Abstract
In vitro 14-day cultures of peripheral blood mononuclear cells from hairy cell leukemia patients consistently showed the presence of hematopoietic stem cells giving rise to multilineage colonies containing a high proportion of lymphoid cells associated with the myeloid and erythroid progenitors. These stem cells are not the hairy cells but appear to be pluripotent lymphomyeloid primitive stem cells persisting in this leukemia. Interferon alpha c or beta 1 did not inhibit the growth of these colonies, as they did the growth of colonies of normal hematopoietic progenitors, but markedly decreased the ratio of lymphoid to myelomonocytic cells, by increasing the formation of monocytes and other nonlymphoid cell types in these multilineage colonies. Interferon gamma did not have the same effects on differentiation.
Full text
PDF
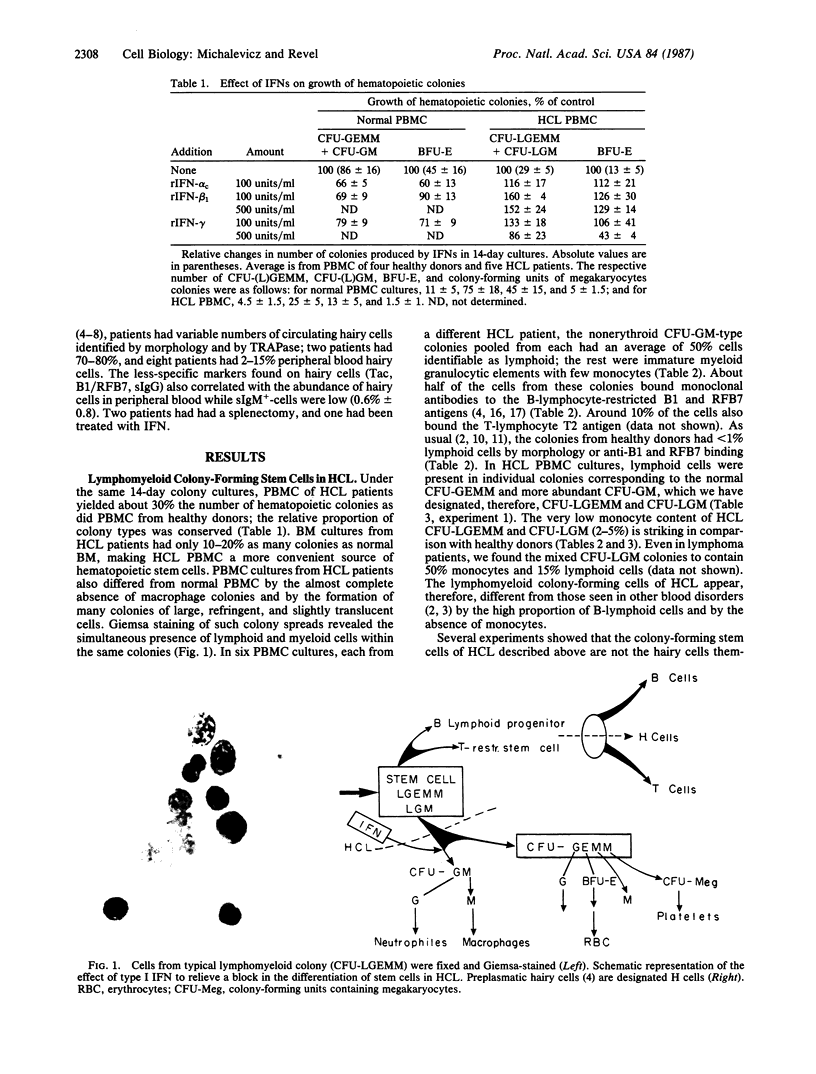
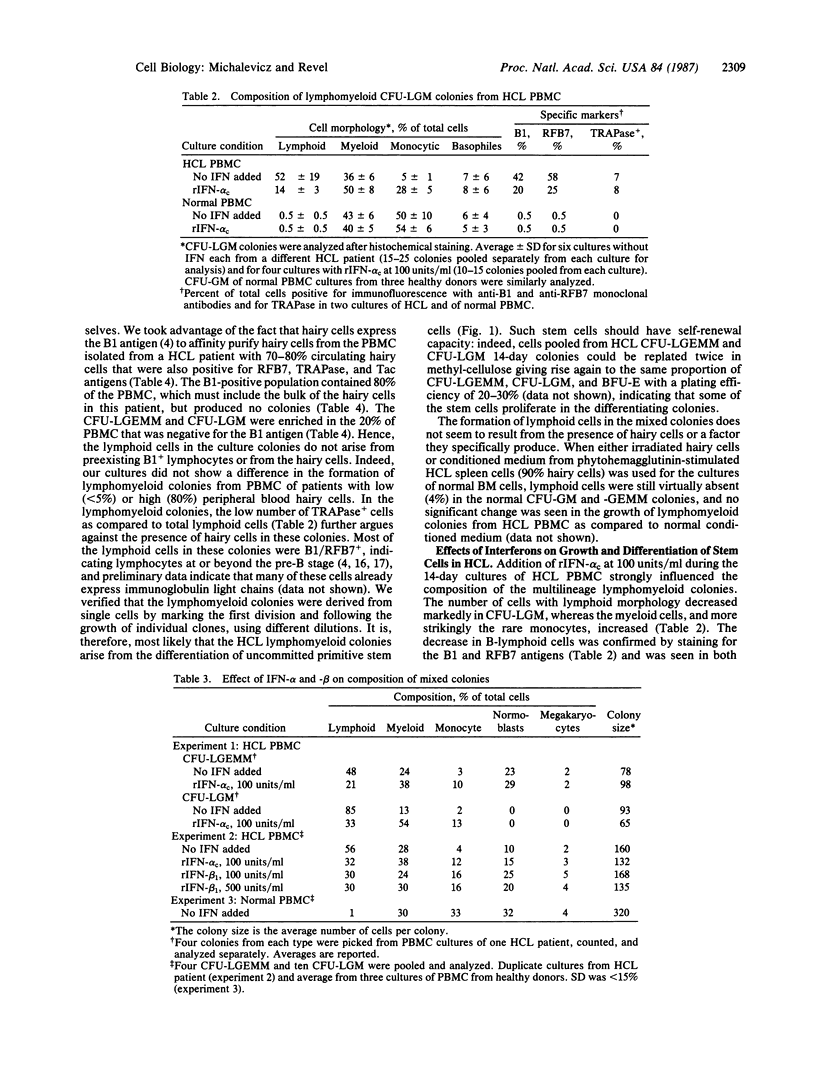
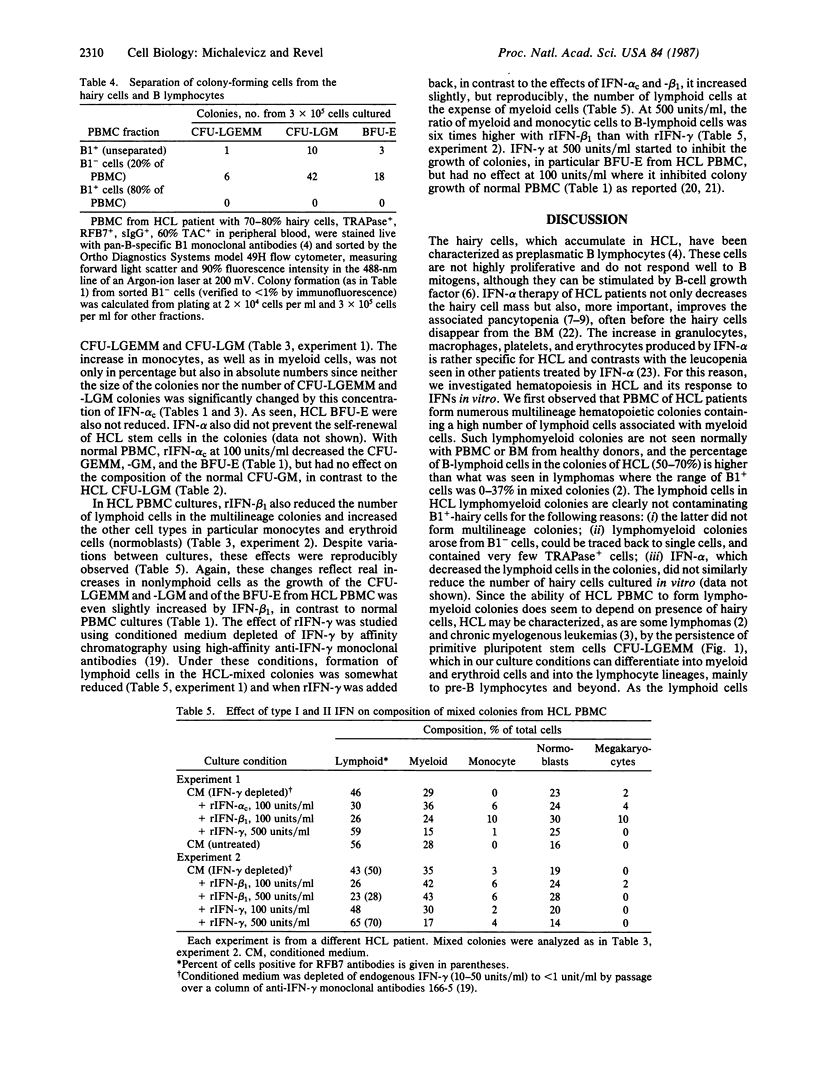

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Levo Y., Rahmani R., Mory Y., Vaks B., Horowitz O., Doerner T., Shoham J., Wallach D., Revel M. Rapid improvement in a terminal case of hairy cell leukemia treated with a new human recombinant interferon, IFN-alpha-C. Isr J Med Sci. 1985 Dec;21(12):977–981. [PubMed] [Google Scholar]
- Anderson K. C., Boyd A. W., Fisher D. C., Leslie D., Schlossman S. F., Nadler L. M. Hairy cell leukemia: a tumor of pre-plasma cells. Blood. 1985 Mar;65(3):620–629. [PubMed] [Google Scholar]
- Chernajovsky Y., Mory Y., Chen L., Marks Z., Novick D., Rubinstein M., Revel M. Efficient constitutive production of human fibroblast interferon by hamster cells transformed with the IFN-beta 1 gene fused to an SV40 early promoter. DNA. 1984 Aug;3(4):297–308. doi: 10.1089/dna.1.1984.3.297. [DOI] [PubMed] [Google Scholar]
- Denis K. A., Witte O. N. In vitro development of B lymphocytes from long-term cultured precursor cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):441–445. doi: 10.1073/pnas.83.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser A. A., Kanz L., Bross K. J., Löhr G. W. T cells and probably B cells arise from the malignant clone in chronic myelogenous leukemia. J Clin Invest. 1985 Mar;75(3):1080–1082. doi: 10.1172/JCI111771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser A. A., Kanz L., Löhr G. W. Identification of B cells in multilineage hematopoietic colonies derived from cells of patients with lymphocytic lymphoma. Proc Natl Acad Sci U S A. 1985 Feb;82(3):883–885. doi: 10.1073/pnas.82.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Fialkow P. J. Cell lineages in hematopoietic neoplasia studied with glucose-6-phosphate dehydrogenase cell markers. J Cell Physiol Suppl. 1982;1:37–43. doi: 10.1002/jcp.1041130409. [DOI] [PubMed] [Google Scholar]
- Flandrin G., Sigaux F., Castaigne S., Billard C., Aguet M., Boiron M., Falcoff E., Degos L. Treatment of hairy cell leukemia with recombinant alpha interferon: I. Quantitative study of bone marrow changes during the first months of treatment. Blood. 1986 Mar;67(3):817–820. [PubMed] [Google Scholar]
- Jacobs A. D., Champlin R. E., Golde D. W. Recombinant alpha-2-interferon for hairy cell leukemia. Blood. 1985 Apr;65(4):1017–1020. [PubMed] [Google Scholar]
- Korsmeyer S. J., Greene W. C., Cossman J., Hsu S. M., Jensen J. P., Neckers L. M., Marshall S. L., Bakhshi A., Depper J. M., Leonard W. J. Rearrangement and expression of immunoglobulin genes and expression of Tac antigen in hairy cell leukemia. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4522–4526. doi: 10.1073/pnas.80.14.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalevicz R., Francis G. E., Price G. M., Hoffbrand A. V. The role of platelet-derived growth factor on human pluripotent progenitor (CFU-GEMM) growth in vitro. Leuk Res. 1985;9(3):399–405. doi: 10.1016/0145-2126(85)90062-1. [DOI] [PubMed] [Google Scholar]
- Mory Y., Ben-Barak J., Segev D., Cohen B., Novick D., Fischer D. G., Rubinstein M., Kargman S., Zilberstein A., Vigneron M. Efficient constitutive production of human IFN-gamma in Chinese hamster ovary cells. DNA. 1986 Jun;5(3):181–193. doi: 10.1089/dna.1986.5.181. [DOI] [PubMed] [Google Scholar]
- Novick D., Eshhar Z., Fischer D. G., Friedlander J., Rubinstein M. Monoclonal antibodies to human interferon-gamma: production, affinity purification and radioimmunoassay. EMBO J. 1983;2(9):1527–1530. doi: 10.1002/j.1460-2075.1983.tb01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund L., Einhorn S., Robèrt K. H., Juliusson G., Biberfeld P. Chronic B-lymphocytic leukemia cells proliferate and differentiate following exposure to interferon in vitro. Blood. 1986 Jan;67(1):152–159. [PubMed] [Google Scholar]
- Paganelli K. A., Evans S. S., Han T., Ozer H. B cell growth factor-induced proliferation of hairy cell lymphocytes and inhibition by type I interferon in vitro. Blood. 1986 Apr;67(4):937–942. [PubMed] [Google Scholar]
- Quesada J. R., Reuben J., Manning J. T., Hersh E. M., Gutterman J. U. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984 Jan 5;310(1):15–18. doi: 10.1056/NEJM198401053100104. [DOI] [PubMed] [Google Scholar]
- Raefsky E. L., Platanias L. C., Zoumbos N. C., Young N. S. Studies of interferon as a regulator of hematopoietic cell proliferation. J Immunol. 1985 Oct;135(4):2507–2512. [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Revel M. F., Kimchi A. Initial characterization of a spontaneous interferon secreted during growth and differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1982 Dec;2(12):1472–1480. doi: 10.1128/mcb.2.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby W. F., Ball E. D., Guyre P. M., Fanger M. W. The effects of recombinant-DNA-derived interferons on the growth of myeloid progenitor cells. Blood. 1985 Apr;65(4):858–861. [PubMed] [Google Scholar]
- Rossi G. B. Interferons and cell differentiation. Interferon. 1985;6:31–68. [PubMed] [Google Scholar]
- Scott G. M. The toxic effects of interferon in man. Interferon. 1983;5:85–114. [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Vainchenker W., Deschamps J. F., Bastin J. M., Guichard J., Titeux M., Breton-Gorius J., McMichael A. J. Two monoclonal antiplatelet antibodies as markers of human megakaryocyte maturation: immunofluorescent staining and platelet peroxidase detection in megakaryocyte colonies and in in vivo cells from normal and leukemic patients. Blood. 1982 Mar;59(3):514–521. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Lam K. W. Tartrate-resistant acid phosphatase isoenzyme in the reticulum cells of leukemic reticuloendotheliosis. N Engl J Med. 1971 Feb 18;284(7):357–360. doi: 10.1056/NEJM197102182840704. [DOI] [PubMed] [Google Scholar]
- Yarden A., Shure-Gottlieb H., Chebath J., Revel M., Kimchi A. Autogenous production of interferon-beta switches on HLA genes during differentiation of histiocytic lymphoma U937 cells. EMBO J. 1984 May;3(5):969–973. doi: 10.1002/j.1460-2075.1984.tb01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]