Abstract
Chronic/recurrent autoimmune (idiopathic) uveitis is difficult to treat and they account for approximately 10% of legal blindness in the Western world. As it has been reported that anti-CD3 antibody can enhance T cell regulatory function, we investigated its effects in vivo on experimental autoimmune uveitis (EAU), a model for autoimmune uveitis in humans. B10RIII mice immunized with an uveitogenic peptide were treated with the F(ab')2 fragment of anti-CD3 mAb either before or at clinical disease onset. Evaluation of EAU and cellular responses showed that disease was inhibited and the activation and expansion of pathogenic T cells selectively reduced, whereas functions of Treg in vivo were enhanced. Moreover, mice treated with anti-CD3 mAb were resistant to a second challenge with antigen and thus protected from recurrence of disease. Our results demonstrate that anti-CD3 mAb is a potent inhibitor of autoimmune uveitis.
Introduction
Uveitis, a common cause of human visual disability and blindness, is associated with chronic and recurrent complications. Animal models of experimental autoimmune uveitis (EAU) have been widely used to dissect the immunopathological mechanisms in uveitis and to develop preventive or therapeutic strategies. EAU can be elicited in rodents either by immunization with retinal antigens, such as retinal S antigen (S-Ag) [34] or interphotoreceptor retinal-binding protein (IRBP)[13;14], or by the adoptive transfer of uveitogenic T cells [1;18;28], suggesting that uveitis is a T cell-mediated, organ-specific autoimmune disease.
Autoimmune processes are related to defects in immunologic tolerance, a state of immune system unresponsiveness to an antigen. Tolerance is maintained by multiple mechanisms including deletion, anergy, and active cellular regulation [26] and strategies to induce immune tolerance are being developed for the treatment of autoimmunity. One such approach is the administration of CD3-specific antibody (Ab), which has shown efficacy in animal models of autoimmunity, including autoimmune diabetes [3;8;9;15;24;33] and experimental allergic encephalomyelitis (EAE) [22;31], and in humans with autoimmune diabetes [4;16;17;21] or psoriatic arthritis [32]. In addition, anti-CD3 Ab treatment is an approved therapy for acute transplant rejection in the clinic [5]. Till now, there have not been any studies of the effects of anti-CD3 monoclonal antibody (mAb) in an autoimmune uveitis model.
Here, we used the model of EAU in B10RIII mice to investigate the effect of the F(ab’)2 non–FC receptor (FcR)-binding fragment of anti-CD3 mAb in the treatment of autoimmune uveitis. The F(ab')2 fragment of anti-CD3 mAb, directed against the invariant CD3e chain of the TCR, is unable to activate complement or interact with type I and III FcRs [7], and so does not activate resting T cells and induces less toxicity due to cytokine release in vivo [7]. We found that this mAb significantly reduced the severity of the disease when administered either before or at disease onset and protected the treated mice from uveitis when they were immunized a second time with IRBP peptide 30 days after the primary immunization. The mechanism of action of the anti-CD3 mAb was further explored.
Materials and Methods
Animals and reagents
Pathogen-free female B10RIII mice (8– to 10-wk-old) were purchased from the Jackson Laboratory and were housed and maintained in the animal facilities of the University of Louisville. Institutional approval was obtained and institutional guidelines regarding animal experimentation were followed.
All T cells were cultured in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% fetal calf serum (FCS) (Hyclone, Logan, Utah), 5 × 10−5 M 2-mercapatoethanol, and 100 μg/ml of penicillin/streptomycin. The human IRBP peptide IRBP161–180 (SGIPYIISYLHPGNTILHVD) was synthesized by Sigma-Aldrich (St. Louis, MO).
Antigen immunization-induced uveitis and anti-CD3 Ab treatment
For active induction of disease in B10RIII, the animals were immunized subcutaneously with 100 μL of emulsion containing human IRBP161–180 (50 μg) and 500 μg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in incomplete Freund’s adjuvant (Sigma, St. Louis, MO), distributed over six spots on the tail base and flank. Concurrently, 0.2 μg of pertussis toxin was injected intraperitoneally (i.p.)[29]. For antigen (Ag) rechallenge experiments, we followed the protocol described by Dr. Caspi’s group[2;30], in which mice were immunized with 50 μg of IRBP161–180 in 0.1 ml at the base of the tail for the primary challenge and re-immunized with 50 μg of the same peptide in 0.1 ml divided between the thighs of the two hindlimbs.
IRBP peptide-immunized mice were injected i.p. (unless otherwise stated) with F(ab')2 anti-CD3 mAb (145–2C11; BioXCell, West Lebanon, NH), control hamster IgG F(ab')2 (Jackson ImmunoResearch Laboratories, Charlestown, MA), or phosphate-buffered saline (PBS) daily for 5 consecutive days[10;36] either prior to appearance of disease (days 6–10 after Ag immunization) or at onset of the disease (days 10–14). In some experiments using anti-CD3 mAb, mice were injected with 500 μg of neutralizing anti–mouse TGF-β or IL-10 Abs (BioXCell) or PBS on days 6–10 after Ag immunization. The clinical course of the disease was assessed by indirect fundoscopy twice a week starting day 6 post immunization. Fundoscopic evaluation for longitudinal follow-up of disease was performed using a binocular microscope after pupil dilation using 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions. The incidence and severity of EAU were graded on a scale of 0–4 in half-point increments using previously described criteria [29], based on the type, number, and size of lesions present. The nature of the disease was confirmed histologically [29]
Preparation of IRBP161–180-specific T cells
Briefly, at 14 days post-immunization with Ag, T cells were isolated from draining lymph node (LN) and spleen cells by passage through a nylon wool column, then 1 × 107 cells in 2 ml of RPMI 1640 medium were added to each well of a 6-well plate (Costar, Corning, NY) and stimulated with 20 μg/ml of IRBP161–180 in the presence of 1 × 107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs). After 2 days, the activated lymphoblasts were isolated by gradient centrifugation on Lymphoprep (Axis-Shield, Oslo, Norway) and cultured in RPMI 1640 medium supplemented with 15% IL-2-containing medium (supernatant from Con A-stimulated rat spleen cells).
Adoptive transfer of EAU
For adoptive transfer, recipient animals of the same strain were injected i.p with 0.2 mL of PBS containing 5 × 106 IRBP161–180-specific T cells isolated from the spleen and LN of mice treated with 50 μg of anti-CD3 mAb or control IgG [27].
Pathological examination
Inflammation of the eye was confirmed by histopathology. Whole eyes were collected, immersed for 1 h in 4% phosphate-buffered glutaraldehyde, then transferred to 10% phosphate-buffered formaldehyde until processed. The fixed and dehydrated tissue was embedded in methacrylate and 5-μm sections were cut through the pupillary-optic nerve plane and stained with hematoxylin and eosin (H&E). Presence or absence of disease was evaluated blind by examining six sections cut at different levels for each eye. Severity of EAU was scored on a scale of 0 (no disease) to 4 (maximum disease) in half-point increments based on the presence of inflammatory cell infiltration of the iris, ciliary body, anterior chamber, and retina, where 0 =normal anterior segment and retinal architecture, with no inflammatory cells in these structures; 1 = mild inflammatory cell infiltration of the anterior segment and retina; 2 = moderate inflammatory cell infiltration of the anterior segment and retina; 3 = massive inflammatory cell infiltration of the anterior segment and retina, disorganized anterior segment and retina; and 4 = as in 3, but with photoreceptor cell damage.
Proliferation assay
T cells prepared from IRBP161–180-immunized B10RIII mice were seeded at 4 × 105 cells/well in 96-well plates, then cultured at 37°C for 60 h in a total volume of 200 μl of medium with or without IRBP161–180 in the presence of irradiated syngeneic spleen APCs (1 × 105), and [3H]thymidine incorporation during the last 8 h was assessed using a microplate scintillation counter (Packard Instrument). The proliferative response was expressed as the mean cpm ± SD of triplicate determinations.
Flow cytometry analysis
Conventional methods were used for analysis of surface molecule expression. Aliquots of 1 × 105 cells were double-stained with combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated mAbs against mouse CD4 or CD25 (all from eBioscience, San Diego, CA).
For intracellular staining of Foxp3, cells were fixed overnight in 1 ml of fixation buffer, washed, and incubated for 30 min at 4°C with anti-mouse-Foxp3 Ab (Milteny Biotec, Auburn, CA).
For intracellular cytokine staining, cells were pretreated for 6 h with 50 ng/ml of phorbol myristic acetate (PMA), 1 μg/ml of ionomycin, and 1 μg/ml of brefeldin A (Sigma, St. Louis, MO), then washed, fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience, San Diego, CA), and intracellularly stained with PE-conjugated-IFN- and FITC-conjugated IL-17 mAbs (BioLegend, San Diago, CA).
Data collection and analysis of samples were performed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences, San Jose, CA).
Isolation of eye-infiltrating cells
Cells were isolated as described previously [23]. Eyes were collected on the last day of experiments after PBS perfusion and a cell suspension was prepared by digestion for 10 minutes at 37°C with collagenase (1 mg/mL) and DNase (100 μg/mL) in RPMI 1640 containing 10% FCS. The cells were washed, resuspended in staining buffer (PBS containing 3% FCS and 0.1% sodium azide), and stained with fluorescent mAb for inflammatory cell identification by flow cytometry.
Real-time quantitative RT-PCR (qPCR) assay for the expression of the transcriptional factors T-bet and RORγt
Total RNA from splenic T cells was extracted using an RNA isolation kit (Invitrogen, Carlsbad, CA), treated with DNase I (GE Healthcare, Piscataway, NJ), and reverse transcribed into cDNA using an MMLV-RT kit (Invitrogen). Each cDNA sample was amplified for the gene of interest and β-actin (TaqMan assays; Mx3000P system; Stratagene, La Jolla, CA). The concentration of the gene of interest was determined using the comparative threshold cycle number and normalized to that of the internal β-actin control. The primers used were: β-actin, forward primer, 5′-ATCTACGAGGGCTATGCTCTCC-3′, reverse primer, 5′-ACGCTCGGTCAGGATCTTCAT-3′; T-bet, forward primer, 5’-GAGTTCCGA GCTGTGAGCATG-3’, reverse primer, 5′-GGAGCCCTGTTCCTCTGAGG-3′; RORγt, forward primer, 5′-TACGGGGTTATCACCTGTGAGG-3′, reverse primer, 5′-CCCTCTGC TTCTTGGACATTCG-3′.
ELISA
Cytokines TGF-β1, IL-10, IL-6, IL-17, and IFN-γ were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
Statistical analysis
Experiments were repeated at least twice, and usually three or more times. An unpaired Student’s t test for two sets of data or one-way ANOVA Dunnet for three or more means at one time or repeated ANOVA for clinical score of uveitis was used for statistical analysis. A P value < 0.05 was considered as significant. Values determined to be significantly different from those for controls are marked with an asterisk in the figures.
Results
Anti-CD3 mAb treatment suppresses uveitis in B10RIII mice immunized with IRBP161–180
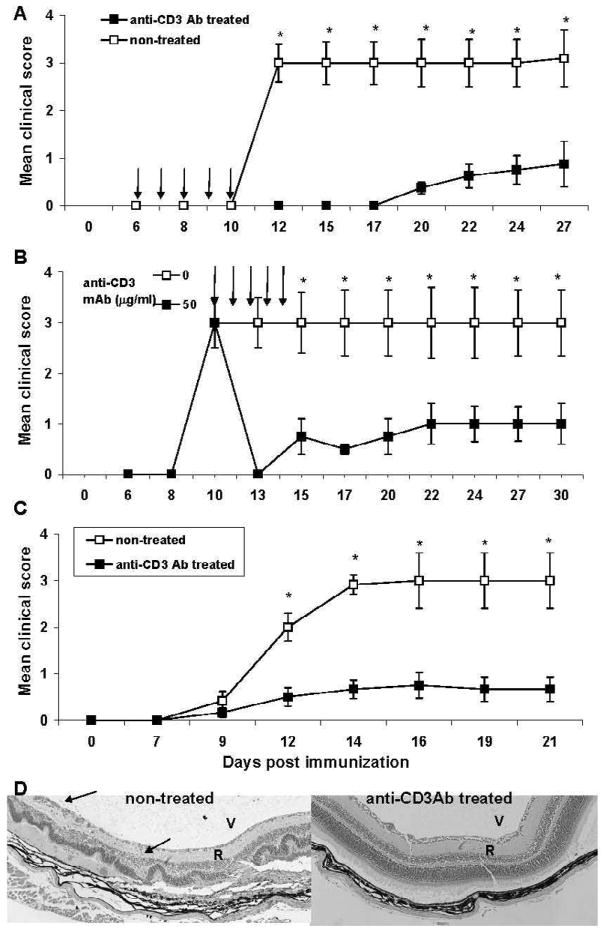
To determine whether anti-CD3 mAb had an effect on IRBP161–180-induced uveitis, IRBP161–180-immunized B10RIII mice received five consecutive i.p. injections of anti-mouse CD3 mAb F(ab’)2 (50 μg/injection) starting on day 6 after IRBP161–180 immunization (day 0), while control B10RIII mice received isotype control. We chose a regimen of 5 consecutive days based on previous protocols for both orally [20] and i.v. [10] administered anti-CD3 mAb. The results showed that, although all control animals developed full disease, anti-CD3 mAb-treated B10RIII mice developed mild disease with a delayed onset (Fig. 1A). We then determined whether delayed injection of anti-CD3 had a similar protective effect. As shown in Fig. 1B, five i.p. injections starting on day 10 (i.e. at disease onset) also had a significant effect on EAU development, indicating that anti-CD3 mAb treatment not only prevents EAU even once IRBP-specific T cells have been primed, but also suppresses EAU when give at early onset of the disease. We also tested the effect of sub-conjunctiva injections of anti-CD3 mAb treatment on EAU. Five consecutive sub-conjunctiva injections of anti-CD3 mAb at 15 μg/injection starting on day 6 post-IRBP161–180 immunization significantly reduced disease severity both clinically and histologically (Fig. 1C&D) compared to that in mice not injected with anti-CD3 mAb. Histopathology of eyes collected on day 21 revealed that control Ab-treated mice showed retinal disorganization; photoreceptor destruction and inflammatory cells in the vitreous, uvea, and retina (Fig. 1D, left). Mice treated with anti-CD3 Ab had completely normal retinal architecture (Fig. 1D, right).
Figure 1.
Systemic and local injection of B10RIII mice with anti-CD3 mAb significantly ameliorates the development of EAU. IRBP161–180-immunized B10RIII mice received (A) five i.p. injections of 50 μg of anti-CD3 mAb on days 6–10 after peptide injection, (B) five i.p. injections of 50 μg on days 10–14 (at disease onset), or (C) five sub-conjunctiva injections of 15 μg on days 6–10. Disease severity was monitored by fundoscopy twice a week starting on day 6 post-immunization and graded as described in the Materials and Methods. The data reflect two combined experiments with five mice per group. Score for each mouse is an average of both eyes. The results shown are the mean ± SD for the 10 mice. (D) Results of pathologic examination of the mice in (C) on day 21. D: Left: Note the loss of the photoreceptor layer, retinal detachment (R), and inflammatory cells (arrows) in the vitreous (V) and subretinal space. Right, Mouse treated with anti-CD3 Ab. Note the well-preserved retinal architecture.
Anti-CD3 mAb treatment results in decreased activation of IRBP-specific T cells
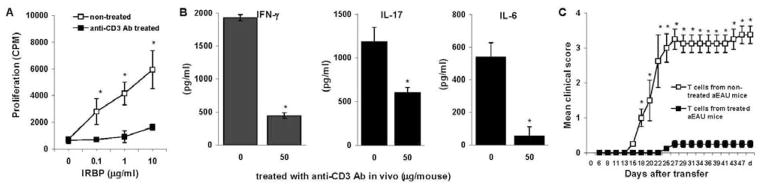
To determine the mechanism by which anti-CD3 mAb injection decreased the development of uveitis, we measured IRBP161–180-specific T cell responses in IRBP161–180-immunized animals left untreated or treated with anti-CD3 mAb. Enriched T cells from the treated mice were stimulated with immunized antigen in the presence of irradiated APCs, then proliferation, cytokine production, and disease-inducing ability of the IRBP-responding T cells were determined. As shown in Fig. 2A and B, anti-CD3 mAb-treated B10RIII mice (50 μg on days 6–10 post-immunization) showed greatly decreased T cell proliferation and the production of the pro-inflammatory cytokines IFN-γ, IL-17, and IL-6 released by IRBP specific Th1 and Th17 cells. Importantly, T cells from anti-CD3 Ab treated mice lost their ability to induce EAU when transferred into naïve animals (Fig. 2C).
Figure 2.
Anti-CD3-treated IRBP161–180-immunized B10RIII mice show a decreased T cell response to the immunizing peptide. (A), Two groups of IRBP161–180-immunized B10RIII mice (n=5) were treated with or without 50 μg of anti-CD3 mAb on days 6–10, then, on day 14, enriched splenic and LN T cells were pooled, stimulated with the immunizing peptide for 2 days in the presence of syngeneic APCs, and tested for their proliferative response in the presence of graded doses of IRBP161–180 and irradiated APCs from B10RIII mice. Shown is the means ± SD, a representative experiment of two with similar results. (B), Supernatants from the above cultures were collected and tested for IFN-γ, IL-17, and IL-6 released by IRBP-specific T cells by ELISA. The results shown are the means ± SD [n=5] and are representative of those in two experiments. (C), Two groups (n =4) of naïve B10RIII mice were adoptively transferred with 5 × 106 in vitro Ag-stimulated T cells isolated on day 14 from IRBP161–180 immunized mice treated with or without anti-CD3 mAb on days 6–10. Disease was monitored and scored twice a week by fundoscopy from day 6 after transfer till day 50. The data reflect two combined experiments with four mice per group. Score for each mouse is an average of both eyes. The results shown are the mean ± SD for the 8 mice.
Anti-CD3 mAb treatment increases the percentage of regulatory T cells and enhances the activity of antigen-specific regulatory T cells
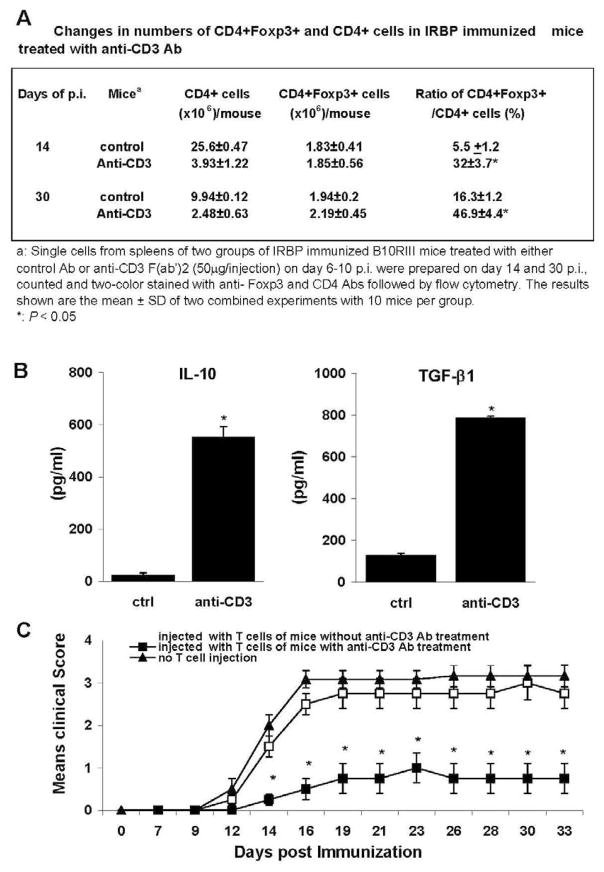
To determine whether anti-CD3 mAb treatment increased the number of regulatory T cells, thus suppressing EAU in recipient animals, we assessed the number of CD4+Foxp3+ T cells on day 14 post-immunization in IRBP161–180-immunized B10RIII mice with and without anti-CD3 treatment. As shown in Fig. 3A, the treated mice had a significantly higher ratio of CD4+Foxp3+ T cells in the spleen at both day 14 and 30 of post-immunization.
Figure 3.
Anti-CD3 mAb treatment increases the percentage of Foxp3+CD4+ T cells and enhances the regulatory activities. (A): Changes in numbers of Foxp3+CD4+ and CD4+ cells in IRBP immunized mice treated with anti-CD3 Ab. (B) Enriched T cells from IRBP immunized mice treated with anti-CD3 mAb (N=5) were stimulated with immunizing peptide and APCs for 2 days as Fig 2A and B described, then IL-10 and TGF-β released into the culture supernatant were measured by ELISA. (C). Stimulated T cells at d13 post-immunization (5 × 106) from IRBP-immunized mice treated with or without anti-CD3 mAb were injected into naïve B10RIII mice (n =4), which were then immunized with a pathogenic dose of IRBP161–180 on the same day as T cell transfer. The control group was naïve B10RIII mice without T cell injection, but immunized with IRBP161–180. Disease was monitored and scored twice a week by funduscopy from day 6 after immunization till day 33. The data reflect two combined experiments with four mice per group. Score for each mouse is an average of both eyes. The results shown are the mean ± SD for the 8 mice.
To determine whether these regulatory T cells from mice treated with anti-CD3 mAb were antigen-specific, we prepared enriched T cells on day 14 post-immunization and stimulated them with IRBP161–180 and APCs for 2 days, then examined their production of immunosuppressive cytokines and their ability to inhibit disease induction by IRBP-specific effector cells. As shown in Fig. 3B, anti-CD3 mAb treatment induced TGF-β and IL-10 secreted regulatory T cells, which, on transferred into naïve mice (3 × 106 Treg/mouse), were able to protect these mice from subsequent EAU induction by Ag immunization on the same day of Treg transfer (Fig. 3C).
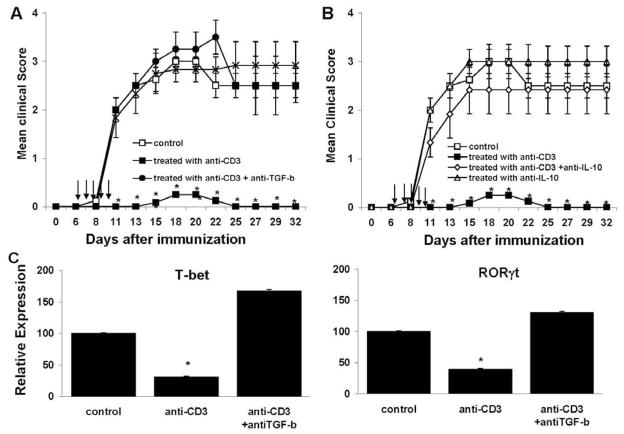
Neutralization of TGF-β or IL-10 in vivo abrogates the effect of anti-CD3 mAb treatment on the development of uveitis
To determine whether TGF-β and IL-10 were involved in preventing EAU progression after anti-CD3 mAb treatment in vivo, we administered neutralizing anti–TGF-β or anti-IL-10 or a control Ab intraperitoneally to IRBP161–180-immunized B10RIII mice treated with anti-CD3 mAb, then checked for disease. Recipients of anti-CD3 mAb had a little detectable eye inflammation, and neutralization of TGF-β or IL-10 abrogated the protective effect on EAU progression (Fig. 4A and B). Injection of anti-TGF-β or IL-10 Abs only did not change the disease course, severity and incidence. In addition, anti-CD3 mAb treatment led to low expression of the transcription factors T-bet and RORγt for Th1 and Th17 differentiation, respectively, and this effect was also prevented by anti–TGF-β Ab (Fig. 4C).
Figure 4.
Neutralization of TGF-β and IL-10 reverses the protective effect of anti-CD3 mAb. (A and B) Four groups of IRBP161–180-immunized B10RIII mice were treated with (A): Isotype control, anti-CD3 (50 μg/injection), anti-TGF-β (500 μg) or both anti-CD3 and anti–TGF-β Abs, or (B) Isotype control, anti-CD3, anti-IL-10 (250 μg) or both anti-CD3 and anti–IL-10 Abs on days 6 –10 post-immunization, then disease was monitored clinically. The data reflect two combined experiments with four mice per group. Score for each mouse is an average of both eyes. The results shown are the mean ± SD for the 8 mice. (C) At 13 days post-immunization, the T cells from mice in (A) groups were stimulated with immunizing Ag for 2 days and assessed for mRNA levels for the transcription factors ROR t and T-bet for Th17 and Th1, respectively, by real time qPCR. Pooled mRNAs for each group were used for the assay; therefore, although data represent a group average of four mice, no error bars could be generated. Shown is a representative experiment of two with similar results.
Anti-CD3 mAb treatment results in long-term tolerance
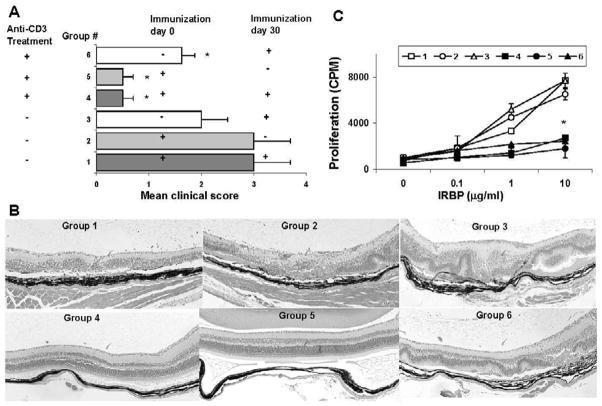
Having shown that anti-CD3 mAb treatment protected mice from a primary episode of EAU, we wished to investigate whether long-term tolerance was achieved as a result of this treatment. To test this, we re-immunized mice on day 30 after the first immunization with Ag (d0), i.e. 20 days after cessation of Ab treatment on days 6–10 (Fig. 5A), then, on day 50 (20 days after re-challenge), harvested the eyes and spleen for examination of pathology and cellular responses. As shown in Fig. 5A, the re-challenged anti-CD3 mAb-treated group 4 had a mild uveitis score on day 50, whereas the corresponding control mice (group 1, without anti-CD3 mAb treatment, but with primary and secondary immunization) showed severe disease. The disease score in the non re-challenged group 5 mice remained mild between days 30 and 50, when the mAb had been cleared away (Fig. 5A). Even after re-challenge, the disease score remained lower in group 4 than in group 3, which received its primary immunization on day 30 and exhibited typical day 21 scores. The clinical score matched the histological presentation in all groups (Fig. 5B).
Figure 5.
Anti-CD3 mAb treatment protects mice from secondary induction of uveitis. (A) EAU score: B10RIII mice were immunized with IRBP161–180 on day 0 and/or day 30 and treated with 50 μg of anti-CD3 mAb or control Ab on days 6–10, then were sacrificed on day 50. + and - indicate whether the group was immunized on the indicated day. The EAU score observed by fundoscopy is two combined experiments with four mice per group. Score for each mouse is an average of both eyes. The results shown are the mean ± SD for the 8 mice. (B) Representative pathological results for each group at day 50. (C) Spleen and LN (n=4) were collected on day 50 and a proliferation assay in response to the indicated concentration of IRBP peptide measured. Shown (mean±SD) is a representative experiment of two with similar results.
Splenic and LN T cells harvested on day 50 from all mice were tested for proliferation in response to IRBP peptide. As with the EAU score, all three anti-CD3 mAb-treated groups (groups 4–6), with or without re-challenge, had weaker proliferative responses than the corresponding control groups not receiving anti-CD3 mAb treatment (group 1–3). Notably, the responses of these anti-CD3 mAb-treated groups were again lower than those in control group 3 mice, which were only immunized on day 30 (Fig. 5C). These data suggest that anti-CD3 mAb blockade not only prevents the primary EAU episode, but also inhibits the generation of a memory response.
Discussion
A new, clinically applicable immunosuppressive regimen that inhibits antigen-specific T cells, increases the number of Treg cells, reverses early-onset uveitis, and prevents secondary induction of ocular inflammation is the ultimate goal in the clinic, since both chronic and recurrent uveitis are refractory to currently available immunosuppressors, the side-effects of which can lead to visual disability after long-term use. Previously, we used antibodies to block the interactions between costimulatory ligands and receptors on T cells and APCs in uveitis models and obtained positive results, but only when the antibodies were given before disease establishment. In this study, we chose short-term anti-CD3 mAb therapy on the base of its successful use in experimental studies in the prevention and treatment of multiple animal models of T cell- and/or Ab-mediated autoimmunity, such as the NOD model of autoimmune diabetes [3;6;8], EAE [25] , and systemic lupus erythematosus [35]. In our study, we focused on therapies targeting uveitis at the early stage of the disease. We also assessed the efficacy of local sub-conjunctiva injection of this mAb. Furthermore, we examined whether this therapy could induce long-term tolerance and prevent secondary induction of the disease. A promising observation in our study is that short-term treatment with anti-CD3 mAb at a time when autoimmune attack of the eye has already been initiated suppressed the disease. Equally encouragingly, this treatment resulted in long-term resistance to secondary induction of uveitis.
The therapeutic effect of anti-CD3 mAb on early-onset uveitis and the secondary induction of the disease is probably related to depletion of the effector CD3+ T cell population, which is made up not only of αβT cells, but also γδ T cells. γδ T cells are critical in promoting the differentiation of Th17 cells, which are involved in tissue chronic inflammation [12]. Anti-CD3 mAb selectively suppressed the activation and expansion of pathogenic Ag-specific Th1 and Th17 cells, resulting in markedly reduced production of IL-17 and IFN-γ by the pathogenic Th1 and Th17 cells. Newly activated effector T cells seem to be more vulnerable to anti-CD3 mAb-mediated cell death, since the antibody was effective when used during IRBP-specific T cell priming and at the early onset of the disease. The specific inhibitory effect of anti-CD3 mAb on newly activated T cells, but not on resting T cells, was also demonstrated in group 6 of Fig 5, in which mice receiving anti-CD3 mAb on d6–10 and immunized with Ag on day 30 developed uveitis.
On the other hand, anti-CD3 mAb treatment significantly increased the percentage of multiple regulatory T cell populations, including CD4+CD25+Foxp3+ (fig 3A) and CD1d-restricted natural killer T (iNKT) cells (data not shown). The increase in ratio of CD4+ over CD4+ Foxp3+ population resulted from a decrease in number of CD4+ T cells; the number of CD4+Fop3+ T cells, however, was not much affected by anti-CD3 Ab (Fig 3A). It has been reported that administration of anti-CD3 mAb can reverse new-onset diabetes in nonobese diabetic mice by the induction and maintenance of immune regulatory IL-10-secreting iNKT cells [11]. Although anti-CD3 mAb induced regulatory CD8+CD25+ T cells in patients with type 1 diabetes [4], we did not detected the increased number of CD8+CD122+ Treg in our anti-CD3 Ab treated mice (data shown).
Adoptive transfer of splenic T cells from anti-CD3 mAb-treated mice protected recipient mice from the development of uveitis induced by immunization with IRBP (Fig 3C), indicating a direct inhibitory effect of Treg cells on antigen-specific effector T cells in vivo. The inhibitory effect of Treg cells on the function of Ag-specific Th1 and Th17 cells might be associated with inhibitory cytokines, such as IL-10 and TGF-β1, secreted by regulatory T cells following administration of anti-CD3 mAb. Neutralization of TGF-β using anti-TGF-β Ab reversed the inhibition of transcription factor ROR T and T-bet activity in the differentiation of Th1 and Th17 cells and the reduction of disease severity, compatible with the reports that TGF-β-induced Foxp3 Treg cells inhibit the differentiation of Th17 and Th1 cells by reducing RORγt and T-bet levels [19;37]. Similarly, neutralization of IL-10 using anti-IL-10 Ab reversed the suppressive effect of anti-CD3 Ab treatment on EAU. These results demonstrate that anti-CD3 Ab induced Treg cells mediate disease suppression in vivo via both IL-10 and TGF-β. Production and action of these two cytokines are interrelated and are likely to involve positive feedback loops in which IL-10 enhances the expression of TGF-β and vice versa.
Two mechanisms have been proposed to explain how anti-CD3 Ab inhibits Ag-specific T effector cells and protects against T cell-mediated autoimmune disease. One is that anti-CD3 mAb primarily induces pathogenic T cell tolerance, apoptosis, or alterations in cell trafficking [7] and the other is that it induces Treg cells, which then downregulate the function of T effector cells by direct cell-cell contact and in a TGF-β-dependent fashion [3]. Our study showed that the action of the anti-CD3 mAb involved direct inhibitory effects on pathogenic T cells and/or the induction of populations of regulatory cells. The suppressive function of anti-CD3-induced Treg cells appeared to depend on both IL-10 and TGF-β. However, why anti-CD3 mAb has opposite effects on effector and regulatory T cells, both of which express CD3, remains to be addressed.
In summary, our study confirms that short-term treatment with a low dose of anti-CD3 mAb in its F(ab’)2 form, either systemically or locally at disease onset, can reduce disease severity and prevent the re-induction of uveitis by a direct action on pathogenic T cells and/or the induction of populations of regulatory cells, and provides a potentially alternative immune therapy in the clinic for patients with acute and chronic/recurrent uveitis.
Acknowledgments
Supported in part by NIH grants NEI EY12974, EY14599, Vision Research Infrastructure Development (R24 EY015636), RPB career development award and Lew R Wasserman Merit Award (HS), and the Commonwealth of Kentucky Research Challenge Trust Fund (HK). The authors thank Tom Barkas for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agarwal RK, Kang Y, Zambidis E, Scott DW, Chan CC, Caspi RR. Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J Clin Invest. 2000;106:245–252. doi: 10.1172/JCI9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagenstose LM, Agarwal RK, Silver PB, Harlan DM, Hoffmann SC, Kampen RL, Chan CC, Caspi RR. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol. 2005;175:124–130. doi: 10.4049/jimmunol.175.1.124. [DOI] [PubMed] [Google Scholar]
- 3.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 4.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos HH, Bach JF, Chatenoud L. Devising murine models to better adapt clinical protocols: sequential low-dose treatment with anti-CD3 and anti-CD4 monoclonal antibodies to prevent fully mismatched allograft rejection. Transplant Proc. 1993;25:798–799. [PubMed] [Google Scholar]
- 6.Chatenoud L. CD3-specific antibodies as promising tools to aim at immune tolerance in the clinic. Int Rev Immunol. 2006;25:215–233. doi: 10.1080/08830180600743032. [DOI] [PubMed] [Google Scholar]
- 7.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 8.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 9.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Han G, Wang J, Wang R, Xu R, Shen B, Qian J, Li Y. Essential roles of TGF-beta in anti-CD3 antibody therapy: reversal of diabetes in nonobese diabetic mice independent of Foxp3+CD4+ regulatory T cells. J Leukoc Biol. 2008;83:280–287. doi: 10.1189/jlb.0707498. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Han G, Wang J, Wang R, Xu R, Shen B, Qian J, Li Y. Induction of active tolerance and involvement of CD1d-restricted natural killer T cells in anti-CD3 F(ab')2 treatment-reversed new-onset diabetes in nonobese diabetic mice. Am J Pathol. 2008;172:972–979. doi: 10.2353/ajpath.2008.070159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK, Kaplan HJ, Sun D. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donoso LA, Merryman CF, Sery T, Sanders R, Vrabec T, Fong SL. Human interstitial retinoid binding protein. A potent uveitopathogenic agent for the induction of experimental autoimmune uveitis. J Immunol. 1989;143:79–83. [PubMed] [Google Scholar]
- 14.Gery I, Wiggert B, Redmond TM, Kuwabara T, Crawford MA, Vistica BP, Chader GJ. Uveoretinitis and pinealitis induced by immunization with interphotoreceptor retinoid-binding protein. Invest Ophthalmol Vis Sci. 1986;27:1296–1300. [PubMed] [Google Scholar]
- 15.Herold KC, Bluestone JA, Montag AG, Parihar A, Wiegner A, Gress RE, Hirsch R. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41:385–391. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- 16.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111:409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu LH, Redmond TM, Sanui H, Kuwabara T, McAllister CG, Wiggert B, Chader GJ, Gery I. Rat T-cell lines specific to a nonimmunodominant determinant of a retinal protein (IRBP) produce uveoretinitis and pinealitis. Cell Immunol. 1989;122:251–261. doi: 10.1016/0008-8749(89)90165-2. [DOI] [PubMed] [Google Scholar]
- 19.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 21.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van D, Crenier VL, De BC, Candon S, Waldmann H, Ziegler AG, Chatenoud L, Pipeleers D. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 22.Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 23.Liao T, Ke Y, Shao WH, Haribabu B, Kaplan HJ, Sun D, Shao H. Blockade of the interaction of leukotriene b4 with its receptor prevents development of autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006;47:1543–1549. doi: 10.1167/iovs.05-1238. [DOI] [PubMed] [Google Scholar]
- 24.Mottram PL, Murray-Segal LJ, Han W, Maguire J, Stein-Oakley AN. Remission and pancreas isograft survival in recent onset diabetic NOD mice after treatment with low-dose anti-CD3 monoclonal antibodies. Transpl Immunol. 2002;10:63–72. doi: 10.1016/s0966-3274(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 25.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso A, Wu H, Chen ML, Gandhi R, Miller A, Maron R, Weiner HL. New immunosuppressive approaches: oral administration of CD3-specific antibody to treat autoimmunity. J Neurol Sci. 2008;274:9–12. doi: 10.1016/j.jns.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz RH. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 27.Shao H, Fu Y, Liao T, Peng Y, Chen L, Kaplan HJ, Sun D. Anti-CD137 mAb treatment inhibits experimental autoimmune uveitis by limiting expansion and increasing apoptotic death of uveitogenic T cells. Invest Ophthalmol Vis Sci. 2005;46:596–603. doi: 10.1167/iovs.04-0835. [DOI] [PubMed] [Google Scholar]
- 28.Shao H, Lei S, Sun SL, Kaplan HJ, Sun D. Conversion of monophasic to recurrent autoimmune disease by autoreactive T cell subsets. J Immunol. 2003;171:5624–5630. doi: 10.4049/jimmunol.171.10.5624. [DOI] [PubMed] [Google Scholar]
- 29.Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Silver PB, Hathcock KS, Chan CC, Wiggert B, Caspi RR. Blockade of costimulation through B7/CD28 inhibits experimental autoimmune uveoretinitis, but does not induce long-term tolerance. J Immunol. 2000;165:5041–5047. doi: 10.4049/jimmunol.165.9.5041. [DOI] [PubMed] [Google Scholar]
- 31.Tran GT, Carter N, He XY, Spicer TS, Plain KM, Nicolls M, Hall BM, Hodgkinson SJ. Reversal of experimental allergic encephalomyelitis with non-mitogenic, non-depleting anti-CD3 mAb therapy with a preferential effect on T(h)1 cells that is augmented by IL-4. Int Immunol. 2001;13:1109–1120. doi: 10.1093/intimm/13.9.1109. [DOI] [PubMed] [Google Scholar]
- 32.Utset TO, Auger JA, Peace D, Zivin RA, Xu D, Jolliffe L, Alegre ML, Bluestone JA, Clark MR. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol. 2002;29:1907–1913. [PubMed] [Google Scholar]
- 33.von Herrath MG, Coon B, Wolfe T, Chatenoud L. Nonmitogenic CD3 antibody reverses virally induced (rat insulin promoter-lymphocytic choriomeningitis virus) autoimmune diabetes without impeding viral clearance. J Immunol. 2002;168:933–941. doi: 10.4049/jimmunol.168.2.933. [DOI] [PubMed] [Google Scholar]
- 34.Wacker WB, Donoso LA, Kalsow CM, Yankeelov JA, Jr, Organisciak DT. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119:1949–1958. [PubMed] [Google Scholar]
- 35.Wu HY, Center EM, Tsokos GC, Weiner HL. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus. 2009;18:586–596. doi: 10.1177/0961203308100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L. TGF-[bgr]-induced Foxp3 inhibits TH17 cell differentiation by antagonizing ROR[ggr]t function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]