Abstract
Regulation of skeletal remodeling appears to influence the differentiation of multipotent mesenchymal stem cells (MSC) resident in the bone marrow. As murine marrow cultures are contaminated with hematopoietic cells, they are problematic for studying direct effects of mechanical input. Here we use a modified technique to isolate marrow-derived MSC (mdMSC) from adult mice, yielding a population able to differentiate into adipogenic and osteogenic phenotypes that is devoid of hematopoietic cells. In pure mdMSC populations, a daily strain regimen inhibited adipogenic differentiation, suppressing expression of PPARγ and adiponectin. Strain increased β-catenin and inhibition of adipogenesis required this effect. Under osteogenic conditions, strain activated β-catenin signaling and increased expression of WISP1 and COX2. mdMSC were also generated from mice lacking caveolin-1, a protein known to sequester β-catenin: caveolin-1(−/−) mdMSC exhibited retarded differentiation along both adipogenic and osteogenic lineages, but retained mechanical responses that involved β-catenin activation. Interestingly, caveolin-1(−/−) mdMSC failed to express bone sialoprotein and did not form mineralized nodules. In summary, mdMSC from adult mice respond to both soluble factors and mechanical input, with mechanical activation of β-catenin influencing phenotype. As such, these cells offer a useful model for studies of direct mechanical regulation of MSC differentiation and function.
Keywords: stem cell, osteoblast, adipocyte, mechanical loading, caveolin-1
INTRODUCTION
Maintenance of the skeleton is a process which must balance diverse inputs that importantly include mechanical information. This information not only influences differentiated cells resident within the bone tissue, but may have critical effects on the fate of multipotent mesenchymal stem cells (MSC) located in the bone marrow. For example, Wnt/β-catenin signaling, associated with promoting bone formation (1), appears to primarily impact early events during MSC lineage determination (2). Similarly, mechanical input activates β-catenin and promotes expression of Wnt/β-catenin target genes in bone cells (3-6), suggesting that loading effects may be most relevant during MSC differentiation. Indeed, recent studies support a mechanical influence on MSC fate in animals: daily exposure to extremely low magnitude mechanical stimulation increases MSC proliferation, with a biasing of the MSC population toward osteogenesis (7), and mice subjected to a climbing regimen have more osteoblasts and fewer adipocytes in the marrow cavity (8). Conversely, hindlimb unloading increases the potential for adipogenesis in ex vivo marrow cultures (9).
In vitro experimentation has shown that mechanical information promotes the osteogenic potential of precursor cells (10). In contrast, daily loading of murine embryonic MSC inhibits adipocyte differentiation (11). Loading of primary marrow cells, which contain MSC, has subtle effects to promote osteogenesis (12) and osteogenic gene expression (13). However, the direct cell target for mechanical input in this mixed population is uncertain. Isolation of murine marrow cells based upon adherence to tissue culture plastic yields a population contaminated with cells of the hematopoietic lineage, in particular an adherent macrophage population (14). A purer marrow-derived MSC model would have great utility for studying mechanisms by which mechanical input influences lineage allocation and osteoprogenitor cell function.
Here we have generated marrow-derived MSC (mdMSC) from adult male C57BL/6 mice isolated by the technique of Peister et al (15) to investigate mechanical effects on skeletal MSC. We show that mdMSC can undergo both adipogenic and osteogenic differentiation and respond to mechanical input. Secondly, we demonstrate that adult mdMSC can provide clues to the effects of genes on differentiation. mdMSC were prepared from mice lacking the membrane protein caveolin-1 (Cav-1). Cav-1 can sequester β-catenin at the membrane (16) and has been implicated in mechanical responses in cardiovascular cells (17, 18). As such, its absence may alter signaling through β-catenin. Additionally, Cav-1(−/−) mice have increased trabecular and cortical bone (19), suggesting an influence of caveolin-1 on MSC differentiation. Cav-1(−/−) mdMSC were able to respond to mechanical input but osteogenic cultures were unable to mineralize. Our results show that both wild-type and Cav-1(−/−) mdMSC respond to mechanical strain with activation of β-catenin signaling, leading to decreased adipogenesis and enhanced osteoblast characteristics. As such, adult murine mdMSC offer a superior and easily achievable model for studies of direct mechanical regulation of MSC differentiation and function.
METHODS
Reagents
Fetal bovine serum (FBS) was from Atlanta Biologicals (Atlanta, GA). Horse serum (HS) was from Hyclone (Logan, UT). Culture medium, L-glutamine, antibiotics, trypsin-EDTA reagent and Taq polymerase were from Invitrogen (Carlsbad, CA). Insulin, indomethacin, L-ascorbic acid 2-phosphate and SB415286 were from Sigma-Aldrich (St. Louis, MO).
mdMSC isolation
mdMSC from 8-10 wk male C57BL/6 mice were prepared after Peister et al (15): tibial and femoral marrow were collected in RPMI-1640, 9% FBS, 9% HS, 100 μg/ml pen/strep and 12μM L-glutamine. After 24 h, nonadherent cells were removed by washing with phosphate-buffered saline and adherent cells cultured for 4 weeks. Passage 1 cells were collected after incubation with 0.25% trypsin/1 mM EDTA × 2 minutes, and replated in a single 175-cm2 flask. After 1-2 weeks, passage 2 cells were replated at 50 cells/cm2 in expansion medium (Iscove modified Dulbecco’s, 9% FBS, 9% HS, antibiotics, L-glutamine). mdMSC were replated every 1-2 weeks for two consecutive passages, then passage 5 mdMSC were frozen. mdMSC were similarly prepared from Cav-1(−/−) mice (20) in a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME).
Culture conditions
Beginning with passage 5, mdMSC were plated at 3000 cells/cm2 in growth medium (α-MEM, 10% FBS, antibiotics) for expansion. For experiments, mdMSC (passage 6 - 15) were plated on collagen-I coated silicone membranes (~10,000 cells/cm2) in growth medium then changed to specific differentiation medium: adipogenic medium consisted of 0.1 μM dexamethasone, 5 μg/ml insulin and 50 μM indomethacin; osteogenic medium was 50 μg/ml ascorbate and 1 μM β-glycerol phosphate, with β-glycerol phosphate increased to 2 mM for analysis of matrix mineralization,.
Mechanical strain
A daily strain regimen (2% magnitude, 0.17Hz, 3600 cycles) was initiated at the beginning of experiments using the Z-strain cell deformation device as previously described (21). For some experiments, mdMSC were cultured in osteogenic medium for 5 days prior to application of strain using the Flexcell FX-4000 system (Flexcell International, Hillsborough, NC).
RNA interference
Cells (~50% confluent) were transfected with siRNA (100nM; Invitrogen) targeting β-catenin or a nonsense siRNA and lipofectamine (25 μl/nmol siRNA) in serum-free OptiMEM overnight before replacing with adipogenic medium and initiating the strain regimen.
Real-time RT-PCR
Total RNA was isolated and reverse transcribed as in (3, 11). Aliquots of cDNA were diluted 5-5000 fold to generate relative standard curves. PPARγ, adiponectin, WISP1, COX2, osterix and 18S primers were as previously described (3, 11). Osteocalcin primers were (5′-3′) ctgacctcacagatgccaa and ggtctgatagctcgtcacaa. Bone sialoprotein primers were (5′-3′) ccggccacgctactttctt and tggactggaaaccgtttcaga. PCR products were normalized to 18S amplicons in the RT sample.
Histochemical staining
Alkaline phosphatase staining was performed with a kit (Sigma-Aldrich). Cultures fixed in 10% formalin were stained with alizarin red S, pH 4.1 (Sigma-Aldrich) to detect calcified matrix. Cultures fixed in 10% formalin were stained with 0.5% oil red O (Poly Scientific, Bay Shore, NY) to detect cytoplasmic triglyceride droplets.
Western blotting
Whole cell lysates and cytoplasmic and nuclear fractionates were prepared as previously described (3, 11) and protein (5-20 μg) separated on a polyacrylamide gel, then transferred to PVDF membrane. Antibodies directed against β-catenin (BD Biosciences), active β-catenin (clone 8E7, Upstate, Temecula, CA), GSK3β (Chemicon, Billerica, MA), phospho-GSK3β (serine-9), phospho-Akt (serine-473), Akt (Cell Signaling, Danvers, MA), PPARγ, adiponectin, caveolin-1, β-tubulin and actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used. The antibody for active β-catenin was specific for the hypo-phosphorylated form of β-catenin (22). Secondary antibody conjugated with horseradish peroxidase was detected by chemiluminescence. Images were acquired with a Hewlett-Packard Scanjet and densitometry determined using NIH ImageJ, 1.37v.
Flow cytometry
Single cell suspensions were prepared, counted, and stained as previously described (23). Directly conjugated primary antibodies included CD45 (eBioscience, San Diego, CA), CD90/Thy-1, CD11b/Mac-1, Sca-1/Ly6A, CD45R/B220, (BD Biosciences, San Jose, CA) and anti-alkaline phosphatase (24). Alternatively, cells were treated with diluted (1:100) primary antibody × 30 min at 4°C (anti-Pref-1, Abcam, Cambridge, MA), washed in staining buffer and treated with FITC conjugated second antibody. Cells were analyzed by flow cytometry using a FACS Caliber (BD Biosciences) and data were analyzed using FloJo software. Analyses were repeated twice.
Statistical analysis
Results are expressed as the mean ± SEM. Significance was evaluated by one–way ANOVA or t-Test (GraphPad Prism). All experiments were replicated at least once. Densitometry data, where given, were compiled from at least three separate experiments.
RESULTS
Adult murine mdMSC differentiate appropriately in response to culture conditions
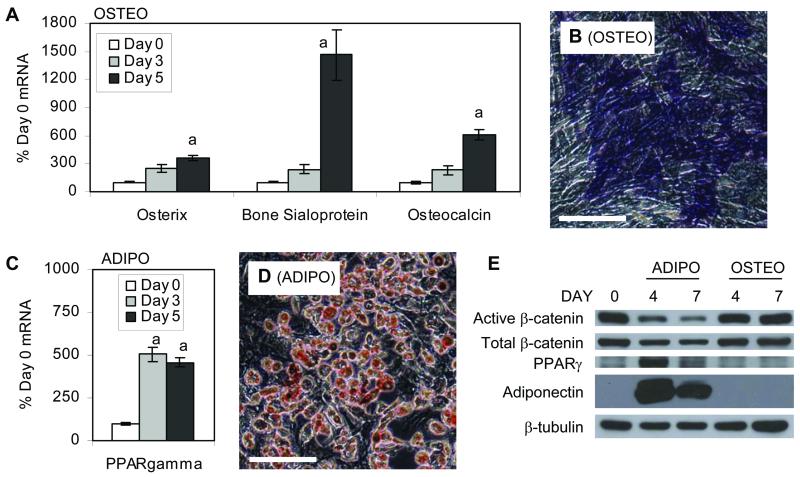
To confirm that mdMSC would undergo controlled differentiation, cells were cultured in either osteogenic or adipogenic medium. mdMSC in osteogenic medium showed significant increases in osterix, osteocalcin and bone sialoprotein expression (Fig. 1A) and positive alkaline phosphatase staining (Fig. 1B). Mineralized nodules stained by alizarin red S were present after three weeks in osteogenic medium (Fig. 5B). mdMSC in adipogenic medium significantly upregulated PPARγ mRNA (Fig. 1C), stained positively for oil red O (Fig. 1D) and expressed PPARγ and adiponectin proteins (Fig. 1E).
Fig. 1. Murine mdMSC differentiate in response to culture conditions.
mdMSC were cultured in osteogenic (OSTEO) or adipogenic (ADIPO) medium. (A) Designated mRNA was amplified by real-time RT-PCR. “a” shows significant difference from day 0, p < 0.01. (B) Alkaline phosphatase staining of day 5 cultures. Scale bar = 200 μm. (C) PPARγ mRNA was amplified by real-time RT-PCR. “a” shows significant difference from day 0, p < 0.01. (D) Oil red O staining of day 5 cultures. Scale bar = 200 μm. (E) Total cellular proteins were immunoblotted for designated proteins, with β-tubulin used as a loading control.
Fig. 5. Osteogenic differentiation is disrupted in mdMSC lacking caveolin-1.
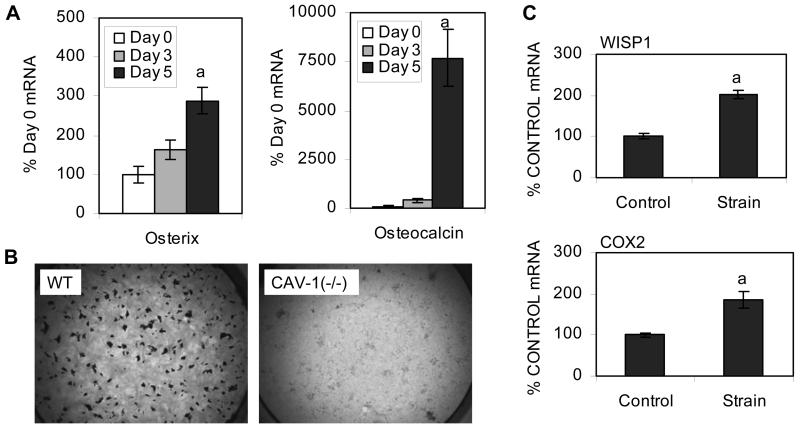
mdMSC were cultured in osteogenic medium. (A) Designated mRNA from Cav-1(−/−) mdMSC was amplified by real-time RT-PCR. “a” shows significant difference from day 0, p < 0.01. (B) Alizarin red S staining of three-week wild-type (WT) and Cav-1(−/−) mdMSC cultures. (C) Strain was applied to day 5 Cav-1(−/−) mdMSC cultures for 6 h. WISP1 and COX2 mRNA were amplified by real-time RT-PCR. “a” shows significant difference from unstrained control, p < 0.01.
The appearance of PPARγ and adiponectin proteins after culture in adipogenic medium was accompanied by a reduction in both active (dephosphorylated) and total β-catenin levels (Fig. 1E) as compared to the levels present in day 0 cultures, an effect that has been reported in other progenitor cells undergoing adipogenesis (11, 25). In contrast, mdMSC cultured in osteogenic medium expressed β-catenin at levels unchanged from day 0 cultures. While β-catenin levels continued to drop during the first week in adipogenic medium, levels of PPARγ and adiponectin peaked at day 4, suggesting that adipocyte lineage commitment occurred early and did not require maintaining peak expression of PPARγ.
Because the cells were isolated from bone marrow, it was important to demonstrate that cultured mdMSC from wild-type C57BL/6 and Cav-1(−/−) mice represented similar and uncontaminated cell populations. Neither wild-type nor Cav-1(−/−) mdMSC expressed CD45, CD90/Thy-1, or CD11b/Mac-1 assessed by FACs, indicating an absence of hematopoetic stem cells. Wild-type mdMSC were 74% Sca-1/Ly6A+, 2% ALP+, and Pref-1−, indicating that the vast majority of cells retained an undifferentiated phenotype. In comparison, Cav-1(−/−) mdMSC were 94% Sca-1/Ly6A+, 7% ALP+, and expressed low levels of Pref-1+, suggesting that although the caveolin-1 negative population differed slightly from wild-type, it similarly represented an uncontaminated early mesenchymal stem cell.
Mechanical strain inhibits mdMSC adipogenesis
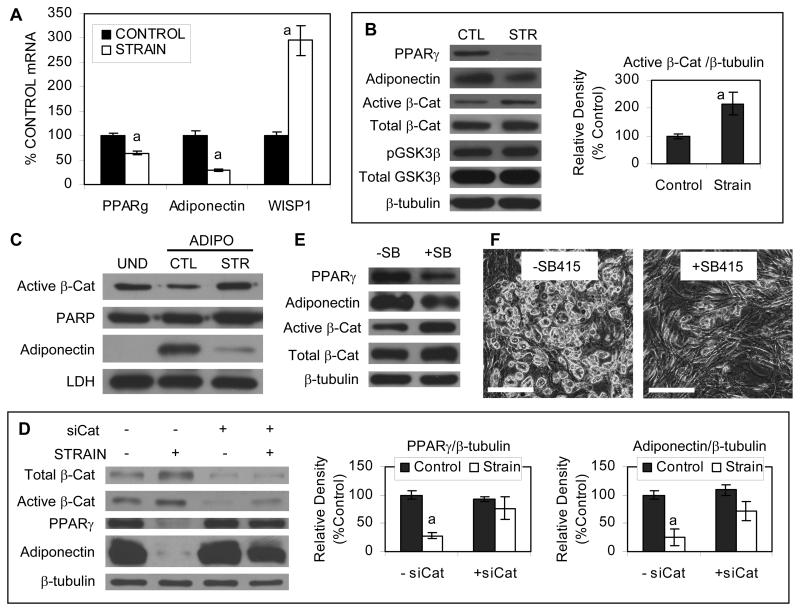
Mechanical strain directly inhibits adipogenesis of the multipotent C3H10T1/2 cell line (11). To evaluate whether this mechanical response pertained to mdMSC, cells were grown in adipogenic medium and exposed to a daily strain regimen (3600 cycles/day). Mechanical strain significantly reduced mRNA for PPARγ to 57 ± 6% and adiponectin to 32 ± 2% of the control response after 3 days (Fig. 2A). Strain-induced reductions in PPARγ and adiponection mRNA were confirmed at the protein level (Fig. 2B).
Fig. 2. Mechanical strain inhibits adipogenesis.
(A) mdMSC in adipogenic medium were exposed to daily strain regimen × 3 d, and designated mRNA was amplified by real-time RT-PCR. “a” shows significant difference from unstrained control, p < 0.01. (B) Total cellular proteins were analyzed by immunoblotting after treatment as in (A) with daily strain × 5 d. CTL = control culture; STR = strained culture. Densitometric analysis of active β-catenin was performed (n = 4 experiments). “a” shows significant difference from unstrained control, p < 0.05. (C) Proteins from nuclear and cytoplasmic fractionates were immunoblotted for active β-catenin and adiponectin, respectively, along with loading controls, after treatment as in (A) with daily strain × 4 d and an undifferentiated (UND) mdMSC control sample was included for comparison. (D) Immunoblots of total cellular proteins from mdMSC treated with nonsense siRNA (− siCat) or siRNA targeting β-catenin (+ siCat), then cultured in adipogenic medium ± daily strain. Densitometric analyses of PPARγ and adiponectin were performed (n = 3 experiments). “a” shows significant difference from unstrained control, p < 0.05. (E) The GSK3β inhibitor SB415286 (SB415, 20 μM) was added to mdMSC in adipogenic medium and proteins were analyzed by immunoblotting on day 4. (F) Oil red O staining after treatment as in (D) for 5 d. Scale bar = 200 μm.
Active and total β-catenin were measured at higher levels in strai control cultures, where β-catenin dropped during adipogenesis (Fig. 2B). Densitometry of active β-catenin bands confirmed that the level was significantly increased in strained cultures as compared to the unstrained adipogenic cultures. Maintenance of β-catenin was accompanied by mechanical inactivation of GSK3β, indicated by increased serine-9 phosphorylation in cultures exposed to strain. Consistent with mechanical activation of β-catenin, strain induced a three-fold increase in the Wnt/β-catenin target gene WISP1 (Fig. 2A).
To measure the change of active β-catenin in the nucleus in response to strain, the nuclear fraction was separated from the cytoplasmic fraction. Consistent with total protein levels, culture of mdMSC in adipogenic medium caused a drop in active β-catenin in the nucleus as compared to the level in undifferentiated mdMSC (Fig. 2C). Application of daily strain prevented the decrease in nuclear active β-catenin, while blocking accumulation of adiponectin in the cytoplasm.
To investigate whether mechanical preservation of β-catenin was critical to preventing adipogenesis in mdMSC, β-catenin was knocked down using siRNA. Densitometry showed that total β-catenin protein was reduced by 73% using siRNA. Silencing β-catenin disrupted the ability of the daily strain regimen to impair adipogenesis, demonstrated as a lack of significant effect by strain to reduce PPARγ and adiponectin protein expression (Fig. 2D). To further support a role for β-catenin in preventing adipogenic differentiation of mdMSC, the GSK3β inhibitor SB415286 (20 μM) was added to adipogenic medium to block β-catenin degradation. In the presence of SB415286, expression of PPARγ and adiponectin was decreased (Fig. 2E) and lipid accumulation was reduced (Fig. 2F). These data show that mechanical regulation of β-catenin is critical to its effect on phenotype in mdMSC.
Strain activates β-catenin signaling in mdMSC grown under osteogenic conditions
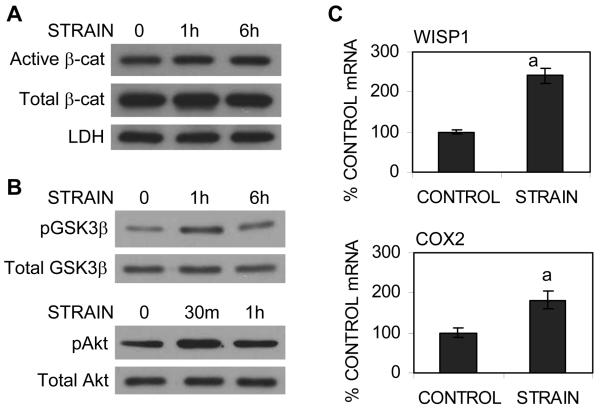
Having confirmed that mechanical strain inhibits adipogenesis of mdMSC, we next wished to ascertain whether mdMSC which had undergone osteogenic transformation would respond to strain with activation of β-catenin, as had been previously demonstrated in osteoblasts (3, 4). mdMSC were cultured in osteogenic medium for 5 days to induce differentiation (see figure 1). As shown in Fig. 3A, cytoplasmic active β-catenin was elevated for up to 6 hours after initiating strain, while total β-catenin was unchanged. The increase in active β-catenin suggested an alteration in GSK3β activity: phosphorylation of GSK3β was increased after 1 hour of strain, returning to basal levels by 6 hours despite continuous strain (Fig. 3B). Activation of Akt, which phosphorylates and inactivates GSK3β, was also studied. Akt activation, measured by phosphorylation at serine-473, peaked at 30 minutes after strain initiation. In response to increased β-catenin activity, WISP1 expression increased with strain (240 ± 20% of control; Fig. 3C). COX2, known to be influenced by mechanical loading in osteoblasts (5) and a downstream target of β-catenin in skeletal lineage cells (26, 27), was also significantly upregulated by strain (181 ± 22% of control) in mdMSC.
Fig. 3. Strain activates β-catenin signaling in osteogenic cultures.
mdMSC were cultured in osteogenic medium for 5 d and strain applied for 30 min to 6 h. (A) The cytoplasmic protein fractionate was analyzed for β-catenin and LDH (loading control). (B) Total cellular proteins were immunoblotted for phosphorylated GSK3β and Akt. (C) WISP1 and COX2 mRNA were amplified by real-time RT-PCR in cultures strained for 6 h. “a” shows significant difference from unstrained control, p < 0.01.
Absence of caveolin-1 does not block strain effects in mdMSC
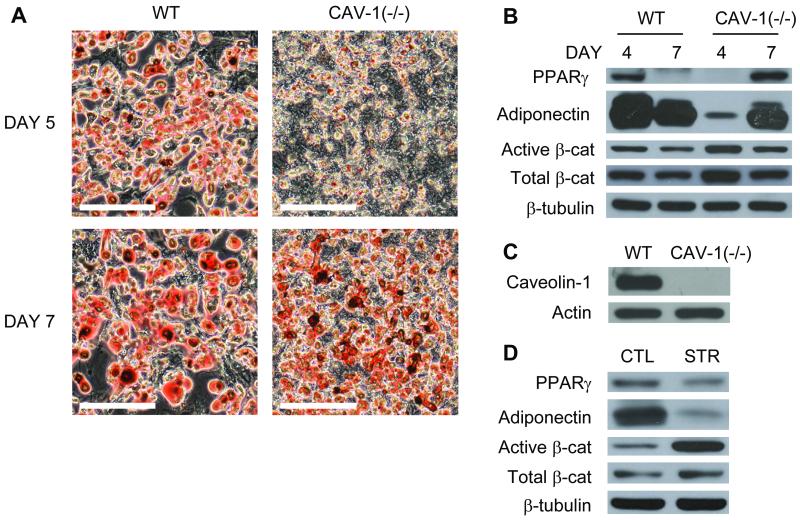
Given that caveolin-1 can alter β-catenin signaling via sequestration (16) and that it has been implicated in mechanical responses in vascular cells (17, 18), mdMSC isolated from Cav-1(−/−) mice were evaluated. Cav-1(−/−) mdMSC in adipogenic medium accumulated lipid (Fig. 4A) and expressed both PPARγ and adiponectin (Fig. 4B). As expected, β-catenin levels fell during adipogenesis. Cav-1(−/−) mdMSC proliferated more rapidly than wild-type mdMSC, with 45% more cells after 2 days in adipogenic medium. Interestingly, despite the slight increase in Pref-1+ cells measured by FACs in Cav-1(−/−) mdMSC, adipogenesis progressed at a slower rate in mdMSC lacking caveolin-1 (Fig. 4C). Expression of PPARγ and adiponectin in Cav-1(−/−) mdMSC required 7 days of culture to reach levels attained by wild-type mdMSC at 4 days (Fig. 4B). This retardation was consistent with the reduced lipid seen at 5 and 7 days in the Cav-1(−/−) cultures, as shown in photomicrographs (Fig. 4A).
Fig. 4. Absence of caveolin-1 does not block strain effects on adipogenesis.
mdMSC were cultured in adipogenic medium. (A) Oil red O staining of wild-type (WT) and Cav-1(−/−) mdMSC. Scale bar = 200 μm. (B) Total cellular proteins were immunoblotted for designated proteins. (C) Total cellular proteins were immunoblotted for caveolin-1 and actin (loading control). (D) Daily strain regimen × 7 d was applied to Cav-1(−/−) mdMSC, and designated proteins were analyzed by immunoblotting. CTL = control culture; STR = strained culture.
Mechanical inhibition of adipogenesis in Cav-1(−/−) mdMSC did not differ significantly from the effect in wild-type mdMSC. A daily strain regimen slowed adipogenic differentiation, resulting in a 48 ± 7% reduction in PPARγ and an 85 ± 4% reduction in adiponectin mRNAs compared to unstrained cultures. Strained cultures also had higher levels of β-catenin and reductions in PPARγ and adiponectin protein (Fig. 4D). Mechanical activation of β-catenin was accompanied by a two-fold increase in WISP1 expression (180 ± 13% of unstrained cultures).
Cav-1(−/−) mdMSC were multipotent, as osterix and osteocalcin expression increased with culture in osteogenic medium (Fig. 5A). However, expression of bone sialoprotein was undetectable in Cav-1(−/−) mdMSC at all cultures times evaluated. Cav-1(−/−) mdMSC cultures also did not generate mineralized nodules after three weeks in osteogenic medium, in contrast to the formation of numerous mineralized nodules in the wild-type mdMSC cultures (Fig. 5B). Although osteogenic differentiation appeared to be dysfunctional in the Cav-1(−/−) mdMSC, these cells were able to respond to mechanical input. Expression of WISP1 and COX2 were both increased with strain (202 ± 10% of control for WISP1 and 187 ± 20% of control for COX2; Fig. 5C), similar to the response measured in wild-type mdMSC (see figure 3).
DISCUSSION
Mechanical information is one of many inputs that regulate skeletal remodeling. This information may target both differentiated cells and multipotent MSC resident in the skeleton. Because murine marrow cultures are contaminated with hematopoietic cells, these cultures are problematic for studies of direct mechanical effects. In this work, a modified technique (15) was used to isolate marrow-derived MSC from adult mice, yielding a population able to differentiate into adipogenic and osteogenic phenotypes and devoid of hematopoietic cells. A unique aspect of the work presented here is the demonstration of mechanical responses in two distinct cell phenotypes generated from the same genetic population of mdMSC. While our work focused on mechanical effects, it promises that adult mdMSC will be useful for detailed studies of lineage commitment and early differentiation events that consider a broad range of factors pertinent to the bone marrow compartment.
Within the marrow, MSC differentiation along osteogenic and adipogenic pathways appears to be reciprocally coordinated (28) and subject to regulation by mechanical input. Signaling through β-catenin is now established as a primary pathway controlling differentiation between these two lineages (2, 11, 29), and as such, may be an important pathway whereby mechanical loading influences MSC fate. Animal experiments have shown that both exercise (12) and constitutive activation of β-catenin (30) increase the proportion of marrow precursors assigned to the osteogenic lineage. These pro-osteogenic effects may be due in part to a blocking of MSC entering the adipogenic pathway, thus preserving a pool of cells available for alternate lineages.
In this work, mechanical strain directly prevented adipogenic differentiation of mdMSC, as evidenced by suppression of PPARγ and adiponectin. This effect was dependent on activation of β-catenin, consistent with the mechanism of the strain response in C3H10T1/2 embryonic mesenchymal cells (11). Increased levels of active and total β-catenin resulted from mechanical inactivation of GSK3β, as recently reported (21). We also demonstrated that mdMSC differentiated along the osteoblast lineage responded to mechanical strain with activation of β-catenin signaling, followed by induction of WISP1 and COX2, genes associated with osteogenesis (31, 32). As such, the adult mdMSC model has allowed confirmation that β-catenin signaling can regulate phenotype in skeletal precursors responding to mechanical input.
A primary benefit in using a marrow-derived MSC population is the ability to isolate cells from adult transgenic mice. mdMSC lacking caveolin-1 indeed responded differently to differentiation protocols, exhibiting retardation of adipogenesis and an incomplete response to osteogenic culture conditions. Adipogenic differentiation was unimpaired in Cav-1(−/−) embryonic fibroblasts (33), suggesting that mdMSC represent a different phenotype. In contrast, the uncontrolled endothelial and fibroblast proliferation in Cav-1(−/−) mice (20, 34) was mirrored in the increased proliferation rate of Cav-1(−/−) mdMSC compared to wild-type. Cav-1(−/−) mdMSC may experience a delayed braking in the switch from proliferation to differentiation.
Cav-1(−/−) mdMSC exhibited dysfunctional osteogenic differentiation, being unable to form mineralized nodules. These cells also did not express bone sialoprotein, a protein abundant in bone and associated with matrix mineralization (35). Importantly, a near complete disruption in mineralized nodule formation occurs in bone marrow stromal cultures from mice lacking bone sialoprotein (36). The deficit in bone sialoprotein in Cav-1(−/−) mdMSC suggests an interaction between caveolin-1 and bone sialoprotein expression which can be compensated by other factors in vivo, allowing ossification to occur in Cav-1(−/−) transgenics.
In summary, murine marrow-derived MSC reliably undergo adipogenesis and osteogenesis, are not contaminated by hematopoietic cells and, as such, offer an efficient and reproducible experimental model for the study of lineage regulation in the marrow compartment. mdMSC can be isolated from adult transgenic mice, allowing study of direct molecular interactions in this population. Our work further demonstrates that mechanical input activates β-catenin signaling during regulation of mdMSC phenotype, directly inhibiting adipogenic differentiation and enhancing expression of osteogenic genes.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH to JR: AR42360 and AR52014.
REFERENCES
- 1.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 3.Case N, Ma M, Sen B, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong VJ, Muzylak M, Sunters A, et al. Wnt/beta -catenin signaling is a component of osteoblastic bone cells’ early responses to load-bearing, and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 5.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. WNT/beta -catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 6.Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menuki K, Mori T, Sakai A, et al. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone. 2008;43:613–620. doi: 10.1016/j.bone.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Pan Z, Yang J, Guo C, et al. Effects of hindlimb unloading on ex vivo growth and osteogenic/adipogenic potentials of bone marrow-derived mesenchymal stem cells in rats. Stem Cells Dev. 2008;17:795–804. doi: 10.1089/scd.2007.0254. [DOI] [PubMed] [Google Scholar]
- 10.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen B, Xie Z, Case N, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David V, Martin A, Lafage-Proust MH, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 13.Rubin J, Murphy TC, Rahnert J, et al. Mechanical Inhibition of RANKL Expression Is Regulated by H-Ras-GTPase. J Biol Chem. 2006;281:1412–1418. doi: 10.1074/jbc.M508639200. [DOI] [PubMed] [Google Scholar]
- 14.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 15.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 16.Galbiati F, Volonte D, Brown AM, et al. Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem. 2000;275:23368–23377. doi: 10.1074/jbc.M002020200. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Go YM, Darji R, et al. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol. 2000;278:H1285–1293. doi: 10.1152/ajpheart.2000.278.4.H1285. [DOI] [PubMed] [Google Scholar]
- 18.Sedding DG, Hermsen J, Seay U, et al. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 19.Rubin J, Schwartz Z, Boyan BD, et al. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res. 2007;22:1408–1418. doi: 10.1359/jbmr.070601. [DOI] [PubMed] [Google Scholar]
- 20.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 21.Sen B, Styner M, Xie Z, et al. Mechanical loading regulates NFATC1 and {beta}-catenin signaling through a GSK3{beta} control node. J Biol Chem. 2009 doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Noort M, Meeldijk J, van der Zee R, et al. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz MC, Fields A, DeMeo D, et al. Expression and regulation of Ly-6 differentiation antigens by murine osteoblasts. Endocrinology. 1994;135:1032–1043. doi: 10.1210/endo.135.3.7520861. [DOI] [PubMed] [Google Scholar]
- 24.Marquez C, De la Hera A, Leonardo E, et al. Identity of PB76 differentiation antigen and lymphocyte alkaline phosphatase. Eur J Immunol. 1990;20:947–950. doi: 10.1002/eji.1830200436. [DOI] [PubMed] [Google Scholar]
- 25.Moldes M, Zuo Y, Morrison RF, et al. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid Shear Stress Induces beta-Catenin Signaling in Osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Im DS, Kim SH, et al. Beta-catenin regulates expression of cyclooxygenase-2 in articular chondrocytes. Biochem Biophys Res Commun. 2002;296:221–226. doi: 10.1016/s0006-291x(02)00824-0. [DOI] [PubMed] [Google Scholar]
- 28.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang S, Bennett CN, Gerin I, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 30.Hu H, Hilton MJ, Tu X, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Schwarz EM, Young DA, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French DM, Kaul RJ, D’Souza AL, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165:855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen AW, Razani B, Schubert W, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 34.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 35.Hunter GK, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: role of glutamic acid-rich sequences in the nucleation of hydroxyapatite by bone sialoprotein. Biochem J. 1994;302(Pt 1):175–179. doi: 10.1042/bj3020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malaval L, Wade-Gueye NM, Boudiffa M, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 2008;205:1145–1153. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]