Abstract
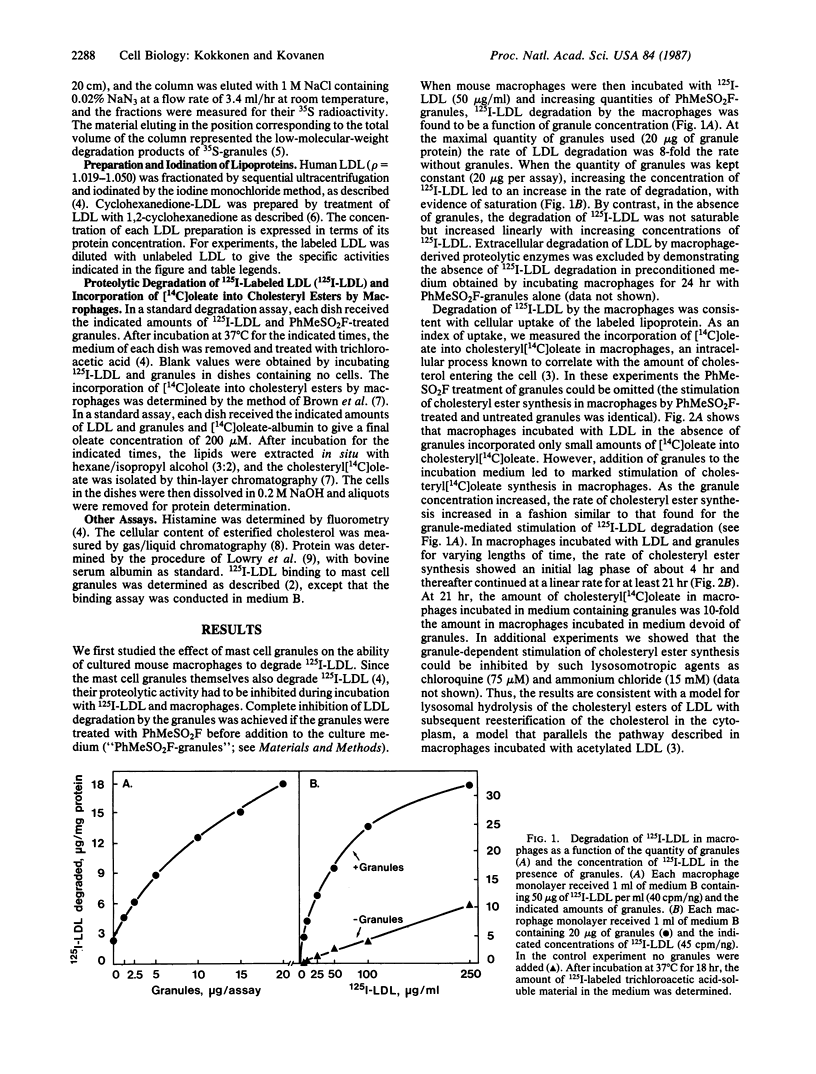
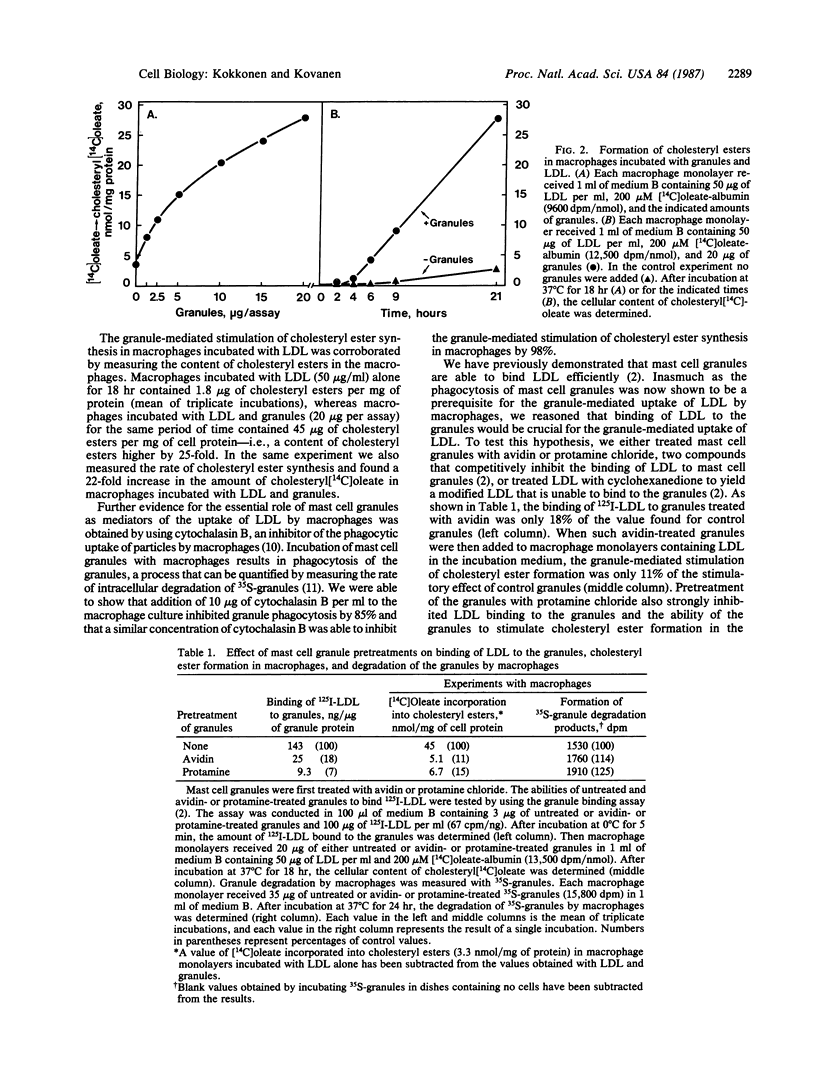
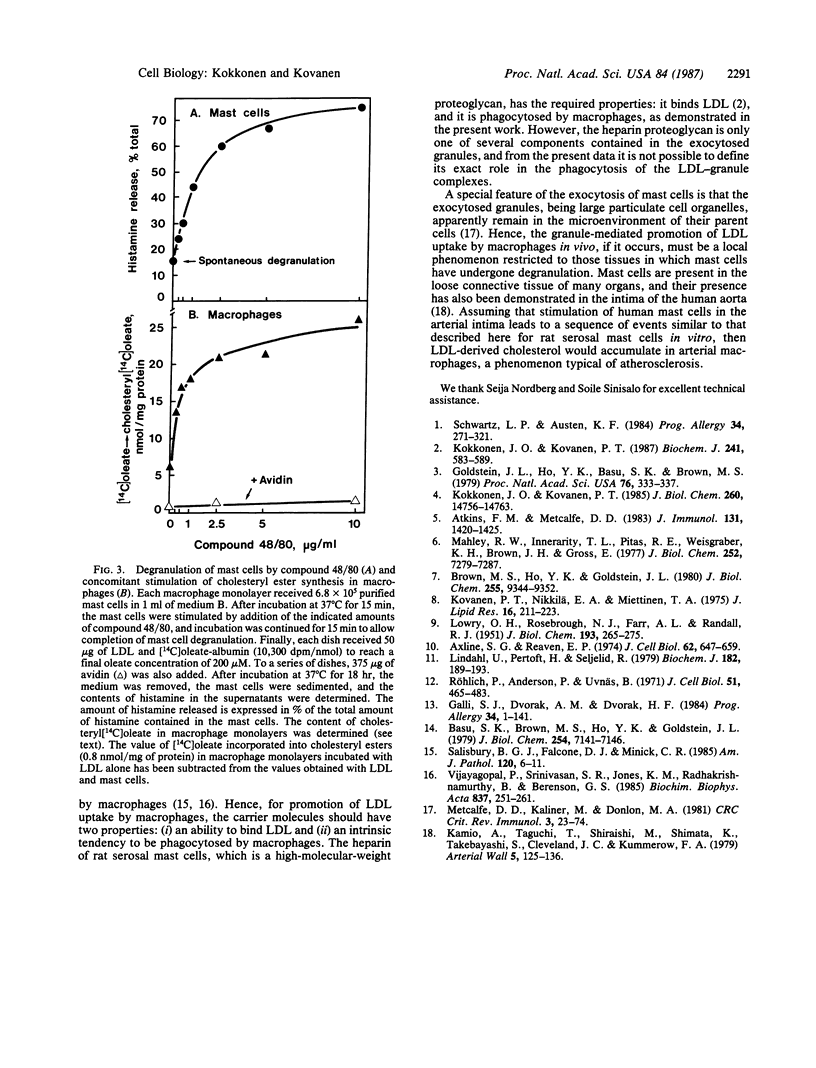
The uptake of low density lipoprotein (LDL) by cultured mouse macrophages was markedly promoted by isolated rat mast cell granules present in the culture medium. The granule-mediated uptake of LDL enhanced the rate of cholesteryl ester synthesis in the macrophages, the result being accumulation of cholesteryl esters in these cells. Binding of LDL to the granules was essential for the granule-mediated uptake of LDL by macrophages, for the uptake process was prevented by treating the granules with avidin or protamine chloride or by treating LDL with 1,2-cyclohexanedione, all of which inhibit the binding of LDL to the granules. Inhibition of granule phagocytosis by the macrophages with cytochalasin B also abolished the granule-mediated uptake of LDL. Finally, mouse macrophage monolayers and LDL were incubated in the presence of isolated rat serosal mast cells. Stimulation of the mast cells with compound 48/80, a degranulating agent, resulted in dose-dependent release of secretory granules from the mast cells and a parallel increase in cholesteryl ester synthesis in the macrophages. The results show that, in this in vitro model, the sequence of events leading to accumulation of cholesteryl esters in macrophages involves initial stimulation of mast cells, subsequent release of their secretory granules, binding of LDL to the exocytosed granules, and, finally, phagocytosis of the LDL-containing granules by macrophages.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins F. M., Metcalfe D. D. Degradation of the heparin matrix of mast cell granules by cultured fibroblasts. J Immunol. 1983 Sep;131(3):1420–1425. [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Goldstein J. L. Degradation of low density lipoprotein . dextran sulfate complexes associated with deposition of cholesteryl esters in mouse macrophages. J Biol Chem. 1979 Aug 10;254(15):7141–7146. [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Dvorak H. F. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio A., Taguchi T., Shiraishi M., Shitama K., Takebayashi S., Cleveland J. C., Kummerow F. A. Mast cells in human aorta. Paroi Arterielle. 1979 Oct;5(3):125–136. [PubMed] [Google Scholar]
- Kokkonen J. O., Kovanen P. T. Low density lipoprotein degradation by rat mast cells. Demonstration of extracellular proteolysis caused by mast cell granules. J Biol Chem. 1985 Nov 25;260(27):14756–14763. [PubMed] [Google Scholar]
- Kokkonen J. O., Kovanen P. T. Low-density-lipoprotein binding by mast-cell granules. Demonstration of binding of apolipoprotein B to heparin proteoglycan of exocytosed granules. Biochem J. 1987 Jan 15;241(2):583–589. doi: 10.1042/bj2410583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Nikkilä E. A., Miettinen T. A. Regulation of cholesterol synthesis and storage in fat cells. J Lipid Res. 1975 May;16(3):211–223. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl U., Pertoft H., Seljelid R. Uptake and degradation of mast-cell granules by mouse peritoneal macrophages. Biochem J. 1979 Jul 15;182(1):189–193. doi: 10.1042/bj1820189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Metcalfe D. D., Kaliner M., Donlon M. A. The mast cell. Crit Rev Immunol. 1981 Sep;3(1):23–74. [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury B. G., Falcone D. J., Minick C. R. Insoluble low-density lipoprotein-proteoglycan complexes enhance cholesteryl ester accumulation in macrophages. Am J Pathol. 1985 Jul;120(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Vijayagopal P., Srinivasan S. R., Jones K. M., Radhakrishnamurthy B., Berenson G. S. Complexes of low-density lipoproteins and arterial proteoglycan aggregates promote cholesteryl ester accumulation in mouse macrophages. Biochim Biophys Acta. 1985 Dec 4;837(3):251–261. doi: 10.1016/0005-2760(85)90048-7. [DOI] [PubMed] [Google Scholar]



