orcokinin immunoreactivity (MC IV contralateral optic lobe projecting medulla cells group 4). Bars 50 μm
Table 3.
Mean numbers of ventral neurons (VNe) of the AMe backfilled from the contralateral optic lobe in the two different colocalization experiments. Values are arithmetical means ± standard deviation; the numbers after the first semicolon is the highest count in the respective group, and the number after the second semicolon is the number of animals contributing to the mean value. For calculations of the means, only those experiments were considered in which at least one neuron was found in the respective group
| Experimental setup | Contralateral VNe with PDF immunoreactivity, without second immunoreactivity | Contralateral VNe with PDF and second immunoreactivity | Contralateral VNe without PDF, FMRFamide, or orcokinin immunoreactivity |
|---|---|---|---|
| PDF + FMRFamide (n = 11) | 1.0 ± 0.0; 1; 9 | 1.9 ± 0.6; 3; 10 | 1.8 ± 1.3; 4; 5 a |
| PDF + orcokinin (n = 14) | 1.0 ± 0.0; 1; 7 b | 2.1 ± 0.9; 3; 10 b | 1.0 ± 0.0; 1; 1 |
aThe high mean number is the result from one preparation with four labeled neurons; the value would be 1.2±0.4; 2; 5 without this specimen
bPreparations with low orcokinin-ir labeling intensity were not counted
The large and medium-sized PDFMe were also identified according to the presence or absence of additional FMRFamide or orcokinin immunoreactivity (Fig. 5d, e). The mean number of neurobiotin-labeled PDFMe in all 24 experiments was 2.9 ± 1.0 (mean ± SD, maximal counts of four neurons in six cases). Of these, maximally one neuron, which was visible in 20 experiments, belonged to the large PDFMe, and this was generally the largest and most proximal PDF-ir neuron (Fig. 5d, e). In one case, this neuron was located among the posterior PDFMe. All other neurobiotin-labeled PDFMe belonged to the medium-sized PDFMe, with a mean number of 2.0 ± 0.7 (mean ± SD, n = 20, maximum counts: three neurons in five cases). Discrimination of large and medium-sized PDFMe was based upon additional FMRFamide or orcokinin immunoreactivity in the medium-sized PDFMe (see Discussion), but in four experiments, the PDFMe could not be assigned either to the large PDFMe or to the medium-sized PDFMe because of low orcokinin immunoreactivity with high background. When the experiments with anti-FMRFamide and anti-orcokinin were evaluated separately, the distribution of backfilled neurons in large and medium-sized PDFMe was similar in both experimental series (Table 3). This implied that next to the largest PDFMe, which only expressed PDF, up to three medium-sized PDFMe that simultaneously expressed FMRFamide and orcokinin immunoreactivity projected to the contralateral optic lobe.
No backfilled neuron was found among the VNe that combined FMRFamide or orcokinin immunoreactivity but lacked PDF. In six cases, backfilled neurons that were considered to belong to the VNe lacked any further immunoreactivity (Table 3; four and two neurons in one case each, one neuron in four cases). As in previous studies (Reischig and Stengl 2002; Reischig et al. 2004), backfilled and contralaterally projecting VNe neurons were assigned to group I of the medulla cells projecting to the contralateral optic lobe (MC I).
The MC II (Fig. 5b) and MC III (Fig. 5c) neurons could be observed in every experiment (n = 25). MC II neurons corresponded to the VMNe group of the AMe, whereas MC III neurons were mostly situated in close vicinity to the posterior PDFMe. Since these neurons were also consistently labeled with rhodamine-dextran in previous experiments (Reischig and Stengl 2002), and since sample counts revealed no differences from the previous experiments, their numbers were not further evaluated in detail here. Approximately three orcokinin-ir neurons were among the contralaterally projecting MC II/VMNe (Table 2) as described previously (Hofer and Homberg 2006a, b). Remarkably, the neurobiotin labeling intensity of MC II and III somata was nearly always higher than that of MC I neurons.
The neurobiotin backfills from optic lobe stumps revealed a new group of labeled neurons that had never previously been observed after similar rhodamine-dextran backfills or injections (Reischig and Stengl 2002; Reischig et al. 2004). These neurons (Fig. 5a, b) appeared among the MNe group with a mean number of 2.2 ± 1.2 SD (n = 18, maximum counts: 5 neurons in two cases). According to the existing nomenclature for medulla neurons projecting to the contralateral optic lobe, they were called MC IV. The MC IV never showed PDF, FMRFamide, or orcokinin immunoreactivity.
Within the AMe, neurobiotin was especially abundant in fibers in the internodular core neuropil and in posterior parts of the nodular core neuropil (Fig. 5b). In the anterior and proximal shell neuropil and internodular core neuropil, they were often additionally PDF-ir and generally belonged to the strongly labeled type of PDF-ir fibers. Many neurobiotin-labeled fibers of the distal shell neuropil and posterior nodular core neuropil were additionally orcokinin-ir. No neurobiotin-labeled fibers were found that showed additional colocalizing FMRFamide immunoreactivity alone, combined PDF and FMRFamide immunoreactivity, or PDF plus orcokinin immunoreactivity.
In the central brain hemisphere contralateral to the backfilled side, neurobiotin-labeled PDF-ir fibers without additional FMRFamide or orcokinin immunoreactivity were detected in nearly all projection areas of the PDF-ir neuron system, namely the superior median protocerebrum (SMP), the SLP (Fig. 3a), inferior lateral protocerebrum (ILP), and the POTu (Fig. 3b). Remarkably, as in the AMe, the PDF/FMRFamide-ir fibers of the dorsal SLP and the POTu showed no additional neurobiotin labeling (Fig. 3a, b). PDF-ir fibers with additional neurobiotin labeling were found in the anterior and posterior optic commissures (AOC and POC, respectively), but we never found additional FMRFamide-ir (Fig. 3c, d) or orcokinin-ir (data not shown) colabeling in these fibers. However, non-PDF-ir backfilled fibers that were FMRFamide-ir were present in the posterior optic commissure paralleling the PDF-ir fibers (Fig. 3d). In no case were all PDF-ir commissural fibers labeled with neurobiotin, even in preparations in which the maximal count of four backfilled PDFMe was reached.
Discussion
We examined which neuronal groups and pathways form direct connections between the two optic lobes and the two AMae as candidates for mutual pacemaker synchronization in the cockroach L. maderae. Backfills with neurobiotin from the stump of one sectioned optic lobe were performed, combined with double-immunolabeling against PDF and FMRFamide or against PDF and orcokinin. FMRFamide-ir neurons, orcokinin-ir neurons, and backfilled neurons in the VNe and MNe groups were quantitatively evaluated in the AMe contralateral to the backfilled PDFMe side. In contrast to former publications (Reischig et al. 2004), the backfills revealed that, in the MC I cell group next to the largest PDFMe and an unstained cell, maximally three medium-sized PDFMe neurons projected to the contralateral optic lobe. In contrast to the large PDFMe neuron, the three medium-sized PDFMe also colabeled with anti-FMRFamide and anti-orcokinin. In addition to the previously described MC II (comprising VMNe cells) and MC III groups (comprising posterior PDFMe cells), the newly described MC IV cells (a subgroup of MNe) projected contralaterally. The double-label studies suggested that, in contrast to medium-sized and small PDFMe, the large anterior PDFMe were only PDF-immunoreactive. The small posterior PDFMe were always FMRFamide-ir but were, in most cases, devoid of additional orcokinin-ir immunolabeling. Intriguingly, double-immunolabeling together with neurobiotin staining of neurites was never observed.
These data indicates that the contralaterally projecting medium-sized PDFMe employ peptide sorting and never release both PDF and orcokinin or PDF and FMRFamides at the same terminal. Only the ipsilateral projections of PDFMe neurons appear to corelease both PDF and FMRFamide at specific release sites in the dorsal SLP, the POTu, and the noduli of the ipsilateral AMe. Thus, the differential release of neuromodulating peptide cocktails might be an important mechanism in circadian pacemaker coupling.
Neuron classification and developmental plasticity in the AMe
In neuroanatomy, the differentiation of neurons in groups and subgroups is indispensable for the characterization of neuronal circuits. Common morphological characters and positions are generally considered to imply functional similarities in neurons. In the AMe of the cockroach, the PDFMe neurons have been classified into three subgroups by their relative position, soma size, and intensity of PDF-ir immunolabeling, thus implying greater morphological and functional commonalities within each subgroup than among them. The identification of these subgroups is reproducible in most animals, and cell counts in various animals have revealed similar group sizes (Reischig and Stengl 2003b). Nevertheless, in some animals, neuron positions diverge considerably from the “standard situation” (Fig. 6a). Intensely labeled PDFMe, which have thus been categorized as "large PDFMe cells" are occasionally dislocated to more posterior VMNe neurons or are smaller than the largest less intensely labeled medium-sized PDFMe. Hence, for some individual neurons, the correct assignment by labeling intensity, location, or size is not unequivocally possible. Here, we suggest that the large PDFMe should be defined as the cell group that lacks immunoreactivity to anti-FMRFamide and anti-Asn13-orcokinin antisera.
Fig. 6.
a Summary representation of the composition of ipsi- and contralaterally projecting PDF-expressing medulla neurons at the accessory medulla (AMe) of the cockroach. Although the numbers of gray circles do not reflect the actual group sizes, numbers of colored circles represent the mean numbers of the respective PDFMe neurons as determined in this work. All small and medium-sized anterior PDFMe and all small posterior PDFMe appear to coexpress peptide(s) of the FMRFamide-related peptide family. Additionally, all medium-sized PDFMe appear to coexpress an Asn13 orcokinin-like peptide. Asn13-orokinin-like immunoreactivity is also present in at least a subpopulation of the small anterior PDFMe, but not in the small posterior PDFMe. However, all large anterior and large posterior PDFMe do not coexpress one of the tested peptides after PDF. Thus, possibly all medium-sized anterior PDFMe and at least a subpopulation of the small anterior PDFMe express three different neuropeptides simultaneously. Together, six anterior and posterior large (including the largest) PDFMe comprise a homogeneous group of neurons that appear to be randomly distributed to anterior or posterior positions during the cockroach’s development; hence, all large PDFMe are here depicted among the anterior AMe neuron groups. The neurobiotin backfills suggest that the largest and three medium-sized PDFMe project to the contralateral AMe for mutual pacemaker coupling (C). Furthermore, an additional contralaterally projecting VNe neuron appears to exist that is not immunoreactive to one of the peptide antisera tested (DFVNe distal group of frontoventral neurons, MFVNe medial group of frontoventral neurons, MNe medial neurons, VNe ventral neurons, VMNe ventromedial neurons, VPNe ventroposterior neurons, di distal, do dorsal). b Representation of pathways and output regions of contralaterally projecting PDFMe (violet lines pathways of the largest PDFMe, blue lines pathways of the contralaterally projecting medium-sized PDFMe, violet/blue circles respective output regions in the neuropil). The large PDFMe appears to connect the two AMae via the anterior and posterior optic commissure (AOC, POC) and terminates in most output regions of the PDF-ir fiber system. Medium-sized contralateral PDFMe terminate at least in the AMe, the dorsal lateral protocerebrum (dSLP), and the posterior optic tubercles (POTu), as judged by the presence of anti-PDF/FMRFamide colabeled terminal arborizations. The medium-sized contralaterally projecting PDFMe appear to project only via the AOC (La lamina, Me medulla, ILP/SLP inferior/superior lateral protocerebrum, SMP superior median protocerebrum)
In addition, the sum of large anterior and large posterior PDFMe has emerged as being exactly 6, these neurons forming one group with a common developmental origin. Apparently, neurons of this group are pulled apart while the optic lobe is growing during development, with those neurons that are shifted posteriorly apparently being decided at random. In accordance with this hypothesis, posterior PDFMe cells have not yet been described in any other insect, although PDF-ir neurons have been demonstrated in more than 50 insect species (e.g., Homberg et al. 1991; Sehadová et al. 2003; Závodská et al. 2003). Thus, neurons with quite different positions might have a common developmental origin, and anterior and posterior PDFMe probably do not differ physiologically. Therefore, we can now include in our definition of “large PDFMe” all anterior and posterior PDFMe that show high labeling intensity and exhibit no FMRFamide or orcokinin immunoreactivity, regardless of their actual size. The shift to a posterior position rarely occurs among the medium-sized and small anterior PDFMe neurons. Furthermore, the small anterior and small posterior PDFMe neurons appear to differ with respect to colocalized immunoreactivities, and small posterior PDFMe are not found in all animals. Whereas the recognition of the large PDFMe is greatly facilitated by the lack of immunoreactivity to both FMRFamide and orcokinin, the differentiation between small and medium-sized anterior PDFMe still relies on differences in labeling intensity and soma size. Accordingly, larger variations in cell counts compared with the large PDFMe cells are found among these groups.
Multiple peptide immunoreactivities in single presumptive pacemaker neurons: distinct outputs from one neuron by peptide sorting?
We have described the colocalization of immunoreactivities against up to three peptide antisera in the small and medium-sized anterior PDFMe for the first time. Furthermore, anti-baratin immunoreactivity (Nässel et al. 2000) has been observed in all medium-sized and in at least some of the small anterior PDFMe neurons (T. Reischig, unpublished). Thus, at least four different neuropeptides appear to be expressed in single circadian pacemaker cells.
How likely is coexpression of three or more neuropeptides in single neurons? The peptide PDF has been characterized in the cockroach L. maderae (Hamasaka et al. 2005), and homologous PDF-ir neurons and respective conserved PDF-peptides have been described in other species (Dircksen et al. 1987; Park and Hall 1998; Chuman et al. 2002; Matsushima et al. 2004; Honda et al. 2006). Therefore, the anti-Drosophila-PDF monoclonal antibody employed in this study most probably recognizes only the PDF peptide in PDFMe neurons, especially as it shows the same labeling pattern as the well-characterized anti-Uca-PDH polyclonal antibody from Dircksen et al. (1987).
Contrarily to the PDF antiserum, the anti-FMRFamide antiserum detects a variety of peptides belonging to the family of FaRPs. More than 100 neuropeptides have been isolated from the central and peripheral nervous systems of invertebrates and vertebrates, and they generally share the C-terminal amidated RF (Arg-Phe-NH2) sequence (for reviews, see Orchard et al. 2001; Nässel 2002; Mercier et al. 2003; Orchard and Lange 2006). These include the N-terminally extended FMRFamides and FL/IRFamides, the short and long extended neuropeptides F (sNPFs and lNPFs, respectively), the myosuppressins (extended FLRFamides such as LMS), and the sulfakinins (extended HMRFamides with a sulfated tyrosine residue). These peptides are commonly referred as FaRPs, although these families are structurally different and are not further related to one another. The FMRFamide antibody employed is known to label a variety of FaRPs in invertebrates (Orchard et al. 2001; Nässel and Homberg 2006; Predel 2006). It has also been used in L. maderae to distinguish AMe neurons (Soehler et al. 2008); in this work, the presence of several FaRPs in neurons of the AMe was demonstrated by mass spectroscopy, and injections of two members of the FaRP family into the optic lobe of free-running cockroaches resulted in time-dependent phase shifts of locomotor activity. Therefore, AMe neurons including the small and medium-sized PDFMe labeled by the FMRFamide antiserum probably express various peptides of the FaRP family.
The third antiserum used was raised against the crustacean neuropeptide Asn13-orcokinin, a member of the orcokinin peptide family (Bungart et al. 1994). In several hemimetabolous insects, this antiserum labels various neurons throughout the brain including the AMe of L. maderae (Hofer et al. 2005; Hofer and Homberg 2006b). Orcokinin-related peptides have been directly identified in the cockroaches L. maderae and B. germanica and in the locust Locusta migratoria (Pascual et al. 2004; Hofer et al. 2005). Like PDF and FaRPs mentioned above, Asn13-orcokinin has been injected at various circadian times into the optic lobes of free-running cockroaches and causes light-like phase response curves. Therefore, the anti-orcokinin antiserum probably also specifically labels neurons expressing orcokinin-related neuropeptides in the AMe. The differences in the cell counts between our study and previous publications (Hofer and Homberg 2006a, b) might be attributable to the different antibody concentrations used here.
In summary, our immunohistochemical evidence indicates that at least three different neuropeptides are expressed in single AMe neurons. Future studies with mass spectroscopy of single identified neurons might challenge this hypothesis. Whereas the coexpression of two neuropeptides in single neurons has been demonstrated earlier (Pollak et al. 2005), to our knowledge, the coexpression of three or more neuropeptides has not previously been shown. Furthermore, whereas coexpression is mostly observed among peptides that are transcribed from one gene and undergo subsequent cleavage and postprocessing in the Golgi apparatus and secretory vesicles, this is obviously not the case for the unrelated PDF, FaRPs, and orcokinin-related peptides. Interestingly, PDF/FMRFamide-ir colocalization in neuronal terminals has been observed only in a few areas (nodular neuropil of AMe, dSLP, POTu) and never for PDF/orcokinin immunoreactivity. Moreover, the colocalization of peptide immunoreactivities has never been observed in the commissural fibers, although PDFMe somata with additional FMRFamide and orcokinin immunoreactivities have been shown by the backfills to project contralaterally. This confirms previous observations (Petri et al. 1995; Hofer and Homberg 2006a) and suggests that either peptide concentrations in the terminals are below the detection threshold, or that peptide sorting occurs in the small and medium-sized PDFMe neurons. Peptide sorting has previously been demonstrated in other species, e.g., by Sossin et al. (1990) in the bag cells of Aplysia californica. In their study, the authors have shown that the prohormone of the egg-laying hormone is sorted into distinct vesicle classes, and that these distinct vesicles are located to separate processes by unknown mechanisms. Future studies should investigate the hypothesis of multiple peptide coexpression and peptide sorting in the PDFMe neurons. Further, in order to examine the interactions of neuropeptides involved in circadian pacemaker output and coupling, the time courses of peptide production, processing, and release should be addressed by additional techniques involving molecular genetics, biochemistry, and electrophysiology.
Significance of the backfill experiments
Since not all backfilled neurons take up the same amount of tracer, the number of contralaterally projecting neurons is better estimated by the maximal cell counts than by the mean values of backfilled somata. Neurobiotin (an amino derivative of biotin), dextran, and horseradish peroxidase (HRP) are appropriate neuronal tracers to reveal pathways connecting the two optic lobes and the two AMae (Reischig and Stengl 2002; Reischig et al. 2004). However, compared with dextran and HRP injections, neurobiotin injections reveal more reproducible results because of the more consistent and stronger labeling. Thus, we can conclude that, in contrast to previous publications, at least four PDFMe neurons, viz., the largest and three medium-sized neurons, interconnect the two optic lobes and, most probably, the two bilaterally symmetric AMae. The projection pattern of the contralaterally projecting MNe neurons of group MC IV, which are here described for the first time, and of the immunocytochemically nonlabeled neuron from group I remains to be determined in single cell injections combined with immunocytochemistry.
Anatomical properties of circadian coupling pathways
This study has not confirmed that a contralateral projecting MC I cell without PDF immunoreactivity is FMRFamide-ir and/or orcokinin-ir. Here, all contralaterally projecting VNe neurons that are labeled by anti-FMRFamide and anti-orcokinin antisera are also PDF-ir (= medium-sized anterior PDFMe), thus indicating that they employ at least three peptides as their output signal. The neuromodulators that are located in the one to four non-PDF-ir MC I VNe somata that have been occasionally seen remain to be examined. The contralaterally projecting VMNe neurons (identical to MC II) generally form a compact cluster posterior to the VNe neurons. However, because single neurons of the VMNe neurons are sometimes displaced to the MC I cluster, the 1-4 non-PDF-ir MC I VNe somata might indeed be displaced VMNe neurons. Thus, the MC I cells possibly consist only of the four contralaterally projecting PDFMe neurons; this hypothesis awaits further clarification.
The exact pathways and termination sites of the contralaterally projecting PDFMe, which pass the brain midline via the AOC and POC, still remain undescribed. In the optic lobe contralateral to the backfilled side, neurobiotin/PDF-ir colabeled fibers have been observed in the AMe, in the fan-shaped arborization of the distal layer of the medulla, and in the proximal lamina of the contralateral optic lobe. Neurobiotin/PDF-ir colabeled fibers occur in most brain termination sites of the PDF-ir neuron system (ipsi- and contralateral SMP, SLP, ILP, and POTu). Interestingly, neurobiotin/PDF-ir colabeled fibers that are additionally FMRFamide-ir or orcokinin-ir have never been detected, not even in the AMe.
Therefore, the most parsimonious conclusion is that all of the neurobiotin/PDF-ir colabeled fibers that have been found in the brain hemisphere contralateral to the backfilled side originate only from the single non-FMRF-ir largest contralaterally projecting PDFMe (Fig. 6b). In this case, neurites of this neuron probably travel through both the AOC and POC and innervate most arborization sites of the PDF-ir neuron system in the ipsi- and contralateral brain hemispheres of the cockroach. This hypothesis is supported by the findings that all neurobiotin-labeled PDF-ir terminals in the contralateral SLP, the SMP, ILP, and POTu and in the distal fiber-fan and shell neuropil of the AMe in contralateral optic lobe are strongly labeled and have large round varicosities. Furthermore, recent three-dimensional studies have also confirmed this hypothesis (Wei et al. 2010). In contrast, fibers from medium-sized PDFMe that express PDF/FMRF-ir colabeling in the nodular neuropil of the AMe, in the dSLP, and in the POTu are more irregularly shaped and showed less intense PDF-ir.
Our experiments could not resolve the termination sites of the remaining medium-sized contralateral PDFMe. We assume that they contribute to the PDF-ir fibers in the AOC, because generally only two thick PDF-ir fibers occur in the POC, probably originating from the single largest PDFMe per hemisphere. However, the PDF-ir AOC fibers do not show colocalization for FMRFamide or orcokinin immunoreactivity. We cannot exclude that the FaRP and orcokinin concentrations lie below the detection threshold in most of the fibers of the medium-sized PDFMe; however, the immunolabeling intensity in fibers of peptidergic neurons is often higher than that in the somata. In addition, we have employed higher antisera concentrations than used previously to determine the staining of any possible weakly labeled processes (Hofer and Homberg 2006a, b). However, we hypothesize that, within the same PDF-ir cells, the different coexpressed peptides are sorted into different processes before reaching the AOC.
According to the data presented herein, we can state that small and/or medium-sized PDFMe arborize at least in the ipsilateral AMe, dSLP, and POTu according to the PDF/FMRFamide-ir colocalization found there, but that these arborizations are always devoid of neurobiotin labeling (Fig. 6b). Nevertheless, medium-sized FMRFamide-ir and orcokinin-ir PDFMe are regularly labeled by contralateral backfills. To resolve this apparent contradiction, we consider that neurobiotin might not be distributed equally in all terminals of every labeled neuron. For example, Heinrich et al. (1998) have shown that neurobiotin in certain neurons appears to be generally transported retrogradely and thus fills only axonal endings but no dendritic regions in backfilled neurons. Thus, uneven tracer transport might be one reason that neurobiotin labeling was never visible in PDF/FMRFamide-ir fibers of the AMe, the dSLP, and the POTu. Additionally, in some cases, neurobiotin labeling was not at all visible in any of the PDF-ir commissural fibers, although somata were successfully labeled, thus supporting the assumption that neurobiotin is often not enriched to detection threshold in all fibers of backfilled neurons. The tracer distribution within neurons therefore appears to depend on several factors including the peculiarities of intracellular transport in given neurons, the site and amount of tracer uptake, and the special properties of the various tracer substances. Hence, arborization patterns, including contralateral terminations of the medium-sized PDF/FMRFamide/orcokinin-ir PDFMe neurons, remain to be clarified by intracellular dye injection.
Mutual pacemaker synchronization: bi- or multi-modal coupling pathways?
Behavioral and anatomical studies have suggested the existence of two neuronal coupling pathways between the two bilaterally symmetric circadian pacemakers in the cockroach: one that transmits light information, and one that transmits circadian phase information for mutual pacemaker coupling (Roth and Sokolove 1975; Page 1978, 1983a, b). The PDF-ir neuron system is postulated to provide at least a part of the circadian coupling pathway (Homberg et al. 1991; Stengl and Homberg 1994). This hypothesis is based upon the discovery that the overt circadian period length in cockroaches is correlated with the number of regenerated commissural PDF-ir fibers that experience bilateral transection of the optic stalks. In contrast to cockroaches, the bilateral pacemakers in crickets can be easily de-coupled in behavioral experiments, and crickets possess only a few or no commissural PDF-ir fibers (Page et al. 1977; Wiedenmann and Loher 1984; Stengl 1995; Ushirogawa et al. 1997). Our study together with previous work (Reischig and Stengl 2002; Reischig et al. 2004) has now revealed four different neuron groups as candidates for mutual coupling pathways between the two AMae. The first group are the MC I with maximally four contralaterally projecting PDFMe. They include the largest of the large PDFMe, maximally three medium-sized PDFMe, and probably one VNe, which is not immunoreactive to any of the antisera employed. In electrophysiological studies, neurons resembling PDFMe with commissural projections respond at most weakly to light stimuli (Loesel and Homberg 2001). Thus, PDFMe do not appear mainly to transmit light information. Phase response curves obtained by extracellular injections of Uca-β-PDH next to one AMe confirm that PDF-ir neurons provide a non-photic input into the AMe, possibly as a circadian coupling pathway (Petri and Stengl 1997). Thus, the contralateral projecting PDFMe neurons probably provide a circadian coupling pathway for the transmission of circadian phase information.
Computer simulations predict that the physiological data can only be modeled via circadian coupling pathways providing both phase delays and advances to the AMe (Petri and Stengl 2001). Therefore, either one coupling neuron provides both an advancing and a delaying signal, or at least two different parallel coupling pathways exist transmitting circadian phase information. Previous immunohistochemical observations suggest parallel coupling pathways: PDF-ir, FMRFamide-ir, and orcokinin-ir contralateral AMe neurons (Petri et al. 1995; Petri and Stengl 2001; Hofer and Homberg 2006a). The contralateral projecting VNe from group MC I, which is not PDF-ir, has been hypothesized to be orcokinin-ir or FMRFamide-ir. Here, we have shown that this MC I cell is not FMRFamide-ir and/or orcokinin-ir. However, since all medium-sized contralateral PDFMe-neurons appear to express three or more modulatory neuropeptides, a single coupling neuron could provide the advancing and the delaying signal from different processes with sorted neuropeptides. Additionally, different postsynaptic cells might carry different phase information, even if they possess the same neuropeptide receptors. Further studies are necessary to resolve this issue.
A second contralateral pathway of the AMe is provided by the 35 MC II neurons, which are identical to the VMNe group (Reischig and Stengl 2002; Reischig et al. 2004) a group previously suggested to form a contralateral pathway in the cockroach (Roth and Sokolove 1975). The VMNe neurons project solely via the POC to the contralateral side. Intracellular single cell labeling in cockroaches, crickets, and locusts has shown that some, but not all, neurons of this group project into the ipsi- and contralateral AMe (Labhart and Petzold 1993; Homberg and Würden 1997; Loesel and Homberg 2001; Yukizane et al. 2002). In the cockroach, the VMNe neurons contain approximately three contralaterally projecting orcokinin-ir neurons (Hofer and Homberg 2006a, b), as confirmed in this study. The VMNe neurons are especially well investigated in crickets, where they are termed medulla bilateral neurons (MBN; for a review, see Tomioka and Abdelsalam 2004). Up to 25 MBN have been counted in the cricket Gryllus bimaculatus (Yukizane et al. 2002). In all investigated animals, the MC II/VMNe/MBN group forms tangential arborizations in the middle layers (but never in the anterior fiber fan) of both medullae via the POC (Labhart and Petzold 1993; Homberg and Würden 1997; Loesel and Homberg 2001; Yukizane et al. 2002). In electrophysiological observations, VMNe neurons often show regular spontaneous spiking activity and are light-sensitive in an intensity-dependent manner (Homberg and Würden 1997; Loesel and Homberg 2001; Yukizane et al. 2002). The MBN of G. bimaculatus exhibits a clear circadian rhythm in their response behavior to light; this can be influenced by the application of serotonin (setting them to a physiological day state) and PDF (setting them to night state; Saifullah and Tomioka 2002, 2003a, b). Therefore, these neurons provide a pathway transferring light information to the contralateral optic lobe in order to maintain stable phase angle relationships between the bilaterally symmetric circadian pacemakers and the external Zeitgeber (for a review, see Tomioka and Abdelsalam 2004). Interestingly, a subgroup of the MBN react to e-vector changes of polarized light (Labhart and Petzold 1993; Homberg and Würden 1997; Loesel and Homberg 2001). Orientation to polarized light in the sky by insects largely relies on precise information about current daytime; this might be one cause for the intercalation of the AMe as putative circadian pacemaker in the polarization vision pathway.
In summary, MC I and MC II both appear to couple the bilateral AMae but subserve different functions. Whereas the PDFMe of the MC I group appear to form circadian coupling pathways via the AOC and POC, the VMNe group appears to provide contralateral light information to the AMe via the POC.
A third and a fourth coupling pathway might be provided by the MC III and the newly discovered MC IV cells, respectively. However, so far, we know nothing about the projection patterns of these neurons, and we are not certain whether they indeed connect the two AMae. Therefore, the mutual circadian synchronization pathway of the cockroach is probably more complex than we appreciate at this time and awaits further investigation.
Acknowledgments
We are extremely grateful to Heinrich Dircksen (University of Stockholm, Sweden) for providing the anti-β-PDH and the anti-Asn13 antisera, and to Eve Marder (Brandeis University, Waltham, USA) for providing the FMRFamide antiserum. The monoclonal anti-PDF antibody originally raised by Justin Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. We thank Dr. Sabine Hofer (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for critical manuscript reading and helpful suggestions regarding the anti-orcokinin labeling.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bungart D, Dircksen H, Keller R. Quantitative determination and distribution of the myotropic neuropeptide orcokinin in the nervous system of astacidean crustaceans. Peptides. 1994;15:393–400. doi: 10.1016/0196-9781(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Chuman Y, Matsushima A, Sato S, Tomioka K, Tominaga Y, Meinertzhagen IA, Shimohigashi Y, Shimohigashi M. CDNA cloning and nuclear localization of the circadian neuropeptide designated as pigment-dispersing factor (PDF) in the cricket Gryllus bimaculatus. J Biochem (Tokyo) 2002;131:895–903. doi: 10.1093/oxfordjournals.jbchem.a003180. [DOI] [PubMed] [Google Scholar]
- Cyran SA, Yiannoulos G, Buchsbaum AM, Saez L, Young MW, Blau J. The double-time protein kinase regulates the subcellular localization of the Drosophila clock protein period. J Neurosci. 2005;25:5430–5437. doi: 10.1523/JNEUROSCI.0263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP. The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean glands: identification by an antiserum against a synthetic PDH. Cell Tissue Res. 1987;250:377–387. [Google Scholar]
- Ehnbom K. Studies on the central and sympathetic nervous system and some sense organs in the head of neuropteroid insects. Opusc Entomol (Suppl) 1948;8:1–162. [Google Scholar]
- Hamasaka Y, Mohrherr CJ, Predel R, Wegener C. Chronobiological analysis and mass spectrometric characterization of pigment-dispersing factor in the cockroach Leucophaea maderae. J Insect Sci. 2005;5:43. doi: 10.1093/jis/5.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R, Jacobs K, Lakes-Harlan R. Tracing of a neuronal network in the locust by pressure injection of markers into a synaptic neuropil. J Neurosci Methods. 1998;80:81–89. doi: 10.1016/s0165-0270(97)00205-7. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Shafer OT, Wülbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- Hofer S, Homberg U. Evidence for a role of orcokinin-related peptides in the circadian clock controlling locomotor activity of the cockroach Leucophaea maderae. J Exp Biol. 2006;209:2794–2803. doi: 10.1242/jeb.02307. [DOI] [PubMed] [Google Scholar]
- Hofer S, Homberg U. Orcokinin immunoreactivity in the accessory medulla of the cockroach Leucophaea maderae. Cell Tissue Res. 2006;325:589–600. doi: 10.1007/s00441-006-0155-y. [DOI] [PubMed] [Google Scholar]
- Hofer S, Dircksen H, Tollback P, Homberg U. Novel insect orcokinins: characterization and neuronal distribution in the brains of selected dicondylian insects. J Comp Neurol. 2005;490:57–71. doi: 10.1002/cne.20650. [DOI] [PubMed] [Google Scholar]
- Homberg U, Würden S. Movement-sensitive, polarization-sensitive, and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol. 1997;386:329–346. [PubMed] [Google Scholar]
- Homberg U, Würden S, Dircksen H, Rao KR. Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res. 1991;266:343–357. [Google Scholar]
- Homberg U, Reischig T, Stengl M. Neural organization of the circadian system of the cockroach Leucophaea maderae. Chronobiol Int. 2003;20:577–591. doi: 10.1081/cbi-120022412. [DOI] [PubMed] [Google Scholar]
- Honda T, Matsushima A, Sumida K, Chuman Y, Sakaguchi K, Onoue H, Meinertzhagen IA, Shimohigashi Y, Shimohigashi M. Structural isoforms of the circadian neuropeptide PDF expressed in the optic lobes of the cricket Gryllus bimaculatus: immunocytochemical evidence from specific monoclonal antibodies. J Comp Neurol. 2006;499:404–421. doi: 10.1002/cne.21112. [DOI] [PubMed] [Google Scholar]
- Labhart T, Petzold J. Processing of polarized light information in the visual system of crickets. In: Wiese K, Gribakin F, Popov AV, Renninger G, editors. Sensory system of arthropods. Basel: Birkhäuser; 1993. pp. 158–169. [Google Scholar]
- Lee C-M, Su M-T, Lee H-J. Pigment dispersing factor: an output regulator of the circadian clock in the German cockroach. J Biol Rhythms. 2009;25:34–43. doi: 10.1177/0748730408327909. [DOI] [PubMed] [Google Scholar]
- Loesel R, Homberg U. Anatomy and physiology of neurons with processes in the accessory medulla of the cockroach Leucophaea maderae. J Comp Neurol. 2001;439:193–207. doi: 10.1002/cne.1342. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL, Nusbaum MP, Trimmer B. Distribution and partial characterization of FMRFamide-like peptides in the stomatogastric nervous systems of the rock crab, Cancer borealis, and the spiny lobster, Panulirus interruptus. J Comp Neurol. 1987;259:150–163. doi: 10.1002/cne.902590111. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Sato S, Chuman Y, Takeda Y, Yokotani S, Nose T, Tominaga Y, Shimohigashi M, Shimohigashi Y. cDNA cloning of the housefly pigment-dispersing factor (PDF) precursor protein and its peptide comparison among the insect circadian neuropeptides. J Pept Sci. 2004;10:82–91. doi: 10.1002/psc.511. [DOI] [PubMed] [Google Scholar]
- Mercier AJ, Friedrich R, Boldt M. Physiological functions of FMRFamide-like peptides (FLPs) in crustaceans. Microsc Res Tech. 2003;60:313–324. doi: 10.1002/jemt.10270. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Progr Neurobiol. 2002;68:1–84. doi: 10.1016/s0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Homberg U. Neuropeptides in interneurons of the insect brain. Cell Tissue Res. 2006;326:1–24. doi: 10.1007/s00441-006-0210-8. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Persson MG, Muren JE. Baratin, a nonamidated neurostimulating neuropeptide, isolated from cockroach brain: distribution and actions in the cockroach and locust nervous systems. J Comp Neurol. 2000;422:267–286. [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J, Pittendrigh C. Central nervous system control of circadian rhythmicity in the cockroach. III. The optic lobes, locus of the driving oscillation? Z Vgl Physiol. 1968;58:14–46. [Google Scholar]
- Orchard I, Lange AB. Insect myosuppressins/FMRFamides and FL/IRFamides/NPFs. In: Kastin AJ, editor. Handbook of biologically active peptides. Burlington: Academic Press; 2006. pp. 193–199. [Google Scholar]
- Orchard I, Lange AB, Bendena WG. FMRFamide-related peptides: a multifunctional family of structurally related neuropeptides in insects. Adv Insect Physiol. 2001;28:267–329. [Google Scholar]
- Page TL. Interactions between bilaterally paired components of the cockroach circadian system. J Comp Physiol [A] 1978;124:225–236. [Google Scholar]
- Page TL. Transplantation of the cockroach circadian pacemaker. Science. 1982;216:73–75. doi: 10.1126/science.216.4541.73. [DOI] [PubMed] [Google Scholar]
- Page TL. Effects of optic tract regeneration on internal coupling in the circadian system of the cockroach. J Comp Physiol [A] 1983;153:353–363. [Google Scholar]
- Page TL. Regeneration of the optic tracts and circadian pacemaker activity in the cockroach Leucophaea maderae. J Comp Physiol [A] 1983;152:231–240. [Google Scholar]
- Page TL, Caldarola PC, Pittendrigh CS. Mutual entrainment of bilaterally distributed circadian pacemakers. Proc Natl Acad Sci USA. 1977;74:1277–1281. doi: 10.1073/pnas.74.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Hall JC. Isolation and chronobiological analysis of a neuropeptide pigment-dispersing factor gene in Drosophila melanogaster. J Biol Rhythms. 1998;13:219–228. doi: 10.1177/074873098129000066. [DOI] [PubMed] [Google Scholar]
- Pascual N, Castresana J, Valero ML, Andreu D, Belles X. Orcokinins in insects and other invertebrates. Insect Biochem Mol Biol. 2004;34:1141–1146. doi: 10.1016/j.ibmb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Petri B, Stengl M. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci. 1997;17:4087–4093. doi: 10.1523/JNEUROSCI.17-11-04087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri B, Stengl M. Phase response curves of a molecular model oscillator: implications for mutual coupling of paired oscillators. J Biol Rhythms. 2001;16:125–141. doi: 10.1177/074873001129001836. [DOI] [PubMed] [Google Scholar]
- Petri B, Stengl M, Würden S, Homberg U. Immunocytochemical characterization of the accessory medulla in the cockroach Leucophaea maderae. Cell Tissue Res. 1995;282:3–19. doi: 10.1007/BF00319128. [DOI] [PubMed] [Google Scholar]
- Pollak E, Eckert M, Molnar L, Predel R. Differential sorting and packaging of capa-gene related products in an insect. J Comp Neurol. 2005;481:84–95. doi: 10.1002/cne.20364. [DOI] [PubMed] [Google Scholar]
- Predel R. Cockroach neuropeptides: sequences, localization, and physiological actions. In: Satake H, editor. Invertebrate neuropeptides and hormones: basic knowledge and recent advances. Kerala: Transworld Research Network; 2006. pp. 1–29. [Google Scholar]
- Reischig T, Stengl M. Morphology and pigment-dispersing hormone immunocytochemistry of the accessory medulla, the presumptive circadian pacemaker of the cockroach Leucophaea maderae: a light- and electron- microscopic study. Cell Tissue Res. 1996;285:305–319. [Google Scholar]
- Reischig T, Stengl M. Optic lobe commissures in a three-dimensional brain model of the cockroach Leucophaea maderae: a search for the circadian coupling pathways. J Comp Neurol. 2002;443:388–400. doi: 10.1002/cne.10133. [DOI] [PubMed] [Google Scholar]
- Reischig T, Stengl M. Ectopic transplantation of the accessory medulla restores circadian locomotor rhythms in arrhythmic cockroaches (Leucophaea maderae) J Exp Biol. 2003;206:1877–1886. doi: 10.1242/jeb.00373. [DOI] [PubMed] [Google Scholar]
- Reischig T, Stengl M. Ultrastructure of pigment-dispersing hormone-immunoreactive neurons in a three-dimensional model of the accessory medulla of the cockroach (Leucophaea maderae) Cell Tissue Res. 2003;314:421–435. doi: 10.1007/s00441-003-0772-7. [DOI] [PubMed] [Google Scholar]
- Reischig T, Petri B, Stengl M. Pigment-dispersing hormone (PDH)-immunoreactive neurons form a direct coupling pathway between the bilaterally symmetric circadian pacemakers of the cockroach Leucophaea maderae. Cell Tissue Res. 2004;318:553–564. doi: 10.1007/s00441-004-0927-1. [DOI] [PubMed] [Google Scholar]
- Roberts SK. Circadian rhythms in cockroaches: effects of optic lobe lesions. J Comp Physiol. 1974;88:21–30. [Google Scholar]
- Roth RL, Sokolove PG. Histological evidence for direct connections between the optic lobes of the cockroach Leucophaea maderae. Brain Res. 1975;87:23–39. doi: 10.1016/0006-8993(75)90776-3. [DOI] [PubMed] [Google Scholar]
- Saifullah AS, Tomioka K. Serotonin sets the day state in the neurons that control coupling between the optic lobe circadian pacemakers in the cricket Gryllus bimaculatus. J Exp Biol. 2002;205:1305–1314. doi: 10.1242/jeb.205.9.1305. [DOI] [PubMed] [Google Scholar]
- Saifullah AS, Tomioka K. 5-HT(7)-like receptors mediate serotonergic modulation of photo-responsiveness of the medulla bilateral neurons in the cricket, Gryllus bimaculatus. Zool Sci. 2003;20:303–309. doi: 10.2108/zsj.20.303. [DOI] [PubMed] [Google Scholar]
- Saifullah AS, Tomioka K. Pigment-dispersing factor sets the night state of the medulla bilateral neurons in the optic lobe of the cricket, Gryllus bimaculatus. J Insect Physiol. 2003;49:231–239. doi: 10.1016/s0022-1910(02)00270-6. [DOI] [PubMed] [Google Scholar]
- Sehadová H, Sauman I, Sehnal F. Immunocytochemical distribution of pigment-dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell Tissue Res. 2003;312:113–125. doi: 10.1007/s00441-003-0705-5. [DOI] [PubMed] [Google Scholar]
- Singaravel M, Fujisawa Y, Hisada M, Saifullah AS, Tomioka K. Phase shifts of the circadian locomotor rhythm induced by pigment-dispersing factor in the cricket Gryllus bimaculatus. Zool Sci. 2003;20:1347–1354. doi: 10.2108/zsj.20.1347. [DOI] [PubMed] [Google Scholar]
- Soehler S, Neupert S, Predel R, Stengl M. Examination of the role of FMRFamide-related peptides in the circadian clock of the cockroach Leucophaea maderae. Cell Tissue Res. 2008;332:257–269. doi: 10.1007/s00441-008-0585-9. [DOI] [PubMed] [Google Scholar]
- Sokolove PG. Localization of the cockroach optic lobe circadian pacemaker with microlesions. Brain Res. 1975;87:13–21. doi: 10.1016/0006-8993(75)90775-1. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Sweet-Cordero A, Scheller RH. Dale’s hypothesis revisited: different neuropeptides derived from a common prohormone are targeted to different processes. Proc Natl Acad Sci USA. 1990;87:4845–4848. doi: 10.1073/pnas.87.12.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengl M. Pigment-dispersing hormone-immunoreactive fibers persist in crickets which remain rhythmic after bilateral transection of the optic stalks. J Comp Physiol [A] 1995;176:217–228. [Google Scholar]
- Stengl M, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. J Comp Physiol [A] 1994;175:203–213. doi: 10.1007/BF00215116. [DOI] [PubMed] [Google Scholar]
- Tomioka K, Abdelsalam S. Circadian organization in hemimetabolous insects. Zool Sci. 2004;21:1153–1162. doi: 10.2108/zsj.21.1153. [DOI] [PubMed] [Google Scholar]
- Ushirogawa H, Abe Y, Tomioka K. Circadian locomotor rhythms in the cricket Gryllodes sigillatus. II. Interactions between bilaterally paired circadian pacemakers. Zool Sci. 1997;14:729–736. doi: 10.2108/zsj.14.729. [DOI] [PubMed] [Google Scholar]
- Wei H, el Jundi B, Homberg U, Stengl M. Implementation of pigment-dispersing factor immunoreactive neurons in a standardized atlas of the brain of the cockroach Leucophaea maderae. J Comp Neurol. 2010;518:4113–4133. doi: 10.1002/cne.22471. [DOI] [PubMed] [Google Scholar]
- Wen CJ, Lee HJ. Mapping the cellular network of the circadian clock in two cockroach species. Arch Insect Biochem Physiol. 2008;68:215–231. doi: 10.1002/arch.20236. [DOI] [PubMed] [Google Scholar]
- Wiedenmann G, Loher W. Circadian control of singing in crickets: two different pacemakers for early-evening and before-dawn activity. J Insect Physiol. 1984;30:145–151. [Google Scholar]
- Yukizane M, Kaneko A, Tomioka K. Electrophysiological and morphological characterization of the medulla bilateral neurons that connect bilateral optic lobes in the cricket, Gryllus bimaculatus. J Insect Physiol. 2002;48:631–641. doi: 10.1016/s0022-1910(02)00091-4. [DOI] [PubMed] [Google Scholar]
- Závodská R, Sauman I, Sehnal F. Distribution of PER protein, pigment-dispersing hormone, prothoracicotropic hormone, and eclosion hormone in the cephalic nervous system of insects. J Biol Rhythms. 2003;18:106–122. doi: 10.1177/0748730403251711. [DOI] [PubMed] [Google Scholar]