Abstract
The glutamatergic synapse in specific brain regions has been shown to be a site for convergence of stress and addictive substances. The bed nucleus of the stria terminalis (BNST), a nucleus that relays between higher order processing centers and classical reward and stress pathways, receives dense noradrenergic inputs that are known to influence behavioral paradigms of both anxiety and stress-induced relapse to drug seeking. α1-Adrenergic receptors (α1-ARs) within this region have been implicated in modulation of the HPA axis and anxiety responses. We found that application of an α1-AR agonist produced a long-term depression (LTD) of excitatory transmission in an acute mouse BNST slice preparation. This effect was mimicked by a 20 min, but not a 10 min, application of 100 µM norepinephrine (NE) in a prazosin-sensitive manner. This α1-AR LTD was independent of N-methyl-d-aspartate receptor (NMDAR) function unlike previously described α1-AR LTD in the hippocampus and visual cortex; however, it was dependent on the activation of L-type voltage gated calcium channels (VGCCs). In addition, α1-AR LTD was induced independently of the activation of mGluR5 which can also induce LTD in this region. Furthermore, α1-AR LTD was intact in mice receiving an intraperitoneal injection of cocaine but was disrupted in α2a-AR and NE transporter (NET) knockout (KO) mice. Thus a loss of this plasticity at glutamatergic synapses in BNST could contribute to affective behavioral phenotypes of these mice.
Keywords: synaptic plasticity, heterosynaptic neuromodulation, catecholamine, norepinephrine, anxiety, depression
INTRODUCTION
Relapse to drug use after extended abstinence remains a troublesome aspect of addiction. Clinical studies have implicated psychological stress as a major factor that induces relapse behavior. Although the noradrenergic system has long been implicated in withdrawal related-behaviors (Aston-Jones and Harris, 2004), recent evidence has highlighted its role in addiction (Weinshenker and Schroeder, 2007), particularly with the stress-induced relapse response (Shaham et al, 2000). For example, administration of an α2-AR agonist or lesioning of the ventral noradrenergic bundle (VNAB) attenuates stress-induced reinstatement to opiate seeking (Erb et al, 2000; Wang et al, 2001) and viral restoration of dopamine β-hydroxylase (DBH, the enzyme that produces norepinephrine (NE)) in the nucleus tractus solitarius (NTS) of mice lacking DBH restores conditioned place preference (CPP) to morphine (Olson et al, 2006).
The bed nucleus of the stria terminalis (BNST), a component of the extended amygdala, receives input from the VNAB, as well as glutamatergic inputs from cortical/limbic areas and sends outputs to stress and reward centers (Forray and Gysling, 2004). The lateral BNST (lBNST), and α2- and β-AR signaling therein, regulates stress-induced relapse behaviors (Wang et al, 2001; Leri et al, 2002). Moreover, blockade of excitatory transmission within this same region also disrupts anxiety-related behavior (Walker and Davis, 1997). Due to these observations, our group characterized NE modulation of glutamatergic signaling in the dorsal and ventral lBNST (dlBNST, vlBNST), focusing on α2- and β-AR signaling (Egli et al, 2005). Studies, however, have also suggested a role for α1-ARs in the BNST, demonstrating that blocking α1-ARs decreases anxiety responses concurrent with reductions in hypothalamic-pituitary-adrenal (HPA) axis activation (Cecchi et al, 2002). Further, activation of α1-ARs also increases spontaneous inhibitory postsynaptic current (IPSC) frequency in the vlBNST of animals exposed to morphine (Dumont and Williams, 2004).
α1-ARs have been reported to modulate glutamatergic transmission in other brain regions. α1-AR activation leads to depression of excitatory transmission that is long lasting in the hippocampus and visual cortex (Kirkwood et al, 1999; Scheiderer et al, 2004) and transient in the caudal NTS (Zhang and Mifflin, 2007; for review see Grueter et al, 2007.) In contrast, in the paraventricular nucleus of the hypothalamus (PVN), α1-AR signaling enhances excitatory transmission through both pre- and postsynaptic mechanisms (Gordon and Bains, 2003, 2005; Gordon et al, 2005).
Here we investigate the impact of α1-AR signaling on excitatory transmission in the lBNST. We find an extended application of NE results in robust long-term depression (LTD), which is dependent on α1-AR activation and that can be mimicked by α1-AR agonists. Intriguingly, the LTD described here differs from the previously described α1-AR LTD in the hippocampus and visual cortex in its induction characteristics. Additionally, because of the relative importance of the BNST in relapse and anxiety paradigms, and adrenergic signaling therein, we sought to determine if chronic alterations in adrenergic signaling would interfere with the expression of α1-AR LTD. We found that BNST α1-AR LTD is intact in animals acutely treated with cocaine, yet, is disrupted in both the α2A-AR knockout (KO) and the NE transporter (NET) KO lines, suggesting that chronic, but not transient, alterations in adrenergic signaling modulate the expression of α1-AR LTD.
METHODS
Animal Care
Male C57BL/6j mice 5–10 weeks old (The Jackson Laboratories, Bar Harbor, ME) and α2A-AR KOs and NET KOs which were both generated from in-house breeding on a C57BL/6 background were used in experiments. All animals were provided with food and water ad libitum and housed in groups within the Vanderbilt Animal Care Facilities. Experiments were performed under Vanderbilt Animal Care and Use committee approved guidelines. Animals receiving cocaine were prehandled for 5 days, receiving saline injections for 4 days.
Brain Slice Preparations
Slicing methods were as previously described in Grueter et al (2006). Briefly, animals were retrieved from the colony and allowed to rest in sound attenuating boxes for a minimum of 1 h after which they were anesthetized (isoflurane) and decapitated in a separate room. 300 µm coronal slices were made on a Leica VT1000S vibratome (Leica Microsystems, Bannockburn, IL) in a 1–4°C, oxygenated (95% O2, 5% CO2), high-sucrose low Na+ artificial cerebral spinal fluid (ACSF in mM: 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, 26 NaHCO3).
Field Potential Recordings
After slicing, whole or hemisected slices were transferred immediately to interface chambers where they rested for at least 30 min in a humidified and oxygenated environment while continuously being perfused with oxygenated and heated (approximately 28–30°C) ACSF (in mM: 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10 glucose, 26 NaHCO3) at a rate of 2 ml/min. Following this initial incubation, 25 µM picrotoxin was added to the bath to block GABAA eceptors and slices were allowed to rest at least another 30 min prior to recording, picrotoxin was included during the entirety of all experiments to isolate excitatory transmission. This concentration has been shown by our group to sufficiently block all inhibitory transmission via the GABAA receptor (Egli and Winder, 2003). Recording electrodes of approximately 1MΩ resistance were pulled on a Flaming/Brown microelectrode puller (Sutter Instruments, Novato, CA) and filled with ACSF. A bipolar Nichrome (A-M Systems, Carlsborg, WA) stimulating electrode was placed dorsally to the recording electrode within the dlBNST such that stimulation of the field resulted in two distinguishable negative shifts in potential: N1 (the TTX sensitive fiber volley estimate) and N2 (CNQX sensitive synaptic response) as previously reported (Weitlauf et al, 2004; Egli et al, 2005; Grueter and Winder, 2005). The amplitude (voltage) of the N2 was measured at a stimulation intensity that resulted in a voltage approximately 50% of the maximum N2 response. Slices were stimulated at a frequency of 0.05 Hz. Field potentials were recorded using Clampex 8.2 (Molecular Devices, Sunnyvale, CA). All drugs were bath applied at their final concentrations.
Whole Cell Recordings
Following slicing, hemisected slices were allowed to rest submerged in a holding chamber filled with oxygenated and heated (28°C) ACSF for at least 30 min. After this incubation time an individual slice was moved to the recording chamber where it was submerged in oxygenated and heated (28°C) ACSF with added picrotoxin (25 µM included for the entirety of all experiments as with the field recordings) to isolate currents evoked by glutamate receptor activation at a rate of 2 ml/min. Stimulating electrodes were the same as for field recordings in dorsal recordings and medial to the lBNST in ventral recordings. Patch electrodes (3–6MΩ) were pulled on a Flaming/Brown microelectrode puller (Sutter Instruments) and filled with either Cs- or K-gluconate intracellular solution (in mM: Cs- or K-gluconate 135, NaCl 5, MgCl2 2, HEPES 10, EGTA 0.6, Na2ATP 4, Na2GTP 0.4; there was no observable difference with either intracellular solution on the LTD effect). In all whole cell experiments, cells were clamped at −70 mV throughout and excitatory postsynaptic currents (EPSCs) were recorded using Clampex 9.2 (Molecular Devices). Series resistance was monitored throughout each experiment and a change greater than 20% resulted in the exclusion of the experiment from the data set. EPSCs were evoked at a frequency of 0.167 Hz and 100–400 pA EPSCs were recorded. Consistent with the field experiments, drugs were bath applied at their final concentrations.
Analysis of Field Recordings
All recorded data were analyzed via Clampfit 9.2 (Molecular Devices). All field recordings contain a 20 min baseline recording prior to agonist application and all data points were normalized to the baseline 5 min prior to the agonist application. Plotted time courses for field experiments are represented as 1 min averages. For the majority of LTD experimental measurements, the LTD measurement was taken 55–60 min post agonist application. The exceptions are the experiments with prazosin (Figure 1c and d), in which the LTD measurement was the final 5 min of each recording.
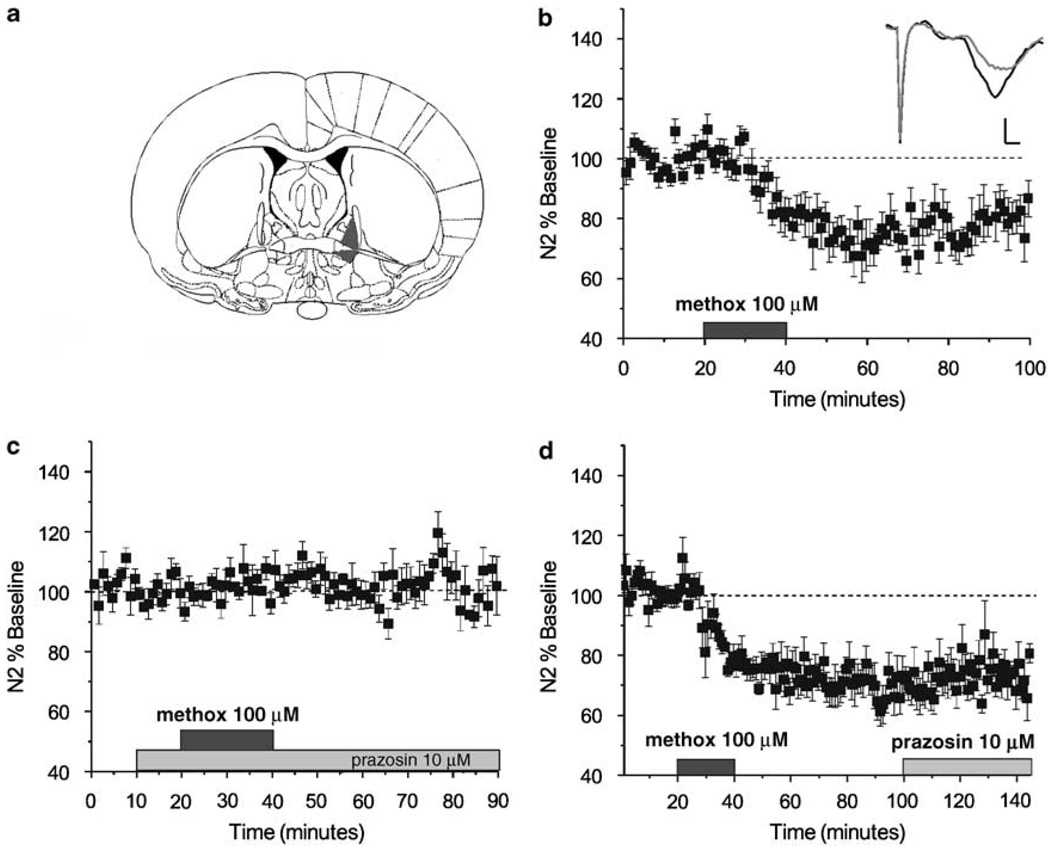
Figure 1.
α1-AR activation induces long-term depression (LTD) in bed nucleus of the stria terminalis (BNST). (a) BNST schematic adapted from Paxinos and Franklin (2001). Gray shading represents the lateral BNST, above anterior commissure dorsal lateral BNST, below anterior commissure ventral lateral BNST. (b) Application of the α1-AR selective agonist methoxamine (100 µM) induces a depression of extracellularly recorded excitatory responses that persists for over 60 min post wash (N = 6). (Inset) Representative traces of N1 and N2, 5 min average of baseline (black) and LTD (gray) (scale bars 0.2 mV by 0.5 ms). (c) The α1-AR antagonist prazosin (10 µM) blocks induction of the depression by methoxamine (100 µM; (N = 6). (d) Prazosin (10 µM) cannot reverse the depression induced by methoxamine (100 µM) when applied in wash (N = 5).
Analysis of Whole Cell Recordings
A 5 min baseline prior was acquired prior to agonist application and all points were normalized to minutes 3–5 within each experiment (with the exception of the low concentration methoxamine experiments where a 10 min baseline was acquired). Points are 30 s averages on plotted time course. For the majority of LTD experiments the LTD measurement is taken at 58–60 min within the experimental time course. The exceptions to this rule are the dual agonist application experiments, where the first LTD measurement is at 28–30 min within the time course and the second measurement is at min 55–57 min within the time course. The experiments with the lower concentration of methoxamine had measurements taken in the last 2 min of recording due to the shorter duration of wash.
Statistics
All data points were reported as the mean ± SEM and significance (determined by paired and unpaired Student’s t-test) is reported in the text and figure legends. Significant differences were defined as having a p < 0.05.
Reagents
(2S)-3-(((1S)-1-(3,4-Dichlorophenyl)ethyl)amino-2-hydroxypropyl) (phenylmethyl) phosphinic acid (CGP 55845, Tocris, Ellisville, MO), Cocaine (Sigma, St Louis, MO), (RS)-3,5-Dihydroxyphenylglycine (DHPG, Tocris), DL-2-amino-5-phosphonopentanoic acid (DL-APV, Sigma), methoxamine-HCl (Sigma), 2-methyl-6-(phenylethynyl) pyridine hydrochloride (MPEP, Tocris), nimodipine (Tocris), picrotoxin (Tocris), prazosin (Tocris). Dimethylsulfoxide (DMSO) was the solvent used for stock solutions of CGP 55845, MPEP, nimodipine, picrotoxin, and prazosin where the maximum final concentration of DMSO was 0.02% by volume.
RESULTS
α1-AR Activation Produces LTD of Excitatory Responses in the BNST
Both dlBNST and vlBNST are activated in response to various stressors (footshock, yohimbine, restraint, etc; Funk et al, 2006). Additionally electrical stimulation of dlBNST and vlBNST produces behavior similar to that observed after experiencing a stressor (Casada and Dafny, 1991). We began our investigation into the modulation of excitatory synapses via α1-AR in the BNST by recording extracellular excitatory potentials in the dlBNST (Figure 1a). We first applied the α1-AR selective agonist methoxamine (100 µM) for 20 min and observed a long lasting depression in excitatory transmission (79.5 ± 4.7%, p < 0.01, N = 6; Figure 1b) that was absent in the presence of the α1-AR antagonist 10 µM prazosin (101.9 ± 5.6%, N = 6; Figure 1c). To verify that this phenomenon was LTD and not the result of a constitutively activated α1-AR, we applied prazosin (10 µM) to slices 60 min after a 20 min application of methoxamine (100 µM) was terminated. This treatment failed to reverse the depression observed after agonist application (67.3 ± 3.0%, p < 0.001, N = 5; Figure 1d) suggesting that the 20 min activation of α1-ARs results in an LTD of excitatory inputs. The BNST, however, is composed of a heterogeneous population of dendrites and cell bodies and therefore extracellular recordings may potentially reflect effects on excitability of the postsynaptic dendrites/cell. We therefore utilized whole-cell voltage clamp recordings to measure isolated EPSCs in dlBNST and vlBNST neurons. Application of methoxamine (10 or 100 µM) for 15 min produced a robust depression of the evoked EPSC that persisted for at least 40 min after washout of agonist (dlBNST: 10 µM, 65.6 ± 6.3%, N = 5, p < 0.001, Figure 2a; 100 µM, 46.1 ± 9.8%, N = 6, p < 0.001, Figure 2b; 10 vs 100 µM was not statistically different p > 0.15; vlBNST 100 µM: 63.9 ± 8.3%, N = 9, p < 0.001, Figure 2c; representative experiment from the dlBNST 100 µM methoxamine recording Figure 2d). We did not see changes in the input resistance (IR) following application of methoxamine in either the dlBNST (10 and 100 µM) nor the vlBNST (100 µM) (97.3 ± 5.5%, p > 0.7, N = 19, Figure 2d representative trace). Additionally, we did not observe a significant change to a second 15 min application of methoxamine in the dlBNST (second methoxamine application vs first methoxamine application p > 0.05, N = 6; Figure 2e). This, along with the extent of the depression, suggests that the initial induction of α1-ARLTD was saturated under our conditions although we cannot rule out that receptors may have become desensitized to the first agonist application.
Figure 2.
Excitatory postsynaptic currents (EPSCs) are depressed by α1-AR agonist application. (a) Whole cell voltage clamp (−70 mV holding potential) experiments were performed to assess long-term depression (LTD) in the dorsal lateral BNST (dlBNST). Methoxamine (100 µM) was applied for 15 min and washed out for 40 min resulting in LTD (N = 6). (b) Under the same conditions 100 µM methoxamine also induced LTD in the ventral lBNST (vlBNST). (N = 8) (c) A lower concentration of methoxamine (10 µM) applied for the same duration also can induce significant LTD in the dlBNST. (d) A representative experiment in the dlBNST: EPSC (pA) = EPSC amplitude, HI (pA) = holding current, RA (MΩ) = Access Resistance, and RI (GΩ) = Input resistance. (d inset) Representative traces of EPSCs, each a 2 min average of baseline (black line) and LTD (gray line; scale bar 40 pA by 5 ms). (e) Applying 100 µM methoxamine after previous induction of LTD fails to further depress EPSCs (N = 6).
Prolonged Exposure to NE Results in α1-AR-Dependent LTD
NE application can elicit LTD of excitatory inputs in the visual cortex and the hippocampus (Kirkwood et al, 1999; Scheiderer et al, 2004). Thus, we decided to test whether NE induces similar LTD in the BNST, a nucleus heavily innervated by adrenergic fibers. Our group has previously reported that a 10 min application of 100 µM NE results in a transient bimodal response (an increase or decrease) in the extracellular field potential that is mediated by α2- and β-ARs (Egli et al, 2005). This application, however, was insufficient to produce a sustained depression in excitatory transmission (98.4 ± 3.4%; Figure 3a, N = 6, Egli et al, 2005) in field recordings. In the BNST and other regions, chronic stressors are thought to promote lasting increases in extracellular NE levels by shifting the firing patterns of noradrenergic cells from phasic firing to burst firing (Forray and Gysling, 2004). We found that increasing the duration of application of NE to 20 min at the same concentration produced a robust LTD of excitatory responses (67.1 ± 7.3%, p < 0.01, N = 6) that persisted for over 60 min after agonist application (Figure 3a). Moreover, the experiments where we applied 100 µM NE for 20 min were significantly different from the experiments where we applied 100 µM NE for 10 min (p < 0.05) during the LTD phase of the recording. With the extended application time we still observed the same bimodal effect to NE in our transient responses. (Two of the six experiments resulted in an initial increase in synaptic efficacy and four of six in an initial decrease in synaptic efficacy.) The sustained depression, however, was observed irrespective of the polarity of the initial response to NE (Figure 3b). To test whether the persistent depression induced by 100 µM NE was due to activation of α1-ARs we applied the α1-AR specific antagonist prazosin (10 µM) to the slice prior to NE application and throughout the experiment. The application of prazosin completely ablated the ability of NE (20 min at 100 µM) to induce a long-lasting depression in excitatory responses (94.6 ± 4.5%, N = 5; Figure 3c); however, we still observed the initial bimodal response (3 of 5 increases in synaptic efficacy, 2 of 5 decreases in synaptic efficacy).
Figure 3.
Norepinephrine (NE) induces α1-AR LTD via a time-dependent mechanism. (a) Expressed as percent of baseline, a 20 min application of NE (100 µM) results in a sustained depression of extracellularly recorded excitatory response that lasts for over 60 min post drug application (open symbols; N = 6). However, responses following a 10 min application of NE (100 µM) fail to induce a sustained depression of the excitatory response (closed symbols; N = 6). (b) Two representative experiments with 20 min applications of NE (100 µM) emonstrate the transient bimodal effect, open symbols: increase in EPSP response followed by LTD, closed symbols: decrease in EPSP response followed by LTD. (c) 10 µM of the α1-AR specific antagonist prazosin blocks the sustained depression of the excitatory field potential (open symbols; N = 5).
α1-AR LTD in the BNST Is Not Dependent on NMDAR Activation or Concurrent Stimulation of Presynaptic Fibers but Is Dependent on L-Type VGCCs
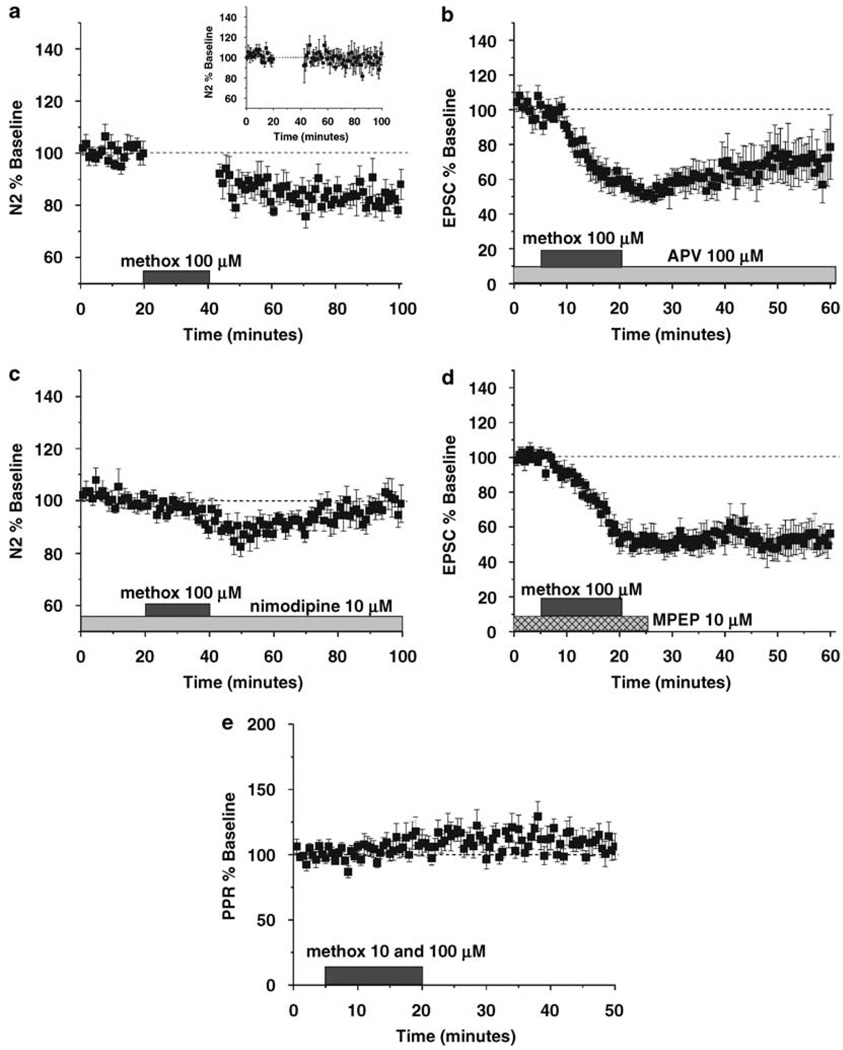
α1-AR LTD has been previously described in the visual cortex and most recently in the hippocampus where it has been shown to require concurrent activation of presynaptic inputs and N-methyl-d-aspartates (NMDARs) (Kirkwood et al, 1999; Scheiderer et al, 2004). We found, however, that applying methoxamine in the absence of stimulation resulted in significant LTD (82.3 ± 2.4%, p < 0.01, N = 6; Figure 4a) that was indistinguishable from that observed with concurrent stimulation. The absence of concurrent stimulation itself, however, had no effect on the amplitude of subsequent field potentials (97.4 ± 9.5%, N = 5; inset, Figure 4a). To examine the influence of NMDAR activation on α1-AR LTD in the BNST, we recorded EPSCs at −70 mV in the presence of 100 µM DL-APV. Again under these conditions, a 15 min application of methoxamine still produced robust LTD (66.7 ± 13.3%, N = 5, p < 0.05, not significantly different from single methoxamine application p > 0.05; Figure 4b). LTD induced by group 1 mGluR receptors (that are coupled to Gq) in other brain regions has been shown to involve L-type voltage gated calcium channels (VGCCs; for review see Grueter et al, 2007). Thus, we hypothesized that α1-AR LTD might also require L-type calcium signals. We applied methoxamine (100 µM) in the presence of the L-type VGCC blocker nimodipine (10 µM) and found that, although there was a small early depression (minutes 47–49, 86.6 ± 3.4%, p < 0.01), LTD was blocked (99.5 ± 4.8%, N = 7; Figure 4c). Our group has previously described LTD in the dlBNST that can be induced by activating the group 1 mGluR, mGluR5 (Grueter et al, 2006). To ensure that α1-ARLTD was not the result of increased glutamate inducing mGluR5-LTD, we applied methoxamine in the presence of the mGluR5 antagonist MPEP (10 µM) at a concentration that prevents the induction of mGluR-LTD (Grueter et al, 2006). We found that MPEP had no effect on α1-AR LTD (54.3 ± 6.7%, p < 0.005, N = 5; Figure 4d). These data thus suggest that α1-ARs heterosynaptically induce LTD via a non-Hebbian mechanism in the dlBNST in an L-type VGCC dependent manner.
Figure 4.
α1-AR LTD (as measured in the dlBNST) is induced independently of evoked glutamatergic synaptic activity but dependent on L-type voltage gated calcium channels (VGCCs). (a) To address the involvement of presynaptic stimulation the stimulus was turned off prior to 100 µM methoxamine application and turned back on 2 min post α1-AR agonist removal. This did not disrupt LTD expression. (N = 7). (a inset) To control for the lack of stimulation interleaved experiments were run without the presence of agonist (N = 5). (b) To assess the role of N-methyl-d-aspartate receptor (NMDAR) activation α1-AR LTD experiments were performed in whole cell voltage clamp (−70 mV holding potential) and DL-APV (100 µM) was included throughout the duration of the experiment (N = 5). (c) The L-type calcium channel blocker nimodipine (10 µM) prevented the induction of α1-AR LTD by 100 µM methoxamine, however it did not prevent a significant transient depression. (N = 7) (d) To verify that α1-AR LTD does not require mGluR5 signaling we applied 100 µM methoxamine in the mGluR5 antagonist MPEP (10 µM; N = 5). (e) Paired pulse ratios do not change in response to methoxamine (10 or 100 µM) in dlBNST (N = 11).
LTD can be maintained at synapses via either a pre- or postsynaptic mechanism. To begin to address questions of the synaptic locus of α1-AR LTD we conducted paired pulse ratio (PPR) analysis. Increases observed in the PPR associated with a decrease in EPSC amplitude are suggestive of a presynaptic mechanism. Evoked EPSCs to two paired stimuli with a 50ms inter-stimulus interval were acquired during whole cell recordings and we analyzed the ratio of the second response to the first response. In the dlBNST we did not observe a change in the PPR upon application of methoxamine (10 and 100 µM) nor at the LTD time point (N = 11, p > 0.15; Figure 4e).
α1-AR LTD Is Disrupted in Mice with Aberrant Noradrenergic Signaling
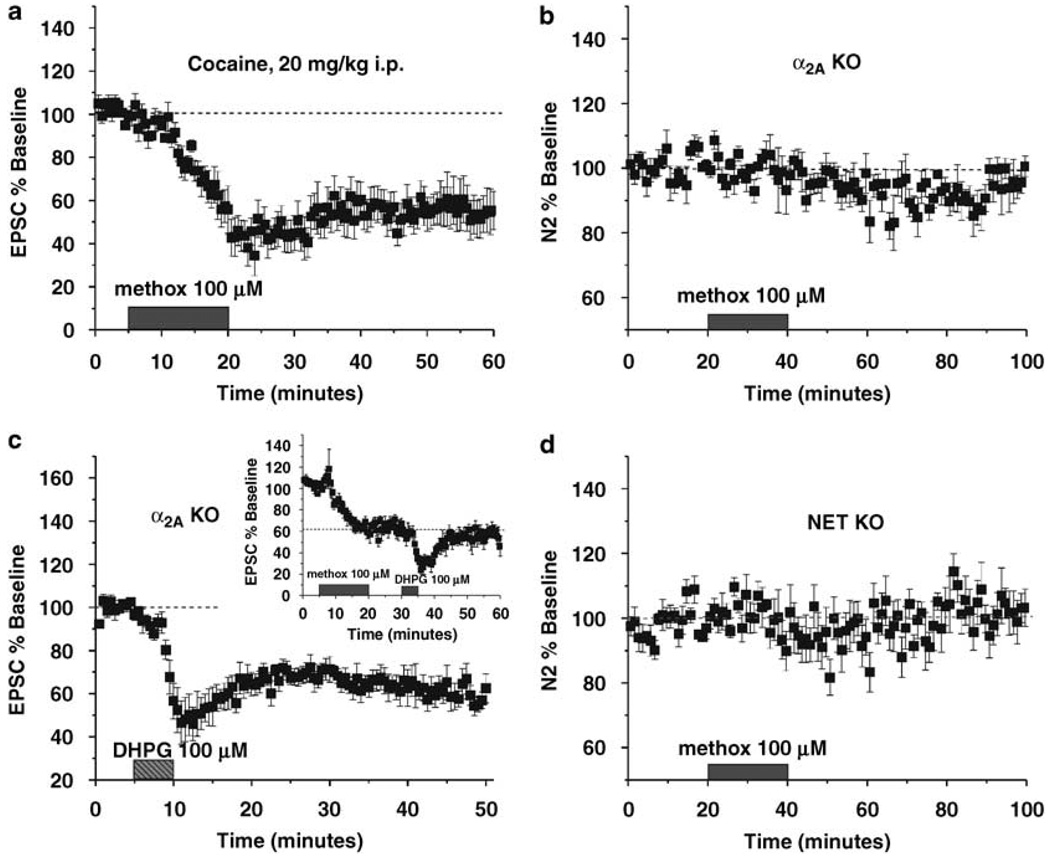
Alterations in noradrenergic signaling may underlie several affective disease states (Raskind et al, 2000; Stone et al, 2007). A single administration of cocaine elicits a transient increase in extracellular NE, while depression, anxiety and alcoholism are thought to involve more chronic alterations in adrenergic tone. Noradrenergic signaling in the BNST has been implicated in anxiety, depression, and drug abuse (Shaham et al, 2000; Forray and Gysling, 2004; Morilak et al, 2005) and synaptic plasticity is altered by multiple substances of abuse and stress in reward nuclei (Saal et al, 2003). We therefore chose to examine α1-AR LTD in the BNST in animals treated with cocaine (20 mg/kg) 30 min prior to slicing, and two animal models of affective disorders, α2A-AR and NET KOs. Both of these KO lines of mice have altered adrenergic systems and behavior (Bohn et al, 2000; Xu et al, 2000; Schramm et al, 2001; Lahdesmaki et al, 2002; Dziedzicka-Wasylewska et al, 2006; Keller et al, 2006). Animals receiving cocaine 30 min prior to slicing still showed robust α1-AR LTD (54.1 ± 9.4%, p < 0.005, N = 5; Figure 5a). In both KO animal models, α1-AR LTD was not observed upon application of methoxamine (α2A-AR KO: 96.0 ± 2.8%, p > 0.05, N = 5; NET KO: 104.2 ± 6.0%, p > 0.05, N = 7; Figure 5b and d); however, application of the mGluR5 agonist DHPG still resulted in robust depression in the α2A-AR KO mouse indicating that LTD via Gαq coupled mechanisms is still intact (57.6 ± 3.7%, p < 0.001, N = 5; Figure 5c). This is additionally intriguing because induction of α1-AR LTD occludes further depression of EPSCs in response to subsequent application of DHPG (100 µM) (p > 0.05, N = 5; Figure 5c inset).
Figure 5.
α1-AR is disrupted in animal models of affective disorders. (a) Cocaine (20 mg/kg) injected 30 min prior to slicing does not prevent the induction/expression of α1-AR LTD (N = 5). (b) A 20 min application of methoxamine (100 µM) fails to induce α1-AR LTD in α2A-AR KO mice (N = 5). (c) DHPG (100 µM) induces mGluR5-LTD in α2A-AR KO mice (N = 5). (d) Methoxamine (100 µM) fails to induce α1-AR LTD in NET KO mice (N = 7).
DISCUSSION
Previously, our group reported on noradrenergic modulation of excitatory synapses within the dlBNST, finding that both α2- and β-ARs contributed to effects observed with a 10 min application of 100 µM NE and that α2-ARs contributed to effects in the vlBNST (Egli et al, 2005). Egli et al found that in the dlBNST, 100 µM NE resulted in either a transient increase or decrease in excitatory transmission, while in the vlBNST it resulted in a transient decrease in excitatory transmission. Here we investigated the possibility that α1-ARs modulate glutamatergic synapses in this region as well. We found that a 20 min, but not a 10 min, 100 µM NE application resulted in an LTD of excitatory transmission that was mediated by the α1-AR and L-type VGCCs, however, this LTD was independent of NMDAR, and mGluR5 activation, or concurrent stimulation. Finally, we found that α1-AR LTD in the dlBNST was disrupted in two KO mice that have genetically manipulated adrenergic systems, and exhibit altered anxiety, depression and reward phenotypes; but, not in animals that received a transient alteration of their adrenergic system—a single intraperitoneal (i.p.) injection of cocaine 30 min prior to slicing.
NE Induces LTD in a Time-Dependent Manner
Our group has shown that while a 10 min application of NE in the dlBNST results in a bimodal transient regulation of the glutamatergic field potential (Egli et al, 2005), it fails to induce LTD via the α1-AR. Doubling the duration of NE application, however, results in α1-AR LTD. This duration-dependent result was an intriguing finding. It is clear that in the 10 min NE application experiments agonist wash-in has occurred and GPCRs are being activated based on data from our group (Egli et al, 2005) and the data shown here. Additionally, Dumont and Williams (2004) demonstrated that a brief (<2 min) application of 100 µM NE (in a submerged recording chamber) activated α1-ARs in vlBNST to transiently increase spontaneous IPSCs, thus, the α1-AR is also presumably activated in our recordings, but at a level below threshold for LTD. The duration-dependence may play a role physiologically, as it may be disadvantageous to induce this plasticity with transient NE increases in the BNST. Therefore this LTD may be activated when the animal experiences a lasting stressor. Microdialysis studies have demonstrated that NE levels remain elevated above the baseline in the BNST over an hour after restraint stress and blocking α1-ARs attenuates stress-induced rises in adrenocorticotropic hormone (ACTH) (Pacak et al, 1995; Cecchi et al, 2002). Moreover, Banihashemi and Rinaman (2006) showed that ablating BNST NE inputs prevents increases in corticosterone to i.p. yohimbine 90 min post injection. Therefore, under stress-producing conditions, α1-AR LTD may be recruited to alter the engagement of the HPA stress axis by the BNST over an hour after the initial stress insult. Thus, this α1-AR LTD may specifically participate in stress responses generated by long-term increases in NE in normal subjects and may be dysregulated in addictive states such as alcoholism and anxiety disorders like post traumatic stress disorder (PTSD).
α1-AR LTD in the BNST Is a Heterosynaptic Form of Plasticity
LTD mediated via group I mGluRs remains the best characterized Gαq-coupled receptor LTD and has been described in the cerebellum, hippocampus, cortex, dorsal and ventral striatum, ventral tegmental area, and BNST (Ito, 2001; Robbe et al, 2002; Malenka and Bear, 2004; Bellone and Luscher, 2005; Grueter et al, 2006, for review see Grueter et al, 2007). An interesting aspect exhibited by group I mGluR-LTD is a degree of promiscuity of mechanism depending on the synapse where the LTD is expressed. This notion can now be extended to the less characterized α1-AR LTD. Unlike in the hippocampus and visual cortex (Kirkwood et al, 1999; Scheiderer et al, 2004), α1-AR LTD within BNST is independent of the activation of NMDARs (in both the induction phase and the maintenance phase of the LTD) and concurrent presynaptic stimulation. Intriguingly, we found that α1-AR LTD expression in the BNST is dependent on L-type VGCC activity. L-type VGCC activity (particularly CaV1.3) is required in the dorsal striatum to induce corticostriatal group I mGluR-LTD (Wang et al, 2006). Furthermore, it has recently been demonstrated in the hippocampus that signaling via phospholipase C can increase the conductance of CaV1.3 at negative potentials (Gao et al, 2006). Consistent with the role of the L-type VGCC in the induction of group 1 mGluR-LTD in the dorsal striatum, postsynaptic cells must be depolarized to −50 mV (Adermark and Lovinger, 2007). In contrast, however, we were able to induce α1-AR LTD holding the cell at −70 mV and in the absence of concurrent stimulation; although, subsequent stimulation, following the activation of α1-AR, may activate L-type channels in the maintenance phase of the LTD. Norepinephrine can modulate cell excitability within BNST, causing depolarization especially in nonprojection cells (Dumont and Williams, 2004) and, therefore, it is possible that the application of methoxamine directly depolarized the cell sufficiently to activate L-type VGCCs. Additionally, the concentrations of our extracellular and intracellular recording solutions may have produced sufficiently depolarizing conditions. These seem unlikely given previous sharp microelectrode studies under identical conditions that indicated resting membrane potentials of −64 to –66 mV (Egli and Winder, 2003) coupled with a lack of change in the input resistance. Another possibility was that α1-AR LTD may be dependent on signaling via mGluR5 downstream of α1-AR activation. We found, however, that blockade of mGluR5 during the induction of α1-AR LTD does not impact the expression of this LTD. This suggests that in this region adrenergic afferents may influence plasticity independently of descending glutamatergic inputs to the BNST from areas like the limbic cortex, hippocampus, and amygdala.
The postulated heterosynaptic mechanism of this α1-AR LTD could be of behavioral significance. A hallmark of some affective disorders is the inherent inability to overcome the disorder by reasoning (ie feelings/urges are beyond the cognitive control of the patient) (DSM-IV). α1-ARs in the BNST modulate ACTH levels in animals who have been exposed to a stressor (Cecchi et al, 2002). Moreover it has recently been shown that the same axon collaterals from the nucleus tractus solitarius that project to the BNST also may project to the PVN (Banihashemi and Rinaman, 2006). NE may therefore modulate this circuitry regardless of input from cortical structures. It is intriguing to think that this dissociation of induction of α1-AR LTD from the glutamatergic input of cognitive centers innervating the BNST potentially contributes to alterations in behavior in a disease state such as generalized anxiety or addiction. Clearly, however, additional work would be needed to provide support for this notion.
α1-AR LTD Is not Observed in Mice with Chronically Altered Adrenergic Signaling
Previously our group reported that a 10 min application of 100 µM NE failed to alter glutamatergic transmission in α2A-AR KOs, mice with altered anxiety phenotypes. It was concluded that the α2A-AR may gate responses to NE within BNST via its interactions with other receptors (Egli et al, 2005). Due to the shorter duration of agonist application, however, contributions by the α1-AR in the α2A-AR KOs may not have been observed. Therefore, we decided to examine α1-AR LTD in the α2A-AR KOs. Surprisingly, α1-AR LTD could not be induced via a 20 min application of methoxamine. This implied that perhaps the lack of LTD was due to functional desensitization of α1-AR or in vivo induction of α1-AR LTD as a result of increased extracellular concentration of NE. These ideas are supported by increased metabolite/transmitter ratios within several brain regions in the α2A-AR KOs (Lahdesmaki et al, 2002), although these ratios can also be interpreted as increases in catabolism and therefore have caveats (Commissiong, 1985). To support our data with the α2A-AR KOs we next used the NET KOs, another mouse model with altered behavioral phenotypes and adrenergic transmission. Fast cyclic voltammetry experiments in the BNST in the NET KOs have demonstrated that NE clearance rates are over six times slower in the KOs as compared to wild-type controls (Xu et al, 2000). As was observed with the α2A-AR KOs, the NET KOs also failed to express LTD after a 20 min exposure to methoxamine. Interestingly, the α2A-AR KOs express mGluR5-LTD after an application of DHPG, demonstrating that signaling via GPCRs, and more specifically those coupled to Gαq, are still intact. Autoradiography data in the NET KOs shows a reduction in the cell surface expression of α1-ARs in several brain regions (Bohn et al, 2000; Xu et al, 2000; Dziedzicka-Wasylewska et al, 2006) but an upregulation of α2A/C-AR within the BNST (Gilsbach et al, 2006). One possibility of our results, taken together with this data, suggest that within these mouse models α1-ARs and/or their signaling pathways are desensitized, preventing induction of α1-AR LTD. An intriguing observation was that α1-AR LTD can occlude mGluR5-LTD in the BNST. This suggests that the two LTDs share a common mechanism as they do in the visual cortex (Choi et al, 2005) There is evidence, however, in dopaminergic cells in the VTA that α1-ARs desensitize group I mGluRs (Paladini et al, 2001). The L-type VGCC experiments provide indirect support for the desensitized receptor hypothesis as well. In these experiments there is a significant transient depression in response to the application of methoxamine that is not observed in either of the mouse models. One possibility for this transient depression would be GABAB receptor activation due to the activation of α1-ARs on interneurons (Dumont and Williams, 2004). This transient depression is not observed in either KO animal suggesting that there may be a tonic reduction in α1-ARs. Additional experiments will need to be conducted to confirm these hypotheses.
Interestingly, both of these KO mice have altered anxiety/depression phenotypes (Schramm et al, 2001; Lahdesmaki et al, 2002; Dziedzicka-Wasylewska et al, 2006; Keller et al, 2006) including increased anxiety-like behavior in the elevated plus maze (α2A-AR KOs), increased response to injection stress (α2A-AR KOs), decreased struggling/mobility in the forced swim and tail suspension tests (NET KOs) and bradycardia to stressful stimuli (NET KOs). In addition the NET KO animals have heightened sensitivity to psychostimulants, enhanced conditioned place preference to cocaine, and increased analgesia to opiates (Bohn et al, 2000; Xu et al, 2000; Hall et al, 2002). Furthermore it has been shown that the BNST sends monosynaptic projections to dopaminergic VTA neurons to modulate reward (Georges and Aston-Jones, 2002). During withdrawal from morphine there is an inhibition of the firing of these dopaminergic cells that can not only be reversed with the α2-AR agonist clonidine, but potentiated by its administration (Georges and Aston-Jones, 2003). It is an interesting notion that the NET KO animals lack a mechanism (α1-AR LTD) that may contribute to the inhibition of the dopaminergic cells in the withdrawal state while simultaneously demonstrating increased behavioral sensitization and reward mediated behaviors to drugs of abuse. A lack of functioning α1-ARs and/or their signaling pathways may impact such behavior. Although α1-AR activation can affect the excitability of cells in various ways, the lack of a long term change in cell function, like synaptic plasticity induced by the α1-ARs, in these animals could have implications for their exhibited behavior.
Clinical data have highlighted the α1-AR as a therapeutic target for anxiety disorders. Raskind and colleagues reported that α1-AR antagonists are efficacious for patients with combat/noncombat induced post-traumatic stress disorder (Raskind et al, 2000, 2002; Taylor and Raskind, 2002; Peskind et al, 2003; Taylor et al, 2006). Additionally, α1-AR antagonists are used to treat ailments like benign prostatic hypertrophy and hypertension and thus specific pharmacological agents are available for further investigation into their benefits in the treatment of affective disorders. Furthermore, the notion that alterations in adrenergic mediated synaptic plasticity within nuclei like the BNST, and others implicated in stress and anxiety, may contribute to the pathological learning (learned fear) that may mediate PTSD and similar disorders remains an interesting possibility.
Previous work has highlighted the role of adrenergic modulation in BNST in behavioral paradigms of stress-induced relapse to drug seeking and anxiety. Our findings showed that NE modulates excitatory synapses in the dlBNST by inducing an LTD that is dependent on the α1-AR and L-type VGCC activation and independent of the NMDAR and stimulation from presynaptic inputs. A crucial element to α1-AR LTD is that the induction depends on the length of exposure to NE, preventing transient increases in NE to elicit plasticity. Furthermore, a lack of this plasticity in animal models of affective disorders may impact their behavioral phenotypes. Together, our results demonstrate a mechanism by which NE may modulate BNST functional output under conditions of psychological stress.
ACKNOWLEDGEMENTS
We thank Thomas Kash and Amanda Vanhoose for critical comments on the manuscript. We also thank David Robertson and Mark Caron for supplying norepinephrine transporter knockout mice. This work was supported by NIDA (DGW) and NIAAA (DGW and ZAM).
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
Ms McElligott declares that except for her graduate student stipend from Vanderbilt University she has not received financial support or compensation from any individual or corporate entity for the past 4 years for research or professional service. Dr Winder has received a distribution from Columbia University for the licensing of transgenic mouse technology to Memory Pharmaceuticals and received a consultancy fee from MEDAcorp. Both authors declare that there are no personal financial holdings that could be perceived as potential conflicts of interest.
REFERENCES
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnosis and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47 Suppl 1:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20:9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Choi SY, Chang J, Jiang B, Seol GH, Min SS, Han JS, et al. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci. 2005;25:11433–11443. doi: 10.1523/JNEUROSCI.4084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissiong JW. Monoamine metabolites: their relationship and lack of relationship to monoaminergic neuronal activity. Biochem Pharmacol. 1985;34:1127–1131. doi: 10.1016/0006-2952(85)90484-8. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Gorecka A, Kusmider M, Drozdowska E, Rogoz Z, Siwanowicz J, et al. Effect of antidepressant drugs in mice lacking the norepinephrine transporter. Neuropsychopharmacology. 2006;31:2424–2432. doi: 10.1038/sj.npp.1301064. [DOI] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, et al. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LA, Salinas GD, Needleman LA, Marshall J. Insulin-like growth factor-1 modulation of CaV1.3 calcium channels depends on Ca2+ release from IP3-sensitive stores and calcium/calmodulin kinase II phosphorylation of the alpha1 subunit EF hand. J Neurosci. 2006;26:6259–6268. doi: 10.1523/JNEUROSCI.0481-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Faron-Gorecka A, Rogoz Z, Bruss M, Caron MG, Dziedzicka-Wasylewska M, et al. Norepinephrine transporter knockout-induced up-regulation of brain alpha2A/C-adrenergic receptors. J Neurochem. 2006;96:1111–1120. doi: 10.1111/j.1471-4159.2005.03598.x. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Priming of excitatory synapses by alpha1 adrenoceptor-mediated inhibition of group III metabotropic glutamate receptors. J Neurosci. 2003;23:6223–6231. doi: 10.1523/JNEUROSCI.23-15-06223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Noradrenaline triggers multivesicular release at glutamatergic synapses in the hypothalamus. J Neurosci. 2005;25:11385–11395. doi: 10.1523/JNEUROSCI.2378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: gatekeepers to addiction? Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, et al. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Keller NR, Diedrich A, Appalsamy M, Miller LC, Caron MG, McDonald MP, et al. Norepinephrine transporter-deficient mice respond to anxiety producing and fearful environments with bradycardia and hypotension. Neuroscience. 2006;139:931–946. doi: 10.1016/j.neuroscience.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Kobilka BK, Fagerholm V, Scheinin M. Behavioral and neurochemical characterization of alpha(2A)-adrenergic receptor knockout mice. Neuroscience. 2002;113:289–299. doi: 10.1016/s0306-4522(02)00185-9. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688:242–246. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Acadamic Press; 2001. [Google Scholar]
- Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16:165–171. doi: 10.1177/0891988703256050. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61:129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Thompson C, Petrie EC, Dobie DJ, Rein RJ, Hoff DJ, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63:565–568. doi: 10.4088/jcp.v63n0705. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J Neurophysiol. 2004;91:1071–1077. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D, Lin Y, Lehmann ML. Central alpha(1)-adrenergic system in behavioral activity and depression. Biochem Pharmacol. 2007;73:1063–1075. doi: 10.1016/j.bcp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22:82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Lowe K, Thompson C, McFall MM, Peskind ER, Kanter ED, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577–581. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. Highfrequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, et al. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin SW. Modulation of synaptic transmission to second-order peripheral chemoreceptor neurons in caudal NTS by & [alpha]1-adrenoreceptors. J Pharmacol Exp Ther. 2007;320:670–677. doi: 10.1124/jpet.106.114033. [DOI] [PubMed] [Google Scholar]