Abstract
Although studies have suggested that a patient’s perceived cost-benefit of a medical intervention could affect his or her utilization of the intervention, the economic value of influenza vaccine from the patient’s perspective remains unclear. Therefore, we developed a stochastic decision analytic computer model representing an adult’s decision of whether to get vaccinated. Different scenarios explored the impact of the patient being insured versus uninsured, influenza attack rate, vaccine administration costs and vaccination time costs. Results indicated that cost of avoiding influenza was fairly low, with one driver being required vaccination time. To encourage vaccination, decision makers may want to focus on ways to reduce this time, such as vaccinating at work, churches, or other normally frequented locations.
Keywords: Influenza Vaccine, H1N1 Patients, Economics
1. INTRODUCTION
Vaccination coverage for influenza among adults has remained well below the desired rates set forth in Healthy People 2010.[1–2] Approximately 32% of the general public and less than 70% of high risk groups identified by the Advisory Committee on Immunization Practices (ACIP) reported being vaccinated against influenza during the 2008–2009 flu season.[1–2] The monovalent 2009 H1N1 influenza vaccine experienced particularly poor uptake, as approximately 80% of the U.S. population remained unvaccinated.[3] Misinformation and lack of knowledge regarding vaccine safety, effectiveness and importance, as well as challenges of cost, location and time have been frequently identified as barriers to achieving higher coverage.[4–6]
While studies have quantified the economic value of influenza vaccination from the societal and third party payor perspectives in a variety of patient populations, few have addressed the economic value of vaccination from the perspective of individual patients.[7–10] Although it is unlikely that patients base their decisions about vaccination purely on economic considerations, better understanding a vaccine’s economic value to patients can assist various types of decision-making. Studies have demonstrated that a patient’s perceived cost-benefit of a medical intervention can affect his or her utilization of the intervention, i.e., medical intervention utilization is fairly price elastic.[11–12] For example, pharmacy copayment increases have been paralleled by reductions in patient use of psychiatric and cardiovascular medications.[13–14] Conversely, decreases in required copayments have been followed by increased utilization of antibiotics.[15–19] A recent survey suggested that higher vaccine cost may dissuade adults from getting vaccinated.[12] As a result, the Task Force on Community Preventive Services recommends reducing client out-of-pocket costs as a means of enhancing access to vaccination services and increasing vaccination coverage.[20]
Therefore, better understanding the costs of vaccination for a patient could be useful for various decision makers associated with vaccination efforts. Health care workers and public health officials may use the information to convey the advantages and disadvantages of vaccination to patients. It could also serve as the basis for vaccine pricing, third party coverage, incentive, and various cost-sharing decisions. For instance, identifying disparities in the economic value for under- or uninsured patients may motivate programs to alleviate their financial burden in getting vaccinated. Value-based insurance design, the idea of promoting the use of a service (such as preventive influenza vaccination)—through lower or no cost immunization—when the clinical benefits exceed its cost, is an approach that may help improve vaccine coverage. Ascertaining the drivers of patient cost can help to identify operations structures and policies amenable to intervention.
To delineate the economic value of influenza vaccination from the adult (between 18 and 49 years old) patient perspective, we developed a computer simulation model representing the decision to get vaccinated. Different scenarios explored the impact of the patient being insured versus uninsured, influenza attack rate, and vaccine administration costs and wait times.
2. METHODS
2.1 Model Structure
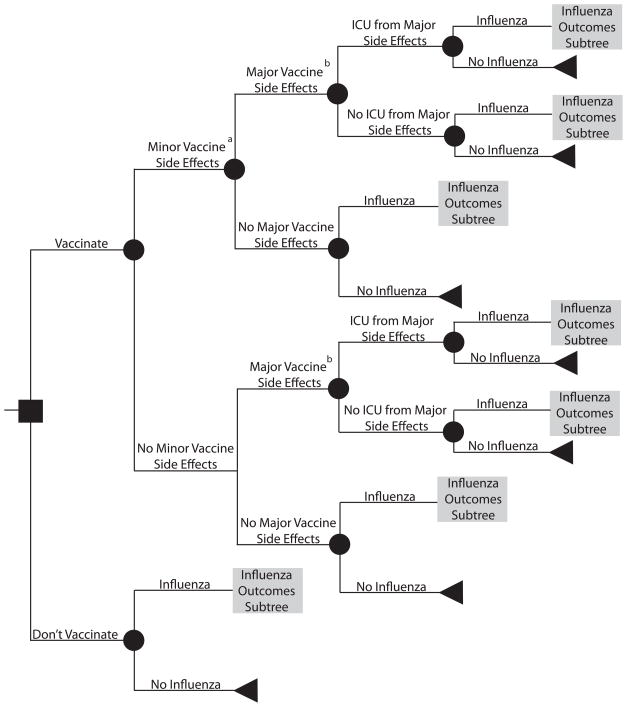
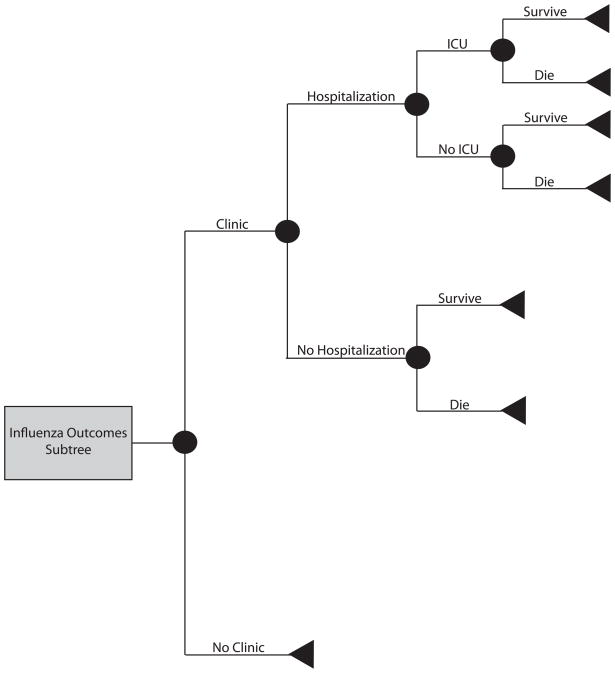
Using TreeAge Pro 2009 (TreeAge Software, Williamstown, Massachusetts), we developed a decision analytic computer simulation model representing an individual’s decision of whether to get vaccinated against influenza. The model focused on standard-risk adults (i.e., not high-risk by ACIP definitions), who comprise the majority of individuals in the 18 to 49 year old population and are eligible for both the TIV and LAIV vaccines. Outcomes data for the models came from an extensive literature review, using the following search terms: [seasonal AND influenza], [2009 H1N1], [influenza AND vaccination], [influenza AND vaccination AND cost], [influenza AND outcomes], and [influenza AND vaccination AND side effects]. The authors judged each article critically on study design and relevance to our target population. Separate scenarios assumed that the patient had commercial insurance or was uninsured. Additional scenarios examined the use of tetravalent inactivated vaccine (TIV) versus live attenuated influenza vaccine (LAIV). Figures 1 and 2 depict the general structure and influenza outcomes subtree of the model, respectively, which assumed a patient perspective.
FIGURE 1.
General structure of the decision model. a Includes local inflammation or minor systemic flu-like symptoms, requiring self-treatment with ibuprofen. b Guillian-Barré Syndrome (GBS)
FIGURE 2.
Influenza outcomes subtree
Patients who received the vaccine then had probabilities of experiencing major or minor side effects or both. Minor side effects included local inflammation or minor systemic flu-like symptoms, requiring self-treatment with ibuprofen. Guillian-Barré Syndrome (GBS), an autoimmune disorder of the peripheral nervous system, was the only major side effect considered; all cost and probability data relating to major side effects from vaccination in the model were therefore GBS- specific. The risk of developing symptomatic influenza depended on the clinical attack rate and whether the patient received a vaccine. Vaccination attenuated the risk of influenza by an amount determined by vaccine efficacy. A patient who developed symptomatic influenza then proceeded through the influenza outcomes sub-tree (Figure 2). A symptomatic patient had a probability of visiting a medical clinic, being hospitalized, requiring admission to the intensive care unit (ICU), and not surviving. Symptomatic patients that did not interface with the health care system relied on over-the-counter medications. Of the patients who visited a clinic, half (50%) received antiviral medication treatment.
Patient costs originated from three sources:
Productivity/Wage Loss: Getting vaccinated, contracting symptomatic influenza, and hospitalization each incurred unique durations of time off from work. Productivity losses were equivalent to the wages that the patient could have earned during the required time off from work.
Medication/Treatment Costs: Vaccines cost the patient money. Symptomatic patients purchased over-the-counter medications. Half of all patients who visited the clinic paid for prescription antivirals. Prescription medication and vaccine costs depended on the patient’s insurance status
Health Care Costs: Patient payments for clinic visits, hospitalization, and ICU stays depended on the patient’s insurance status.
For each simulation run, the equation below computed the cost per influenza case averted by the vaccine:
| (1) |
The following equation calculated the cost per influenza death averted by the vaccine:
| (2) |
2.2 Data Inputs
Table 1 organizes the data inputs for our model into costs, probabilities, and relevant time durations and lists their ranges and distributions. Cost and efficacy parameters were specific to the type of vaccine. Table 1 also lists the sources of each parameter input. The amounts paid by each individual depended on his or her insurance type, where uninsured patients had to cover medical costs (not charges).[21] Hospitalization costs came from the healthcare utilization project’s (HCUP) national inpatient survey (state inpatient data from Arizona, Florida, Massachusetts, Maryland, New Jersey, New York, Texas and Washington), using ICD-9 codes 480 and 357 for hospitalization and ICU admittance from influenza and major side effects such as Guillain-Barré respectively. Clinic costs originated from the Centers for Medicare and Medicaid Services (CMS). Medication costs were derived from their average wholesale prices, as drawn from the PDR Red Book.[22] The 2008 national median wage, retrieved from the Bureau of Labor and Statistics, was used to create a distribution of daily wages.[23]
TABLE 1.
Data Inputs for Model Variables
| Variable (units) | Mean | Standard Deviation | 95% Range | Distribution Type | Source | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Costs (US$) | ||||||
| Influenza | ||||||
| Home Treatment | 15.61 | - | 11.7 | 19.52 | Δ | [22] |
| Antiviral Medication (Oseltamivir) | 99.32 | 21.99 | - | - | γ | [22] |
| Vaccine (LAIV) | 25 | - | - | - | - | [22] |
| Vaccine TIV) | 15 | - | - | - | - | [22] |
| Clinic Cost | ||||||
| Commercial Insurance | 20.26 | - | - | - | - | [24–25, 40] |
| Uninsured | 13.53 | - | - | - | - | [24–25, 40] |
| Hospitalization | ||||||
| Commercial Insurance | 1,013.41 | 1,195.84 | - | - | γ | [24, 40] |
| Uninsured | 2,512.62 | 3,433.98 | - | - | γ | [24–25, 40] |
| ICU (in addition to hospitalization) | ||||||
| Commercial Insurance | 1,450.66 | 1299.34 | - | - | γ | [24, 40] |
| Uninsured | 4,721.98 | 3,956.70 | - | - | γ | [24–25, 40] |
| Daily Wage | 128.30 | - | 66.08 | 313.04 | Δ | [23] |
| Vaccine Side Effects | ||||||
| Minor Side Effects | 15.61 | - | 11.7 | 19.52 | Δ | [22] |
| Hospitalization for Major Side Effects | ||||||
| Commercial Insurance | 1,555.01 | 1,529.14 | - | - | γ | [24, 40] |
| Uninsured | 5,247.99 | 5,117.31 | - | - | γ | [25, 40] |
| ICU | ||||||
| Commercial Insurance | 2610 | 2571.57 | - | - | γ | [24, 40] |
| Uninsured | 10,577.82 | 10,382.13 | - | - | γ | [25, 40] |
| Probabilities (%) | ||||||
| Seasonal Influenza | ||||||
| Influenza | 6.6 | 1.7 | - | - | β | [26] |
| Influenza Outcomes | ||||||
| Hospitalization (given clinic visit) | 0.4 | - | 0.1 | 0.7 | Δ | [26, 41] |
| Case Fatality Rate (given hospitalization) | 0.9 | 0.3 | - | -- | β | [26] |
| Clinic Visit (given symptomatic infection) | 31.3 | 1.4 | β | [26] | ||
| ICU (given hospitalization) | 15 | - | - | - | - | [42] |
| H1N1 Influenza | - | |||||
| Influenza | 11.5 | - | 6.5 | 19.4 | Δ | [29] |
| Influenza Outcomes | - | |||||
| Hospitalization (given clinic visit) | 0.56 | - | - | - | - | [43] |
| Case Fatality Rate (given hospitalization)a | 0.01 | - | 0.007 | 0.016 | Δ | [30] |
| Clinic Visit (given symptomatic infection) | 31.3 | 1.4 | - | - | β | [26] |
| ICU (given hospitalization) | 20 | - | - | - | - | [44] |
| Vaccines | ||||||
| LAIV | ||||||
| Major Side Effects | 0.002 | - | - | - | - | [32] |
| Major Side Effects ICU | 33 | - | - | - | - | [45] |
| Minor Side Effects | 5 | - | - | - | - | [46] |
| Seasonal Vaccine Efficacy | 62 | - | 45 | 73 | Δ | [47] |
| H1N1 Vaccine Efficacy (LAIV) | 83 | - | - | - | - | [48–49] |
| TIV | ||||||
| Major Side Effects | 0.0003 | - | - | - | - | [31] |
| Major Side Effects ICU | 33 | - | - | - | - | [45] |
| Minor Side Effects | 5 | - | - | - | - | [46] |
| Seasonal Vaccine Efficacy (TIV) | 74 | - | 50 | 80 | Δ | [47] |
| H1N1 Vaccine Efficacy (TIV) | 78 | - | - | - | - | [48–49] |
| Vaccine Reduces Hospitalization | 56.5 | - | 45 | 68 | Δ | [46] |
| Vaccine Reduces Mortality | 21 | - | 0 | 42 | Δ | [46] |
| Time (Days) | ||||||
| Work Absenteeismb | 1.03 | - | 0.5 | 1.5 | Δ | [26, 50–51] |
| Hospitalization (due to influenza) | 3 | - | - | - | - | [55] |
Data presents median and interval estimate
Number of days absent from work due to symptomatic influenza infection
By contrast, the model made the following assumptions about the commercially insured based on benefit design information obtained from the Henry J. Kaiser Family Foundation, Health Research & Educational Trust Survey of Employer-Sponsored Health Benefits, 2009:
36.3% had no annual deductible [24]
The distribution of plan types was as follows: 20% health maintenance organization (HMO), 60% preferred provider organization (PPO), 10% point of service (POS), and 10% high deductible health plan (HDHP/SO) [24]
18% of those with no deductible had coinsurance [24]
Those who paid a deductible (63.7%) paid $809.90. [24]
Based on a report using data collected through the Medical Expenditure Panel Surveys, uninsured individuals paid approximately one third of their total medical costs. [25] All costs were expressed in 2009 U.S. dollars with a 3% discount rate converting costs from other years.
2.3 Sensitivity Analyses
Probabilistic (first and second-order) sensitivity analyses with microsimulation simultaneously varied the values of each variable across the ranges listed in Table 1, i.e., each parameter value from distributions listed in Table 1 and then individuals proceeded down each branch of the tree based on stochastic dice rolls against the probability values drawn from the distributions. Specific analyses explored the effects of ranging vaccination cost, which included both the cost of vaccine and other ancillary costs (i.e., travel and/or childcare expenses). We utilized a baseline cost of $15 (TIV) and $25 (LAIV) for scenarios where vaccination costs were equivalent to only that the vaccine itself as well as $0, $50, and $150 for both TIV and LAIV. The $0 vaccination cost accounts for scenarios where vaccine may be administered during a routine visit and does not incur an additional cost; higher vaccine costs ($50 and $150) allow for the inclusion of potential transportation costs needed to get to a health care facility. We additionally varied the hours missed from work to get vaccinated (1 hour to 4 hours), the probability of major vaccine side effects (i.e., Guillain-Barré), the clinical attack rate of influenza, and the probability of influenza resulting in a hospital and ICU admission.
Baseline attack rates for seasonal influenza drew from a beta distribution (estimated from a mean of 6.6% and standard deviation of 1.7%). One-way sensitivity analyses also evaluated ranging this attack rate from flat values of 2% up to 15%.[26–27] As limited U.S based data exists regarding the incidence of 2009 H1N1 during the 2009–2010 influenza season, the H1N1 baseline attack rate for the US was estimated using a seroprevalence reports conducted in England during this time period; additional simulations utilized an attack rate of 6.5%, which is comparable to incidence given in a brief U.S. based report.[28–29] While baseline influenza risks were drawn from studies reporting data from a limited number of influenza seasons, these distributions of influenza risk are likely the most probable. Varying the attack rate higher and lower than these expected distributions was not meant to represent a likely risk of flu, but was instead intended to quantitatively illustrate the effect of an increased or decreased risk of influenza on the cost of avoiding a case of influenza by vaccination. For seasonal flu, the probability of ICU admittance ranged from 5% to 20%; the probability of ICU care being received as a result of H1N1 ranged from 15% to 25%. Two case fatality rates for H1N1 were evaluated; the first distribution (triangular, likeliest value: 0.16% range: 0.066–0.333%) was based on the symptomatic influenza-specific case fatality, while the second was a case fatality rate (triangular, likeliest value: 0.01% range: 0.007–0.016%) calculated out of the overall self-reported symptomatic influenza-like illness reports.[30] Baseline runs used 0.002% and 0.0003% for the probability of major side effects from LAIV and TIV vaccines respectively.[31–32] Additional analyses examined the effects of doubling the probability of ICU admittance and major side effects.
3. RESULTS
3.1 Simulation Parameters
The median age used in the model was 30 years for uninsured patients and 40 years for commercially insured patients. Additional analyses evaluated ages 40 and 49 for uninsured and commercially insured populations, respectively, as 49 is the oldest age at which administering LAIV is still recommended. Median age data was generated with data from the Bureau of Labor and Statistics and a recent paper on the cost and coverage of healthcare among the uninsured.[25, 33] Each simulation run sent 1,000 hypothetical individuals, each with a unique set of characteristics (each individual drew from the wage distribution to represent the diversity of jobs, geographic locations, and sociodemographic characteristics that may affect wages; those who developed side effects drew from the cost of side effects distribution and those who became ill drew from the clinic and hospitalization cost distributions to reflect the sociodemographic and clinical diversity that exists among adults), through the model 1,000 times for a total of 1,000,000 patient outcomes for each simulation run. (Note: the parameters drew independently from each respective distribution and were not correlated.)
3.2 Measurements of Stochasticity
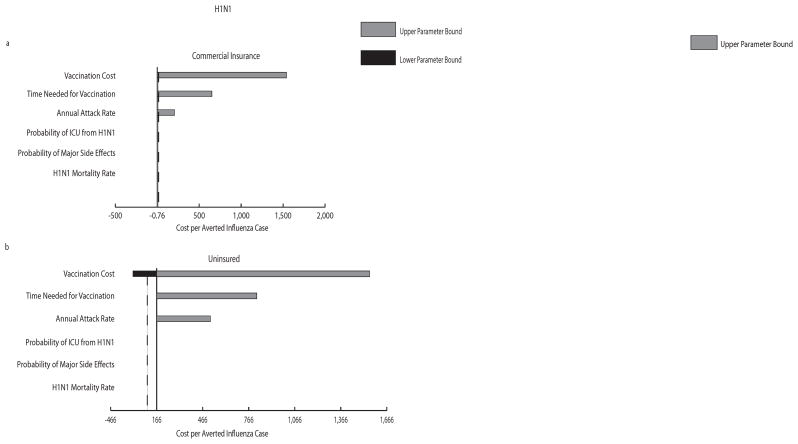
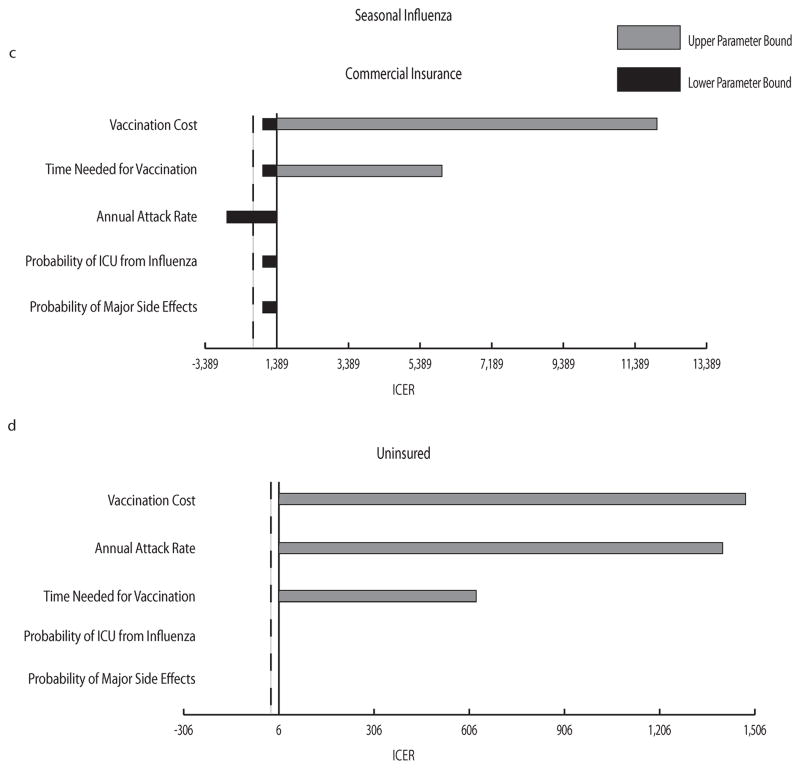
Tornado diagrams in Figure 3 demonstrate the impact that each variable included in our sensitivity analysis has on the incremental cost per averted case. As shown in the figure, ranging vaccination cost, the time needed for vaccination, and attack rate had the most impact on the incremental cost for all scenarios, with vaccination cost having the greatest effect; the probability of ICU or mortality from influenza and the probability of major vaccine side effects had little to no impact on the cost averted by preventing an influenza case.
FIGURE 3.
a–d: Tornado Diagrams. a–b H1N1 vaccination scenarios for commercially insured (a) and uninsured (b) groups; c–d seasonal influenza vaccination scenarios for commercially insured (c) and uninsured (d) groups. The central axis represents the resulting incremental cost per averted influenza case when all parameters listed were set to their midpoint value of their explored ranges: vaccine cost (range: $0 – $150), attack rate (range: 6.5%–12.5% for H1N1 and 2%–15% for seasonal influenza), time needed for vaccination (range: 1–4 hours), major vaccine side effects (range: 0.0003%–0.0006%), ICU from influenza (15%–25% for H1N1 and 5%–20% for seasonal influenza), case fatality rate (0.011%–0.186% for H1N1). Table 1 shows baseline values for each parameter. The dashed vertical line marks where the incremental cost per averted case = 0. Negative incremental costs occurred when vaccination was economically dominant compared to no vaccination.
Scatter plots (see Supplementary Figure 1) depict the variability of cost and influenza occurrence within a particular simulation. While vaccinating was associated with higher cost compared to no vaccination, the vaccination group had substantially fewer influenza cases as well as lower variation in influenza occurrence. Relatively little difference in cost and influenza cases was observed between the commercial insurance and uninsured groups for both H1N1 and seasonal influenza. Seasonal influenza scenarios illustrated a lower variation in the occurrence of influenza in the no vaccination group (but not in the vaccination group), which is likely a product of a narrower attack rate baseline distribution (see Table 1).
3.3 Seasonal Influenza Scenario
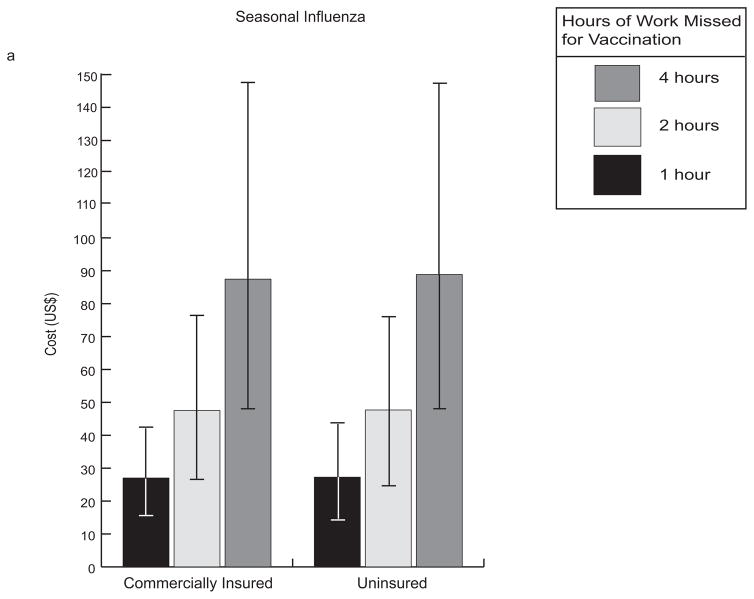
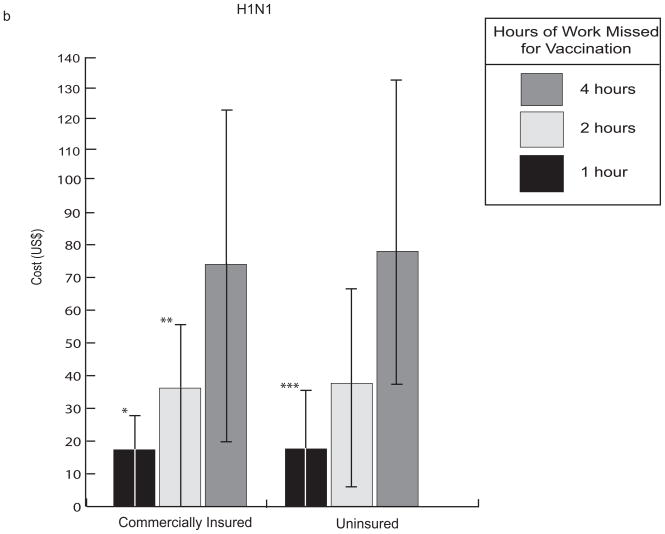
Table 2 presents the median and 95% confidence interval (both 2.5th and 97.5th percentiles) for the cost per averted influenza case from our seasonal influenza model assuming a 15% baseline probability of ICU (given hospitalization) as well as a 0.0003% and 0.002% probability of major side effects from TIV and LAIV vaccine formulations, respectively. As mentioned above, the value of vaccination was fairly sensitive to vaccination cost, the time required for vaccination, and influenza attack rate. When vaccination took no more than one hour, TIV generated marginal (when compared to no vaccination) costs only slightly higher than that of the vaccine itself (approximately $27 for seasonal influenza vaccine and $17 for H1N1 vaccine) shown in Figure 4.
TABLE 2.
Cost per influenza case avoided through vaccination with the seasonal influenza vaccine for the commercially insured and uninsureda
| Commercial Insurance | Uninsured | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time for Vaccination (hrs) | Attack Rate | Attack Rate | |||||||
| Baseline Scenariob, c | Sensitivity Analyses | Baseline Scenariob, c | Sensitivity Analyses | ||||||
| 2%d | 10%e | 15%f | 2%d | 10%e | 15%f | ||||
| Vaccine Cost = $0 | |||||||||
| 1 hr | LAIV | 352 (−14–1,150) | 1,660 (588–4,371) | 161 (−85–511) | 40 (−175–270) | 418 (−29–1,287) | 1,633 (582–4,561) | 168 (−112–507) | 48 (−186–299) |
| TIV | 291 (−50–993) | 1,400 (519–3,549) | 116 (−124–410) | 10 (−182–195) | 126 (−40–1,033) | 2,833 (1,113–7,562) | 123 (−117–428) | 204 (−71–535) | |
| 2 hrs | LAIV | 863 (233–2,562) | 3,300 1,350–8,529 | 501 (99–1,180) | 265 (−42–717) | 1,018 (267–2,490) | 3,409 (1,289–9,044) | 511 (99–1,108) | 273 (−26–684) |
| TIV | 736 (198–2,139) | 2,945 (1,214–7,492) | 415 (61–972) | 201 (−46–563) | 895 (−999–2,344) | 2,956 (1,229–7,310) | 454 (47–989) | 227 (−50–605) | |
| 4 hrs | LAIV | 1,963 (718–5,013) | 6,931 (2,935–16,806) | 1,229 (438–2,486) | 739 (225–1,501) | 2,163 (690–4,614) | 6,925 (3,012–17,771) | 1,207 (465–2,542) | 736 (215–1,561) |
| TIV | 1,669 (617–4,352) | 6,120 (2,522–14,729) | 1,029 (374–2,130) | 599 (150–1,334) | 6,768 (2,643–15,347) | 6,104 (2,643–15,347) | 1,006 (372–2,153) | 597 (152–1,329) | |
| Vaccine Cost =$15 | |||||||||
| 1 hr | TIV | 617 (207–1,606) | 2,505 (1,284–6,285) | 332 (81–662) | 156 (−51–386) | 698 (210–1,614) | 2,604 (1,263–6,496) | 344 (65–702) | 164 (−57–384) |
| 2 hrs | TIV | 1,117 (409–2,754) | 4,012 (1,939–9,024) | 625 (262–1,232) | 348 (113–757) | 1,261 (421–2,874) | 4,172 (2,065–9,882) | 649 (251–1,238) | 363 (57–769) |
| 4 hrs | TIV | 2,016 (787–4,830) | 7,030 (3,235–16,220) | 1,195 (586–2,432) | 743 (307–1,512) | 2,312 (841–5,104) | 6,982 (3,347–17,018) | 1,249 (841–5,104) | 756 (307–1,451) |
| Vaccine Cost = $25 | |||||||||
| 1 hr | LAIV | 1,004 (437–2,417) | 3,674 (2,037–8,845) | 583 (259–1,007) | 315 (107–606) | 1,119 (416–2,394) | 3,698 (1,987–8836) | 575 (262–1,016) | 324 (80–606) |
| 2 hrs | LAIV | 1,545 (682–3,526) | 5,545 (2,894–11,989) | 911 (479–1,699) | 548 (227–1000) | 1,765 (712–3,885) | 5,480 (2,834–12,487) | 910 (470–1,662) | 554 (227–1,026) |
| 4 hrs | LAIV | 2,696 (155–6,160) | 8,886 (4,370–21,334) | 1,605 (834–2,889) | 1,003 (456–1,853) | 2,872 (1,123–6,322) | 9,002 (4,271–20,466) | 1,589 (858–2991) | 992 (495–1,877) |
| Vaccine Cost = $50 | |||||||||
| 1 hr | LAIV | 1,653 (859–4,041) | 5,964 (3,322–12,703) | 1,003 (605–1,584) | 594 (335–974) | 1,813 (773–3,583) | 5,956 (3,375–13,085) | 1,001 (605–1,549) | 560 (334–960) |
| TIV | 1,496 (708–3,206) | 5,101 (2,985–10,843) | 863 (537–1,351) | 497 (256–797) | 1,582 (678–3,202) | 5,170 (3,029–10,765) | 863 (504–1,366) | 506 (250–824) | |
| 2 hrs | LAIV | 2,202 (859–4,041) | 7,602 (4,341–17,141) | 1,332 (807–2,086) | 834 (482–1,357) | 2,441 (1,076–4,836) | 7,618 (4,132–17,052) | 1,371 (844–2,233) | 840 (449–1,382) |
| TIV | 1,897 (925–4,402) | 6,718 (3,823–14,282) | 1,153 (707–1,880) | 710 (392–1,179) | 2,082 (942–4,372) | 6,732 (3,695–14,217) | 1,161 (712–1,878) | 717 (414–1,175) | |
| 4 hrs | LAIV | 3,315 (1,534–7,428) | 11,601 (5,917–26,883) | 1,999 (1,202–3,398) | 2,180 (726–2,180) | 3,639 (1,570–7,637) | 11,119 (5,606–26,161) | 2,026 (1,147–3,456) | 1,268 (749–2,210) |
| TIV | 2,946 (1,448–6,833) | 9,702 (5,015–20,958) | 1,769 (1,047–2,997) | 1,094 (621–1,882) | 3,187 (1,351–6,869) | 9,941 (5,199–20,658) | 1,756 (1,012–2,959) | 1,125 (587–1,972) | |
| Vaccine Cost = $150 | |||||||||
| 1 hr | LAIV | 4,185 (2,371–8,709) | 14,426 (9,076–32,850) | 2,644 (1,899–3,933) | 1,706 (1,264–2,454) | 4,648 (2,371–9,300) | 14,123 (8,603–30,352) | 2,671 (1,941–3,845) | 1,703 (1,233–2,433) |
| TIV | 3,782 (2,070–8,197) | 12,678 (7,815–25,186) | 2,323 (1,707–3,323) | 1,465 (1,101–1,465) | 4,101 (2,015–7,950) | 12,474 (7,624–27,624) | 2,331 (1,712–3,290) | 1,490 (1,098–2,084) | |
| 2 hrs | LAIV | 4,817 (2,643–10,472) | 15,823 (9,451–34,041) | 2,992 (2,186–4,380) | 1,954 (1,386–2,810) | 5,123 (2,541–9,764) | 16,027 (9,206–36,616) | 3,015 (2,138–4,504) | 1,947 (1,422–2,842) |
| TIV | 4,147 (2,211–8,919) | 14,173 (5,526–29,202) | 2,620 (1,850–4,028) | 1,660 (1,204–2,484) | 4,587 (2,295–8,884) | 13,976 (8,397–29,671) | 2,682 (1,885–3,852) | 1,720 (1,198–2,380) | |
| 4 hrs | LAIV | 6,043 (3,196–12,655) | 19,286 (11,257–42,528) | 3,703 (2,501–5,683) | 2,395 (1,701–3,602) | 6,445 (3,125–13,004) | 19,622 (11,200–42,461) | 3,723 (2,573–5,660) | 2,393 (1,659–3,685) |
| TIV | 5,137 (2,776–11,535) | 17,159 (10,200–35,421) | 3,207 (2,267–4,999) | 2,116 (1,466–3,182) | 5,651 (10,208–36,849) | 17,204 (10,208–36,849) | 3,283 (2,262–5,119) | 2,091 (1,453–3,095) | |
median (95% confidence interval)
Beta distribution: mean = 6.6%, standard deviation = 1.7%
Most likely attack rate
Based on a distribution generated using data from Molinari et al, the probability of a 2% attack rate of seasonal influenza is < 2.5%
Based on a distribution created using data from Molinari et al, the probability of an influenza attack rate of 10% or greater is ≤ 2.5%
Based on a distribution generated using data from Molinari et al, the probability of a 15% attack rate of seasonal influenza is < 2.5%
FIGURE 4.
Comparison of median costs for influenza vaccination with 95% confidence interval (2.5th and 97.5th percentiles between uninsured and commercially insured patients for (A) seasonal influenza and (B) 2009 H1N1 influenza. This data assumes a clinical attack rate of 10%, TIV cost of $15, and baseline probabilities shown in Table 1. Negative values indicate cost-savings. * 2.5th percentile = −20.9 ** 2.5th percentile = −7.01 *** 2.5th percentile = −7.78
A 4 hour vaccination administration time, which would correspond to a patient traveling to a physician’s clinic, was almost three times as expensive as a one-hour administration time, which may correspond to a patient traveling to either a closer location or a location that has less of a wait time (e.g., a dedicated immunization clinic). Although varying vaccination costs over the range explored in the sensitivity analyses had the greatest effect on the economic benefit of vaccination, a slight increase in influenza attack rate results in a greater impact on vaccination benefit. For example, while increasing attack rate from baseline to 15% (approximately 2 times the mean at baseline) resulted in a 3 fold decrease in the cost of averting an influenza case, increasing vaccination cost by the same amount (from $25 to $50) resulted in a 2 fold increase in cost per averted case (see Table 2).
TIV consistently provided greater economic value than LAIV, as the added efficacy of LAIV did not overcome the higher cost of the vaccine. The economic value of vaccination was fairly comparable for insured and uninsured patients, mainly because influenza hospitalization was relatively infrequent among adults and insurance status had little impact on the cost of over-the-counter medications and missing work.
3.4 2009 H1N1 Influenza
H1N1 vaccination provided relatively more economic benefit than seasonal influenza vaccination, mainly because H1N1 had slightly higher morbidity and mortality than seasonal influenza among the younger adult population (i.e., the population that comprises much of the commercially insured and uninsured). Table 3 demonstrates the effect of vaccine cost, administration time, and attack rate on the median cost of avoiding an influenza case, where baseline probability of H1N1 influenza was a triangular distribution (min=6.5%, likeliest= 11.5%, max=19.4%) ICU from H1N1 (given hospitalization) = 20%, case fatality rate was a triangular distribution (min= 0.007%, likeliest= 0.01%, max= 0.016%), and the probabilities of major side effects from TIV and LAIV vaccine formulations were 0.0003% and 0.002%, respectively. While the economic benefit of vaccination was comparably dependent on both the time off from work needed for vaccination and influenza attack rate, varying vaccine cost over the range evaluated through sensitivity analyses had the greatest effect on the cost averted by vaccination. However, as with seasonal influenza vaccination, a greater impact results with decreased influenza risk than with increased vaccination cost of similar magnitude. No significant difference was present between insured and uninsured individuals. Hospitalization rates for H1N1 influenza were still not high enough to result in differences by insurance status. Additionally, there was little difference between the LAIV and TIV vaccines even if the individual were responsible for higher total vaccination costs (vaccine costs for the 2009 H1N1 vaccination program were fully subsidized by the federal government.
TABLE 3.
Cost per influenza case avoided through vaccination with the H1N1 vaccine for the commercially insured and uninsureda
| Commercial Insurance | Uninsured | ||||
|---|---|---|---|---|---|
| Time for Vaccination (hrs) | Attack Rate | Attack Rate | |||
| Baseline Scenariob, c | Sensitivity Analyses 6.5%d | Baseline Scenariob, c | Sensitivity Analyses 6.5%d | ||
| Vaccine Cost = $0 | |||||
| 1 hr | LAIV | 16 (−187–275) | 202 (−61–548) | 14 (−206–263) | 204 (−115–576) |
| TIV | 27 (−180–295) | 225 (−48–575) | 29 (−228–273) | 238 (−79–624) | |
| 2 hrs | LAIV | 240 (−56–690) | 604 (164–1,170) | 231 (−90–729) | 601 (143–1,256) |
| TIV | 256 (−50–728) | 664 (196–1,294) | 256 (−73–762) | 657 (158–1,319) | |
| 4 hrs | LAIV | 675 (169–1,573) | 1,407 (582–2,591) | 665 (137–1,528) | 1,391 (556–2,647) |
| TIV | 720 (158–1,648) | 1,487 (574–2,734) | 723 (155–1,638) | 1,502 (605–2,920) | |
| Vaccine Cost = $15 | |||||
| 1 hr | TIV | 193 (−54–508) | 527 (196–919) | 723 (155–1,638) | 528 (168–943) |
| 2 hrs | TIV | 426 (54–986) | 969 (451–1,678) | 427 (64–983) | 957 (413–1,682) |
| 4 hrs | TIV | 868 (307–1,837) | 1,791 (837–3,182) | 865 (250–1,734) | 1,792 (831–3,185) |
| Vaccine Cost = $25 | |||||
| 1 hr | LAIV | 270 (22–654) | 675 (365–1,077) | 277 (5–650) | 675 (318–1,100) |
| 2 hrs | LAIV | 492 (133–1,021) | 1,089 (588–1,750) | 484 (115–1,009) | 1,069 (549–1,780) |
| 4 hrs | LAIV | 904 (320–1,822) | 1,896 (1,016–3,202) | 911 (334–1,793) | 1,860 (966–3,164) |
| Vaccine Cost = $50 | |||||
| 1 hr | LAIV | 523 (212–989) | 1,140 (144–1,652) | 519 (195–978) | 1,155 (713–1,704) |
| TIV | 584 (208–1,110) | 1,222 (790–1,783) | 573 (223–1,127) | 1,244 (777–1,824) | |
| 2 hrs | LAIV | 746 (317–1,382) | 1,535 (961–2,288) | 742 (316–1,434) | 1,571 (1,000–2,360) |
| TIV | 824 (365–1,534) | 1,663 (1,051–2,558) | 821 (358–1,550) | 1,675 (1,038–2,534) | |
| 4 hrs | LAIV | 1,141 (519–2,128) | 2,342 (1,382–3,724) | 1,162 (515–2,257) | 2,377 (1,339–3,874) |
| TIV | 1,274 (527–2,488) | 2,591 (1,526–3,908) | 1,268 (572–2,440) | 2,534 (1,441–4,014) | |
| Vaccine Cost = $150 | |||||
| 1 hr | LAIV | 1,543 (874–2,590) | 3,027 (2,256–4,091) | 1,563 (882–2,605) | 3,046 (2,249–4,182) |
| TIV | 1,645 (953–2,786) | 3,217 (2,406–4,320) | 1,657 (937–2,823) | 3,265 (2,374–4,384) | |
| 2 hrs | LAIV | 1,769 (986–2,995) | 3,430 (2,460–4,668) | 1,750 (1,013–2,897) | 3,425 (2,479–4,820) |
| TIV | 1,837 (1,067–3,165) | 3,688 (2,683–5,250) | 1,870 (1,094–3,281) | 3,683 (2,628–5,090) | |
| 4 hrs | LAIV | 2,187 (1,221–3,901) | 4,201 (2,959–5,927) | 2,217 (1,182–3,921) | 4,188 (2,903–5,968) |
| TIV | 2,356 (1,302–4,174) | 4,533 (3,109–6,322) | 2,387 (1,305–4,201) | 4,481 (3,049–6,368) | |
median (95% confidence interval)
Triangular distribution: Min = 6.5%, Likeliest = 11.5%, Max = 19.4
Most likely attack rate
Based on a distribution generated from data collected by Miller et al, the probability of a 6.5% attack rate of seasonal influenza is approximately <1
4. DISCUSSION
Compared to the number of published studies from the societal and third party payor perspectives, relatively few economic studies have looked at medical interventions from the patient perspective. The vast majority of health economic studies focus on the societal perspective, summing costs across all key stakeholders, or have evaluated whether a large policy-making entity (e.g., government) should make an intervention available or a third party payor (e.g., Medicare or commercial insurance) should cover an intervention. However, when the objective is to increase historically low influenza vaccination rates, accounting for only these two perspectives is myopic and may fail to account for key driving forces or stakeholders. Cost is certainly not the only (or even the primary) factor in a patient’s decision to get vaccinated, but its role is salient. As direct-to-consumer marketing grows more pervasive in health care, the patient economic perspective may be increasing in importance.
A trend over the past two decades has been casting patients as “consumers,” who must choose among goods or interventions offered by traditional and non-traditional health care providers.[34] Commercials and advertisements tout the “value” of health care goods and services. While this value is sometimes quantified in terms of clinical efficacy, it is often not quantified monetarily. In many other situations where consumers have a choice (e.g., purchasing automobiles, food, or clothing), understanding the economic value of a good or service from the consumer’s perspective can help both the consumers and providers of the good or service. Consumers may realize that a good is either more or less valuable than anticipated, which may in turn affect their decision-making (e.g., is it worthwhile to miss work to get vaccinated). Providers may be able to tailor the good or service to assist the consumer (e.g., making it more convenient for patients to get vaccinated or adjusting the price of the vaccine).
Although economic indicators and models cannot fully capture all the advantages and disadvantages of a medical intervention, they can offer important benchmarks, perspectives, and insights. In our model, the strongest drivers of the vaccine’s economic value were the clinical attack rate followed by vaccination cost and the time required (i.e. lost productivity) by the individual to get vaccinated. This suggests that in a seasonal or a relatively mild pandemic scenario such as the 2009 H1N1 pandemic, making influenza vaccination less costly and more convenient may be an important priority and prerequisite for improved vaccination coverage.[4, 35–36] For example, providing vaccination at locations the patient already frequents (e.g., work, churches, retail shops and clinics, concerts, athletic events, and other public gatherings) may be a useful cost-saving measure for individuals. Alternatively, in more severe epidemics, making a special trip and queuing for vaccination may be relatively less costly for an individual.
The relative parity in cost of vaccination observed between uninsured and insured individuals is informative as well. Finding a significant difference between the two would be expected based on reports that insurance status often dictates the decision to get vaccinated, and also would suggest that more tailored approaches to promote vaccination in each group may be needed.[4, 37] Instead, the two groups seem to be facing similar costs for vaccination, i.e., lack of insurance coverage may not actually be a barrier for vaccination.
4.1 Limitations
All computer models make simplifying assumptions and cannot represent all possible outcomes that may arise from influenza vaccination or disease. Additionally, they cannot account for the vast diversity in the socio-demographic and health characteristics of the adult population. Our model only considered the healthy adult population which comprises the majority of the population. High-risk individuals (e.g., those with major co-morbidities such as diabetes, pregnant women, or health care workers) may either have higher risk for influenza-associated morbidity and, therefore, could reap more benefits (i.e., greater cost-savings) from vaccination or could have additional reasons for being immunized (e.g., protecting neonates or patients). Future studies may focus on different categories of high-risk individuals. Although all data came from referenced sources identified from an extensive review of the literature and widely-used databases, the choice of probability distributions could bias the results. Costs drew from gamma distributions, frequently utilized for health care related costs that tend to cluster in the lower end of the distribution and have relatively fewer higher costs trail off into the upper tail. Probability parameters drew from beta distributions, frequently used to represent continuous variables that have values between 0 and 1. Lastly, parameters with relatively sparse observations drew from triangular distributions, built from a minimum, maximum, and likeliest value.[38–39] Moreover, it is difficult to quantify (both monetarily and in terms of health benefits) all facets of vaccine value, such as convenience, patient preference (for either injection or nasal spray, where indicated) and comfort, and the relative worth of LAIV and TIV for all scenarios. The decision to get the influenza vaccine and the type of vaccine administered should be determined on a case-by-case basis.
4.2 Conclusions and Future Directions
Understanding the cost-benefit of vaccination from the patient perspective can provide interesting insights for vaccination policy. While cost is certainly not the only driving factor in a patient’s decision to get vaccinated, studies have suggested that a patient’s perceived cost-benefit of a medical intervention could affect his or her utilization of the intervention. Aside from vaccine cost and attack rate, one of the biggest drivers of vaccination costs was a patient’s time required for vaccination, so decision makers may want to focus on ways to reduce this time, such as bringing vaccination to individuals (e.g., at work, churches, or other normally frequented locations). Ideally, reducing the time demand and cost of immunization for patients will help improve influenza vaccine coverage.
Supplementary Material
SUPPLEMENTARY FIGURE 1: Scatter plots a–d depict the variation in costs and occurrence of influenza cases for H1N1 (a–b) and seasonal influenza (c–d) scenarios across both insurance groups, where TIV vaccine cost = $15 and all other variables remained at their baseline values shown in Table 1. The black diamonds on the graph represent the vaccination group, while the grey squares signify the unvaccinated group. Both groups consist of 1,000 data points (individuals), with each point derived from the average cost and number of influenza cases over 1,000 trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Influenza vaccination coverage among children and adults - United States, 2008–09 influenza season. MMWR Morb Mortal Wkly Rep. 2009 Oct 9;58(39):1091–5. [PubMed] [Google Scholar]
- 2.State-specific influenza vaccination coverage among adults aged > or =18 years--United States, 2003–04 and 2005–06 influenza seasons. MMWR Morb Mortal Wkly Rep. 2007 Sep 21;56(37):953–9. [PubMed] [Google Scholar]
- 3.Interim results: influenza A (H1N1) 2009 monovalent vaccination coverage --- United States, October–December 2009. MMWR Morb Mortal Wkly Rep. 2010 Jan 22;59(2):44–8. [PubMed] [Google Scholar]
- 4.Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008 Jul;121(7 Suppl 2):S28–35. doi: 10.1016/j.amjmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Mirza A, Subedar A, Fowler SL, Murray DL, Arnold S, Tristram D, et al. Influenza vaccine: awareness and barriers to immunization in families of children with chronic medical conditions other than asthma. South Med J. 2008 Nov;101(11):1101–5. doi: 10.1097/SMJ.0b013e318182ee8d. [DOI] [PubMed] [Google Scholar]
- 6.Chapman GB, Coups EJ. Predictors of influenza vaccine acceptance among healthy adults. Prev Med. 1999 Oct;29(4):249–62. doi: 10.1006/pmed.1999.0535. [DOI] [PubMed] [Google Scholar]
- 7.Nichol KL, Goodman M. Cost effectiveness of influenza vaccination for healthy persons between ages 65 and 74 years. Vaccine. 2002 May 15;20( Suppl 2):S21–4. doi: 10.1016/s0264-410x(02)00124-x. [DOI] [PubMed] [Google Scholar]
- 8.Lee PY, Matchar DB, Clements DA, Huber J, Hamilton JD, Peterson ED. Economic analysis of influenza vaccination and antiviral treatment for healthy working adults. Ann Intern Med. 2002 Aug 20;137(4):225–31. doi: 10.7326/0003-4819-137-4-200208200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Prosser LA, Bridges CB, Uyeki TM, Rego VH, Ray GT, Meltzer MI, et al. Values for preventing influenza-related morbidity and vaccine adverse events in children. Health Qual Life Outcomes. 2005;3:18. doi: 10.1186/1477-7525-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis. 2009 Dec 15;49(12):1784–92. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. Sep 25;2009 doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvani AP, Reluga TC, Chapman GB. Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5692–7. doi: 10.1073/pnas.0606774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med. 2003 Dec 4;349(23):2224–32. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 14.Goldman DP, Joyce GE, Escarce J, Pace JE, Solomon MD, Laouri M, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291(19):2344–50. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Donohue JM, Lave JR, O’Donnell G, Newhouse JP. The impact of the Medicare Part D drug benefits on pharmacy and medical care spending. N Engl J Med. 2009;361(1):52–61. doi: 10.1056/NEJMsa0807998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin W, Basu A, Zhang JX, Rabbani A, Meltzer DO, Alexander GC. The effect of the Medicare Part D prescription benefit on drug utilization and expenditures. Ann Intern Med. 2008 January 7;148(3):169–77. doi: 10.7326/0003-4819-148-3-200802050-00200. [DOI] [PubMed] [Google Scholar]
- 17.Ketcham JD, Simon KI. Medicare Part D’s effects on elderly patients’ drug costs and utilization. Am J Manag Care. 2008 Nov;14(11 Suppl):SP14–22. [PubMed] [Google Scholar]
- 18.Doshi JA, Zhu J, Lee BY, Kimmel SE, Volpp KG. Impact of a prescription copayment increase on lipid-lowering medication adherence in veterans. Circulation. 2009 Jan 27;119(3):390–7. doi: 10.1161/CIRCULATIONAHA.108.783944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Lave JR, Newhouse JP, Donohue JM. How the Medicare Part D Drug Benefit Changed the Distribution of Out-of-Pocket Pharmacy Spending among Older Beneficiaries. J Gerontol B Psychol Sci Soc Sci. doi: 10.1093/geronb/gbp111. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Marketing. The Community Guide: Vaccinations for Preventable Diseases (Universally Recommended Vaccines) 2009 [cited 2010 February 28]; Available from: http://www.thecommunityguide.org/vaccines/universally/index.html.
- 21.U.S. Department of Health and Human Services AfHRaQ. HCUPnet definitions. U.S. Department of Health and Human Services; [Google Scholar]
- 22.Physicians’ Desk Reference Inc. Red Book. Montvale, NJ: Thompson Reuters; 2009. [Google Scholar]
- 23.Labor USDo. Occupational Employment Statistics: May 2008 National Occupational Employment and Wages Estimates United States. 2009. [Google Scholar]
- 24.Claxton G, DiJulio B, Finder B, Lundy J, McHugh M, Osei-Anto A, Whitmore H, Pickreign J, Gabel J. Employer Health Benefits 2009 Annual Survey. Chicago, Illinois, Menlo Park, California: Henry J. Kaiser Family Foundation, Health Research & Educational Trust; 2009. [Google Scholar]
- 25.Hadley J, Holahan J, Coughlin T, Miller D. Covering The Uninsured In 2008: Current Costs, Sources Of Payment, And Incremental Costs. Health Affairs. 2008:w399–w415. doi: 10.1377/hlthaff.27.5.w399. [DOI] [PubMed] [Google Scholar]
- 26.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 28;25(27):5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010 Aug 6;59(RR-8):1–62. [PubMed] [Google Scholar]
- 28.Morens DM, Taubenberger JK, Fauci AS. The 2009 H1N1 Pandemic Influenza Virus: What Next? MBio. 2010;1(4) doi: 10.1128/mBio.00211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010 Mar 27;375(9720):1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 30.Presanis AM, De Angelis D, Hagy A, Reed C, Riley S, Cooper BS, et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009 Dec;6(12):e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine. 2009 Mar 26;27(15):2114–20. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 32.Izurieta HS, Haber P, Wise RP, Iskander J, Pratt D, Mink C, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005 Dec 7;294(21):2720–5. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- 33.Bureau of Labor and Statistics USDoL. The Employment Situation- October 2009. 2009. [Google Scholar]
- 34.Donohue J. A history of drug advertising: the evolving roles of consumers and consumer protection. Milbank Q. 2006;84(4):659–99. doi: 10.1111/j.1468-0009.2006.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris KM, Maurer J, Lurie N. Is getting influenza vaccine coverage data out during mid-season feasible? Evidence from a national survey of U.S. adults. Vaccine. 2009 Jun 8;27(28):3697–9. doi: 10.1016/j.vaccine.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Lee BY, Mehrotra A, Burns RM, Harris KM. Alternative vaccination locations: who uses them and can they increase flu vaccination rates? Vaccine. 2009 Jul 9;27(32):4252–6. doi: 10.1016/j.vaccine.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logan JL. Disparities in influenza immunization among US adults. J Natl Med Assoc. 2009 Feb;101(2):161–6. doi: 10.1016/s0027-9684(15)30830-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee BY, Bailey RR, Wiringa AE, Assi TM, Beigi RH. Antiviral medications for pregnant women for pandemic and seasonal influenza: an economic computer model. Obstet Gynecol. 2009 Nov;114(5):971–80. doi: 10.1097/AOG.0b013e3181bdbfed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002 Jul–Aug;22(4):290–308. doi: 10.1177/0272989X0202200408. [DOI] [PubMed] [Google Scholar]
- 40.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project: Nationwide Inpatient Sample. 2005 [cited; Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 41.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000 Mar;181(3):831–7. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Meltzer MI, Wortley PM. FluSurge--a tool to estimate demand for hospital services during the next pandemic influenza. Med Decis Making. 2006 Nov–Dec;26(6):617–23. doi: 10.1177/0272989X06295359. [DOI] [PubMed] [Google Scholar]
- 43.Reed C, Angulo FJ, Swerdlow DL, Lipsitch M, Meltzer MI, Jernigan D, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April–July 2009. Emerg Infect Dis. 2009 Dec;15(12):2004–7. doi: 10.3201/eid1512.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Hospitalized Patients with Novel Influenza A (H1N1) Virus Infection — California, April–May, 2009. Morbidity and Mortality Weekly Report. 2009 May 22;58:16–21. [PubMed] [Google Scholar]
- 45.Dhar R, Stitt L, Hahn AF. The morbidity and outcome of patients with Guillain-Barre syndrome admitted to the intensive care unit. J Neurol Sci. 2008 Jan 15;264(1–2):121–8. doi: 10.1016/j.jns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Basta NE, Halloran ME, Matrajt L, Longini IM., Jr Estimating influenza vaccine efficacy from challenge and community-based study data. Am J Epidemiol. 2008 Dec 15;168(12):1343–52. doi: 10.1093/aje/kwn259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010 Jan 2;375(9708):41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 50.Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine. 2003 May 16;21(17–18):2207–17. doi: 10.1016/s0264-410x(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 51.Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA. 2000 Oct 4;284(13):1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 52.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002 Sep;113(4):300–7. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 53.Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effectiveness analysis. Am J Med. 2005 Jan;118(1):68–77. doi: 10.1016/j.amjmed.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 54.Rothberg MB, Bellantonio S, Rose DN. Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy. Ann Intern Med. 2003 Sep 2;139(5 Pt 1):321–9. doi: 10.7326/0003-4819-139-5_part_1-200309020-00007. [DOI] [PubMed] [Google Scholar]
- 55.Levit KTR, Wier LTR, Stranges ETR, Ryan KTR, Elixhauser AA. HCUP facts and figures: statistics on hospital-based care in the United States, 2007. 2009 [cited 2009 September 15]; Available from: http://www.hcup-us.ahrq.gov/reports.jsp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1: Scatter plots a–d depict the variation in costs and occurrence of influenza cases for H1N1 (a–b) and seasonal influenza (c–d) scenarios across both insurance groups, where TIV vaccine cost = $15 and all other variables remained at their baseline values shown in Table 1. The black diamonds on the graph represent the vaccination group, while the grey squares signify the unvaccinated group. Both groups consist of 1,000 data points (individuals), with each point derived from the average cost and number of influenza cases over 1,000 trials.