Abstract
Background
The management of low-grade (LGD) and indefinite dysplasia (IND) in patients with ulcerative colitis (UC) remains controversial, as outcomes after a diagnosis of LGD or IND in previous studies vary widely.
Methods
All patients evaluated were from a single institution referral center who had a history of UC and a diagnosis of either LGD or IND between 1994 and 2008 as confirmed by 2 expert gastrointestinal (GI) pathologists. Data were collected by chart review of electronic and paper medical records. All patients who did not undergo a colectomy within 90 days of their dysplasia diagnosis were included in the final analysis. Hazard ratios for risk factors as well as incidence rates and Kaplan–Meier estimates were used to calculate the progression to high-grade dysplasia (HGD) or colorectal cancer (CRC).
Results
Thirty-five patients were included in the analysis, of whom 2 patients with IND and 2 patients with LGD developed HGD or CRC over a mean duration of 49.8 months. In total, the incident rate for advanced neoplasia for all patients was 2.7 cases of HGD or CRC per 100 person-years at risk. For flat and polypoid LGD the incident rate of advanced neoplasia was 4.3 and 1.5 cases per 100 person-years at risk, respectively. Patients with primary sclerosing cholangitis (PSC) had an incident rate of 10.5 cases per 100 years of patient follow-up.
Conclusions
We report a low rate of progression to HGD or CRC in patients who underwent surveillance for LGD or IND; polypoid dysplasia showed less risk of progression than flat dysplasia.
Keywords: ulcerative colitis, dysplasia, colorectal cancer
Patients with long-standing ulcerative colitis (UC) are at increased risk for colorectal cancer (CRC).1–4 Because it is believed that nearly all CRCs develop from dysplastic lesions, the current recommendation is for patients with chronic ulcerative colitis (UC) to undergo frequent surveillance for dysplasia in order to prevent cancer through early identification of neoplastic lesions.5–8 This secondary approach to cancer prevention is fraught with logistical and technical challenges, however, and the field as well as our approach to these precancerous lesions are evolving.
Microscopically, dysplastic lesions are classified as indefinite for dysplasia (IND), low-grade dysplasia (LGD), or having high-grade dysplasia (HGD).9 For patients with HGD, colectomy has been nearly universally recommended because the risks of harboring a synchronous adenocarcinoma as well as the apparent progression to cancer over time are significantly elevated.11
Conversely, the management of patients with IBD-associated IND or LGD remains controversial, especially in recent years.12 There is significant heterogeneity among previous studies examining the progression of low-grade lesions to cancer, with reported rates varying from 16%–54%.10–15 Furthermore, progression to adenocarcinoma in patients with IND has not been well defined, as there are limited surveillance studies with few patients. As such, there is currently no consensus regarding monitoring with endoscopic surveillance or recommending surgery after a diagnosis of IND or LGD. The aim of this study was to determine the likelihood of progression of IND or LGD to HGD or CRC in patients with chronic UC seen at our single referral center.
Materials and Methods
Patients
The study was approved by the Institutional Review Board at the University of Chicago Medical Center. Patients were identified retrospectively from the previously described University of Chicago Endoscopy Database.16 Patients were included if they had chronic UC, as defined by a duration of disease over 8 years, as well as either IND or LGD found during screening or surveillance colonoscopy at our institution.
Approach to Surveillance
At our institution, surveillance colonoscopies are performed after 8–10 years of left-sided or extensive colitis. Repeat exams are done every 1–3 years based on previous endoscopic findings and clinical risk factors. During these exams, random biopsies are obtained every 5–10 cm with targeted biopsies of visible lesions. At the time in which these patients underwent surveillance, chromoendoscopy was not used.17
Data Collection
Data were collected by review of electronic and paper-based medical records from 1994 through 2008. All patients with UC and a diagnosis of either IND or LGD were included in the analysis. Collected data included: index date, age, duration of disease, pathology, colectomy date, presence of primary sclerosing cholangitis (PSC), endoscopic findings as stated in the endoscopy report, number of repeat colonoscopies after the index date, time to colectomy, and time of follow-up. Index date was defined as the date at which dysplasia was first identified at our institution. Patients were classified as having a immediate colectomy if they had surgery within 90 days from their diagnosis of dysplasia. All procedures and pathology records were reviewed for neoplasia from the index date until colectomy, or November 2008.
Dysplasia Definitions
Grades of dysplasia at our institution were all confirmed by 2 expert GI pathologists. “Prevalent dysplasia” was defined as dysplasia found during the patients first screening colonoscopy at our institution. “Incident dysplasia” was defined as dysplasia found after an initial negative screening or surveillance examination and then during subsequent surveillance colonoscopies. Flat dysplasia was defined as dysplasia diagnosed histologically but not noted as suspicious for dysplasia by the endoscopist. Dysplasia associated lesion or mass (DALM) was defined as a visible raised lesion with irregular mucosa that was deemed endoscopically unresectable and in which dysplasia was confirmed on biopsy, diagnosed histologically, and noted as suspicious for dysplasia by the endoscopist. Polypoid dysplasia was defined as a discrete raised lesion located in an area involved by either quiescent or active colitis as confirmed by pathology that was endoscopically resected with biopsy confirmation of dysplasia and had morphology similar to that of a sporadic adenoma.
Statistical Analysis
Statistical analyses were calculated with Stata 10.2 (College Station, TX). P-values and odds ratios were calculated in univariate analysis for the development of HGD/CRC using the chi-squared test, Student's t-test, and Cox proportional hazard modeling. The duration of follow-up was calculated as the lapsed time from the index date to the diagnosis of HGD or CRC, the last date of follow-up, or the date of colectomy, whichever occurred earlier. Incidence rates of HGD or CRC along with its exact 95% confidence intervals were calculated on the basis of the Poisson distribution. The Kaplan–Meier method was used to estimate the cumulative risk of progression to HGD or CRC. The log-rank test was used to explore factors that affect the risk of progression. No multivariate analysis was conducted because the number of patients who progressed to advanced neoplasia was small.
Results
Patient Characteristics
Forty-one patients were identified as having IND or LGD. Of these, 6 patients underwent immediate colectomy within 90 days of diagnosis of dysplasia and the remaining 35 patients were included in the study for further analysis. For the 35 patients who did not undergo an immediate colectomy, the mean duration of UC was 21.7 years with a mean age at diagnosis of CRC of 48.7 years. Five patients had PSC (14%). Seven patients had IND, and 28 patients had LGD and at index colonoscopy. Of the patients with IND, all 7 lesions were flat. Of the patients with LGD, 12 lesions were polypoid, 13 were flat, and 3 patients had DALMs (Table 1).
TABLE 1. Patient Characteristics.
| IND (7) | LGD (28) | All Patients (35) | |
|---|---|---|---|
| Development of HGD/CRC | 2 | 2 | 4 |
| Age at diagnosis of ulcerative colitis in years, mean ± SD | 20.9 ± 8.4 | 28.3 ± 13.0 | 27.0 ± 12.5 |
| Age at diagnosis of low-grade or indefinite dysplasia in years, mean ± SD | 46.6 ± 17.3 | 49.3 ± 12.6 | 48.7 ± 13.4 |
| Duration of disease in years, mean ± SD | 25.7 ± 14.3 | 20.8 ± 10.4 | 21.7 ± 11.2 |
| Follow up time (years) | 3.9 ± 3.2 | 4.2 ± 2.8 | 4.2 ± 2.8 |
| Number of procedures, median (IQR) | 2 (1-3) | 3 (2-4) | 3 (1-4) |
| Number who had dysplasia: | |||
| Flat | 7 | 13 | 20 |
| Polypoid | 0 | 12 | 12 |
| DALM | 0 | 3 | 3 |
| PSC | 2 | 3 | 5 |
Advanced Neoplasia
Two patients with IND and 2 patients with LGD developed HGD or CRC over a mean duration of 49.8 months from their diagnosis of dysplasia. Seven patients with IND had a mean 46.8 months follow-up, during which 2 patients (28.5%) with IND progressed to advanced neoplasia (1 HGD, 1 CRC) in 117.7 and 62.9 months, respectively. Twenty-eight patients with LGD had a mean of 50.4 months of follow-up, during which only 2 patients (1 flat and 1 polypoid) progressed to HGD or CRC in 15.2 and 36.3 months, respectively.
Two additional patients with IND later developed flat LGD and subsequently underwent colectomy. Three patients with IND had no progression to LGD or HGD with a mean follow-up of 30.7 months. One patient with flat LGD dysplasia later developed polypoid LGD, which was removed by endoscopic polypectomy and then had an additional focus of flat LGD at a separate site on a third colonoscopy; the patient had a subsequent colectomy with no dysplasia detected. All 3 patients classified as having DALMs underwent colectomy before the development of advanced neoplasia (Table 1).
At the end of a mean of 50.4 months of follow-up, 12 of 13 patients with flat LGD and 11 of 12 patients with polypoid LGD had not progressed to HGD or CRC. Of the patients who did not undergo colectomy for either a diagnosis of dysplasia or disease progression, 9 patients with flat LGD had no progression to HGD or CRC with a mean follow-up of 52.8 months and 11 patients with polypoid LGD remained cancer-free with a mean follow-up of 72 months.
Incidence Rates
In total, the incident rate for advanced neoplasia for all of these patients was 2.7 cases of HGD or CRC per 100 person-years at risk. For patients with LGD, the incident rate of advanced neoplasia was 1.7 cases per 100 person-years at risk. Patients diagnosed with IND had a calculated incident rate of advanced neoplasia of 7.3 cases per 100 person-years at risk. For flat and polypoid LGD, the incident rate of advanced neoplasia was 4.3 and 1.5 cases per 100 person-years at risk, respectively. No incident rates of advanced neoplasia were calculated for DALMS, given the limited number of patients and follow-up time. Patients with PSC had an incident rate of 10.5 cases per 100 years of person-years at risk (Table 2).
TABLE 2. Incidence Rates and Hazard Ratios of High-grade Dysplasia or Colorectal Cancer.
| Variable | Person-years at Risk | Number of HGD/CRC Developed | Incidence per 100 Person-years (95% CI) |
Hazard Ratio (95% CI) |
P-value |
|---|---|---|---|---|---|
| All patients | 145.6 | 4 | 2.7 (0.7-7.0) | ||
| Low-grade dysplasia | 118.4 | 2 | 1.7 (0.2-6.1) | 1.0 (ref.) | 0.24 |
| Indefinite dysplasia | 27.2 | 2 | 7.3 (0.9-26.5) | 3.32 (0.42-26.1) | |
| Flat | 70.3 | 3 | 4.3 (0.9-12.5) | 1.0 (ref.) | 0.48 |
| Polypoid | 67.3 | 1 | 1.5 (0.04-8.3) | 0.31 (0.03-3.08) | |
| DALM | 8.0 | 0 | 0.0 (0.0-46.0) | ||
| No PSC | 126.5 | 2 | 1.6 (0.2-5.7) | 1.0 (ref.) | 0.06 |
| PSC | 19.1 | 2 | 10.5 (1.3-37.8) | 10.4 (0.94-115) | |
| Incident dysplasia | 118.5 | 3 | 2.5 (0.5-7.4) | 1.0 (ref.) | 0.63 |
| Prevalent dysplasia | 27.2 | 1 | 3.7 (0.1-20.5) | 1.81 (0.16-20.3) | |
| Age at diagnosis of ulcerative colitis | |||||
| <30 years old | 81.3 | 1 | 1.2 (0.03-6.9) | 1.0 (ref.) | 0.37 |
| ≥30 years old | 64.3 | 3 | 4.7 (1.0-13.6) | 2.84 (0.26-31.5) | |
| Age at diagnosis of low-grade or indefinite dysplasia | |||||
| <50 years old | 61.4 | 1 | 1.6 (0.04-9.1) | 1.0 (ref.) | |
| ≥50 years old | 84.2 | 3 | 3.6 (0.7-10.4) | 1.56 (0.14-17.5) | 0.71 |
| Duration of ulcerative colitis | |||||
| <20 years | 70.5 | 2 | 2.8 (0.3-10.2) | 1.87 (0.17-20.9) | |
| ≥20 years | 75.1 | 2 | 2.7 (0.3-9.6) | 1.0 (ref.) | 0.61 |
Discussion
In summary, we report a low rate of progression to HGD or CRC in patients with LGD or IND. In patients who were followed for longer than 90 days after a diagnosis of LGD or IND, only 4 of 35 developed advanced neoplasia after a mean follow-up of 50.4 months. This low frequency of progression was true for both flat and polypoid lesions. Because the 3 patients with DALMs underwent colectomy prior to the development of advanced neoplasia with limited follow-up time, we were unable to assess the risk of progression in these patients.
Aside from a history of PSC, there were no obvious predictors for who would progress to advanced neoplasia in this cohort. Statistical analysis of the rates of progression to advanced neoplasia between IND and LGD were not possible because of the small sample size. However, we did observe that a diagnosis of IND carries a risk of progression to advanced neoplasia.
Several previous studies have examined outcomes in patients diagnosed with IND and LGD with disparate results. In comparison to previous published reports, our study has similar numbers of total patients, those with PSC, as well as a comparable duration of disease, and age of cancer diagnosis.11,14,18 In patients who were followed with surveillance colonoscopies after a diagnosis of LGD or IND, our study found similar rates of progression to CRC (11%) as Leed's analysis (10%), in which a 10-year follow-up was performed.18 Our study had the additional advantage of examining outcomes based on the endoscopic appearance of the dysplastic lesion.
In contrast, several previous studies have demonstrated higher rates of progression than what we reported. Although the retrospective analysis from Mt. Sinai examined only flat LGD, the authors reported a significantly higher rate of progression to advanced neoplasia (30%).11 This may be secondary to the fact that our study found far fewer patients with multiple foci of LGD, examined patients with polypoid lesions as well, and had younger patients overall. Additionally, 2 prospective endoscopic studies, 1 Swedish cohort and the other from a referral center in London, the St. Mark's Hospital, reported rates of progression from LGD to HGD or CRC of 30% and 33%, respectively.12,19 The higher rates of progression to advanced neoplasia in these studies may be partially explained by the fact that the data were collected when different therapies and surveillance strategies existed. This may be supported by the observation that the incidence of CRC decreased over time in the St. Marks study. Additionally, these prospective studies also included a higher percentage of patients with DALMs, which are felt to harbor a higher risk of progression to advanced neoplasia.
Our analysis supports the belief that polypoid dysplasia is a lower-risk lesion than flat dysplasia. Because polypoid lesions can be removed endoscopically and resemble sporadic adenomas, they are felt to behave differently than flat lesions. In fact, there is mounting evidence that polypoid dysplasia can be managed with endoscopic polypectomy similar to a sporadic adenoma in patients without colitis.20,21 While our data indicates the risk of progression to advanced neoplasia is higher than studies examining average risk persons with a sporadic adenoma, it was indeed significantly less than flat dysplastic lesions.22
The major limitation of this study was that it was a single-institution retrospective analysis, which allows for bias in endoscopic surveillance, recommendations for surgery, and pathologic interpretation. Additionally, given the relative infrequency of LGD and IND, as with all such studies, there were small numbers of patients included in the analysis. Because there is no standard of care for the management of IND and LGD, the decision to go to surgery was based on provider recommendations and patient preferences. As such, there was a wide span of time in which patients underwent surgical management, which makes the time survival data more difficult to interpret.
Overall, we demonstrated a low rate of progression to advanced neoplasia in patients with flat and polypoid LGD or IND undergoing surveillance. Based on the results of this analysis coupled with previous studies, consideration of a surveillance program is preferred for management of polypoid LGD and should be considered for flat-low grade and IND. However, given the small sample size and retrospective nature of this and other similar studies, we must collect prospective data and continue to define the biology of neoplasia in chronic colitis as technologies improve our ability to visualize dysplasia in vivo and we choose to follow a larger group of patients.
FIGURE 1.
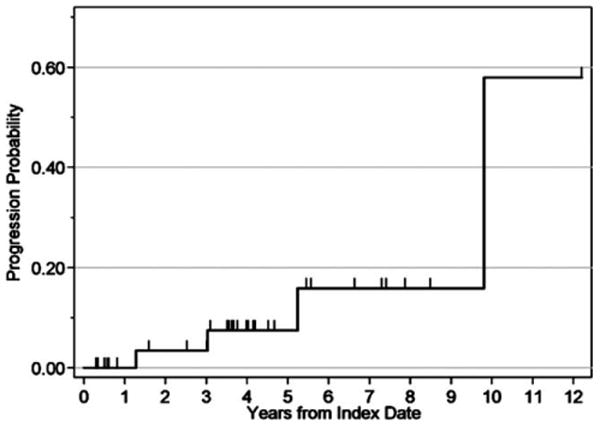
Kaplan–Meier curve of progression to high-grade dysplasia or colorectal cancer in patients with low grade or indefinite dysplasia.
Acknowledgments
Funding in part from the Cancer Research Foundation, Chicago, IL, and the Gastro-Intestinal Research Foundation Associates' Board, Chicago, IL.
References
- 1.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.Askling J, Dickman PW, Karlen P, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356–1362. doi: 10.1053/gast.2001.24052. [DOI] [PubMed] [Google Scholar]
- 4.Shetty K, Rybicki L, Brzezinski A, et al. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:1643–1649. doi: 10.1111/j.1572-0241.1999.01156.x. [DOI] [PubMed] [Google Scholar]
- 5.Karlen P, Kornfeld D, Brostrom O, et al. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–714. doi: 10.1136/gut.42.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lashneer BA, Kane SV, Hanauer SB. Colon cancer surveillance in chronic ulcerative colitis. Am J Gastroenterol. 1995;95:1083–1087. [PubMed] [Google Scholar]
- 7.Choi PM, Nugent FW, Schoetz DJ, et al. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–424. doi: 10.1016/0016-5085(93)90715-o. [DOI] [PubMed] [Google Scholar]
- 8.Eaden J, Abrams K, Ekbom A, et al. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 9.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–74. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 11.Ullman T, Croog V, Harpaz N, et al. Progression of flat low grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–1319. doi: 10.1016/j.gastro.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Connell, Lennard-Jones JE, Williams CB, et al. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934–944. doi: 10.1016/0016-5085(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 14.Jess T, Loftus EV, Velayos FS, et al. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis. 2006;12:669–676. doi: 10.1097/00054725-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ullman TA, Loftus E, Kakar S, et al. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol. 2002;97:922–927. doi: 10.1111/j.1572-0241.2002.05610.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DT, Rothe JA, Hetzel JT, et al. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998–1004. doi: 10.1016/j.gie.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 18.Lim CH, Dixon MF, Vail A, et al. Ten year follow up of ulcerative colitis patients with and without dysplasia. Gut. 2003;52:1127–1132. doi: 10.1136/gut.52.8.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg J, Stenling R, Palmquivst R, et al. Efficiency of colorectal cancer surveillance in patients with ulcerative colitis: 26 years' experience in a patient cohort from a defined population area. Scand J Gastroenterol. 2005;40:1076–1080. doi: 10.1080/00365520510023224. [DOI] [PubMed] [Google Scholar]
- 20.Rubin PH, Friedman S, Harpaz N, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295–300. doi: 10.1016/s0016-5085(99)70279-9. [DOI] [PubMed] [Google Scholar]
- 21.Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288–1294. doi: 10.1016/s0016-5085(99)70278-7. [DOI] [PubMed] [Google Scholar]
- 22.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–662. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]


