Abstract
Objective
The aim of this study was to compare currently proposed sets of pediatric metabolic syndrome criteria for the ability to predict elevations in “surrogate” factors that are associated with metabolic syndrome and with future cardiovascular disease and type 2 diabetes mellitus. These surrogate factors were fasting insulin, hemoglobin A1c (HbA1c), high-sensitivity C-reactive protein (hsCRP), and uric acid.
Methods
Waist circumference (WC), blood pressure, triglycerides, high-density lipoprotein cholesterol (HDL-C), fasting glucose, fasting insulin, HbA1c, hsCRP, and uric acid measurements were obtained from 2,624 adolescent (12–18 years old) participants of the 1999–2006 National Health and Nutrition Examination Surveys. We identified children with metabolic syndrome as defined by six commonly used sets of pediatric metabolic syndrome criteria. We then defined elevations in the surrogate factors as values in the top 5% for the cohort and calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each set of metabolic syndrome criteria and for each surrogate factor.
Results
Current pediatric metabolic syndrome criteria exhibited variable sensitivity and specificity for surrogate predictions. Metabolic syndrome criteria had the highest sensitivity for predicting fasting insulin (40–70%), followed by uric acid (31–54%), hsCRP (13–31%), and HbA1c (7–21%). The criteria of de Ferranti (which includes children with WC >75th percentile, compared to all other sets including children with WC >90th percentile) exhibited the highest sensitivity for predicting each of the surrogates, with only modest decrease in specificity compared to the other sets of criteria. However, the de Ferranti criteria also exhibited the lowest PPV values. Conversely, the pediatric International Diabetes Federation criteria exhibited the lowest sensitivity and the highest specificity.
Conclusions
Pediatric metabolic syndrome criteria exhibit moderate sensitivity for detecting elevations in surrogate factors associated with metabolic syndrome and with risk for future disease. Inclusion of children with more modestly elevated WC improved sensitivity.
Introduction
Pediatric obesity continues at critical levels and threatens to shorten the life span of the current generation of children.1 Many of the medical sequelae that are seen in the setting of longstanding obesity, including cardiovascular disease (CVD) and type 2 diabetes (T2DM), are associated with the metabolic syndrome.2 This cluster of clinical components is comprised of insulin resistance, hypertension, low high-density lipoprotein (HDL), and hypertriglyceridemia, findings that appear to be linked by poorly understood underlying processes related to inflammation and visceral adiposity.3 Because of the association between childhood metabolic syndrome and risk for sequelae in adulthood,4 many have proposed using a diagnosis of metabolic syndrome in childhood as a trigger for early intervention to decrease body fat and increase exercise.5,6
However, our ability to diagnose metabolic syndrome during childhood has been hampered by a lack of knowledge of how the biological processes behind metabolic syndrome are manifest in children to place them at greater risk for future disease, leading to a lack of consensus regarding which of a number of criteria to use to diagnose metabolic syndrome in children5,7–10 (Table 1). Before such a consensus is established, further investigation is warranted into how well the current sets of pediatric metabolic syndrome criteria identify children with evidence of insulin resistance and other biological processes that contribute to metabolic syndrome.
Table 1.
Proposed Criteria for the Diagnosis of the Metabolic Syndrome in Children
| Risk factors | Obesity | Hypertension | Hypertriglyceridemia (mg/dL) | Low HDL (mg/dL) | Elevated fasting glucose (mg/dL) | |
|---|---|---|---|---|---|---|
| Cook (2003)7 | ≥3 | WC ≥90% | SBP or DBP ≥90% for age, sex, height | TG ≥110 mg/dL | HDL ≤ 40 | ≥110 |
| DeFerranti (2004)8 | ≥3 | WC >75% | SBP or DBP ≥90% for age, sex, height | TG ≥ 97 mg/dL | HDL: | ≥110 |
| Males 15-19: <45 | ||||||
| All others: < 50 | ||||||
| Ford (2007)9 | ≥3 | WC≥90% | SBP or DBP ≥90% for age, sex, height | TG ≥110 mg/dL | HDL ≤40 | ≥100 |
| IDF | ≥2a | 6–10 y.o.: WC ≥90% | ≥130/85 mmHg | TG ≥150 mg/dL | HDL < 40 | ≥100 |
| (Zimmet 2007)5 | ≥2a | 10–16 y.o.: WC ≥90% | ≥130/85 mmHg | TG ≥150 mg/dL | HDL: | ≥100 |
| Males <40 | ||||||
| Females < 50 | ||||||
| Jolliffe (2007) (ATP III)10,b | ≥3 | 12 y.o. boys: 94.2 cm | 12 y.o. boys: 121/76 mmHg | 12 y.o. boys: 127 | 12 y.o. boys: 43.7 | ≥100 |
| 12 y.o. girls: 79.5 cm | 12 y.o. girls: 121/80 mmHg | 12 y.o. girls: 142 | 12 y.o. girls: 39.8 | |||
| 20 y.o. boys: 101.8 cm | 20 y.o. boys: 130/85 mmHg | 20 y.o. boys: 150 | 20 y.o. boys: 48.3 | |||
| 20 y.o. girls: 88 cm | 20 y.o. girls: 130/85 mmHg | 20 y.o. girls: 150 | 20 y.o. girls: 50.3 | |||
| Jolliffe (2007) (IDF)10,a,b | ≥2 | 12 y.o. boys: 85.1 cm | Same as Joliffe ATP III | Same as Joliffe ATP III | Same as Joliffe ATP III | Same as Joliffe ATP III |
| 12 y.o. girls: 72.5 cm | ||||||
| 20 y.o. boys: 94 cm | ||||||
| 20 y.o. girls: 80 cm |
Elevated WC is a prerequisite and is not counted toward the number of components needed for diagnosis.
Values of each component are adjusted gradually on a gender-specific basis between childhood levels (starting at 12 y.o.) and levels in ATP III or IDF (at 18 y.o.).
Abbreviations: HDL, High-density lipoprotein; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; IDF, International Diabetes Federation; ATP, Adult Treatment Panel III; y.o., years old.
In the absence of outcomes studies with sufficiently long follow up (i.e., children followed prospectively to identify factors predictive for T2DM and CVD), we used a cross-sectional database—the National Health and Nutrition Evaluation Survey (NHANES)—to analyze six proposed sets of pediatric metabolic syndrome criteria for their ability to predict elevations in four factors that are associated with metabolic syndrome: Fasting insulin,11–14 hemoglobin A1c (HbA1c),15–17 high-sensitivity C-reactive protein (hsCRP),18–25 and uric acid9,26–28 (Table 2). Fasting insulin, although not itself a criterion used to diagnose metabolic syndrome, is significantly correlated with insulin resistance in children.12 HbA1c is a glycosylation product of hemoglobin that rises with average blood sugars. Low-grade inflammation, as assessed by levels of hsCRP, is associated with obesity and insulin resistance in children,24,25,29–31 and in adults is linked to long-term risk for T2DM23,32 and myocardial infarction.18,33,34 High levels of uric acid are strongly associated with metabolic syndrome in children9 and appear to play a role in causing hypertension.35–37
Table 2.
Metabolic Syndrome Associations and Predictive Value of Fasting Insulin, HbA1c, hsCRP, and Uric Acid
| Association with metabolic syndrome in childhood | Association with carotid IMT in childhood | Predictive value for future CAD among adults | Predictive value for future T2DM among adults | |
|---|---|---|---|---|
| Fasting insulin | Related to insulin resistance, the sin qua non of metabolic syndrome | Associated with increased IMT11 | Predicts progression of coronary artery calcification over a 2-year period, adjusted for metabolic syndrome components13 | RR 6.0 (highest 10% vs. lowest 10%, adjusted for insulin sensitivity, body fat, age, sex; median follow up 7.1 years)14 |
| HbA1c | Higher in nondiabetic children and adolescents with metabolic syndrome; independent predictor of metabolic syndrome with OR 3.2515 | No known associations in the nondiabetic range | RR 2.36 for 1% increase HbA1c in nondiabetics, adjusted for other CHD factors17 | RR 3–3.7 for future development of diabetes for HbA1c 5.07–5.22 vs. <4.8 over 10.1 years16 |
| C-reactive protein | Associated with insulin resistance24,25 and with increasing number of metabolic syndrome risk factors19,22 | Independently associated with increased IMT20,21 | RR 1.49 (top 1/3 vs. bottom 1/3; adjusted for other CHD risk factors; 12 years mean follow up)18 | RR 4.2 (highest vs. lowest quartile; adjusted for T2DM risk factors; 4 years median follow up)23 |
| Uric acid | RR risk of metabolic syndrome is 5.4 for children in the highest quartile vs. lowest quartile of uric acid9 | Independently associated with increased IMT28 | RR 1.46 (highest vs. lowest quintile; adjusted for renal function; 6.2 years mean follow up)26 | RR 1.17 per 1.0 mg/dL increase in uric acid (meta-analysis, 2–13 years follow-up)27 |
Abbreviations: HbA1c, Hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein; IMT, intima media thickness; RR, relative risk; OR, odds ratio.
In the ranges of values studied here, these factors are linked among adolescents to increased risk of carotid intima media thickness, a marker for early atherosclerotic disease,11,20,21,28 and/or among adults to increased risk for the development of future CVD and T2DM (Table 2). As such, we termed these factors “surrogate” markers of the biological processes linking metabolic syndrome with future disease risk. Our ultimate goal is to identify children who may benefit most from lifestyle intervention. In the current experiment, we essentially treated a diagnosis of metabolic syndrome as a screening test for detecting elevations in these surrogate factors. We set out to determine which of the current pediatric metabolic syndrome criteria demonstrated the best sensitivity with acceptable specificity (≥80%) in predicting elevations in these surrogates.
Methods
Data were obtained from the NHANES, 1999–2006, a complex, multistage probability sample of the U.S. population. These annual surveys are conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (CDC) with data released every 2 years (www.cdc.gov/nchs/nhanes.htm). The NCHS ethics review board reviewed and approved the survey, and participants were provided with informed consent prior to participation. Waist circumference (WC), blood pressure, and laboratory measures of triglycerides, HDL cholesterol (C), and glucose were obtained using standardized protocols and calibrated equipment. All laboratory values used for analyses were obtained from participants asked to fast for 8 h prior to the blood draw.
Diagnosis of metabolic syndrome
We defined metabolic syndrome using six previously published sets of criteria (Table 1). We excluded subjects with known diabetes and pregnancy. We also excluded children taking antidiabetic medication, growth hormone, glucocorticoids, sex hormones, lipid-lowering agents, and antihypertensives, given that each of these is known to affect the metabolic parameters tested. We included only children 12–18 years old in our data analyses, given that fasting values for triglycerides and glucose were only obtained in participants 12 years and older. We also excluded children whose CRP was greater than 10 mg/L, as performed in previous analyses.38
Statistical methods
Statistical analysis was performed using SUDAAN (version 10; Research Triangle Institute, Research Triangle Park, NC), which accounts for the survey design when estimating standard errors. Population-based estimates were obtained by assigning sampling weights to each sampled child for whom an interview was completed. We combined all data sets for statistical analyses, thereby increasing our total sample size and power. Pearson r correlation coefficients were computed to assess the degree of linear association between each component of metabolic syndrome and each of the examined surrogates. Prevalence rates of the various definitions of metaoblic syndrome were calculated by gender and race/ethnicity (non-Hispanic white, non-Hispanic black, and Mexican American) and compared via chi-squared tests. Mean surrogate levels were also compared in a similar fashion via t-tests (gender) and analysis of variance (race/ethnicity). “High” levels of the surrogates (fasting insulin, HbA1c, hsCRP, and uric acid) were determined to be the top 5% for each surrogate in the sample. The overall abilities of each proposed set of metabolic syndrome criteria to predict “high” levels of these surrogates were assessed by computing sensitivity and specificity. Sensitivity and specificity with respect to prediction of having one or more elevated level of these three surrogates were also computed, with corresponding 95% confidence intervals. Statistical significance was defined as a P value <0.05.
Results
Characteristics
Table 3 shows the percentage of subjects with metabolic syndrome by each of the six criteria. The proportion of adolescents with metabolic syndrome ranged from 4.0% by the IDF criteria to 15.1% by the de Ferranti criteria. Whereas there was a tendency for metabolic syndrome to be more common among males than females, this reached statistical significance for only three of the six criteria. Non-Hispanic white and Mexican-American adolescents had similar levels of metabolic syndrome, whereas non-Hispanic blacks had lower prevalence. There were no trends in metabolic syndrome diagnosis by NHANES cycle over the time period studied.
Table 3.
NHANES 1999–2006 Characteristics: Children 12–18 Years Old with Data on All Outcomes of Interest (N = 2,624)
| |
|
% with metabolic syndrome |
Insulin (uU/L) |
HbA1c (%) |
hsCRP (mg/L) |
Uric Acid (mg/dL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IDF | Cook | Ford | Jolliffe (ATP) | Jolliffe (IDF) | DeFerranti | Mean (95% CI) | 95th Percentile | Mean (95% CI) | 95th Percentile | Mean (95% CI) | 95th Percentile | Mean (95% CI) | 95th Percentile | |
| Overall | 2,624 | 4.0% | 6.6% | 8.1% | 6.8% | 8.4% | 15.1% | 11.9 (11.4, 12.4) | 28.0 | 5.15 (5.13, 5.17) | 5.50 | 1.0 (0.9,1.1) | 4.0 | 5.1 (5.0, 5.2) | 7.3 |
| By gender | |||||||||||||||
| Males | 1,400 | 5.4% | 8.6% | 10.8% | 7.6% | 9.0% | 17.0% | 11.7 (11.0, 12.4) | 29.4 | 5.17 (5.15, 5.19) | 5.55 | 0.9 (0.8, 1.1) | 4.0 | 5.7 (5.6, 5.8) | 7.7 |
| Females | 1,224 | 2.4% | 4.4% | 5.0% | 5.8% | 7.9% | 12.8% | 12.2 (11.5, 12.8) | 27.2 | 5.12 (5.10, 5.15) | 5.50 | 1.0 (0.9, 1.2) | 4.3 | 4.5 (4.5, 4.6) | 6.0 |
| P valuea | 0.009 | 0.009 | 0.003 | 0.242 | 0.575 | 0.060 | 0.286 | 0.001 | 0.237 | <0.001 | |||||
| By ethnicity | |||||||||||||||
| NHW | 752 | 4.3% | 7.1% | 8.7% | 7.0% | 8.9% | 15.8% | 11.4 (10.7, 12.1) | 26.8 | 5.12 (5.10, 5.15) | 5.50 | 0.9 (0.8, 1.0) | 3.7 | 5.2 (5.1, 5.3) | 7.4 |
| MA | 1007 | 4.7% | 7.3% | 9.3% | 7.7% | 10.1% | 17.2% | 13.4 (12.7, 14.2) | 29.6 | 5.15 (5.13, 5.17) | 5.50 | 1.2 (1.1, 1.3) | 4.8 | 5.0 (4.9, 5.1) | 7.1 |
| NHB | 865 | 2.2% | 4.0% | 4.3% | 4.9% | 5.2% | 9.8% | 13.1 (12.5, 13.8) | 32.2 | 5.25 (5.23, 5.28) | 5.69 | 1.1 (1.0, 1.2) | 4.9 | 4.8 (4.7, 4.9) | 6.7 |
| P valueb | 0.012 | 0.019 | 0.001 | 0.073 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| By year | |||||||||||||||
| 99–00 | 676 | 3.7% | 7.0% | 7.3% | 4.9% | 6.2% | 15.7% | 12.6 (11.6, 13.5) | 27.8 | 5.03 (4.96, 5.11) | 5.46 | 1.0 (0.8, 1.2) | 3.8 | 5.1 (5.0, 5.3) | 7.2 |
| 01–02 | 691 | 3.0% | 6.2% | 9.0% | 8.1% | 10.0% | 15.2% | 12.2 (11.3, 13.2) | 25.0 | 5.17 (5.14, 5.19) | 5.48 | 0.9 (0.7, 1.0) | 3.6 | 5.1 (5.0, 5.3) | 7.6 |
| 03–04 | 643 | 4.3% | 6.5% | 7.6% | 5.6% | 8.7% | 17.0% | 11.3 (10.3, 12.4) | 31.1 | 5.18 (5.16, 5.20) | 5.48 | 1.1 (0.9, 1.3) | 4.6 | 5.2 (5.1, 5.3) | 7.3 |
| 05–06 | 614 | 5.2% | 6.8% | 8.2% | 8.1% | 8.5% | 12.5% | 11.6 (10.4, 12.8) | 28.2 | 5.20 (5.17, 5.23) | 5.58 | 1.0 (0.8, 1.2) | 3.7 | 5.1 (4.9, 5.3) | 7.0 |
| P valueb | 0.733 | 0.985 | 0.949 | 0.542 | 0.555 | 0.750 | 0.304 | 0.001 | 0.454 | 0.757 | |||||
Chi-squared test comparing metabolic syndrome prevalences, t-test comparing outcomes of interest.
Chi-squared test comparing metabolic syndrome prevalences, ANOVA comparing outcomes of interest (overall difference among the groups).
Abbreviations: NHANES, National Health and Nutrition Examination Survey; IDF, International Diabetes Federation; ATP, Adult Treatment Panel III; HbA1c, hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein; CI, confidence interval; NHW, non-Hispanic white; MA, Mexican-American; NHB, non-Hispanic black.
Regarding levels of metabolic syndrome–associated factors, Table 3 gives the mean and 95th percentile values. Although there was not a gender difference for levels of insulin or hsCRP, among adolescent females versus males there were lower mean levels of HbA1c (5.12% vs. 5.17%) and uric acid (4.5 mg/dL vs. 5.7 mg/dL, P < 0.001). Regarding racial/ethnic differences, insulin and hsCRP mean values were lower among non-Hispanic white adolescents than either Mexican Americans or non-Hispanic blacks. Conversely, mean uric acid levels were lowest among non-Hispanic black adolescents. There was a significant trend toward increasing HbA1c values with time (Table 3).
Association with metabolic syndrome components
The metabolic syndrome–associated surrogate factors in our analysis showed a high degree of linear association with the components of metabolic syndrome (Table 4). In particular, fasting insulin, hsCRP, and uric acid were related to WC, systolic blood pressure (SBP), triglycerides, and HDL. HbA1c was correlated with SBP; and fasting insulin, HbA1c, and uric acid were correlated with fasting glucose. Diastolic blood pressure (DBP) was negatively correlated with HbA1c.
Table 4.
Correlations Between Metabolic Syndrome Components and Surrogate Measures of Cardiovascular Disease and Type 2 Diabetes Mellitus
| |
Pearson's r (P value) |
|||||
|---|---|---|---|---|---|---|
| Surrogate | WC | SBP | DBP | Triglycerides | HDL | Fasting glucose |
| Fasting insulin | 0.58 | 0.28 | 0.04 | 0.39 | −0.26 | 0.28 |
| (<0.01) | (<0.01) | (0.20) | (<0.01) | (<0.01) | (<0.01) | |
| HbA1c | 0.06 | 0.06 | −0.09 | 0.01 | 0.03 | 0.25 |
| (0.07) | (0.01) | (<0.01) | (0.84) | (0.36) | (<0.01) | |
| hsCRP | 0.42 | 0.14 | 0.00 | 0.14 | −0.12 | 0.02 |
| (<0.01) | (<0.01) | (0.91) | (0.02) | (<0.01) | (0.59) | |
| Uric acid | 0.45 | 0.33 | −0.00 | 0.30 | −0.35 | 0.20 |
| (<0.01) | (<0.01) | (0.96) | (<0.01) | (<0.01) | (<0.01) | |
Abbreviations: WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein; IDF, International Diabetes Federation; ATP, Adult Treatment Panel III; HbA1c, hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein.
Sensitivity and specificity
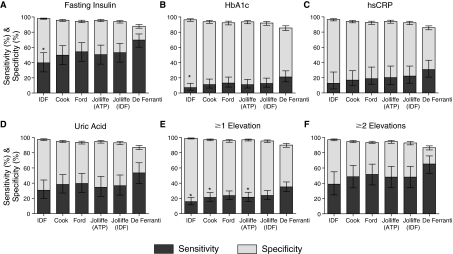
Figure 1 shows the sensitivity and specificity for each of the criteria in predicting elevations in the metabolic syndrome–associated factors. In general, sensitivity and specificity for the prediction of metabolic syndrome–related surrogate factors mirrored the rates of metabolic syndrome diagnosis. For each of the surrogates, the International Diabetes Federation (IDF) criteria had the lowest sensitivity and positive predictive value (PPV) and the highest specificity and negative predictive value (NPV) (Table 5). Also for each surrogate, the set of criteria with highest prevalence of metabolic syndrome diagnosis (de Ferranti) had the highest sensitivity and NPV and the lowest specificity and PPV.
FIG. 1.
Sensitivity and specificity for predicting elevations in surrogate factors in adolescents. Sensitivity (dark grey bars) and specificity (light grey bars) are shown with confidence intervals for each of six sets of pediatric metabolic syndrome criteria regarding the ability of a diagnosis of metabolic syndrome in an adolescent to predict the presence of elevations in surrogate factors that are associated with metabolic syndrome and with future risk for T2DM and CVD. Surrogate elevations (A–D) were defined as the top 5% of values for the entire adolescent cohort (age 12–18). Also shown is the ability of each set of criteria to detect at least one (E) or at least two (F) elevated surrogate values. IDF, International Diabetes Federation; ATP, Adult Treatment Panel III; HbA1c, hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein. (*) P < 0.05 for sensitivity compared to de Ferranti criteria.
Table 5.
Overall Positive Predictive Value and Negative Predictive Value (95% Confidence Intervals) Comparisons
| |
High fasting insulin (>28.0) (n = 156) |
High HbA1c (>5.5) (n = 305) |
High hsCRP (>4.0) (n = 170) |
High uric acid (>7.3) (n = 116) |
One or more elevations (n = 609) |
Two or more elevations (n = 108) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set of Metabolic syndrome criteria | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV |
| IDF | 50.0 | 96.8 | 15.4 | 91.6 | 16.9 | 95.2 | 40.1 | 96.2 | 80.0 | 82.1 | 30.5 | 98.0 |
| (38.7, 61.3) | (95.8, 97.6) | (7.1, 30.1) | (89.6, 93.3) | (7.2, 34.7) | (93.7, 96.3) | (26.4, 55.6) | (95.0, 97.1) | (67.9, 88.3) | (79.7, 84.3) | (20.0, 43.5) | (97.2, 98.5) | |
| Cook | 38.0 | 97.3 | 14.5 | 91.8 | 13.6 | 95.3 | 30.4 | 96.5 | 65.7 | 82.8 | 23.1 | 98.3 |
| (28.1, 49.0) | (96.3, 98.0) | (7.7, 25.7) | (89.7, 93.4) | (8.1, 21.8) | (93.9, 96.3) | (21.8, 40.7) | (95.4, 97.4) | (53.9, 75.8) | (80.4, 85.1) | (15.9, 32.4) | (97.6, 98.8) | |
| Ford | 34.0 | 97.5 | 14.1 | 91.8 | 12.4 | 95.3 | 25.7 | 96.6 | 59.8 | 83.1 | 20.2 | 98.3 |
| (25.6, 43.7) | (96.6, 98.2) | (7.6, 24.7) | (89.8, 93.5) | (7.5, 20.0) | (93.9, 96.4) | (17.4, 36.3) | (95.4, 97.4) | (48.9, 69.8) | (80.6, 85.3) | (13.6, 28.8) | (97.7, 98.8) | |
| Jolliffe(ATP III) | 37.8 | 97.3 | 14.1 | 91.7 | 15.9 | 95.4 | 26.9 | 96.3 | 64.9 | 82.9 | 22.4 | 98.2 |
| (29.1, 47.3) | (96.3, 98.0) | (7.7, 24.4) | (89.7, 93.4) | (8.8, 27.1) | (94.0, 96.6) | (17.5, 38.9) | (95.0, 97.3) | (54.3, 74.2) | (80.4, 85.0) | (14.8, 32.3) | (97.6, 98.7) | |
| Jolliffe(IDF) | 31.8 | 97.4 | 12.9 | 91.7 | 14.0 | 95.5 | 22.8 | 96.4 | 57.7 | 83.1 | 17.9 | 98.2 |
| (24.5, 40.1) | (96.5, 98.1) | (7.2, 22.1) | (89.7, 93.4) | (8.1, 23.2) | (94.1, 96.5) | (14.8, 33.4) | (95.1, 97.3) | (48.1, 66.6) | (80.6, 85.3) | (11.8, 26.3) | (97.5, 98.7) | |
| DeFerranti | 23.4 | 98.2 | 12.1 | 92.0 | 10.9 | 95.7 | 18.6 | 97.1 | 47.4 | 84.4 | 13.7 | 98.7 |
| (17.6, 30.3) | (97.7, 98.6) | (7.8, 18.2) | (89.7, 93.8) | (7.2, 16.2) | (94.4, 96.7) | (12.9, 26.2) | (96.0, 98.0) | (39.9, 55.1) | (81.9, 86.7) | (9.8, 18.7) | (98.1, 99.1) | |
Abbreviations: HbA1c, hemoglobin Ale; hsCRP, high-sensitivity C-reactive protein; PPV, positive predictive value; NPV, negative predictive value; IDF, International Diabetes Federation; ATP, Adult Treatment Panel III.
Regarding the prediction of an elevated insulin level, sensitivities ranged from 40% to 70% with specificities of 88% to 98% and PPV values of 23% to 50% (Fig. 1, Table 5). Results were similar when the 95th percentile of the homeostasis model of insulin resistance (HOMA-IR) was tested, either for lean individuals or for the entire set (data not shown). For predicting HbA1c, sensitivities ranged from 7% to 21%, with specificities of 86% to 96% and PPV values of 12% to 15%. Regarding the prediction of an elevated hsCRP, sensitivity rates for pediatric metabolic syndrome criteria ranged from 13% to 31%, whereas specificity rates ranged from 86% to 97% and PPV values of 11% to 17%. For predicting an elevated uric acid, sensitivity ranged from 31% to 54% with specificities of 87% to 98% and PPV values of 19% to 40%.
In considering the ability to predict an elevation in at least one of the surrogates, sensitivity ranged from 16% to 35% with specificities of 90% to 99% and of PPV values of 47% to 80%, whereas the ability to predict elevations in at least two of the surrogates had sensitivities 39% to 65%, specificities of 87% to 97%, and PPV values of 14% to 31% (Fig. 1, Table 5).
Discussion
We have demonstrated that currently proposed pediatric metabolic syndrome criteria exhibit a variable sensitivity (7% to 70%) for predicting elevations in four factors that are associated with metabolic syndrome and that are themselves independent predictors in adults of CVD and T2DM, suggesting their involvement with the biological processes underlying metabolic syndrome. In making these statements regarding metabolic syndrome, we are differentiating between somewhat arbitrary rules used to diagnose children with metabolic syndrome (a series of cut-off values of the involved components) and the presence in some children of possible underlying processes—related to in part to genetics and obesity—that may link these particular components of metabolic syndrome. Given the differences in cut-off values for the various pediatric metabolic syndrome criteria, some sets of criteria may perform better at diagnosing children who are affected by processes that drive the association of insulin resistance, hypertension, and lipid abnormalities and may be at higher risk for future disease.
To be fair, the biological processes underlying metabolic syndrome have not been well delineated and are likely to involve overlapping pathways with elements of inflammation, dysregulation of adipose-derived hormonal factors, and changes in activity of insulin signaling.3,39–42 The value of metabolic syndrome has thus been questioned as an entity that is more important than the sum of its parts.43 Nevertheless, the tight associations linking the components of metabolic syndrome44 and their ability to predict adult disease4,45–48 make pediatric metabolic syndrome an intriguing way to identify children who would most benefit from early interventions to prevent future sequelae. The better we can identify children with these future risks, the more confident we will be in targeting these children for interventions targeting weight loss and physical activity.
An increasing number of studies are using long-term cohort data to confirm the utility of a childhood diagnosis of metabolic syndrome in predicting future disease.4,45–47 Unfortunately, as a tool for comparing current metabolic syndrome criteria, these studies are frequently limited by a lack of adequate information (including a lack of WC measurements4) collected at recruitment in childhood or by relatively small numbers of subjects who have developed T2DM or coronary artery disease (CAD) events. Because of these limitations of long-term data, we chose to evaluate current pediatric metabolic syndrome criteria by looking for elevations in four factors that are not directly a part of the diagnostic criteria of metabolic syndrome itself but are strongly associated with metabolic syndrome. These factors were fasting insulin, HbA1c, hsCRP, and uric acid (Table 2). We chose these factors because they are associated with metabolic syndrome and linked to the long-term sequelae that make metabolic syndrome a concerning condition; therefore, they may also be related to the underlying insulin resistance and inflammatory processes involved in metabolic syndrome. This approach provides the means of comparing current metabolic syndrome criteria for sensitivity in predicting elevations in factors that may identify processes underlying metabolic syndrome.
Insulin resistance is the sin qua non of metabolic syndrome. We used fasting insulin values as our estimate of insulin resistance, given their association with metabolic syndrome and risk of later T2DM.14,49,50 We chose fasting insulin instead of the HOMA-IR (based on fasting insulin and glucose51) because each of the criteria that we evaluate use fasting glucose values as part of the basis of the diagnosis of metabolic syndrome. Prior studies have shown that fasting insulin closely approximates HOMA-IR because of wide differences in insulin variability (53-fold) compared to minor differences in fasting glucose variability (1.8-fold).52 When we evaluated sensitivity and specificity of the sample using HOMA-IR as a surrogate instead of insulin, we obtained nearly identical results.
Given the central nature of insulin resistance in metabolic syndrome, it is notable that the current sets of criteria exhibited better sensitivity for detecting elevated fasting insulin (40% to 70%) than the other surrogate factors. Nevertheless, current pediatric metabolic syndrome criteria missed detection of 30% to 60% of children that comprised the top 5% of fasting insulin values, suggesting either that a fair number of subjects have isolated insulin resistance without other metabolic abnormalities or that our criteria are not identifying all children with metabolic syndrome.
Uric acid is strongly associated with metabolic syndrome and future risk of T2DM and CVD.9,53 Although its role is not known regarding the biological processes underlying metabolic syndrome, elevated uric acid levels have been identified as a primary cause of hypertension.37,54–60 Our analysis using the 95th percentile for uric acid (7.3 mg/dL, close to the most commonly used cut off of 7.0 to identify risk associated with uric acid9,55,56,61) showed that current metabolic syndrome criteria performed better at predicting uric acid elevations than they did for any of the other surrogates besides insulin. This ability to predict uric acid elevations occurred even though overall uric acid was not as tightly linked to individual metabolic syndrome component as was insulin (Table 4).
CRP is an acute-phase reactant that is produced in inflammatory processes but can also be elevated in children24,25,29–31 and adults18,23,32–34 during low levels of inflammation produced as a result of obesity and the metabolic syndrome. Although hsCRP values in our sample were significantly correlated with the components of metabolic syndrome (Table 4), the correlation with hsCRP was weaker than noted for fasting insulin and uric acid. In addition, none of the pediatric metabolic syndrome criteria exhibited impressive sensitivity in predicting hsCRP elevations (Fig. 1, all <31%), which is either indicative of a high level of non- metabolic syndrome-related elevations in hsCRP (such as due to minor infections62) or difficulties in our current criteria in identifying underlying inflammation due to metabolic syndrome or, more likely, a combination of these. Although the hsCRP values in children and adolescents are not significantly different from those seen in adults,63 the weaker association of hsCRP with metabolic syndrome components may underscore a less precise role for prediction of future disease using hsCRP among children than is observed in adults. Future long-term studies are needed to better define the relationship of childhood hsCRP levels and risk for adult disease.
The sensitivity for detecting mild elevations in HbA1c was poorest for all of the surrogates, and HbA1c also had the weakest correlation with metabolic syndrome components, suggesting that mild elevations in HbA1c may not be related to metabolic syndrome.64 There was a trend toward increasing HbA1c over time. Given that metabolic syndrome itself was stable over this time period, the significance of this increasing trend is uncertain, although it may indicate a subtle worsening of blood sugar values over the time period.
Overall, there was significant variability in the ability of these pediatric metabolic syndrome criteria to identify individuals with elevations in the surrogate risk factors. In particular, the criteria of de Ferranti et al. exhibited the best sensitivity and the IDF criteria exhibited the poorest sensitivity in predicting elevations in the factors. This was true for each of the surrogates, as well as for the ability to pick up at least one or two surrogates. The reason for this improved sensitivity for the de Ferranti criteria likely lies in its inclusion of adolescents with elevated WC (a surrogate for visceral adiposity) from 75% to 90%, when each of the other sets of criteria used 90% as a cut-off value. This inclusion of additional individuals with milder degrees of elevated WC resulted in more overall diagnoses of metabolic syndrome, as well as improved sensitivity and decreased specificity in prediction of surrogates for underlying processes related to metabolic syndrome. This happened despite the de Ferranti criteria having the most stringent cut off for fasting glucose, 110 mg/dL, which was based on a previous recommendation by the American Diabetes Association (ADA).65 It is not surprising that this inclusion of further individuals with elevated WC improved the sensitivity of predicting these elevations, because each of the surrogates, with the exception of HbA1c, was positively correlated with WC (Table 4). WC is itself a surrogate for visceral adipose tissue, which is felt to drive many of the processes underlying metabolic syndrome.66
The positive predictive values were highest for the IDF criteria and lowest for the de Ferranti criteria, which is largely indicative of the relative amounts of metabolic syndrome diagnoses yielded by each set of criteria. The sets of criteria that yielded more metabolic syndrome diagnoses overall were more likely to include individuals with surrogate elevations, but were even more likely to pick up additional metabolic syndrome (+) individuals without surrogate elevations, diluting the specificity of a metabolic syndrome diagnosis for detecting these elevations. Used as a test to detect individuals with elevations in these factors associated with increased risk in adults, some sets of criteria have more false positives (e.g., the de Ferranti criteria) for these surrogates whereas others produce fewer false negatives (e.g., the IDF critieria). In considering the utility of a test to appropriately trigger a given work up or intervention, it is important to take into account whether the population would be better served by including a higher number of false positives for work up and intervention (and thus incur increased costs and anxiety) or to inappropriately exclude false negatives (and thus fail to intervene on certain individuals at higher risk). Given the intervention that we would propose to be triggered (i.e., effective lifestyle intervention) is labor-intensive for health-care practitioners, but is likely to provide cardiovascular benefits to most overweight adolescents, we would submit that it is better to have a higher level of false positives.67 This is particularly true because these surrogates are not the only markers of underlying disease risk but merely are suggestive of the presence of biological processes that drive the associations of metabolic syndrome.
In conclusion, current pediatric metabolic syndrome criteria exhibit a range of sensitivity, specificity, PPV and NPV values in the prediction of surrogates for the underlying processes behind metabolic syndrome. In particular, the currently proposed sets of metabolic syndrome criteria exhibited high NPV, excluding children who did not have elevated levels of these surrogates. The sets of criteria did not perform as well with respect to PPV, meaning that a large proportion of adolescents diagnosed with metabolic syndrome did not have significant elevations in the factors we tested for, potentially indicating that they exhibited a less-advanced condition of metabolic syndrome or otherwise revealing constraints of this means of analysis. Should a diagnosis of metabolic syndrome be used to trigger interventions for individuals at elevated risk for future disease, missing as few as possible, the de Ferranti criteria demonstrated the best sensitivity with adequate specificity (all >85%). Further experiments using longitudinal data sets will be needed to define how these sets of criteria perform in the prediction of future disease risk, a more important means to judge their adequacy.
Acknowledgment
This work was supported by National Institutes of Health grant NIH HD060739-01 (to M.D.D.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Narayan KM. Boyle JP. Thompson TJ. Sorensen SW. Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JA. Friedman LA. Wang P. Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Zimmet P. Alberti G. Kaufman F. Tajima N. Silink M. Arslanian S. Wong G. Bennett P. Shaw J. Caprio S. International Diabetes Federation Task Force on Epidemiology Prevention of Diabetes. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 6.Lieb DC. Snow RE. DeBoer MD. Socioeconomic factors in the development of childhood obesity and diabetes. Clin Sports Med. 2009;28:349–378. doi: 10.1016/j.csm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook S. Weitzman M. Auinger P. Nguyen M. Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 8.de Ferranti SD. Gauvreau K. Ludwig DS. Neufeld EJ. Newburger JW. Rifai N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES. Li C. Cook S. Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 10.Jolliffe CJ. Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the Adult Treatment Panel III and International Diabetes Federation criteria. J Am Coll Cardiol. 2007;49:891–898. doi: 10.1016/j.jacc.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 11.Beauloye V. Zech F. Tran HT. Clapuyt P. Maes M. Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92:3025–3032. doi: 10.1210/jc.2007-0619. [DOI] [PubMed] [Google Scholar]
- 12.Gungor N. Saad R. Janosky J. Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 13.Lee KK. Fortmann SP. Fair JM. Iribarren C. Rubin GD. Varady A. Go AS. Quertermous T. Hlatky MA. Insulin resistance independently predicts the progression of coronary artery calcification. Am Heart J. 2009;157:939–945. doi: 10.1016/j.ahj.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Weyer C. Hanson RL. Tataranni PA. Bogardus C. Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: Evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49:2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Capape M. Alonso M. Colino E. Mustieles C. Corbaton J. Barrio R. Frequency of the metabolic syndrome in obese Spanish pediatric population. Eur J Endocrinol. 2006;155:313–319. doi: 10.1530/eje.1.02206. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan AD. Rifai N. Buring JE. Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007;120:720–727. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E. Coresh J. Golden SH. Brancati FL. Folsom AR. Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: The atherosclerosis risk in communities study. Arch Intern Med. 2005;165:1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 18.Danesh J. Wheeler JG. Hirschfield GM. Eda S. Eiriksdottir G. Rumley A. Lowe GD. Pepys MB. Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 19.de Ferranti SD. Gauvreau K. Ludwig DS. Newburger JW. Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: Findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem. 2006;52:1325–1330. doi: 10.1373/clinchem.2006.067181. [DOI] [PubMed] [Google Scholar]
- 20.Giannini C. de Giorgis T. Scarinci A. Ciampani M. Marcovecchio ML. Chiarelli F. Mohn A. Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis. 2008;197:448–456. doi: 10.1016/j.atherosclerosis.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Jarvisalo MJ. Harmoinen A. Hakanen M. Paakkunainen U. Vlikari J. Hartiala J. Lehtimaki T. Simell O. Raitakar OT. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 22.Patel DA. Srinivasan SR. Xu JH. Li S. Chen W. Berenson GS. Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: The Bogalusa Heart Study. Metabolism. 2006;55:699–705. doi: 10.1016/j.metabol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan AD. Manson JE. Rifai N. Buring JE. Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 24.Wu DM. Chu NF. Shen MH. Wang SC. Obesity, plasma high sensitivity C-reactive protein levels and insulin resistance status among school children in Taiwan. Clin Biochem. 2006;39:810–815. doi: 10.1016/j.clinbiochem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T. Kaneshi T. Shimabukuro T. Sunagawa M. Ohta T. Serum C-reactive protein and its relation to cardiovascular risk factors and adipocytokines in Japanese children. J Clin Endocrinol Metab. 2006;91:2133–2137. doi: 10.1210/jc.2005-2856. [DOI] [PubMed] [Google Scholar]
- 26.Brodov Y. Chouraqui P. Goldenberg I. Boyko V. Mandelzweig L. Behar S. Serum uric acid for risk stratification of patients with coronary artery disease. Cardiology. 2009;114:300–305. doi: 10.1159/000239860. [DOI] [PubMed] [Google Scholar]
- 27.Kodama S. Saito K. Yachi Y. Asumi M. Sugawara A. Totsuka K. Saito A. Sone H. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer AA. Kundt G. Steiner M. Schuff-Werner P. Kienast W. Impaired flow-mediated vasodilation, carotid artery intimamedia thickening, and elevated endothelial plasma markers in obese children: The impact of cardiovascular risk factors. Pediatric. 2006;117:1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 29.Ford ES. Ajani UA. Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005;28:878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES. Galuska DA. Gillespie C. Will JC. Giles WH. Dietz WH. C-reactive protein and body mass index in children: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Pediatr. 2001;138:486–492. doi: 10.1067/mpd.2001.112898. [DOI] [PubMed] [Google Scholar]
- 31.Visser M. Bouter LM. McQuillan GM. Wener MH. Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 32.Freeman DJ. Norrie J. Caslake MJ. Gaw A. Ford I. Lowe GD. O'Reilly DS. Packard CJ. Sattar N. West of Scotland Coronary Prevention Study. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 33.Danesh J. Collins R. Appleby P. Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM. Rifai N. Rose L. Buring JE. Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 35.Feig DI. Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feig DI. Rodriguez-Iturbe B. Nakagawa T. Johnson RJ. Nephron number, uric acid, and renal microvascular disease in the pathogenesis of essential hypertension. Hypertension. 2006;48:25–26. doi: 10.1161/01.HYP.0000223447.53155.d5. [DOI] [PubMed] [Google Scholar]
- 37.Siu YP. Leung KT. Tong MK. Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Ford ES. Giles WH. Myers GL. Rifai N. Ridker PM. Mannino DM. C-reactive protein concentration distribution among US children and young adults: Findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 39.Lago F. Gomez R. Gomez-Reino JJ. Dieguez C. Gualillo O. Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci. 2009;34:500–510. doi: 10.1016/j.tibs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Guilherme A. Virbasius JV. Puri V. Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahn BB. Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeBoer MD. Underdiagnosis of the metabolic syndrome in non-hispanic black adolescents: A call for ethnic-specific criteria. Curr Cardiovas Risk Rep. 2010;4:302–310. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahn R. Metabolic syndrome: Is it a syndrome? Does it matter? Circulation. 2007;115:1806–1810. doi: 10.1161/CIRCULATIONAHA.106.658336. discussion 1811. [DOI] [PubMed] [Google Scholar]
- 44.Li C. Ford ES. Is there a single underlying factor for the metabolic syndrome in adolescents? A confirmatory factor analysis. Diabetes Care. 2007;30:1556–1561. doi: 10.2337/dc06-2481. [DOI] [PubMed] [Google Scholar]
- 45.Burns TL. Letuchy EM. Paolos R. Witt J. Childhood predictors of the metabolic syndrome in middle-aged adults: The Muscatine Study. J Pediatr. 2009;155:e17–e126. doi: 10.1016/j.jpeds.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franks PW. Hanson RL. Knowler WC. Moffett C. Enos G. Infante AM. Krakoff J. Looker HC. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56:2964–2972. doi: 10.2337/db06-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang TT. Nansel TR. Belsheim AR. Morrison JA. Sensitivity, specificity, and predictive values of pediatric metabolic syndrome components in relation to adult metabolic syndrome: The Princeton LRC follow-up study. J Pediatr. 2008;152:185–190. doi: 10.1016/j.jpeds.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wannamethee SG. Shaper AG. Lennon L. Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 49.Hanley AJ. Williams K. Gonzalez C. D'Agostino RB., Jr Wagenknecht LE. Stern MP. Haffner SM. San Antonio Heart Study, Mexico City Diabetes Study, Insulin Resistance Atherosclerosis Study. Prediction of type 2 diabetes using simple measures of insulin resistance: Combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 50.Hanson RL. Pratley RE. Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 51.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz B. Jacobs DR., Jr. Moran A. Steinberger J. Hong CP. Sinaiko AR. Measurement of insulin sensitivity in children: Comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–788. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 53.Ebrahimpour P. Fakhrzadeh H. Heshmat R. Bandarian F. Larijani B. Serum uric acid levels and risk of metabolic syndrome in healthy adults. Endocr Pract. 2008;14:298–304. doi: 10.4158/EP.14.3.298. [DOI] [PubMed] [Google Scholar]
- 54.Alper AB., Jr. Chen W. Yau L. Srinivasan SR. Berenson GS. Hamm LL. Childhood uric acid predicts adult blood pressure: The Bogalusa Heart Study. Hypertension. 2005;45:34–38. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 55.Mellen PB. Bleyer AJ. Erlinger TP. Evans GW. Nieto FJ. Wagenknecht LE. Wofford MR. Herrington DM. Serum uric acid predicts incident hypertension in a biethnic cohort: The atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 56.Perlstein TS. Gumieniak O. Williams GH. Sparrow D. Vokonas PS. Gaziano M. Weiss ST. Litonjua AA. Uric acid and the development of hypertension: The normative aging study. Hypertension. 2006;48:1031–1036. doi: 10.1161/01.HYP.0000248752.08807.4c. [DOI] [PubMed] [Google Scholar]
- 57.Sundstrom J. Sullivan L. D'Agostino RB. Levy D. Kannel WB. Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 58.Khosla UM. Zharikov S. Finch JL. Nakagawa T. Roncal C. Mu W. Krotova K. Block ER. Prabhakar S. Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 59.Mazzali M. Hughes J. Kim YG. Jefferson JA. Kang DH. Gordon KL. Lan HY. Kivlighn S. Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 60.Mazzali M. Kanellis J. Han L. Feng L. Xia YY. Chen Q. Kang DH. Gordon KL. Watanabe S. Nakagawa T. Lan HY. Johnson RJ. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 61.Kawada T. Otsuka T. Katsumata M. Suzuki H. Serum uric acid is significantly related to the components of the metabolic syndrome in Japanese workingmen. J Cardiometab Syndr. 2007;2:158–162. doi: 10.1111/j.1559-4564.2007.06596.x. [DOI] [PubMed] [Google Scholar]
- 62.Melbye H. Hvidsten D. Holm A. Nordbo SA. Brox J. The course of C-reactive protein response in untreated upper respiratory tract infection. Br J Gen Pract. 2004;54:653–658. [PMC free article] [PubMed] [Google Scholar]
- 63.Ford ES. Giles WH. Mokdad AH. Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50:574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 64.Jansen H. Wijga AH. Smit HA. Scholtens S. Kerkhof M. Koppelman GH. de Jongste JC. Stolk RP. HbA(1c) levels in nondiabetic Dutch children aged 8–9 years: The PIAMA birth cohort study. Diabet Med. 2009;26:122–127. doi: 10.1111/j.1464-5491.2008.02641.x. [DOI] [PubMed] [Google Scholar]
- 65.Genuth S. Alberti KG. Bennett P. Buse J. Defronzo R. Kahn R. Kitzmiller J. Knowler WC. Lebovitz V. Lernmark A. Nathan D. Palmer J. Rizza R. Saudek C. Shaw J. Steffes M. Stern M. Tuomilehto J. Zimmet P. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 66.de Ferranti S. Mozaffarian D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 67.Walker SE. Gurka MJ. Oliver MN. Johns DW. DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]