SUMMARY
Purpose
To examine the role of innate immunity in a novel viral infection-induced seizure model.
Methods
C57BL/6 mice, mouse strains deficient in interleukin (IL)-1RI, IL-6, tumor necrosis factor (TNF)-RI, or myeloid differentiation primary response gene 88 (MyD88), or transgenic mice (OT-I) were infected with Theiler’s murine encephalomyelitis virus (TMEV) or mock-infected. Mice were followed for acute seizures. Tissues were examined for neuron loss, the presence of virus (viral RNA and antigen), perivascular cuffs, macrophages/microglia and gliosis, and mRNA expression of IL-1, TNF-α and IL-6.
Results
IL-1 does not play a major role in seizures as IL-1RI and MyD88 deficient mice displayed a comparable seizure frequency relative to controls. In contrast, TNF-α and IL-6 appear to be important in the development of seizures as only 10% and 15% of TNF-RI and IL-6 deficient mice showed signs of seizure activity, respectively. TNF-α and IL-6 mRNA levels also increased in mice with seizures. Inflammation (perivascular cuffs, macrophages/microglia and gliosis) was greater in mice with seizures. OT-I mice (virus persists) had a seizure rate that was comparable to controls (no viral persistence) thereby discounting a role for TMEV-specific T–cells in seizures.
Discussion
We have implicated the innate immune response to viral infection, specifically TNF-α and IL-6, and concomitant inflammatory changes in the brain as contributing to the development of acute seizures. This model is a potential infection-driven model of mesial temporal lobe epilepsy with hippocampal sclerosis.
Keywords: Theiler’s murine encephalomyelitis virus, Seizures, Innate immune response, Inflammation, Temporal lobe epilepsy
INTRODUCTION
Viral infections within the central nervous system (CNS) can cause encephalitis (inflammation within the brain), which is often associated with seizures (Annegers et al., 1988; Hauser & Hesdorffer, 1990; Hosoya et al., 1997; Labar & Harden, 1997; Hosoya et al., 2001; Getts et al., 2008). We previously reported the induction of behavioral seizures in C57BL/6 mice using the neurotropic virus Theiler’s murine encephalomyelitis virus (TMEV) (Libbey et al., 2008). Infection of a susceptible strain of mouse with the Daniels (DA) strain of TMEV causes a late chronic inflammatory demyelinating disease (used as a model for multiple sclerosis) at around 1 month postinfection (p.i.) characterized by persistence of the virus (Tsunoda & Fujinami, 1999). The C57BL/6 strain of mouse has been considered to be resistant to TMEV-induced demyelination. Infected C57BL/6 mice do develop an acute disease, and have the ability to clear the virus. These mice do not go on to develop the late disease (Lipton & Dal Canto, 1979; Chamorro et al., 1986; Lindsley & Rodriguez, 1989; Pena-Rossi et al., 1991). We previously reported that more than 50% of C57BL/6 mice infected with TMEV develop acute seizures between days 3 and 10 p.i. (Libbey et al., 2008). The seizures appear to be limbic in nature and are characterized by forelimb clonus with rearing and falling; i.e., a Racine scale stage 5 seizure (Racine, 1972). Following DA viral infection, neurons, particularly in the hippocampal CA1 and CA2 regions, were lost due to direct viral infection and/or bystander cell death (Libbey et al., 2008). In general, no mouse was observed to have seizures before day 3 p.i., the peak of seizure activity occurred around day 6 p.i. and the majority of seizures had a seizure score of 3 and above (Libbey et al., 2008).
A distinct advantage of this viral infection-induced seizure model is that the animals survive the acute seizures, the seizures resolve by day 10 p.i, and thus the animals are available for studies investigating the development of epilepsy later in life. In fact by following the mice that had acute seizures for several months, an unsymptomatic period was observed to be followed by the development of spontaneous seizures/epilepsy (Stewart et al., 2008). Thus this model mimics the initial insult, latent period and development of spontaneous seizures often reported for mesial temporal lobe epilepsy (TLE) patients.
Because acute seizures developed on day 3 p.i., we hypothesized that the innate immune response to viral infection contributed to the development of seizures (Libbey et al., 2008). The innate immune system is made up of effector cells and a variety of proteins that have anti-viral activity or participate in the inflammatory response to infection. The effector cells, including neutrophils, macrophages and natural killer (NK) cells, produce many of the proinflammatory cytokines that lead to inflammation and the anti-viral state. Cytokines are also produced, in some instances, by cells within the CNS, particularly the microglial cells and astrocytes (Rubio & Sierra, 1993; Raivich et al., 1998; Konsman et al., 2007). It is thought that the major producers of these cytokines are the infiltrating mononuclear cells (MNCs) (Konsman et al., 2007); however, both infiltrating cells and resident CNS cells participate. Tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6 are all produced by macrophages.
The second part of the immune system is the adaptive immune response which consists of T–cells and antibody molecules (not discussed further). The adaptive immune response takes days to respond to infection, while the innate immune response occurs within hours. CD8+ T–cells recognize antigenic peptides complexed to major histocompatibility complex (MHC) class I molecules through the T-cell receptor (TCR) and kill virus-infected cells. CD4+ T–cells that recognize antigenic peptides complexed to MHC class II molecules via TCRs perform several tasks. CD4+ T–cells secrete proinflammatory cytokines, such as interferon-γ or IL-17, and have several effector functions.
In this study we describe inflammatory changes that occur in the brains of C57BL/6 mice in a novel viral infection-induced seizure model. In addition, we find that modulation of the innate inflammatory response can markedly decrease the number of mice that have seizures and that TNF-RI engagement and IL-6 are important for the development of these seizures.
METHODS
Animals and infection
C57BL/6 inbred mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice deficient in IL-1RI, IL-6 and TNF-RI, all on a C57BL/6 background, were purchased from the Jackson Laboratory. Myeloid differentiation primary response gene 88 (MyD88) deficient mice on a C57BL/6 background were kindly provided by Janis Weis, University of Utah. Male and female OT-I (CD8+, ovalbumin-specific TCR) transgenic mice on a C57BL/6 background were kindly provided by Matt Williams, University of Utah.
On day 0, 5- 6-week old mice were anesthetized with isoflurane and infected intracerebrally (i.c.), in the post parietal cortex of the right cerebral hemisphere to a depth of 2 mm [posterior (caudal) and medial of the right eye], with 2 × 104 plaque forming units (pfu) of the DA strain of TMEV or mock-infected with 20 µl of phosphate-buffered saline (PBS). The DA strain of TMEV was propagated as previously described (Zurbriggen & Fujinami, 1989).
The care and use of the above-mentioned mice was in accordance with the guidelines prepared by the committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council.
Observations
To monitor seizure activity, mice were observed continuously for 2 h, randomly between the hours of 9:00 a.m. and 5:00 p.m. each day p.i. Seizure activity was graded using the Racine scale as follows: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; and stage 5, rearing and falling (Racine, 1972; Benkovic et al., 2004).
Histology
In order to observe the pathological manifestations of the seizures, additional mice were euthanized with isoflurane on days 1, 2, 3, 4, 5, 6, 7, 14, 21 and 35 p.i. Animals were perfused with PBS, followed by a buffered 4% paraformaldehyde solution. Brains were harvested and fixed in 4% paraformaldehyde, divided into five coronal slabs per brain and embedded in paraffin. Multiple 4-µm-thick tissue sections, containing sections from all five coronal slabs per brain, were cut, mounted on slides and stained via various methods as described below. The tissue section of only one of the five coronal slabs represented per slide contained the hippocampal/dentate gyral regions of the brain.
Luxol fast blue stained slides were used to evaluate neuron loss in the hippocampus in a blinded fashion using one slide per brain (N = 4 to 13 brains per experimental group). Neuron loss in the pyramidal cell layer of the hippocampus, from CA1 to CA3, was scored as follows: score 0, no damage; score 1, 10–29% cell loss (10–29% of the pyramidal cell layer from CA1 to CA3 is missing); score 2, 30–59% cell loss; and score 3, >60% cell loss. An undamaged control brain represented a score of 0 with no cell loss and thus 100% of the cells present. A score was given for each of the two hippocampi present in a brain and then the scores were summed so the highest possible score for cell loss per brain could be 6 (the highest score, 3, for two regions of the brain). Cytoarchitecture of the hippocampus was referenced to Figs. 43–49 in The Mouse Brain in Stereotaxic Coordinates (Franklin & Paxinos, 1997).
Immunohistochemistry
DA viral antigen positive cells and astrocytes were detected on paraffin sections using hyperimmune rabbit serum against TMEV and glial fibrillary acidic protein (GFAP) antibody (DAKO Corp., Carpinteria, CA, USA), respectively, as previously described (Tsunoda et al., 1997; Tsunoda et al., 2001). The slides were labeled using the avidin-biotin peroxidase complex technique with 3,3'-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO, USA) in 0.01% hydrogen peroxide (Sigma) in PBS. Specificity of antibody binding was confirmed by parallel staining minus the hyperimmune serum or the GFAP antibody, respectively.
Enumeration of DA viral antigen positive cells was performed in a blinded fashion with a light microscope using one slide per brain and evaluating tissue sections from all five coronal slabs represented per slide (N = 4 to 5 brains per experimental group). DA viral antigen positive cells were enumerated and summed in the following brain regions in OT-I mice: frontal lobe, olfactory bulb, septum, caudoputamen, hippocampus, thalamus, hypothalamus, midbrain, cortex, cerebellum and brain stem. DA viral antigen positive cells were enumerated in the following brain regions in C57BL/6 mice: septum, hippocampus and cortex. No DA viral antigen was detected in the additional eight brain regions of C57BL/6 mice.
The extent of gliosis was semi-quantified by scoring GFAP+ activated astrocytes in the hippocampus and dentate gyrus in a blinded fashion using one slide per brain (N = 5 to 11 brains per experimental group). Activated astrocytes have larger cell bodies, fatter processes and stain more intensely than quiescent astrocytes. Gliosis was given a graded score as follows: score 0, no damage (<50 activated astrocytes present); score 1, mild (50–350 activated astrocytes present); score 2, moderate (351–700 activated astrocytes present); and score 3, severe (>700 activated astrocytes present). A score was given for each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain and then the scores were summed so the highest possible score for gliosis per brain could be 12 (the highest score, 3, for four regions of the brain). As a control (labeled PBS) for GFAP staining, untreated mice (N = 2) and PBS-treated mice (N = 3; one each sacrificed on days 3, 5 and 7 p.i.) were evaluated as described and the scores were averaged.
Ricinus communis agglutinin (RCA)-I lectin histochemistry
Activated microglia and macrophages were identified by biotinylated RCA-I (Vector Laboratories Inc., Burlingame, CA, USA) as previously described (Suzuki et al., 1988; Tsunoda et al., 1996; Tsunoda et al., 2003; Tsunoda et al., 2007). One slide per brain for three to ten brains per experimental group was examined in a blinded fashion. RCA-I+ cells in each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain were enumerated and summed. As a control (labeled PBS) for RCA-I staining, PBS-treated mice (N = 3; one each sacrificed on days 3, 5 and 7 p.i.) were evaluated as described and averaged.
PCR arrays
Five- to 6-week old C57BL/6 mice (three to four per group) infected with 2 × 104 pfu DA virus or injected with PBS were euthanized with isoflurane on days 2 and 6 p.i. and brains were harvested and frozen. Brains from naïve mice were used as a normal control. RNA was isolated by homogenizing the brains in Trizol reagent (Invitrogen, San Diego, CA, USA), performing a chloroform extraction and then further purifying the RNA by means of the RNeasy Maxi Kit (Qiagen, Chatsworth, CA, USA). From the RNA, cDNA was made using M-MLV (Moloney Murine Leukemia Virus) Reverse Transcriptase (Invitrogen) according to the manufacturer’s recommendations and using random primers. cDNAs from three to four brains were pooled from the PBS, day 2; infected, day 2; PBS, day 6; no seizures (infected), day 6; and seizures (infected), day 6 groups. cDNA was assayed on a LC480 Light Cycler (Roche, Indianapolis, IN, USA) 96-well block, via a polymerase chain reaction (PCR) array specific for mouse inflammatory cytokines and receptors (SABiosciences, Frederick, MD, USA) as per the manufacturer’s instructions.
Quantitative real-time (QRT)-PCR
Template for QRT-PCR for the detection of cytokines was the individual cDNAs as generated for the PCR arrays but without pooling the cDNAs (three per group) from the PBS, day 6; no seizures (infected), day 6; and seizures (infected), day 6 groups. QRT-PCR was performed using a LC480 Light Cycler and the SYBR Green method of amplicon detection. β-actin was used to normalize the data. The level (L) of gene expression was determined using the formula: L = (2−Ct)108, where Ct = cycle threshold. A relative normalized expression level for each sample was determined by dividing the L of the gene of interest by the L of β-actin.
QRT-PCR for the detection of DA viral RNA was performed as above with the following modifications. Infected 5- 6-week old mice were euthanized with isoflurane between days 12 and 17 p.i. for OT-I mice and on days 12 and 14 p.i. for C57BL/6 mice, and their brains were harvested and frozen. RNA was isolated and cDNA was generated as described above for the PCR arrays/QRT-PCR for cytokines. β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used to normalize the data. Primers were designed that amplified within the 2C region of the TMEV genome.
Statistical analysis
The StatView program (SAS Institute Inc., Cary, NC) was used for all statistical analyses performed. Analysis of variance (ANOVA), followed were indicated by the Fisher’s Protected Least Significant Difference (Fisher’s PLSD) post hoc test, was performed to determine group differences for continuous data. The chi-square test was utilized for nominal data (seizures: yes or no). Finally, the unpaired two group Mann-Whitney U test was performed for all nonparametric analyses (neuron loss and gliosis).
RESULTS
Viral antigen and neuron loss
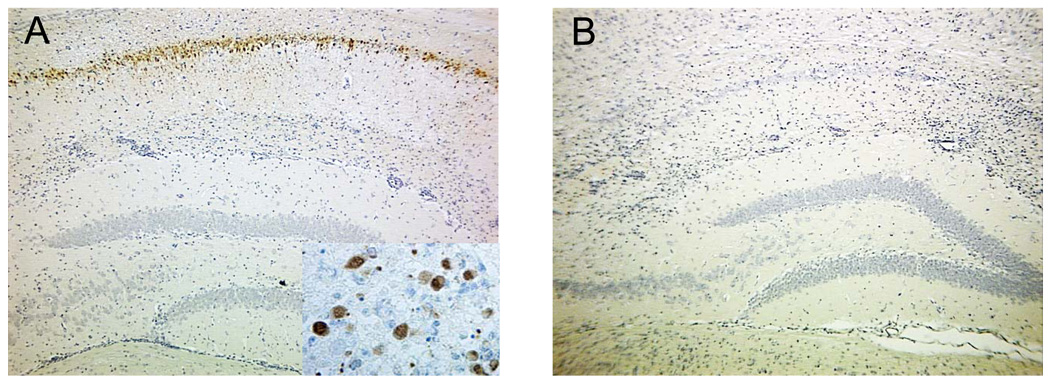
Evaluation of brains from DA virus-infected C57BL/6 mice demonstrated viral infection of neurons and neuron loss within the hippocampus. DA viral antigen positive cells were detected by immunohistochemistry (Tsunoda et al., 2001). Neurons within the hippocampus of mice with acute seizures were clearly DA viral antigen positive on day 7 p.i. (Fig. 1A), but viral antigen was largely undetectable by day 14 p.i. (Fig. 1B).
Figure 1.
Daniels (DA) virus infects neurons during the acute seizures but viral antigen is subsequently cleared. DA viral antigen-positive cells as determined by immunohistochemistry using hyperimmune rabbit serum against Theiler’s murine encephalomyelitis virus (TMEV) are clearly visible in pyramidal neurons of the hippocampus at day 7 postinfection (p.i.) in mice with seizures (A), but the viral antigen is cleared by day 14 p.i. in mice with seizures (B). Images are representative of DA virus-infected mice with seizures at the respective time points. Inset in (A) is higher magnification of DA viral antigen positive cells.
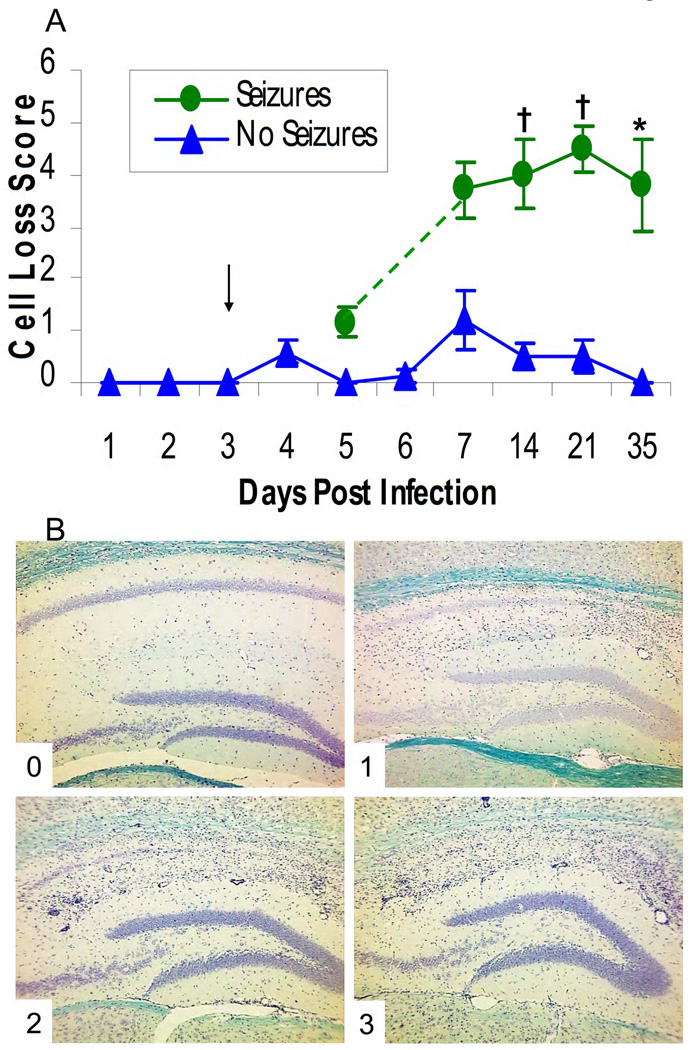
We previously reported that there was marked neuron death following DA virus infection in the hippocampus, particularly in the CA1 to CA3 regions (Libbey et al., 2008). In the current study we quantified the extent of neuron loss within the hippocampus of infected mice. Prior to and including day 5 p.i., the extent of neuron loss was very minimal for those animals that had seizures and for those infected animals without seizures (Fig. 2A). By day 7 p.i. and continuing through day 35 p.i., there was a clear segregation between the infected mice that displayed behavioral seizures (Seizures) and those that did not (No Seizures). Mice with seizures had a significant increase in neuron loss compared to mice that did not show seizures for days 14, 21 and 35 p.i. (P < 0.001 for days 14 and 21, P < 0.01 for day 35, Mann-Whitney U test, Fig. 2A). This neuron death was focused in the pyramidal layer resulting in extensive loss of CA1 to CA2 neurons and some CA3 neurons, with little or no cell loss in the dentate gyrus. Representative images for each cell loss score (described in the Methods), which is noted in the lower left corner of each image (0–3), are shown in Fig. 2B.
Figure 2.
Neuron loss within the hippocampus of mice infected with Daniels (DA) virus. Neuron loss was scored as described in the Methods. (A) Neuron loss was significantly greater in the mice with seizures compared to the mice without seizures for days 14, 21 and 35 p.i (days on which mice were sacrificed). *, p < 0.01; †, P < 0.001 (Mann-Whitney U test). The arrow marks the earliest day on which seizures were observed. Results are mean ± standard error of the mean (SEM) of groups with 4–13 mice per group. (B) Representative images are shown for each cell loss score, which is noted in the lower left corner of each image (0–3).
TNF-α and IL-6 are important cytokines for seizures
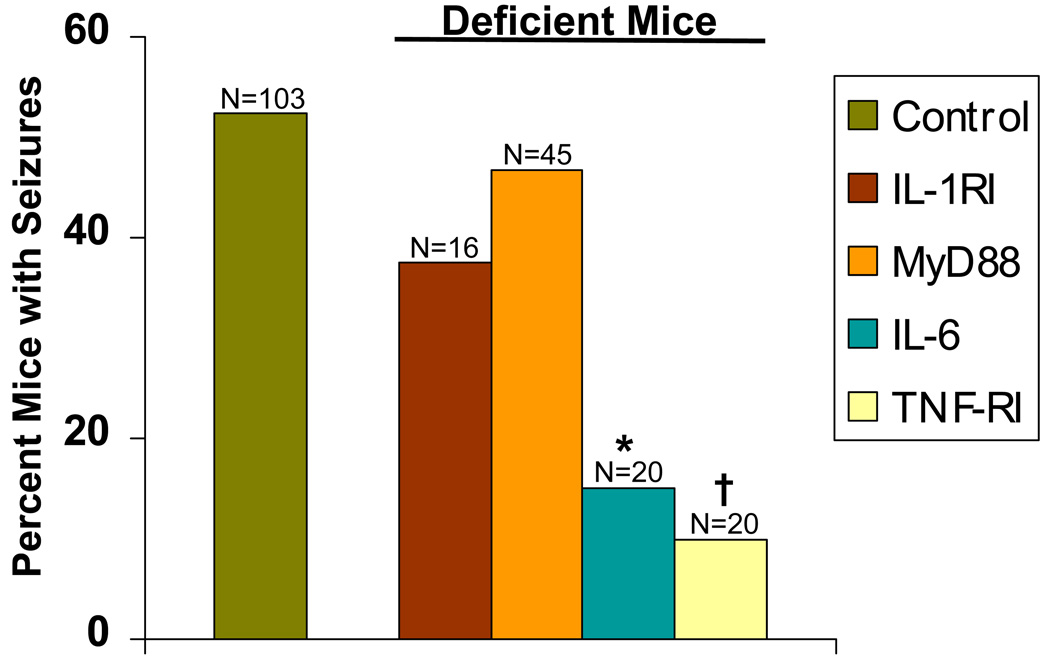
We tested how proinflammatory cytokines produced early after infection could influence whether infected mice developed seizures. IL-1, TNF-α and IL-6 are cytokines produced early within the CNS following viral infection. Therefore, we tested various mice deficient in these cytokines or their receptors for the development of seizures. Infected IL-1RI deficient mice or wild-type mice were observed for the development of seizures through day 21 p.i. Thirty-eight percent of mice lacking IL-1 receptor signaling developed seizures (Racine scale stage 3–5) compared to wild-type infected mice (difference not significant, chi-sSquare) (Fig. 3). This was somewhat surprising in that previous studies using other systems have reported the importance of IL-1 in seizures (Gahring et al., 1997; Bartfai et al., 2007; Vezzani & Baram, 2007). Since IL-1 signals through MyD88, we infected mice deficient in MyD88 as well as wild-type control mice. Forty-seven percent of MyD88 deficient mice also developed seizures (Racine scale stage 3–5) compared to wild-type mice (difference not significant, chi-square), indicating that IL-1 signaling through MyD88 was not important for mice to develop seizures. In contrast, when TNF-RI deficient mice and IL-6 deficient mice were infected with DA virus, significantly fewer mice, 10% (P < 0.001, chi-square) and 15% (P < 0.01, chi-square) respectively, developed seizures (Racine scale stage 3–5) compared to wild-type control mice (Fig. 3). These data indicate that cytokines, particularly IL-6 and TNF-α, display modulating effects and that cytokine signaling within the CNS contributes to the development of seizures. Therefore, seizures arise independently of IL-1 signaling through the adaptor protein MyD88.
Figure 3.
Seizure (Racine scale stage 3–5) frequency in deficient mice. Thirty-eight percent of IL-1RI deficient and 47% of myeloid differentiation primary response gene 88 (MyD88)- deficient mice developed seizures compared to wild-type C57BL/6 control mice (differences not significant). In contrast, significantly fewer tumor necrosis factor (TNF)-RI-deficient and IL-6-deficient mice, 10% and 15% respectively, developed seizures compared to wild-type control mice. *, p < 0.01; †, P < 0.001 (chi-square). The total number of mice infected is shown as N over the individual bars of the graph. Percent mice with seizures (y-axis) is calculated as follows: (number of mice with seizures/total number of mice infected) × 100.
mRNA expression levels for TNF-α and IL-6 correlate with seizure expression
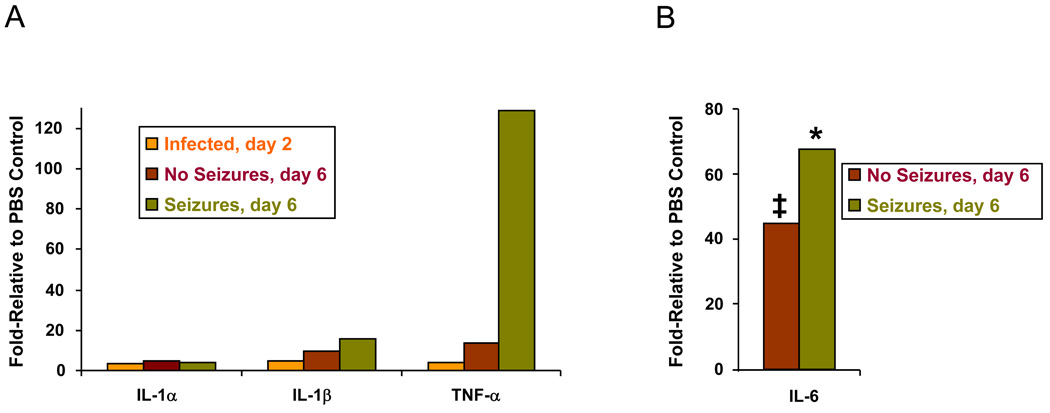
Our data indicate that IL-1 does not play a major role in seizure expression in the TMEV model but that TNF-α signaling correlates with seizures. In order to confirm this result, mRNA expression levels for IL-1α/β and TNF-α were assayed via PCR arrays as described in the Methods section. mRNA expression was compared between PBS-injected and DA virus-infected mice for day 2 p.i., prior to the development of seizures, and day 6 p.i., when seizures were clearly evident. PBS-injected day 2 and PBS-injected day 6 were not different from normal control brains. For day 6, we observed an increase in IL-1β mRNA expression in mice with seizures (Seizures, day 6) and mice without seizures (No Seizures, day 6) of 16-fold and 9-fold relative to PBS control, respectively (Fig. 4A). In contrast, there was a marked increase in TNF-α mRNA expression in the mice with seizures (128.5-fold relative to PBS control) with a much smaller increase in the No Seizures group (13.5-fold relative to PBS control) (Fig. 4A). These changes in TNF-α were confirmed using QRT-PCR (data not shown). IL-1α mRNA expression increased only 4.3-fold for the Seizures and 4.7-fold for the No Seizures groups relative to PBS control (Fig. 4A). In addition, although IL-6 was not included in the PCR array, we did perform QRT-PCR monitoring IL-6 mRNA expression levels and found that mice both with and without seizures had a significant increase in IL-6 mRNA expression compared to PBS control (P < 0.01 and P < 0.05, respectively, ANOVA, Fisher’s PLSD post hoc test). IL-6 mRNA expression levels were increased by day 6 in mice with seizures by 67-fold relative to PBS control and in mice without seizures by 44.5-fold relative to PBS control (Fig. 4B).
Figure 4.
mRNA expression levels for mouse inflammatory cytokines. (A) mRNA expression was compared, using polymerase chain reaction (PCR) arrays specific for mouse inflammatory cytokines, between Daniels (DA) virus-infected mice for day 2 postinfection (p.i.) (Infected, day 2) and day 6 p.i. (No Seizures, day 6 and Seizures, day 6), relative to phosphate-buffered saline (PBS)-injected control mice. Interleukin (IL)-1β mRNA expression increased in mice with seizures (Seizures, day 6) and mice without seizures (No Seizures, day 6) by 16-fold and 9-fold, respectively, relative to PBS control. In contrast, tumor necrosis factor alpha (TNF-α) mRNA expression increased in mice with seizures (Seizures, day 6) and mice without seizures (No Seizures, day 6) by 128.5-fold and 13.5-fold, respectively, relative to PBS control. IL-1α mRNA expression increased by only 4.3-fold and 4.7-fold relative to PBS control for the mice with seizures (Seizures, day 6) and mice without seizures (No Seizures, day 6), respectively. cDNAs from three to four brains per group were pooled for the assay. (B) mRNA expression was compared, using quantitative real-time (QRT)-PCR specific for IL-6, between DA virus-infected mice for day 6 p.i. (No Seizures, day 6 and Seizures, day 6), relative to PBS-injected control mice. IL-6 mRNA expression increased in mice with seizures (Seizures, day 6) and mice without seizures (No Seizures, day 6) by 67-fold and 44.5-fold relative to PBS control, respectively. *, p < 0.01; ‡, P < 0.05 (ANOVA).
Role of inflammation in acute seizures
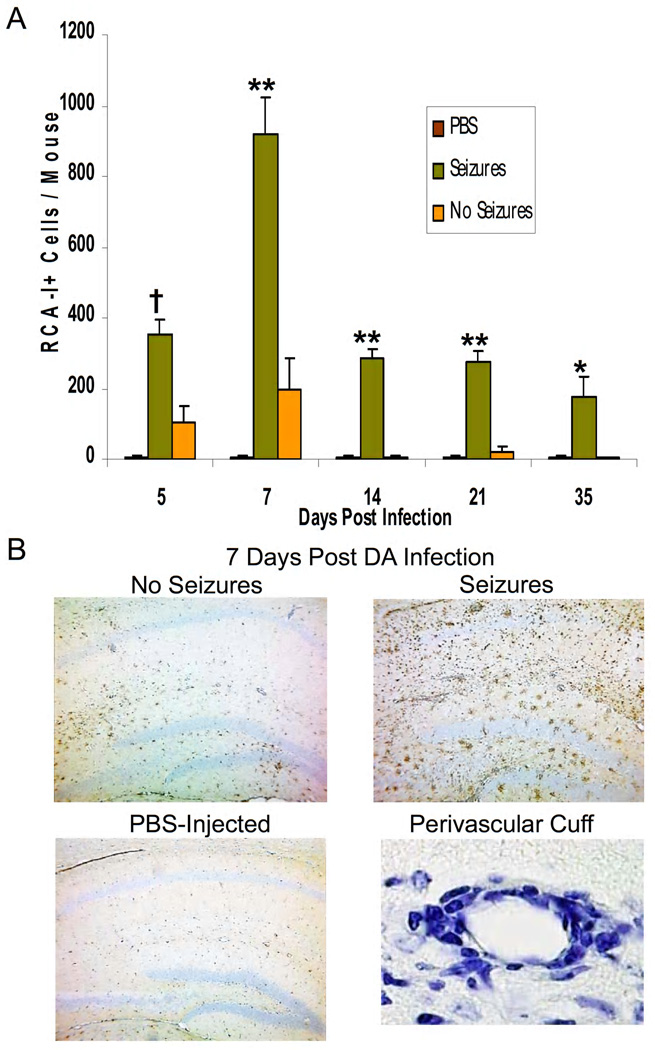
The above data suggest that the innate immune response to viral infection contributes to seizures. To examine further the pathological manifestations of the viral infection and seizures, we examined the extent of gliosis, infiltration of macrophages and/or activation of microglial cells and perivascular cuffing (comprised of infiltrating MNCs). As early as day 5 and continuing on through day 35 p.i., perivascular cuffing within the hippocampus was increased in mice that had seizures relative to mice in the No Seizures and control groups (PBS-injected). This was due to the continued presence of inflammatory cells in the brains of mice with seizures, while infiltrating cells in the brains of animals without seizures started to decline and very few if any perivascular cuffs were seen by day 14 p.i. in the No Seizures group. This was confirmed by staining brain sections with RCA-I (macrophage/activated microglia marker). Mice displaying seizures had a significantly higher number of RCA-I+ cells in the hippocampus and dentate gyrus compared to both PBS control and mice not observed to have seizures for days 5, 7, 14, 21 and 35 (P < 0.01 for day 35, P < 0.001 for day 5, P < 0.0001 for days 7, 14 and 21, ANOVA, Fisher’s PLSD post hoc test, Fig. 5A). The Seizures group had approximately 900 RCA-I+ cells/mouse (compared to 200 for No Seizures) by day 7 p.i. which decreased to 200–300 RCA-I+ cells/mouse (compared to 20 or less for No Seizures) for days 14, 21 and 35 p.i. Representative images for RCA-I lectin histochemistry on brain sections from PBS-injected mice and DA virus-infected mice with or without seizures at 7 days p.i. and for perivascular cuffing (Luxol fast blue stain) are shown in Fig. 5B.
Figure 5.
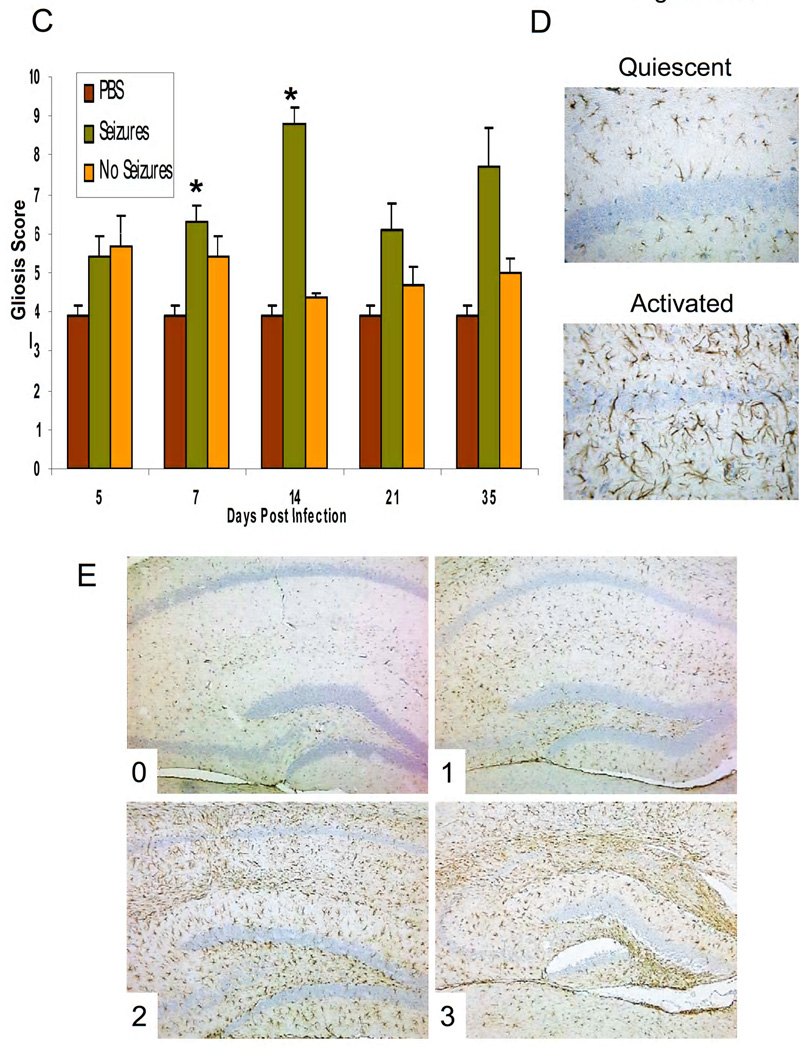
Inflammation within the hippocampus and dentate gyrus of mice infected with Daniels (DA) virus. (A,B) Activated microglia/macrophages, detected through Ricinus communis agglutinin (RCA)-I lectin histochemistry, (B) perivascular cuffing, detected through Luxol fast blue staining, and (C–E) activated astrocytes (gliosis), detected through glial fibrillary acidic protein (GFAP) immunohistochemistry, are a measure of inflammation. (A) The numbers of RCA-I+ cells were significantly greater in the mice with seizures compared to both phosphate-buffered saline (PBS) control and mice without seizures for days 5, 7, 14, 21 and 35 postinfection (p.i.) *p < 0.01; †p < 0.001; **p < 0.0001 (analysis of variance, ANOVA). Results are mean + standard error of the mean (SEM) of groups with 3–10 per group. (B) Representative images of RCA-I lectin histochemistry on brain sections from PBS-injected mice and DA virus-infected mice with or without seizures at 7 days p.i. and an example of a perivascular cuff (Luxol fast blue stain). (C) Gliosis was scored as described in the Methods. Gliosis was significantly greater in the mice with seizures compared to both PBS control and mice without seizures on day 14. Gliosis was significantly greater in the mice with seizures compared to only PBS control mice on day 7 p.i. *p < 0.01 (Mann-Whitney U test). Results are mean + SEM of groups with 5–11 mice per group. (D) Representative images are shown for both quiescent and activated astrocytes at high magnification. (E) Representative images are shown for each gliosis score, which is noted in the lower left corner of each image (0–3).
By scoring GFAP+ activated astrocytes in the hippocampus and dentate gyrus we were able to semi-quantify the extent of gliosis using a graded score (see Methods). As with RCA-I+ cells, gliosis was greater in the Seizures group on days 7, 14, 21 and 35 p.i. compared to PBS control and No Seizures groups (Fig. 5C). The gliosis was significantly greater in the mice with seizures compared to both PBS control and mice without seizures on day 14 (P < 0.01, Mann-Whitney U test, Fig. 5C) and was significantly greater compared to only PBS control mice on day 7 p.i. (P < 0.01, Mann-Whitney U test, Fig. 5C). Representative images for both quiescent and activated astrocytes at high magnification are shown in Fig. 5D. Representative images for each gliosis score (described in the Methods), which is noted in the lower left corner of each image (0–3), are shown in Fig. 5E.
Role of virus-specific CD8+ T ells in acute seizures
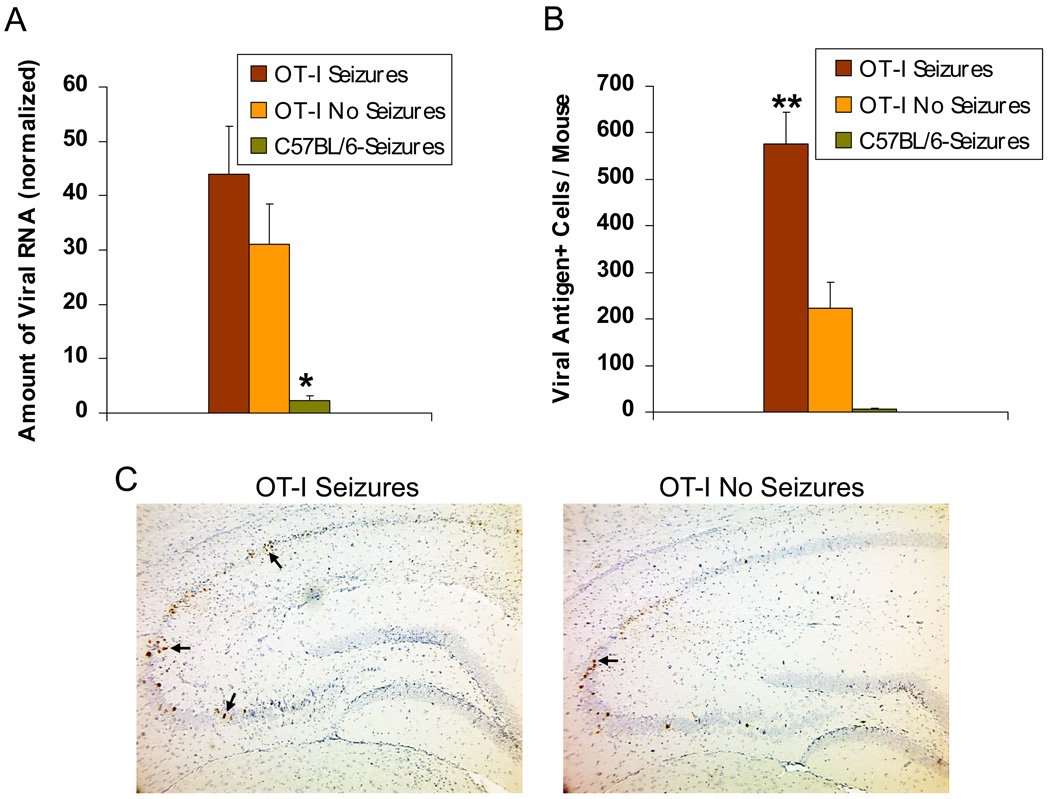
OT-I mice, which are transgenic C57BL/6 mice in which the majority of the CD8+ T–cells are specific for ovalbumin, were observed to experience seizures at a rate that was not significantly different from infected wild-type C57BL/6 mice when monitored for seizures through day 21 p.i. (38% versus 50%, chi-square test). As seen with wild-type mice, no seizures were seen in the infected OT-I mice after day 10 p.i. Unlike wild-type mice which clear viral antigen by day 14 p.i. (Fig. 1B), DA virus was found to persist in the OT-I mice upon examination of the OT-I mouse brains for the presence of DA viral RNA (Fig. 6A) and DA viral antigen (Fig. 6B). DA viral RNA was found to be significantly less in the C57BL/6-Seizures mice compared to both the OT-I Seizures and OT-I No Seizures mice (P < 0.01, ANOVA, Fisher’s PLSD post hoc test, Fig. 6A). DA viral antigen was found to be significantly higher in the OT-I Seizures mice compared to both the OT-I No Seizures mice and the C57BL/6-Seizures mice (P < 0.0001, ANOVA, Fisher’s PLSD post hoc test, Fig. 6B). Due to the inability of the OT-I mice to clear the virus, these mice developed hind limb paralysis and all died or were euthanized by day 12 to 17 p.i. Representative images of TMEV immunohistochemistry with DA viral antigen positive cells (arrows) on OT-I Seizures and OT-I No Seizures mouse brain sections are shown in Fig. 6C.
Fig. 6. Daniels (DA) virus persistence in transgenic OT-I mouse brains.
(A) DA viral RNA as determined by quantitative real-time-polymerase chain reaction (QRT-PCR) and (B,C) DA viral antigen-positive cells as determined by immunohistochemistry demonstrate the presence of DA viral RNA and protein in the brains of OT-I mice, both with and without seizures, 12–17 days postinfection (p.i.). C57BL/6 mice with seizures were assayed at days 12 and 14 p.i. for comparison. (A) Viral RNA was normalized against β-actin and GAPDH mRNA levels. DA viral RNA was significantly less in the C57BL/6 mice with seizures compared to both the OT-I mice with seizures and OT-I mice without seizures. *p < 0.01 (ANOVA). (B) DA viral antigen was significantly greater in the OT-I mice with seizures compared to both the OT-I mice without seizures and the C57BL/6 mice with seizures. **p < 0.0001 (ANOVA). Results are mean + standard error of the mean (SEM) of groups with three to five mice per group. (C) Representative images of Theiler’s murine encephalomyelitis virus (TMEV) immunohistochemistry showing DA viral antigen positive cells (arrows) on OT-I Seizures and OT-I No Seizures mouse brain sections.
DISCUSSION
Seizures induced chemically, electrically, audiogenically or by kindling induce the rapid-onset production of proinflammatory cytokines (IL-1β, TNF-α, IL-6) in microglia and astrocytes in the rodent brain [reviewed in (Vezzani & Granata, 2005; Vezzani et al., 2008a; Vezzani et al., 2008b)]. This inflammatory response occurs in regions of the brain (i.e., hippocampus) involved in the onset and propagation of epileptic activity. Injuries to the CNS caused by seizures or infections may induce transient changes in the permeability of the blood-brain barrier; thereby allowing an inflammatory state which, possibly through neuronal hyperexcitability and/or neuron loss, can lead to the onset of epilepsy [reviewed in (Vezzani & Granata, 2005; Vezzani et al., 2008a; Vezzani et al., 2008b)].
The importance of CNS inflammation in the development of seizures has recently been demonstrated using the pilocarpine model of status epilepticus (chemically-induced) in C57BL/6 mice (Fabene et al., 2008). The occurrence of several cell adhesion molecules was shown to increase in brain endothelial cells shortly after pilocarpine administration. Antibody blockade of, or genetic interference with, these specific cell adhesion molecules or their corresponding ligands, cell surface integrins on leukocytes (including neutrophils, lymphocytes and monocytes/macrophages), which disrupts leukocyte-endothelial interactions, resulted in a reduction in seizure activity. More specifically, neutrophil depletion resulted in a reduction in seizure activity whereas treatment with monoclonal antibody against CD4 had no effect. These findings demonstrate the importance of leukocyte recruitment, a hallmark of tissue inflammation, in the development of seizures (Fabene et al., 2008).
Still others have demonstrated the importance of peripheral inflammation in seizure susceptibility (Riazi et al., 2008). The susceptibility of Sprague-Dawley rats to pentylenetetrazole-induced seizures was found to be increased in those animals pretreated with 2,4,6-trinitrobenzene sulfonic acid to induce inflammatory bowel disease when compared to saline-treated controls. This increase in seizure susceptibility was found to be a microglial cell-dependent TNF-α-mediated increase in CNS excitability in the hippocampus (Riazi et al., 2008).
Here we demonstrate the importance of proinflammatory cytokines (TNF-α and IL-6) and inflammation (activated astrocytes, microglia and/or infiltrating macrophages) in the development of acute seizures in the TMEV infection-induced seizure model, and we are developing plans to investigate the importance of proinflammatory cytokines and inflammation in the development of epilepsy using this same animal model. A possible functional relationship between infection and seizures/epilepsy is as follows: microglia are activated very early after CNS infection; proinflammatory cytokines (TNF-α and IL-6) are released by activated microglia or infiltrating MNCs; these cytokines can modulate glutamate homeostasis by regulating glutamate receptors and transporters on astrocytes; impaired handling of extracellular glutamate by activated astrocytes could result in excessive extracellular glutamate levels around neurons leading to neuroexcitability and excitotoxic neuronal damage; seizures and epilepsy result [reviewed in (Choi & Koh, 2008)].
TNF-α is secreted by NK, mast and activated T-cells, in addition to macrophages, and has the ability to directly lyse cells and induce apoptosis (neurotoxic) or can lead to the expression of mediators of inflammation and anti-apoptotic proteins, thus protecting cells from apoptosis (neuroprotective), depending on which of two TNF receptors (TNF-RI and II), present on most cells, are engaged by the cytokine (Aggarwal, 2003; Krishnaswamy et al., 2006). Thus, there is a balance or regulatory process which functions to keep TNF-α activity in check (Aggarwal, 2003). It has been reported that increased brain levels of TNF-α, achieved through either intrahippocampal injection of recombinant murine TNF-α in C57BL/6 mice or astrocytic overexpression of TNF-α, under regulatory control of the GFAP gene promoter which targets the expression to astrocytes, in transgenic mice (C57BL/6 × SJL), significantly inhibited seizures induced by means of kainic acid (Balosso et al., 2005). Additionally, C57BL/6 mice that lacked just TNF-RII or both TNF-RI and II showed prolonged/enhanced seizures, whereas mice that lacked just TNF-RI showed reduced seizures in the kainic acid seizure model (Balosso et al., 2005). Here we demonstrate that mice lacking TNF-RI showed reduced seizures in our model. As the lack of TNF-RI had similar effects on seizures in both the kainic acid and TMEV infection-induced seizure models, the mode of induction of seizures by this chemical and virus infection may be the same. We have yet to examine the role of TNF-RII in our seizure model.
IL-6 which is synthesized in response to other cytokines (IL-1 and TNF-α) signals through its receptor leading to gene transcription that promotes neuron survival (de Araujo et al., 2009). It has been reported that overexpression of IL-6 in the CNS, under the GFAP gene promoter, induced spontaneous seizures in (C57BL/6J × SJL)F1 hybrid mice that were high expressor GFAP-IL-6 mice (Campbell et al., 1993), and increased the sensitivity of low expressor GFAP-IL-6 mice (which did not spontaneously develop seizures) to both kainic acid-and N-methyl-D-aspartate-induced seizures (Samland et al., 2003). Also, IL-6 mRNA was shown to be induced in the hippocampus by seizures in the lithium-pilocarpine model of status epilepticus (Rosell et al., 2003). Finally, increased concentrations of IL-6 have been reported in the cerebrospinal fluid and serum of patients following tonic-clonic seizures and in hippocampal neurons in rats following electrically-induced seizures [reviewed in (Juttler et al., 2002)]. Endogenous IL-6 has been shown to reduce the susceptibility of mice (129Sv) to seizures induced with kainic acid as IL-6 knockout mice (C57BL/6 × 129Sv) were observed to have seizures at a higher percentage, had more convulsions, higher mortality and increased susceptibility to kainic acid-induced brain damage (Penkowa et al., 2001). We now report that C57BL/6 mice which lacked IL-6 showed reduced seizures in the TMEV infection-induced seizure model. The resulting opposite effect of the lack of IL-6 in these two cases may be due to either the mode of induction, kainic acid versus TMEV infection, or may be due to a genetic difference in the strains of mice.
The goal in including the OT-I mice was to address three questions: 1) Are TMEV antigen-specific T–cells involved in the seizures?; 2) Do the seizures stop at day 10 p.i. due to the clearance of virus by T–cells?; and 3) Does viral persistence contribute to seizures? Based on the observation that OT-I mice (ovalbumin-specific CD8+ T–cells) still display behavioral seizures, we can conclude that the seizures are not likely influenced by TMEV-specific CD8+ T–cells. The CD8+ T-cell response in OT-I mice is overwhelmingly against ovalbumin, and thus there is no CD8+ T-cell response to DA viral infection, and yet the mice were still observed to experience seizures. Since the OT-I mice stop seizing by day 10 p.i. in the presence of viral persistence (RNA and antigen), we can conclude that it is not the clearance of virus by the T-cell response that causes the seizures to stop. This observation of viral persistence in the CNS is consistent with DA virus infection of SJL/J mice which do not develop seizures but virus persists in the CNS (Libbey et al., 2008).
The TMEV infection-induced seizure model is characterized by neuron loss and gliosis. The neuron loss resulting from TMEV infection has been demonstrated to be largely restricted to the hippocampus (Tsunoda et al., 1997; Libbey et al., 2008; Buenz et al., 2009). This leads us to believe that this model is similar to mesial TLE with hippocampal sclerosis which is characterized by gliosis and a loss of neurons (Sharma et al., 2007). Mesial TLE is thought to be a multifactorial disease involving multiple susceptibility genes and environmental factors, including CNS infections (van Gassen et al., 2008). Large-scale gene expression profiling (microarray) studies of human TLE have identified genes encoding chemokines (expressed by microglia and astrocytes in the brain), thereby implicating the immune system (van Gassen et al., 2008), and many genes associated with immune and inflammatory functions, as well as genes associated with activated astrocytes (Lee et al., 2007). Our seizure model displays neuropathology, neuron loss, gliosis and astrocyte activation, similar to that of human TLE with hippocampal sclerosis, and as such may be a novel model of infection-induced TLE. In addition, a recent study found that T–cells of the adaptive immune response were not present in the brain parenchyma of TLE patients with hippocampal sclerosis (Ravizza et al., 2008). We also have discounted a role for TMEV-specific T–cells in this seizure model, thus lending additional support to the suggestion that this model is a good model for infection-induced TLE with hippocampal sclerosis.
In summary we provide evidence that TNF-RI and IL-6 contribute to the development of acute seizures following viral infection. We feel that the innate immune response to viral infection within the CNS, not viral persistence or virus-specific CD8+ T–cells, is the major driving force for these seizures. This model is a potential infection-driven model of TLE and serves as a unique model to study the impact of inflammatory processes on neuron death, astrocytes and epileptogenesis.
Acknowledgement
We wish to thank Faris Hasanovic, BS, Daniel J. Doty and Krystal D. Porter, BS, for excellent technical assistance. We thank Ikuo Tsunoda, MD, PhD, for many helpful discussions. We wish to acknowledge Kathleen Borick for the outstanding preparation of the manuscript. This work was supported by funding from the Margolis Foundation, CURE, the Emma Mary Deland Foundation and NIH (NS065714).
Footnotes
Disclosure of Conflict of Interest: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors have no conflict of interest to disclose.
REFERENCES
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407–1410. doi: 10.1212/wnl.38.9.1407. [DOI] [PubMed] [Google Scholar]
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-α inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Sanchez-Alavez M, Andell-Jonsson S, Schultzberg M, Vezzani A, Danielsson E, Conti B. Interleukin-1 system in CNS stress: Seizures, fever, and neurotrauma. Ann N Y Acad Sci. 2007;1113:173–177. doi: 10.1196/annals.1391.022. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Buenz EJ, Sauer BM, Lafrance-Corey RG, Deb C, Denic A, German CL, Howe CL. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol. 2009;175:668–684. doi: 10.2353/ajpath.2009.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro M, Aubert C, Brahic M. Demyelinating lesions due to Theiler's virus are associated with ongoing central nervous system infection. J Virol. 1986;57:992–997. doi: 10.1128/jvi.57.3.992-997.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo EG, da Silva GM, Dos Santos AA. Neuronal cell survival: the role of interleukins. Ann N Y Acad Sci. 2009;1153:57–64. doi: 10.1111/j.1749-6632.2008.03974.x. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Mora GN, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San. Diego: Academic Press; 1997. [Google Scholar]
- Gahring LC, White HS, Skradski SL, Carlson NG, Rogers SW. Interleukin-1α in the brain is induced by audiogenic seizure. Neurobiol Dis. 1997;3:263–269. doi: 10.1006/nbdi.1996.0123. [DOI] [PubMed] [Google Scholar]
- Getts DR, Balcar VJ, Matsumoto I, Müller M, King NJC. Viruses and the immune system: their roles in seizure cascade development. J Neurochem. 2008;104:1167–1176. doi: 10.1111/j.1471-4159.2007.05171.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences (Epilepsy Foundation of America) New York: Demos Publications; 1990. [Google Scholar]
- Hosoya M, Honzumi K, Suzuki H. Detection of enterovirus by polymerase chain reaction and culture in cerebrospinal fluid of children with transient neurologic complications associated with acute febrile illness. J Infect Dis. 1997;175:700–703. doi: 10.1093/infdis/175.3.700. [DOI] [PubMed] [Google Scholar]
- Hosoya M, Sato M, Honzumi K, Katayose M, Kawasaki Y, Sakuma H, Kato K, Shimada Y, Ishiko H, Suzuki H. Association of nonpolio enteroviral infection in the central nervous system of children with febrile seizures. Pediatrics. 2001;107:E12. doi: 10.1542/peds.107.1.e12. [DOI] [PubMed] [Google Scholar]
- Juttler E, Tarabin V, Schwaninger M. Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity. Neuroscientist. 2002;8:268–275. doi: 10.1177/1073858402008003012. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Drukarch B, Van Dam A-M. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112:1–25. doi: 10.1042/CS20060043. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods Mol Biol. 2006;315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- Labar DR, Harden C. Infection and inflammatory diseases. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook, First Edition. Philadelphia: Lippincott-Raven; 1997. pp. 2587–2596. [Google Scholar]
- Lee TS, Mane S, Eid T, Zhao H, Lin A, Guan Z, Kim JH, Schweitzer J, King-Stevens D, Weber P, Spencer SS, Spencer DD, de Lanerolle NC. Gene expression in temporal lobe epilepsy is consistent with increased release of glutamate by astrocytes. Mol Med. 2007;13:1–13. doi: 10.2119/2006-00079.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Lindsley MD, Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989;142:2677–2682. [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Susceptibility of inbred mice to chronic central nervous system infection by Theiler's murine encephalomyelitis virus. Infect Immun. 1979;26:369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Rossi C, Cash E, Aubert C, Coutinho A. Role of the humoral immune response in resistance to Theiler's virus infection. J Virol. 1991;65:3895–3899. doi: 10.1128/jvi.65.7.3895-3899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Molinero A, Carrasco J, Hidalgo J. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience. 2001;102:805–818. doi: 10.1016/s0306-4522(00)00515-7. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CUA, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell DR, Nacher J, Akama KT, McEwen BS. Spatiotemporal distribution of gp130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 2003;122:329–348. doi: 10.1016/s0306-4522(03)00593-1. [DOI] [PubMed] [Google Scholar]
- Rubio N, Sierra A. Interleukin-6 production by brain tissue and cultured astrocytes infected with Theiler's murine encephalomyelitis virus. Glia. 1993;9:41–47. doi: 10.1002/glia.440090106. [DOI] [PubMed] [Google Scholar]
- Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–999. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Stewart K-AA, Wilcox KS, Fujinami RS, White HS. A novel model of infection-induced epilepsy: Chronic seizures and neuronal cell loss in Theiler's virus infected C57BL/6 mice. Epilepsia. 2008;49 suppl. 7:323–324. [Google Scholar]
- Suzuki H, Franz H, Yamamoto T, Iwasaki Y, Konno H. Identification of the normal microglial population in human and rodent nervous tissue using lectin-histochemistry. Neuropathol Appl Neurobiol. 1988;14:221–227. doi: 10.1111/j.1365-2990.1988.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. Theiler’s murine encephalomyelitis virus. In: Ahmed R, Chen ISY, editors. Persistent viral infections. Chichester, West Sussex, England: John Wiley & Sons, Ltd; 1999. pp. 517–536. [Google Scholar]
- Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler's murine encephalomyelitis virus. Acta Neuropathol (Berl) 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am J Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, McCright IJ, Kuang L-Q, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gassen KL, de Wit M, Koerkamp MJ, Rensen MG, van Rijen PC, Holstege FC, Lindhout D, de Graan PN. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia. 2008;49:1055–1065. doi: 10.1111/j.1528-1167.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008a;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Baram TZ. New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008b;49 Suppl 2:24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- Zurbriggen A, Fujinami RS. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989;63:1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]