Abstract
Background:
Several cervical laminectomies and instrumented posterior cervical fusions utilize iliac autograft supplemented with demineralized bone matrix, or bone morphogenetic protein, but few utilize artificial bone graft expanders. Here we analyzed whether posterior cervical fusions could effectively utilize iliac autograft supplemented with an artificial bone graft expander, Beta Tricalcium Phosphate [B-TCP]
Materials and Methods:
Fifty-three severely myelopathic patients [average Nurick Score 4.1], averaging 65.3 years of age, underwent posterior cervical laminectomies [average 2.3 levels] and multilevel instrumented fusions [average 7.5 levels] utilizing iliac crest autograft and B-TCP. Pathology addressed included multilevel spondylosis accompanied by ossification of the posterior longitudinal ligament [24 patients], ossification of the yellow ligament [27 patients], and instability [53 patients]. Fusion rates [dynamic X-ray, two-dimensional computerized axial tomography (2D-CT) and outcomes [Nurick Grades, Odom's Criteria, SF-36] were assessed at 3, 6, and 12 months postoperatively.
Results:
Fusion was confirmed by two independent neuroradiologists utilizing dynamic X-ray studies [100% of patients] and 2D-CT studies [86.8% of patients] an average of 5.4 months postoperatively. Although there were no symptomatic pseudarthroses, three smokers exhibited delayed fusions [8 postoperative months]. Within 1 postoperative year, patients improved an average of 2.7 Nurick Grades [Nurick Score 1.4], Odom's criteria revealed 48 good/excellent, and 5 fair/poor outcomes, and improvement on all 8 SF-36 Health Scales [maximal on Bodily Pain [+21.96].
Conclusions:
High fusion rates and improved neurological outcomes were achieved within one year for 53 patients undergoing multilevel level cervical laminectomies with posterior instrumented fusions utilizing iliac autograft supplemented with B-TCP.
Keywords: Artificial Bone Graft Expander, Beta TriCalcium Phosphate, Cervical Laminectomy, Iliac Autograft, Multilevel Instrumented Fusion
INTRODUCTION
Select patients with cervical myelopathy attributed to spinal stenosis and other pathology, with adequate preservation of the cervical lordotic curvature, may be successfully managed with cervical laminectomies and multilevel posterior instrumented fusions. The majority of lateral fusion masses have consisted of lamina and/or iliac crest autograft supplemented with allograft bone graft expanders [e.g., demineralized bone matrix [DBM] or bone morphogenetic protein derivatives [BMP]. However, DBM may adversely transmit viral infections [e.g., HIV, Hepatitis, etc.] or promote allergic/immunologic reactions, while BMP may contribute to cervical cord swelling. This prompted a search for an artificial bone graft expander that could safely be utilized in the cervical spine without complications. Such an artificial bone graft expander, Beta Tricalcium phosphate [B-TCP; Vitoss, OrthoVita, Malvern, PA, USA] was utilized to supplement iliac crest autograft in this series of 53 patients undergoing multilevel cervical laminectomies and posterior cervical fusions. Over the course of the first postoperative year, fusion rates were assessed utilizing both dynamic X-rays and 2D-CT studies, while outcomes were evaluated based upon Odom's Criteria, Nurick Grades, and SF-36 questionnaires.
MATERIALS AND METHODS
Clinical Data: In this series, 53 patients averaged 65.3 years of age [range 4782]; 37 patients [70%] were over 60 years of age [Table 1]. There were 32 males and 21 females. No patient in this series had prior cervical surgery. Patients exhibited multiple comorbidities, namely, hypertension [33 patients], osteoporosis [28 patients], obesity [26 patients], anxiety/depression [12 patients], diabetes [4 patients], and ethanol abuse [3 patients] [Table 1]. All 53 patients exhibited magnetic resonance imaging [MR] /CT-documented stenosis/spondylosis with/without ossification of the yellow ligament [OYL; 27 patients], ossification of the posterior longitudinal ligament [OPLL; 24 patients], olisthesis [12 patients], and instability [53 patients] [Figures 1–3]. Patients underwent multilevel fusions, typically extending several segments cephalad and caudad to the levels of laminectomy and/or the presence of accompanying instability/olisthesis [Figure 4]. Furthermore, in the presence of OPLL, extended levels of fusion were performed to avoid/halt cephalad/caudad progression of OPLL. In addition, 32 patients exhibited hyperintense intrinsic cord signals on T2-weighted MR images. The average preoperative Nurick Score was 4.1 [moderate/severe myelopathy].
Table 1.
Clinical Data for Patients Undergoing Cervical Laminectomies/Multilevel Posterior Fusions
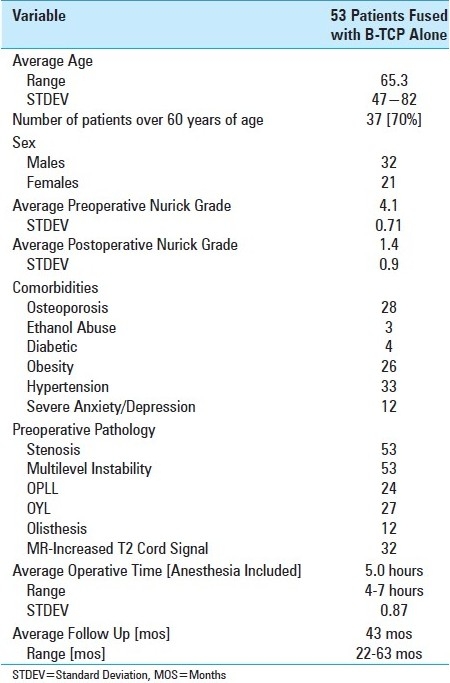
Figure 1.
A midline sagittal CT documents multilevel ventral and dorsal/lateral cord compromise/stenosis at the C3-C7 levels. Compression was attributed to both ventral spondylosis/OPLL and dorsolateral OYL. Following laminectomies [C4, C5, C6, and undercutting of C7] with posterior rod/eyelet fusion [C2-T2], the patient's preoperative myelopathy resolved [Grade IV to Grade I].
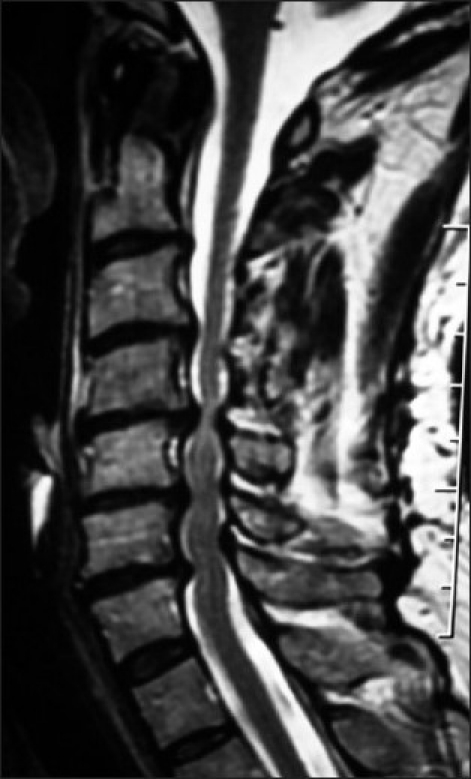
Figure 3.
A T2 sagittal MR study [same patient in Figure 2] revealed moderate focal dorsolateral C3-C4 OYL with mild ventral spondylosis resulting in moderate cord compression. More significantly, severe dorsolateral shingling of the C6 and C7 laminae accompanied by marked OYL enfolding at C5-C6 [moderate], and C6-C7 [marked] levels contributed to marked cord compromise.
Figure 4.
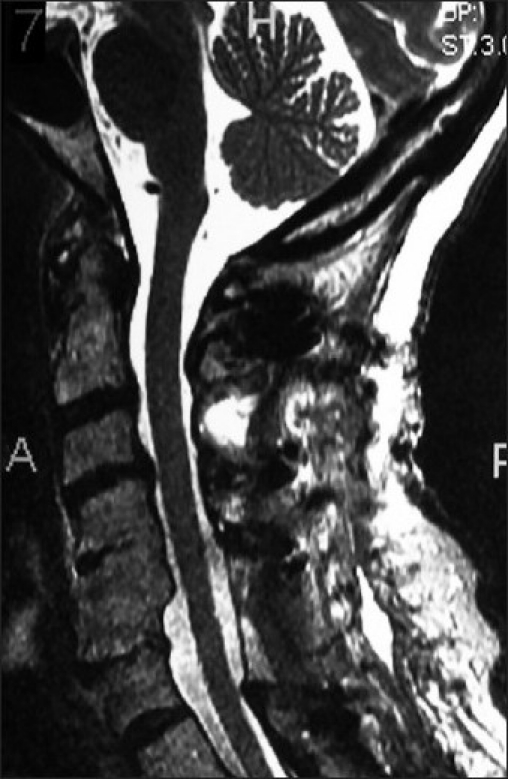
A postoperative T2-weighted MR study [same patient as Figure 2] revealed dorsolateral laminectomies involving the C3, C6, C7 levels. Observe the adequate degree of cord decompression on this study.
Figure 2.
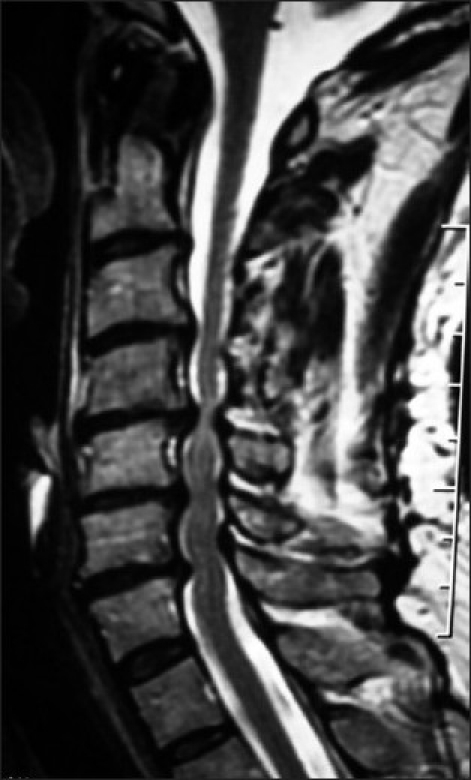
A midline sagittal 2D-CT study shows marked ventral degenerative spondylotic changes at the C5 and C6 level. What was not seen on this study, but visualized on the MR was marked OYL contributing to dorsolateral cord compression at the C3-C4, C5-C6, C6-C7, and the C7-T1 levels.
Operative Data: Patients underwent 1-3 level laminectomies [average 2.3] and multilevel posterior fusions [average 7.5] [Table 2] [Figures 4,5]. Fusions utilized a bilateral titanium rod-eyelet construct [Vertex Rod/Eyelet System; Medtronic, Memphis, TN, USA] and braided titanium cables passed through the base of the intact spinous processes, hand-tightened in a “cerclage fashion” [Figures 5,6]. Harvested laminar and iliac crest autograft were applied bilaterally over the decorticated laminae [cephalad and caudad to the laminectomy site] and facet joints at all levels. The fusion mass on each side was comprised of half of the harvested lamina and iliac crest autograft supplemented with 1/2 [12.5 mm] of 2.5 cm × 10 cm strip [cut longitudinally] of B-TCP impregnated with 10 cc of iliac crest bone marrow aspirate. Typically, the strips opposite the laminectomy site were cut to 1/4 of the width of the sheet [6.25 mm]; the residual B-TCP was applied over the intact laminae cephalad and caudad to this site. The average operative time was 5.0 hours. Patients were followed an average of 43 postoperative months [range 2263 mos].
Table 2.
Surgical Data
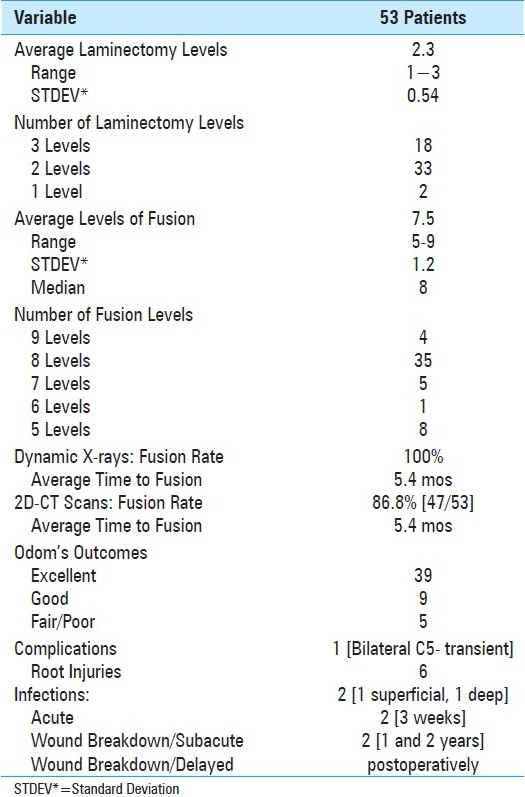
Figure 5.
This photograph demonstrates the application of the eyelets on the rods prior to positioning and placement. Utilizing the rod bender, two similarly configured, mirror-image rods have eyelets applied opposite the retained spinous processes. The rods are then applied dorsally, with the eyelets affixed ventrally. This is followed by passage of a braided titanium cable through the base of the spinous processes; each side of the wire is then brought up through the eyelets and then tightened in a cerclage fashion over the dorsal/superficial rods.
Figure 6.
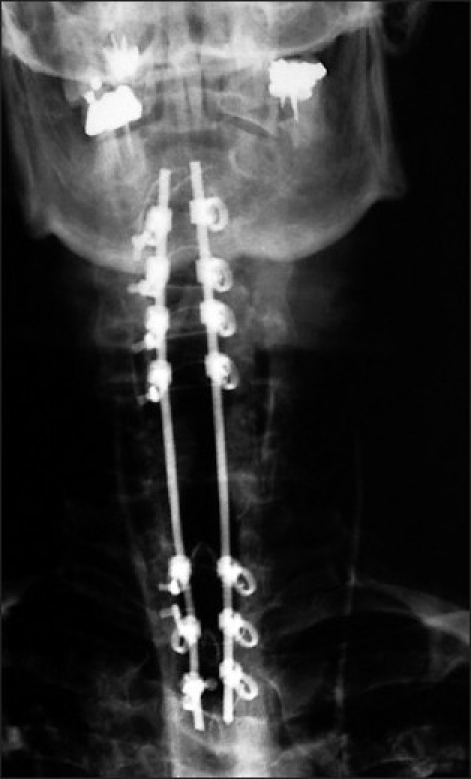
This AP X-ray, following a laminectomy of C5 and C6 demonstrates a rod/eyelet fusion construct involving the C2, C3, C4 and the C7, T1, and T2 levels. The eyelets are located ventrally, while the crimped wires and rods are found dorsally.
Outcomes Assessment: Odom's Criteria, Nurick Grades, and SF-36 questionnaire data were evaluated preoperatively, and at 6 weeks, 3 months, 6 months, and 12 months postoperatively. To simplify the presentation of SF-36 data, they are presented in 3 groups; mild improvement [05 points], moderate improvement [>5 to 10 points], and marked improvement [>10 points].
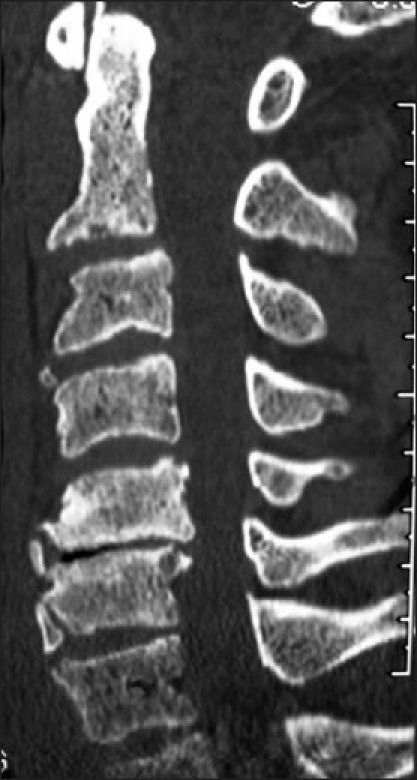
X-ray and 2D-CT Fusion Criteria: Confirmation of fusion utilizing both dynamic X-rays and 2D-CT evaluations was independently performed by two neuroradiologists blinded to the study design [Figures 7,8]. Each reviewed both studies performed 3, 6, and up to the time of fusion [12 months postoperatively]. Dynamic X-ray fusion criteria included the documentation of bridging trabecular bone/lack of lucency between the fusion mass and laminae and/or facet joints, combined with the lack of motion [translation, angulation, and less than 1 mm of motion between adjacent spinous processes]. Fusion criteria on 2D-CT scans required the demonstration of bridging trabecular bone/lack of lucency between the fusion mass and underlying lamina, and/or facet joints [Figures 7,8].
Figure 7.
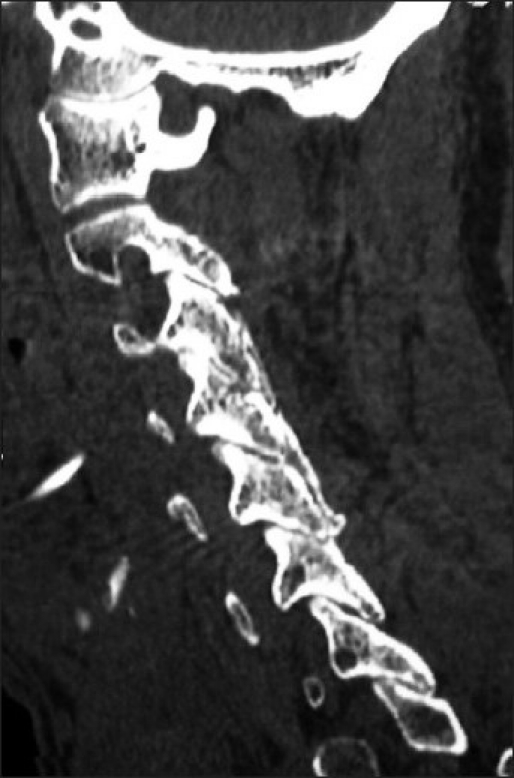
Six months following C3 and C4 laminectomies with C2- -C6 fusion, the parasagittal 2D-CT scan demonstrated bony trabeculation/continuity of the dorsolateral fusion mass with the underlying facets.
Figure 8.
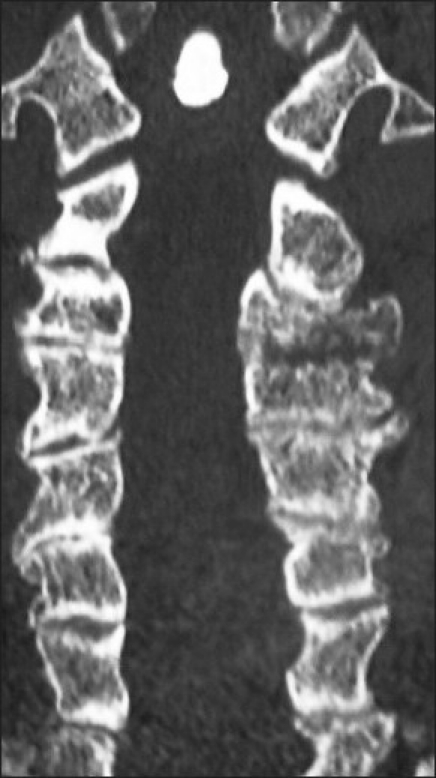
On this 6-month postoperative coronal 2D-CT scan, facet fusion is seen at multiple levels. Note the presence of bony trabeculation and lack of lucency at multiple facet interfaces.
RESULTS
Fusion Rates: At an average of 5.4 months postoperatively, the dynamic X-ray fusion rate was 100%, while the 2D-CT documented fusion rate was 86.8% [Table 2]. Although three smokers exhibited delayed fusions that occurred 8 months postoperatively, no patients required secondary surgery for symptomatic postoperative pseudarthrosis.
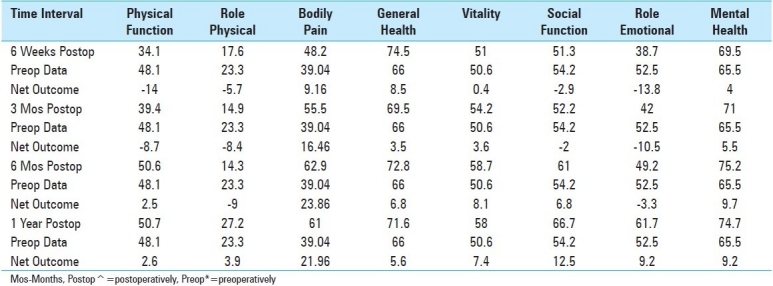
Outcomes: Outcomes were assessed at 1 postoperative year utilizing three different scoring techniques [Tables 1,3,4]. At 1 postoperative year, patients improved to an average Nurick Score of 1.4 [mild/moderate residual myelopathy], reflecting an average of 2.7 Nurick Grades of improvement. At 1 postoperative year, Odom's criteria revealed that 39 patients exhibited excellent, 9 had good, and 5 demonstrated fair/poor outcomes. Also within the first postoperative year, 53 patients improved on all 8 SF-36 Health Scales [Tables 3,4]. Within the first 6 weeks, transient deterioration was observed on four health scales Physical Function [PF], Role Physical [RP], Social Function [SF], and Role Emotional [RE]. Mild improvement [<05 points] was observed on 2 health scales Vitality [V], Mental Health [MH], and moderate improvement was seen on 2 health scales Bodily Pain [BP], and General Health [GH]. By the third postoperative month, transient deterioration was still noted on 4 health scales [PF, RP, SF, and RE], while mild improvement was seen on 2 [GH, V], moderate improvement on 1 [MH], and marked improvement on 1 health scale [BP]. At 6 postoperative months, transient deterioration was still noted on 2 health scales [RP, RE], mild improvement on 1 [PF], moderate improvement on 4 [GH, V, SF, MH], and marked improvement on 1 health scale [BP]. Finally, at one postoperative year, mild improvement was observed for 2 [PF, RP], moderate for 4 [GH, V, RE, MH], and marked improvement on 2 health scales [BP, SF].
Table 3.
SF-36 Outcomes for 53 Patients Undergoing Cervical Laminectomy and Multilevel Posterior Fusions
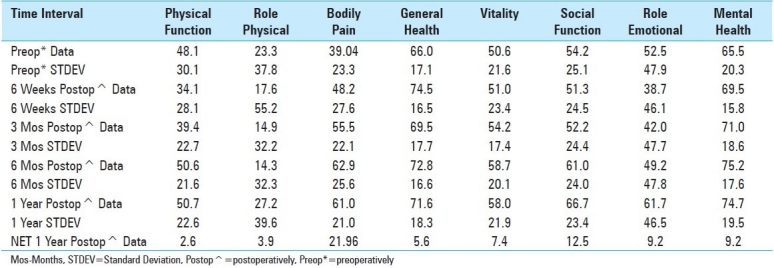
Table 4.
Comparison of 8 Health Scales over the 4 Postoperative Time Intervals For 53 Patients
Complications: Postoperative morbidity was limited, and there were no mortalities among the 53 patients undergoing cervical laminectomies and multilevel-instrumented fusions utilizing iliac crest autograft, and the artificial bone graft expander, B-TCP. One patient, a smoker and ethanol abuser in his mid-fifties, developed transient bilateral C5 nerve root palsies; the right occurred on postoperative day 3, the left on postoperative day 6. Both deficits fully resolved within 3 postoperative months. Two other patients developed postoperative wound infections. One infection was superficial, while the other was deep [Staphylococcus aureus; Methicillin sensitive] requiring 6 weeks of intravenous antibiotic therapy. Additionally, 4 patients developed wound breakdowns [two at 3 weeks (on Aspirin/Plavix), one at 1 year (on Warfarin), one at 2 years (on Warfarin)]. However, all were attributed to antiplatelet aggregants/anticoagulants as none exhibited wound infections [all cultures negative].
DISCUSSION
Fusion Techniques: Although cervical laminectomies alone may provide short-term improvement for cervical spondylotic myelopathy (CSM), they are associated with a 15%-37.5% incidence of postoperative instability/kyphosis, and accompanying delayed deterioration.[3,6,9] Such postoperative kyphosis following laminectomies [average 4.6 segments] in patients with CSM and/or OPLL may be avoided by performing lateral mass plate fixation.[5] Reports indicate a 07.7% incidence of neural, and 00.6% frequency of vertebral artery injuries [up to 1.4%] associated with the utilization of lateral mass screws.[1,2,4,7,8,10,11] Nevertheless, in a cadaveric study, utilizing stealth CT guidance, lateral mass screw placement was associated with a 10.6% incidence of critical breaches, and a 13.4% frequency of non-critical breaches.[12] Furthermore, in clinical studies, critical breaches associated with lateral mass screw placement ranged from 1.4%9%, non-critical breaches ranged from 2131%, while other screw-related complications were observed in up to 1.4% of cases.[1,2,4,7,8,10,11] In this series, utilization of the bilateral titanium rod/eyelet construct without lateral mass screws, but utilizing spinous process based cerclage wires, resulted in neither neural nor vascular injuries.
Fusion Rates: Other series employing lateral mass screw/rod fixation and allograft/DBM attained similar fusion rates varying from 8998.6% [typically utilizing dynamic X-ray criteria alone] for “open procedures, with some minimally invasive techniques reporting 100% fusion rates utilizing varying fusion criteria”.[4,8,11,12] In this series, fusion rates utilizing a rod/eyelet/cable system with autograft and the artificial bone graft expander B-TCP, documented on both dynamic X-rays and 2D-CT studies [100% dynamic X-rays, 86.6% with 2D-CT] proved comparable to those achieved with lateral mass screws/rods and allograft/DBM. Notably, none of these 53 patients developed symptomatic pseudarthrosis, and none required secondary fusion surgery. Furthermore, the utilization of B-TCP avoided the inherent risks of allograft/DBM, which included infection [e.g., HIV, hepatitis, slow viruses, etc.], allergic, or immunologic reactions.
CONCLUSION
High rates of fusion [X-ray 100%, 2D-CT 86.6%] were achieved following cervical laminectomies accompanied by multilevel posterior rod/eyelet/cable fusions utilizing lamina autograft and an artificial bone graft expander, B-TCP. Fusion rates proved comparable to those achieved with allograft/DBM, while avoiding the inherent risks of other allografts.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/15/76458
REFERENCES
- 1.Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25:962–9. doi: 10.1097/00007632-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Deen HG, Birch BD, Wharen RE, Reimer R. Lateral mass screw-rod fixation of the cervical spine: A prospective clinical series with 1-year follow-up. Spine J. 2003;3:489–95. [PubMed] [Google Scholar]
- 3.Guigi P, Benoist M, Deburge A. Spinal deformity and instability after multilevel cervical laminectomy for spondylotic myelopathy. Spine. 1998;23:440–7. doi: 10.1097/00007632-199802150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Heller JG, Silcox DH, Sutterlin CE. Complications of posterior cervical plating. Spine. 1995;20:2442–8. doi: 10.1097/00007632-199511001-00013. [DOI] [PubMed] [Google Scholar]
- 5.Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: Effects of cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081–7. [PubMed] [Google Scholar]
- 6.Kaptain GJ, Simmons NE, Replogle RE, Pobereskin L. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:199–204. doi: 10.3171/spi.2000.93.2.0199. [DOI] [PubMed] [Google Scholar]
- 7.Kast E, Mohr K, Richter HP, Borm W. Complications of transpedicular screw fixation in the cervical spine. Eur Spine J. 2006;15:327–49. doi: 10.1007/s00586-004-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muffoletto AJ, Hadjipavlou AG, Jensen RE, Nauta HJ, Necessary JT, Norcross-Nechay K. Techniques and pitfalls of cervical lateral mass plate fixation. Am J Orthop. 2000;29:897–903. [PubMed] [Google Scholar]
- 9.Mummaneni PV, Kaiser MG, Matz PG, Anderson PA, Groff MW, Heary RF, et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130–41. doi: 10.3171/2009.3.SPINE08728. [DOI] [PubMed] [Google Scholar]
- 10.Pateder DB, Carbone JJ. Lateral mass screw fixation for cervical spine trauma: Associated complications and efficacy in maintaining alignment. Spine J. 2006;6:40–3. doi: 10.1016/j.spinee.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Sekhon LH. Posterior cervical lateral mass screw fixation: Analysis of 1026 consecutive screws in 143 patients. J Spinal Disord Tech. 2005;18:297–303. doi: 10.1097/01.bsd.0000166640.23448.09. [DOI] [PubMed] [Google Scholar]
- 12.Wang MY, Levi AD. Minimally invasive lateral mass screw fixation in the cervical spine: Initial clinical experience with long-term follow-up. Neurosurgery. 2006;58:907–12. doi: 10.1227/01.NEU.0000209929.38213.72. [DOI] [PubMed] [Google Scholar]


