Abstract
Recent work identified novel progestin signaling molecules, including progesterone receptor membrane component 1 (Pgrmc1), Pgrmc2, serpine mRNA binding protein 1 (Serbp1), progestin and adiponectin receptors 7 (Paqr7) and Paqr8. These molecules mediate rapid progesterone (P4) effects in non-neural tissue and we recently mapped their expression in the brain. Many rapid effects of P4 require 17β-estradiol (E2) and P4 priming; therefore, we examined the effects of ovarian hormones on the expression of these non-classical progestin signaling molecules. We focused specifically on the anteroventral periventricular nucleus (AVPV), the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the ventrolateral portion of the ventromedial nucleus (VMNvl). These brain nuclei are important for female reproduction. Ovariectomized adult female rats were implanted with capsules containing sesame oil or E2, and injected 48 hours later with sesame oil or P4. Brains were collected eight hours later and RNA was isolated from the AVPV, SDN-POA and VMNvl. We assessed the effects of ovarian hormones on mRNA levels using quantitative polymerase chain reaction (QPCR). In the AVPV, Serbp1 mRNA levels were increased by P4 in the presence of E2, and Paqr8 was downregulated by P4 alone. In the SDN-POA, combined E2 and P4 increased Pgrmc1 and Serbp1 mRNA levels, and E2 alone increased Paqr8 mRNA levels. Finally, in the VMNvl, P4 increased mRNA levels encoding Pgrmc1, Pgrmc2 and Serbp1, and the combination of E2 and P4 increased Pgrmc1 and Serbp1 mRNA levels. Paqr7 was not regulated by E2 or P4 in any brain region examined. In summary, we showed that ovarian hormones regulate novel progestin signaling molecules in brain regions important for the neuroendocrine control of reproduction.
Keywords: Estradiol, progesterone, neuroendocrine, non-classical signaling, receptor
INTRODUCTION
Progesterone (P4) signaling in the female brain regulates several facets of reproduction including the neural control of ovulation and the expression of feminine sex behaviors. The molecular mechanisms underlying these P4 actions have been primarily attributed to activation of the progestin receptor (Pgr), a ligand-dependent transcription factor. This classical model of steroid hormone action has been revised to include rapid non-genomic effects of Pgr activation on diverse signaling systems, such as MAPK and c-Src pathways (Richer et al., 1998, Boonyaratanakornkit et al., 2001). However, this model may still be incomplete because many cells that lack Pgr retain rapid P4-elicited responses (Ehring et al., 1998, Bar et al., 2000, Frye et al., 2006). These findings may be explained by recent discoveries of novel progestin signaling molecules that mediate diverse responses to P4 in non-neural tissues (Falkenstein et al., 1999, Zhu et al., 2003b, Peluso et al., 2004).
We recently mapped the expression of several of these progestin signaling molecules in the rat forebrain (Intlekofer and Petersen, in press). Genes encoding progesterone receptor membrane component 1 (Pgrmc1), Pgrmc2 and serpine mRNA binding protein 1 (Serbp1) were particularly abundant in neuroendocrine nuclei important for female reproduction. We confirmed and extended findings on the distribution pattern of Pgrmc1 (Krebs et al., 2000, Sakamoto et al., 2004, Meffre et al., 2005), and showed that the pattern overlaps closely with that of its binding partner, Serbp1, and Pgrmc1 homologue, Pgrmc2. Few studies have examined the role of these molecules in neural function, but Pgrmc1 and Serbp1 have been implicated in the rapid effects of P4 observed in ovarian cells and sperm (Correia et al., 2007, Peluso et al., 2009). While Pgrmc2 has not been studied in the context of rapid P4 signaling, it may mediate P4 actions in the ovary (Nilsson et al., 2006). Together these findings suggest that Pgrmc1, Pgrmc2 and Serbp1 may mediate non-classical P4 signaling in the brain as in other tissues.
Other P4 signaling molecules include progestin and adipoQ receptor 7 (Paqr7) and Paqr8, G-protein-like receptors that bind P4 and regulate cAMP levels in several fish species (Zhu et al., 2003a, Zhu et al., 2003b, Hanna et al., 2006). Although controversy surrounds their role in mammalian cells (Fernandes et al., 2008), mRNAs encoding Paqr7 and Paqr8 have been detected in mammalian reproductive tissues (Zhu et al., 2003a). Our recent work showed that Paqr7 and Paqr8 gene expression is present in the hypothalamus (Intlekofer and Petersen, in press), though expression appears lower compared with that of Pgrmc1, Pgrmc2 and Serbp1. Other evidence suggests that Paqr7 and Paqr8 mediate P4 signaling and couple to inhibitory G-proteins in immortalized gonadotropin-releasing hormone (GnRH) neurons (Sleiter et al., 2009). Despite these significant advances, neither the regulation nor the functions of Paqr7 and Paqr8 in the brain are known.
In the female rodent, many of the rapid P4 signaling events require 17β-estradiol (E2) activation of estrogen receptor 1 (Esr1) (Edwards, 2005). This is partially due to E2 induction of Pgr (Kastner et al., 1990), a nuclear transcription factor that also activates rapid intracellular kinase cascades (Leonhardt et al., 2003). In regions of high Esr1 expression, such E2-induced effects result in greater P4-sensitivity. For example, in the preoptic area (POA) and ventromedial nucleus (VMN) of the hypothalamus, E2 exposure lowers cell signaling activation thresholds for P4, resulting in greater P4-sensitivity (Balasubramanian et al., 2008). In view of our recent findings that Pgrmc1, Pgrmc2, Serbp1, Paqr7 and Paqr8 are found in regions that contain Esr1 and Pgr, it is possible that E2 and/or P4 regulation of these molecules may be important in non-classical P4 signaling. This idea is supported by findings that Pgrmc1 expression is regulated by P4 in E2-primed rats (Krebs et al., 2000), uterine levels of Pgrmc2 mRNA vary across the estrus cycle (Zhang et al., 2008) and Paqr7 and Paqr8 ovarian expression is regulated by E2 (Karteris et al., 2006). It is unclear whether Pgrmc1 is regulated by steroids in brain regions other than the VMN, and no studies have tested the effects of ovarian steroids on Pgrmc2, Serbp1, Paqr7 and Paqr8 in the brain.
To address these issues, we examined the effects of E2, P4 and the combination of E2 and P4 (E2+P4) on levels of mRNA encoding Pgrmc1, Pgrmc2, Serbp1, Paqr7 and Paqr8. We focused specifically on the anteroventral periventricular nucleus (AVPV), the sexually dimorphic nucleus of the POA (SDN-POA) and the ventrolateral portion of the VMN (VMNvl). These nuclei have abundant expression of Esr1 and Pgr, are sexually dimorphic and are important for female reproduction (Dugger et al., 2007, Sakuma, 2009). In addition, we recently found expression of Pgrmc1, Pgrmc2, Serbp1, Paqr7 and Paqr8 in these nuclei (Intlekofer and Petersen, in press). We now report that ovarian steroid hormones regulate these putative progestin signaling molecules, and do so in a region-specific manner.
EXPERIMENTAL PROCEDURES
Animals
All protocols and post-operative care were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts, and animals were housed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Twenty-eight adult female Sprague-Dawley rats (200–250 g, Harlan, Madison, WI) were housed individually on a 14:10 light:dark cycle with food and water provided ad libitum. Animals were anesthetized with isofluorane and bilateral ovariectomies were performed through very small (5-mm) flank incisions that minimized tissue trauma. They were observed for respiratory distress and bleeding for 6 h postoperatively and examined again at 12, 24 and 48 h to ensure that they were freely moving and had no significant weight loss. One week later (Day 0), animals were implanted s.c. with Silastic capsules (Dow Corning, Midland, MI; 1.57 mm, o.d., 3.8 mm; 30 mm length) containing either sesame oil vehicle or E2 (150 μg/ml 17β-estradiol in sesame oil) as described previously (Petersen and LaFlamme, 1997). At 0900 H on Day 2, animals were injected s.c. with either sesame oil vehicle or 50 mg P4. Eight hours later, animals were anesthetized with CO2 and brains were rapidly frozen on powdered dry ice, wrapped in Parafilm (American Can Co., Greenwich, CT) and stored at −80 °C.
Tissue preparation
Coronal cryosections that contained the AVPV, SDN-POA and VMNvl were acquired using a Leica CM3000 cryostat (Nussloch, Germany). These sections were taken from the rostral AVPV (bregma -0.02 mm), SDN-POA (bregma -0.4 mm) and VMNvl (bregma -0.20 mm) (Swanson, 1998). We obtained tissue punches from these sections using a 1.0-mm diameter Harris Uni-Core tissue needle (Ted Pella Inc., Redding, CA) from a single 300-μm section as illustrated in Fig. 1, and used this tissue for quantitative polymerase chain reaction (QPCR).
Figure 1.
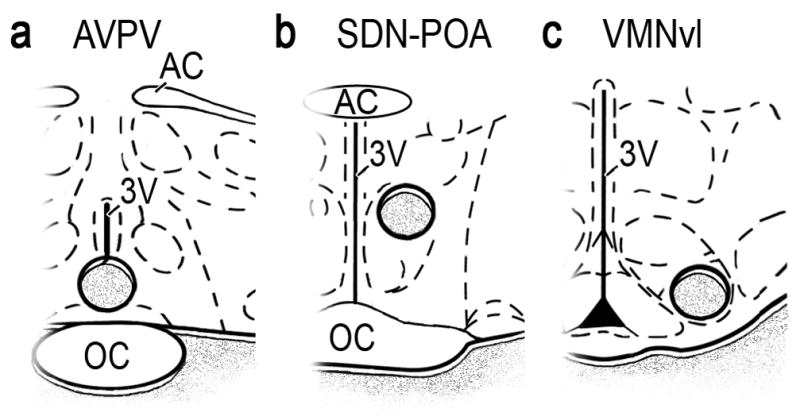
Diagrams of brain sections containing the a) AVPV, b) SDN-POA and c) VMNvl modified from the atlas of Swanson (1998). Circles indicate regions from which tissue was excised for analysis. OC, optic chiasm; 3V, third ventricle; AC, anterior commissure.
RNA preparation and QPCR
RNA was isolated from tissue punches using the RNeasy kit (Qiagen, Valencia, CA) and reverse transcribed with the QuantiTect Reverse Transcription Kit (Qiagen), using manufacturer’s protocol. QPCR was performed in a Stratagene Mx3000P thermocycler (Agilent Technologies, Wilmington, DE) programmed as follows: 95 °C, 10 min; 40 cycles of 95 °C for 15 sec; and 60 °C for 60 sec. Reactions contained reagents from QuantiTect SYBR Green Kit, following manufacturer’s protocol (Roche Diagnostics, Indianapolis, IN). Specific primer sets were obtained from Integrated DNA Technologies (Coralville, Iowa), and sequences are listed in Table 1. The efficiency of each primer set was validated over a range of cDNA concentrations and samples with no cDNA were included as negative controls. Primer specificity was verified using melting curve analyses and confirmation of a single fluorescence peak in each QPCR reaction. Melting curve analyses were performed by heating samples to 95 °C for two min, 55 °C for 15 sec, and recording fluorescence measurements during incremental increases of 0.5 °C for 80 cycles. Primer specificity was also validated using 2% agarose gel electrophoresis to verify single products following the QPCR reaction. Fluorescence measurements were detected using MxPro QPCR analysis software (Agilent Technologies). We verified that levels of mRNA encoding β-actin did not differ among treatments; therefore, we used it as an internal control (primers as indicated in Table 1). The ΔΔCt method was used to analyze the data (Livak and Schmittgen, 2001).
Table 1.
Primers Used in QPCR Studies
| NCBI Gene Name and Refseq ID# | Primer sequences 5′- 3′ | Antisense to bases | Amplicon (bp) |
|---|---|---|---|
| Actb NM_031144 |
GGGAAATCGTGCGTGACATT | 698–717 | 76 |
| GCGGCAGTGGCCATCTC | 773–757 | ||
| Esr1 NM_012689 |
AGTGAAGCCTCAATGATGGG | 1236–1255 | 146 |
| ATCTCCAACCAGGCACACTC | 1381–1362 | ||
| Pgr NM_022847 |
GGTGGAGGTCGTACAAGCAT | 2261–2280 | 214 |
| AGGCCTTCCAAAGGAATTGT | 2474–2455 | ||
| Pgrmc1 NM_021766 |
CTGCCGAACTAAGGCGATAC | 321–340 | 247 |
| TCCCAGTCATTCAGGGTCTC | 567–548 | ||
| Pgrmc2 NM_001008374 |
AGCAGCTGCGCCAGTACGAC | 306–325 | 139 |
| GAGGCGTCCCTGCCAGCAAA | 444–425 | ||
| Serbp1 NM_145086 |
GAAACACCCGAAGGTGAAGA | 789–808 | 190 |
| TTTTCCATTGTCCATCAGCA | 978–959 | ||
| Paqr7 NM_001034081 |
GTGCACCGCATCATAGTGTC | 661–680 | 230 |
| TGATAGTCCAGCGTCACAGC | 890–871 | ||
| Paqr8 NM_001014099 |
CTGCAGCCTCTTGGCCCACC | 825–844 | 179 |
| CAGCCGCCGGCAGGAAGAAA | 1003–984 |
Statistics
All data are expressed as mean ± SEM. Effects of E2, P4 and E2+P4 on mRNA levels were detected using a one-way ANOVA, followed by pair-wise comparisons using t-tests with Bonferroni correction.
RESULTS
QPCR reaction specificity
First, melting curve analyses verified a single peak of fluorescence, and the size of each product was confirmed by gel electrophoresis. In addition, the PCR amplification efficiency calculated from the standard curve was between 96–100% for all primer sets used. Consistent with our previous in situ hybridization findings (Intlekofer and Petersen, in press), QPCR verified that the genes of interest were expressed in the AVPV, SDN-POA and VMNvl.
Esr1 and Pgr mRNA levels
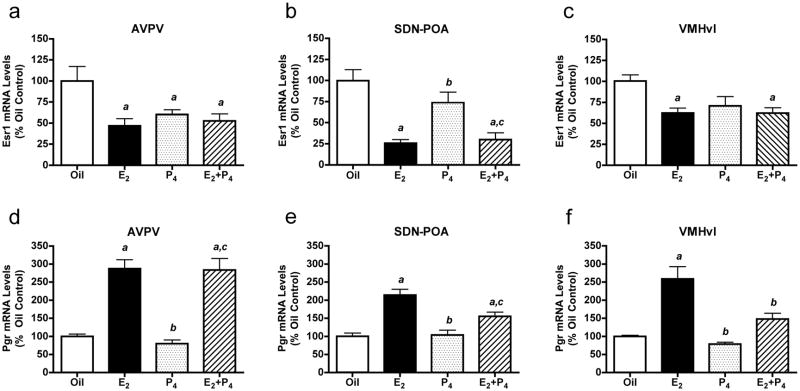
In ovariectomized adult rats, exposure to E2 reduced Esr1 mRNA levels in the AVPV, SDN-POA and VMNvl (Fig. 2a, b and c, respectively). P4 administration significantly decreased Esr1 mRNA levels in the AVPV (Fig. 2a), but not in the SDN-POA (Fig. 2b) or VMNvl (Fig. 2c). In all three areas examined, E2+P4 reduced levels of mRNAs encoding Esr1 (Fig. 2a, b and c). E2 markedly increased levels of mRNA encoding Pgr in all brain regions examined (Fig. 2d, e and f), and in the AVPV and SDN-POA, E2+P4 increased Pgr mRNA levels.
Figure 2.
Levels of mRNAs encoding Esr1 and Pgr in ovariectomized rats treated with oil, E2, P4 or E2+P4. Esr1 mRNA levels in the AVPV (a), SDN-POA (b) and VMNvl (c), and Pgr mRNA levels in the AVPV (d), SDN-POA (e) and VMNvl (f) were determined by QPCR. Bars = means ± SEM. a Significantly different from oil-treated controls; b significantly different from E2-treated animals; c significantly different from P4-treated animals; values considered significantly different if p < 0.05 in post-hoc analyses. One-way ANOVA results: a) F(3,22)=4.72 1, p < 0.010; b) F(3,20)=11.81, p < 0.0002; c) F(3,20)=8.14, p < 0.001; d) F(3,20)=20.83, p < 0.0001; e) F(3,20)=17.14, p < 0.0001; f) F(3,20)=41.49, p < 0.0001.
Pgrmc1, Serbp1 and Pgrmc2 mRNA levels
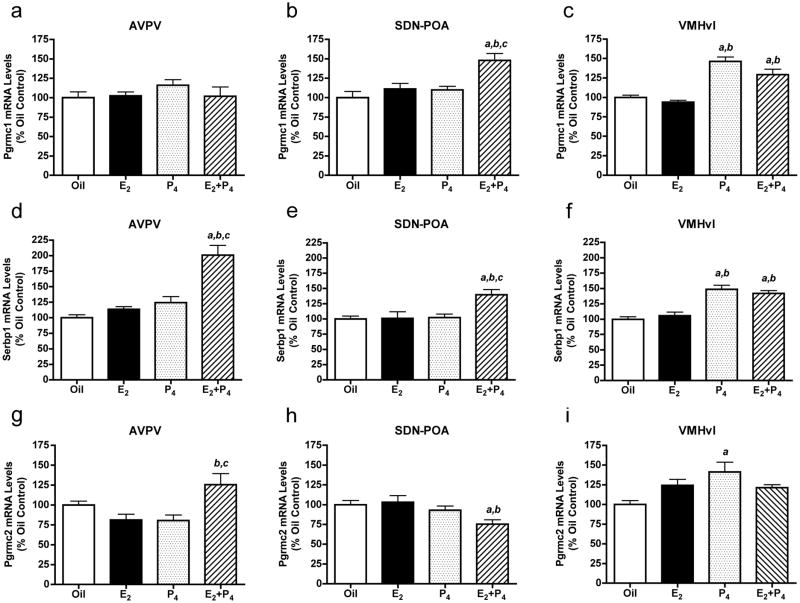
In the AVPV, mRNA levels of Pgrmc1 were unaltered by treatment with E2, P4 or E2+P4 (Fig. 3a). Serbp1 and Pgrmc2 mRNA levels were both increased by E2+P4 in the AVPV (Fig. 3d and g). Within the SDN-POA, E2+P4 increased Pgrmc1 and Serbp1 levels (Fig. 3b and e) and decreased Pgrmc2 mRNA levels (Fig. 3h). In the VMNvl, P4 increased Pgrmc1, Pgrmc2 and Serbp1 mRNA levels (Fig. 3c, f and i). Levels of mRNA encoding Pgrmc1 and its binding partner, Serbp1, were also increased by E2+P4 in the VMNvl (Fig. 3c and f).
Figure 3.
Levels of mRNAs encoding Pgrmc1, Pgrmc2 and Serbp1 in ovariectomized rats treated with oil, E2, P4 or E2+P4. Pgrmc1 mRNA levels in the AVPV (a), SDN-POA (b) and VMNvl (c), Pgrmc2 mRNA levels in the AVPV (d), SDN-POA (e) and VMNvl (f), and Serbp1 mRNA levels in the AVPV (g), SDN-POA (h) and VMNvl (i) were determined by QPCR. Bars = means ± SEM. a Significantly different from oil-treated controls; b significantly different from E2-treated animals; c significantly different from P4-treated animals; values considered significantly different if p < 0.05 in post-hoc analyses. One-way ANOVA results: a) F(3,22)= 2.32, p < 0. 103; b) F(3,20)= 8.56, p < 0. 0.001; c) F(3,22)= 28.59, p < 0.0001; d) F(3,22)= 21.79, p < 0.0001; e) F(3,22)=6.56, p < 0.002; f) F(3,22)= 21.13, p < 0.0001; g) F(3,22)=4.65, p < 0.01; h) F(3,22)=4.55, p < 0.01; i) F(3,22)=6.16, p < 0.003.
Paqr7 and Paqr8 mRNA levels
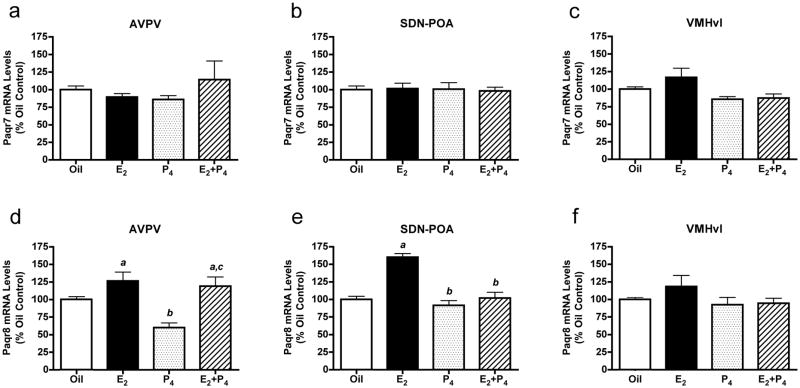
Ovarian steroids did not regulate Paqr7 mRNA levels in any brain region examined (Fig. 4a, b and c). In contrast, Paqr8 mRNA levels were repressed by treatment with P4 in the AVPV and increased by E2+P4 (Fig. 4d). Paqr8 mRNA levels were increased by E2 in the AVPV and SDN-POA (Fig. 4e), but not altered in the VMNvl (Fig. 4f).
Figure 4.
Levels of mRNAs encoding Paqr7 and Paqr8 in ovariectomized rats treated with oil, E2, P4 or E2+P4. Paqr7 mRNA levels in the AVPV (a), SDN-POA (b) and VMNvl (c), and Paqr8 mRNA levels in the AVPV (d), SDN-POA (e) and VMNvl (f) were determined by QPCR. Bars = means ± SEM. a Significantly different from oil-treated controls; b significantly different from E2-treated animals; c significantly different from P4-treated animals; values considered significantly different if p < 0.05 in post-hoc analyses. One-way ANOVA results: a) F(3,22)=0.65, p < 0.59; b) F(3,22)=0.037, p < 0.99; c) F(3,20)=3.67, p < 0.03; d) F(3,22)=10.39, p < 0.0002; e) F(3,22)=33.87, p < 0.0001; f) F(3,20)=1.35, p < 0.29.
DISCUSSION
These results are the first to show that E2 and P4 regulate non-classical progestin signaling molecules in the AVPV, SDN-POA and VMNvl. Importantly, E2+P4 increased Serbp1 mRNA levels in all brain regions examined, but increased expression of its putative binding partners, Pgrmc1 and Pgrmc2, in a region-specific manner. These findings are consistent with the idea that Serbp1 availability is the key factor determining P4 responsiveness of Pgrmc1 complexes (Peluso et al., 2004). Ovarian steroid regulation of Paqr8 also varied by brain region; however, the closely-related Paqr7 was not regulated in any region examined. Together these findings support the hypothesis that Pgrmc1, Pgrmc2, Serbp1 and Paqr8 mediate rapid P4 signaling within neuroendocrine nuclei important for female reproduction.
Our findings that E2 decreased Esr1 and increased Pgr in the AVPV and VMNvl are similar to results of previous studies (Lauber et al., 1990, Simerly and Young, 1991). We now report that the same pattern exists in the SDN-POA. Interestingly, in the VMNvl, P4 abrogated the effects of E2 on Pgr mRNA levels. Although this was not seen in other brain regions examined herein, P4 blocks E2 induction of Pgr in non-neural cells (Kraus and Katzenellenbogen, 1993).
We found that E2+P4 increased Pgrmc1 mRNA levels in the SDN-POA but not the AVPV; however, in both regions Serbp1 expression was increased, and this may be sufficient for rapid P4 effects. This idea is supported by findings that P4 responses mediated by the Pgrmc1/Serbp1 complex depend upon Serbp1 levels in non-neural cells (Peluso et al., 2005). Interestingly, E2+P4 increased Pgrmc2 mRNA levels in the AVPV, though no studies have tested whether Serbp1 binds Pgrmc2 to form a functional complex. These mechanisms are of particular interest as the AVPV is required for induction of the preovulatory luteinizing hormone surge (Wiegand et al., 1980, Ronnekleiv and Kelly, 1986, Petersen et al., 1995, Chappell and Levine, 2000). The AVPV and SDN-POA have dense projections to GnRH neurons (Simonian et al., 1999), and show abundant Pgrmc1, Pgrmc2 and Serbp1 mRNA levels (Intlekofer and Petersen, in press). Thus, further studies are warranted to determine whether these signaling molecules mediate rapid P4 effects in the AVPV and SDN-POA.
P4 and E2+P4 increased Pgrmc1 and Serbp1 mRNA levels in the VMNvl, a region in which P4 facilitates lordosis (Pfaff and Sakuma, 1979, Pfaff et al., 1994, Frye and Vongher, 1999, Frye, 2001). These rapid P4 effects are partially due to activation of cGMP-dependent protein kinase (DeBold and Frye, 1994, Lydon et al., 1995). This is especially interesting because Pgrmc1 is involved in P4 induction of cGMP-dependent protein kinase (Peluso and Pappalardo, 2004), and the C-terminus of Pgrmc1 contains several putative kinase binding sites (Cahill, 2007). Other researchers examining the entire VMN also found that Pgrmc1 was regulated by ovarian steroids; however, in that study E2 alone increased Pgrmc1 mRNA levels (Krebs et al., 2000). Factors that may explain these differences include dosage, duration of treatment, and region examined. Overall, these findings suggest a link between Pgrmc1/ Serbp1 and the rapid facilitation of feminine sex behavior.
Our findings are the first to show that ovarian steroids regulate Paqr8 mRNA levels in the brain. Similar to studies in myometrial cells, we found that E2 increased both Paqr8 and Pgr mRNA levels in the AVPV and SDN-POA. These results are interesting in light of evidence that Paqr8 cross-talks with Pgr through coupling to inhibitory G-proteins and decreasing Pgr transactivation (Karteris et al., 2006). In contrast to Paqr8, ovarian steroid exposure did not alter Paqr7 mRNA levels in any brain region examined. In other reproductive tissues, E2 and P4 also have variable effects on these signaling molecules (Cai and Stocco, 2005, Fernandes et al., 2005). Thus, despite their structural similarities, Paqr7 and Paqr8 may be regulated differently by ovarian steroids and may have functionally distinct roles.
Though reproductive functions coordinated by ovarian hormones have been studied extensively, the underlying molecular events are unclear. The present studies identified several steroid-inducible progestin signaling molecules that may mediate rapid P4 actions in the neuroendocrine control of reproduction. The functional relevance and specific role(s) of these novel signaling molecules will be the topic of future research.
Highlights.
Non-classical progesterone signaling molecules are regulated by ovarian steroids in the brain
Rapid progestin signaling molecules are found in brain nuclei that regulate female reproduction
Ovarian steroids regulate Pgrmc1, Pgrmc2, Serbp1, and Paqr8 in a brain nuclei-specific manner
Acknowledgments
The authors would like to thank Leah Aggison and Dr. Paula Moura for editorial assistance. Funding for this research was provided by NIH ES013885 and HD027305 to SLP and T32 MH020051 to KAI.
Abbreviations
- AVPV
anteroventral periventricular nucleus
- E2
17-β estradiol
- Esr1
estrogen receptor 1
- GnRH
gonadotropin releasing hormone
- P4
progesterone
- Paqr7
progestin and adipoQ receptor 7
- Paqr8
progestin and adipoQ receptor 8
- Pgr
progestin receptor
- Pgrmc1
progesterone receptor membrane component 1
- Pgrmc2
progesterone receptor membrane component 2
- QPCR
quantitative polymerase chain reaction
- SDN-POA
sexually dimorphic nucleus of the preoptic area
- Serbp1
serpine mRNA-binding protein 1
- VMNvl
ventrolateral portion of the ventromedial nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology. 2008;149:5518–5526. doi: 10.1210/en.2008-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar J, Lahav J, Hod M, Ben-Rafael Z, Weinberger I, Brosens J. Regulation of platelet aggregation and adenosine triphosphate release in vitro by 17beta-estradiol and medroxyprogesterone acetate in postmenopausal women. Thromb Haemost. 2000;84:695–700. [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Correia JN, Conner SJ, Kirkman-Brown JC. Non-genomic steroid actions in human spermatozoa. "Persistent tickling from a laden environment". Semin Reprod Med. 2007;25:208–219. doi: 10.1055/s-2007-973433. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Frye CA. Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Ehring GR, Kerschbaum HH, Eder C, Neben AL, Fanger CM, Khoury RM, Negulescu PA, Cahalan MD. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- Fernandes MS, Brosens JJ, Gellersen B. Honey, we need to talk about the membrane progestin receptors. Steroids. 2008;73:942–952. doi: 10.1016/j.steroids.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Fernandes MS, Pierron V, Michalovich D, Astle S, Thornton S, Peltoketo H, Lam EW, Gellersen B, Huhtaniemi I, Allen J, Brosens JJ. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J Endocrinol. 2005;187:89–101. doi: 10.1677/joe.1.06242. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Lydon JP, O'Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3alpha,5alpha-THP-facilitated lordosis. Psychopharmacology (Berl) 2006;185:423–432. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Res. 1999;815:259–269. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and adipoQ receptor family members 7 and 8 in rat forebrain. Neuroscience. doi: 10.1016/j.neuroscience.2010.10.051. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL, Katzenellenbogen BS. Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology. 1993;132:2371–2379. doi: 10.1210/endo.132.6.8504742. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci U S A. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Mobbs CV, Pfaff DW. Estradiol Regulation of Estrogen Receptor Messenger Ribonucleic Acid in Rat Mediobasal Hypothalamus: An in situ Hybridization Study. J Neuroendocrinol. 1990;2:605–611. doi: 10.1111/j.1365-2826.1990.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–770. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Meffre D, Delespierre B, Gouezou M, Leclerc P, Vinson GP, Schumacher M, Stein DG, Guennoun R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J Neurochem. 2005;93:1314–1326. doi: 10.1111/j.1471-4159.2005.03127.x. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Stanfield J, Skinner MK. Interactions between progesterone and tumor necrosis factor-alpha in the regulation of primordial follicle assembly. Reproduction. 2006;132:877–886. doi: 10.1530/REP-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab. 2009;94:2644–2649. doi: 10.1210/jc.2009-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A. Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14-3-3sigma. Biol Reprod. 2004;71:1870–1878. doi: 10.1095/biolreprod.104.031716. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Fernandez G, Wu CA. Involvement of an unnamed protein, RDA288, in the mechanism through which progesterone mediates its antiapoptotic action in spontaneously immortalized granulosa cells. Endocrinology. 2004;145:3014–3022. doi: 10.1210/en.2004-0067. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M. Expression and function of PAIRBP1 within gonadotropin-primed immature rat ovaries: PAIRBP1 regulation of granulosa and luteal cell viability. Biol Reprod. 2005;73:261–270. doi: 10.1095/biolreprod.105.041061. [DOI] [PubMed] [Google Scholar]
- Petersen SL, LaFlamme KD. Progesterone increases levels of mu-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Brain Res Mol Brain Res. 1997;52:32–37. doi: 10.1016/s0169-328x(97)00194-0. [DOI] [PubMed] [Google Scholar]
- Petersen SL, McCrone S, Keller M, Shores S. Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology. 1995;136:3604–3610. doi: 10.1210/endo.136.8.7628399. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neil E, editors. The physiology of reproduction. 1994. pp. 107–220. [Google Scholar]
- Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ. Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology. 1986;43:564–576. doi: 10.1159/000124583. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ukena K, Takemori H, Okamoto M, Kawata M, Tsutsui K. Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell. Neuroscience. 2004;126:325–334. doi: 10.1016/j.neuroscience.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Sakuma Y. Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol. 2009;21:410–414. doi: 10.1111/j.1365-2826.2009.01856.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain: a laboratory guide with printed and electronic templates for data, models, and schematics. 2 Elsevier; 1998. [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kanda Y, Roberts DJ, Ecker JL, Losel R, Wehling M, Peluso JJ, Pru JK. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol. 2008;287:81–89. doi: 10.1016/j.mce.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003a;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003b;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]