Abstract
The Achilles tendon is the most frequently ruptured tendon. Both acute and chronic (neglected) tendon ruptures can dramatically affect a patient’s quality of life, and require a prolonged period of recovery before return to pre-injury activity levels. This paper describes the use of an adhesive-coated biologic scaffold to augment primary suture repair of transected Achilles tendons. The adhesive portion consisted of a synthetic mimic of mussel adhesive proteins that can adhere to various surfaces in a wet environment, including biologic tissues. When combined with biologic scaffolds such as bovine pericardium or porcine dermal tissues, these adhesive constructs demonstrated lap shear adhesive strengths significantly greater than that of fibrin glue, while reaching up to 60% of the strength of a cyanoacrylate-based adhesive. These adhesive constructs were wrapped around transected cadaveric porcine Achilles tendons repaired with a combination of parallel and three-loop suture patterns. Tensile mechanical testing of the augmented repairs exhibited significantly higher stiffness (22–34%), failure load (24–44%), and energy to failure (27–63%) when compared to control tendons with suture repair alone. Potential clinical implications of this novel adhesive biomaterial are discussed.
1. Introduction
The Achilles tendon is ruptured more frequently than any other tendon. It accounts for 40–60% of all operative tendon repairs, with 75% of these procedures stemming from sports-related activities [1-3]. The number of ruptures has increased over the last several decades, and the rate has doubled nearly every 10 years [4-6]. The aging population, increased popularity of recreational sports among the middle aged, and medical advances that enable an aging population to participate in recreational sports all contribute to this increase [1, 7].
Primary suture repair is the current standard of care, and many different suture techniques are available (e.g., Krackow, Bunnell, Kessler, three-loop pulley, epitendinous suture augmentation) [8-13]. However, primary repair of ruptured Achilles tendons has resulted in partial or complete re-ruptures in over 5% of patients [2, 14, 15]. Causes of re-rupture have been reportedly due to traumatic episodes, patient non-compliance with post-surgical regimens, and premature return to athletic activity. The suture–tendon junction is usually the weak link in primary tendon repairs. The strength between the tendon fibers is much less than that of the fibers themselves, so sutures can tear through the tendon when force is applied [16].
To reduce the rate of re-rupture and accelerate rehabilitation, primary suture repair is sometimes reinforced with biologic scaffolds or grafts (e.g., bovine pericardium, small intestinal submucosa (SIS), or acellular human or porcine dermal matrix) [17, 18]. In addition to improved mechanical support, these biologic materials provide an extracellular matrix for the in-growth of tissue so that they become well incorporated into the tendon. Patients augmented with biologic grafts have been able to undergo an aggressive rehabilitation program with early return to activity without re-rupture or complications [19, 20]. However, these grafts are secured to the tendon with sutures which can cause local impairment of circulation with compromised healing [21, 22]. Regardless of the treatment method, complete regeneration of the tendon is never achieved [23].
In previous development work, a synthetic, degradable bioadhesive film was coated onto biologic scaffolds, creating an adhesive construct (AC) that adhered tightly to wetted tissue substrates [24]. This adhesive is a synthetic mimic of mussel adhesive proteins (MAPs), which marine mussels utilize to bind tenaciously to various substrates in a wet, turbulent, and saline environment [25, 26]. This paper describes the use of the biologically inspired adhesive to affix biologic scaffolds to Achilles tendons. Two scaffolds, bovine pericardium and a commercial porcine dermal tissue (Biotape™, Wright Medical Technology, Inc.) were pre-coated with an adhesive film (figure 1). The adhesive properties of these adhesive constructs were characterized using lap shear testing. Additionally, tensile failure testing was performed on transected porcine Achilles tendons that had received primary suture repair with and without augmentation with these adhesive constructs. It was hypothesized that tensile failure properties of augmented repairs will be significantly greater than repairs with suture alone.
Figure 1.
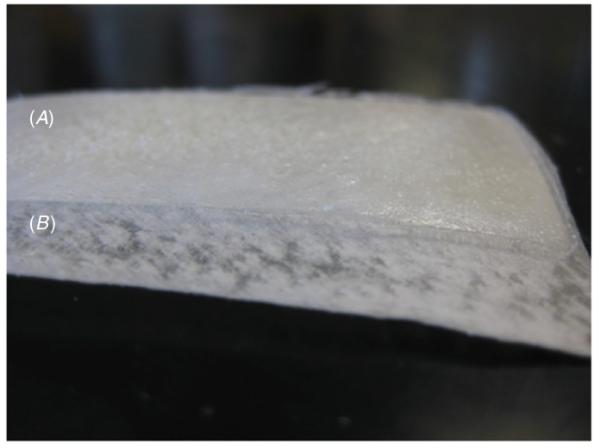
Photograph of a 4 cm × 8 cm adhesive film (A) coated onto a 6 cm × 8 cm segment of Biotape (B).
2. Materials and methods
2.1. Materials
The preparation and characterization of the adhesive polymer has been previously described [24]. Bovine pericardium was obtained from the Nirod Corporation (Ames, IA), while Biotape™ was purchased from Wright Medical Technology, Inc. (Arlington, TN). Porcine tendons (rear leg deep flexor) were purchased from Spear Products (Coopersburg, PA).
2.2. Coating and testing adhesive-coated biologic scaffolds
Solutions of the adhesive polymer (100 mg mL−1 in chloroform) were cast over bovine pericardium and Biotape at a coating density of 150 g m−2, and then dried under vacuum overnight to create the adhesive-coated constructs denoted as AC1 and AC2, respectively. To capture images of the construct using scanning electron microscope (SEM), AC2 specimens were cut into 0.5 cm × 1.0 cm pieces, mounted onto an aluminum stud, and coated with gold in a Denton Desk II Sputter Coater for 30–40 s, resulting in a coating thickness of approximately 20 nm. The images of the samples were recorded by a computer-controlled, high resolution Schottky field emission SEM (LEO DSM-1530, Carl Zeiss NTS, Inc., Peabody, MA) at an accelerating voltage of 3 kV.
Lap shear testing was performed as previously described [24]. Briefly, wetted bovine pericardium was used as the tissue substrate. Prior to forming the adhesive joint, the adhesive was activated with a solution of NaIO4 (20 mg mL−1, 40 μL), and the adhesive construct was brought into contact with the tissue substrate with a 1 cm × 2.5 cm overlap as measured by a ruler. The adhesive joint was compressed with a 100 g weight for 10 min, and further conditioned in phosphate buffered saline (PBS, pH 7.4, 37 °C) for an hour before testing. The adhesive joints were gripped and pulled to failure at a rate of 10 mm min−1 using a materials test machine (Admet, Inc., Norwood, MA). The maximum load needed to separate the adhesive joints was recorded, and the lap shear strength was determined by normalizing the maximum load to failure with the initial area of contact. Commercially available tissue adhesives, Dermabond® (Ethicon Inc., Somerville, NJ) and Tisseel™ (Baxter Healthcare Corporation, Deerfield, IL), were included as controls. These adhesives were applied in situ between the two tissue substrates with an overlap of 1 cm × 2.5 cm, and prepared for testing as described above. The sample size was six in each test group.
2.3. Mechanical testing of repaired tendons
Tensile failure testing was performed on transected tendons repaired using suture with and without augmentation with the proposed adhesive constructs. Transected porcine tendons were sutured with both parallel (Polysorb™ braided lactomer™, 4-0, Covidien, Dublin, Ireland) and three-loop pulley (Maxon™ monofilament polyglyconate, 0, Covidien, Dublin, Ireland) suture patterns (figure 2). The adhesive construct was first secured to the tendon with three stay sutures. A solution of NaIO4 (20 mg mL−1) was then sprayed onto the adhesive prior to wrapping the construct around the tendon, thereby activating the adhesive. AC1 was wrapped around the tendon twice to increase the footprint of the adhesive. AC2 was wrapped around the tendon once with 1 cm of overlap as recommended by the manufacturer of Biotape. The wrapped tendons were held tightly for 10 min and incubated at 37 °C (PBS, pH 7.4) for 1 h prior to testing. The two ends of the repaired tendons were secured with a 1 kN Vise grip equipped with jaws coated with synthetic diamonds (Admet, Inc., Norwood, MA), with a grip-to-grip length of 6 cm. After preconditioning the repaired tendons (cycled ten times between 2 and 10 N), both sutured and adhesive-wrapped tendons were loaded to failure at a rate of 25 mm min−1, and load versus displacement data were recorded. Each test was video taped to document the failure mechanism. The failure load was defined as the load where irreversible damage to the repair occurred—failure of the adhesive in the AC-augmented repairs, and failure of the parallel sutures in the non-augmented repairs. To determine the stiffness of the repair, the slope of the linear portion of the load/displacement curve prior to failure was determined by linear regression (R2 ≥ 0.999). The energy to failure was determined from the area under the curve up to the failure load. The percent elongation of the specimen was calculated as one hundred times the displacement (i.e., change in length) divided by the initial grip length of 6 cm. The sample size was ten for each test group.
Figure 2.
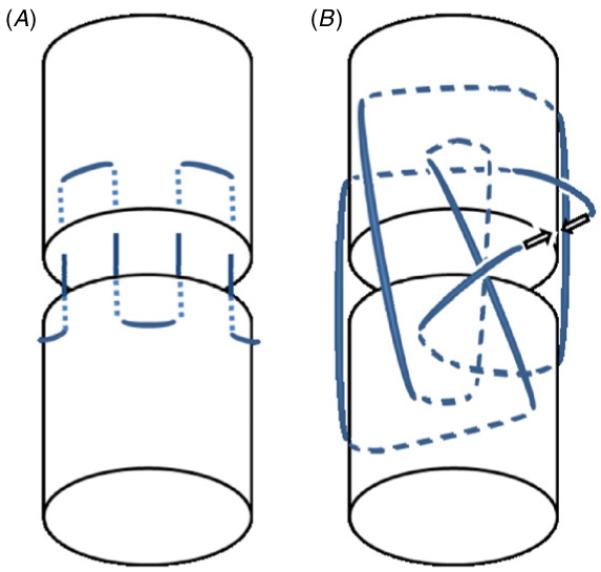
Schematics of parallel (A) and three-loop pulley (B) suture patterns used to repair transected tendons. Solid and dashed lines indicate sutures on the surface of and within the tendon, respectively. The parallel pattern is repeated around the circumference of the tendon. The arrows in the three-loop pulley indicate the two ends of the sutures where the knot will be tied.
2.4. Statistical analysis
Statistic analysis was performed on the lap shear adhesion testing (two proposed construct treatments and two controls) and tensile failure testing of tendons (two proposed construct treatments with suture, and suture alone controls) using one-way analysis of variance (ANOVA) and Tukey post hoc analysis with a significance level of p = 0.05.
3. Results
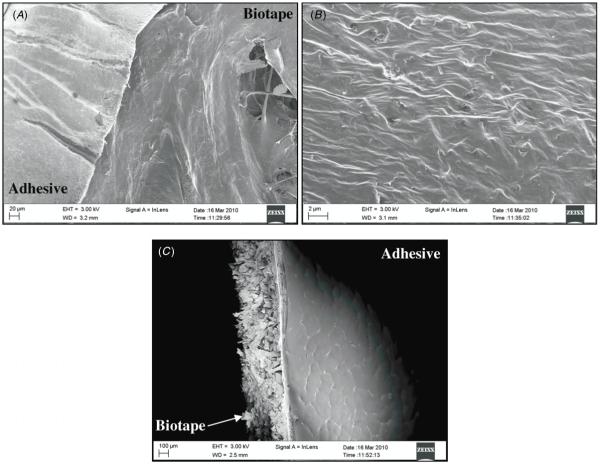
The novel adhesive polymer was solvent cast onto two representative biological scaffolds, bovine pericardium (AC1) and Biotape (AC2), a commercial porcine dermal tissue. The adhesive coating on Biotape was clearly visible from both photographed (figure 1) and scanning electron microscopy (figure 3) images. The cross-sectional view (figure 3(C)) of the adhesive construct revealed an adhesive layer tightly bound to the surface of the scaffold with a coating thickness of around 20 μm. AC1 (106 ± 22.9 kPa) and AC2 (73.4 ± 24.4 kPa) exhibited strong adhesive properties to both the wetted tissue substrate and biologic scaffold, compared to the fibrin glue (figure 4). The lap shear strengths of these constructs were 28–40 times greater than that of Tisseel (2.58 ± 1.76 kPa). Among the adhesives tested, Dermabond exhibited the highest adhesive strength (181 ± 33.4 kPa). AC1 and AC2 were not statistically different from each other. These constructs were used in subsequent testing to augment the suture repair of transected tendons.
Figure 3.
Scanning electronic microscopy images showing the edge of the adhesive film coated onto Biotape (A), a close-up of the adhesive coating (B), and a cross-section of the adhesive construct (C).
Figure 4.
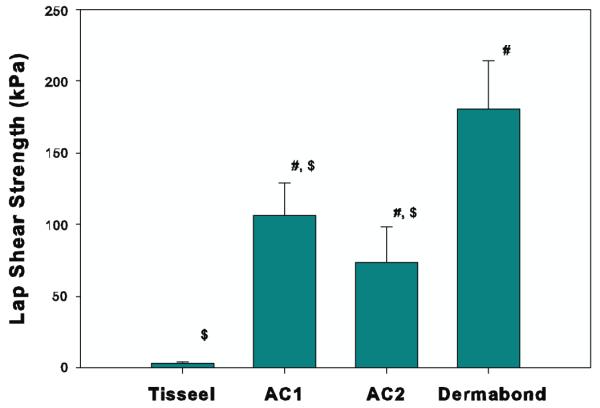
Maximum lap shear strength using bovine pericardium as a test substrate. Both Tisseel and Dermabond were applied in situ to adhere two pieces of bovine pericardium together. # and $ indicate a statistically significant difference from Tisseel and Dermabond, respectively (p < 0.05). Differences in adhesive strength between AC1 and AC2 were not statistically significant.
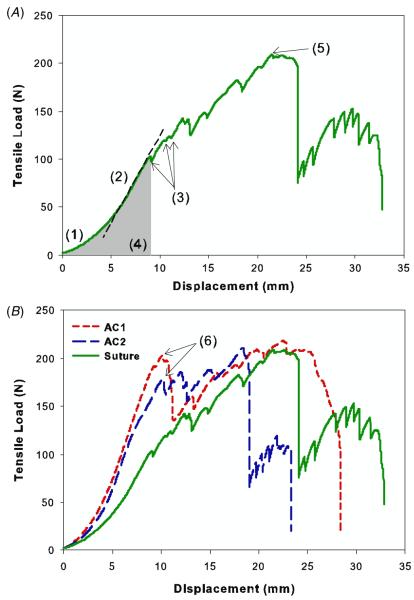
Figure 5(A) demonstrates a representative load versus displacement curve for a sutured tendon, which contains typical features that were evident in all test groups (figure 5(B)); (1) nonlinear toe region where the fibers are being recruited as the tendon is stretched, (2) linear region representing the stiffness of the repaired tendon, (3) arrows pointing to reduction in the load corresponding with the parallel sutures being pulled off the tendon, with the first of these instances being considered as the irreversible failure of the repair (failure load), (4) energy to failure calculated as the area under the load versus displacement curve up to the failure load, and (5) peak load where the three-loop pulley began to fail as it is pulled through the tendon. For repairs augmented with an AC, irreversible failure occurred when the adhesive layer failed cohesively (i.e., the adhesive remained attached to both the scaffold and tendon surfaces). Both AC1- and AC2-augmented tendons exhibited greater failure load (24–44%), stiffness (22–34%), and energy to failure (27–63%), compared with suture-only controls (table 1). These differences were statistically significant for AC1 (p < 0.05). Suture-only tendons readily formed a gap at the transected site at loads as low as 10 N, with the gap typically increasing to around 3 mm at 100 N (figure 6). However, the repair site was not easily visible through the adhesive constructs, so gap formation was not used as a criterion for failure in this study. The percent elongation at failure and peak loads were not statistically different between the three test groups.
Figure 5.
Load versus displacement curves for Achilles tendon repaired with suture alone (A), and representative curves for each type of repaired tendon (B). (1) Toe region, (2) dashed line indicating the stiffness of the repaired tendon, (3) arrows indicating the first parallel suture being pulled off, which was considered to be failure of the repair (failure load), (4) energy to failure as calculated by the area under the curve up to the failure load, (5) peak load where the three-loop suture began to fail, and (6) failure of AC-augmented repairs occurring at the interface of the adhesive.
Table 1.
Tensile structural properties of repaired tendons
| Suture Only | AC1 | AC2 | |
|---|---|---|---|
| Stiffness (N/mm) | 18.1 ± 3.60 | 24.3 ± 4.23* | 22.1 ± 5.32# |
| Failure Load (N) | 105 ± 25.1 | 151 ± 37.4* | 130 ± 45.5# |
|
% Elongation at
Failure Load |
15.8 ± 2.08 | 15.9 ± 3.18 | 15.9 ± 2.98 |
| Energy to Failure (J) | 0.386 ± 0.131 | 0.630 ± 0.194* | 0.492 ± 0.236 |
| Peak Load (N) | 217 ± 45.7 | 231 ± 35.6 | 245 ± 35.8 |
|
% Elongation at
Peak Load |
35.6± 6.02 | 37.0 ± 6.12 | 38.0 ± 6.06 |
p < 0.05 compared to suture only
p < 0.15 compared to suture only
n = 10 replicates per treatment.
Figure 6.
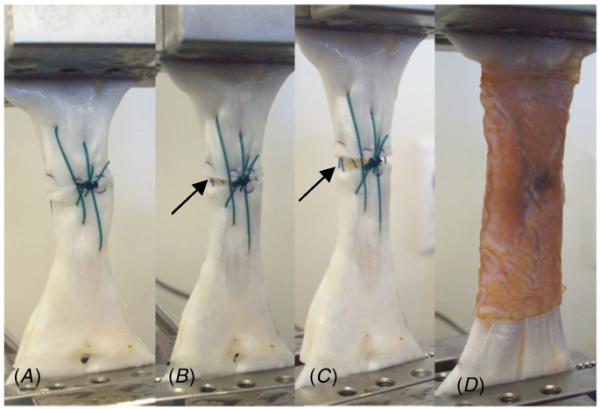
Close-up images of the gap formation during tensile testing of sutured tendons loaded at 0 N (A), 50 N (B) and 100 N (C), and a sutured tendon augmented with AC1 and loaded at 100 N (D). Solid arrows indicate gap formation for tendons repaired with suture alone.
4. Discussion
A synthetic bioadhesive was utilized as an adhesive coating for securing surgical graft material to repair Achilles tendons. This coating contains an active adhesive functional group, dopamine, which resembles the adhesive catecholic side chain 3,4-dihydroxyphenylalanine (DOPA) that marine mussels utilize to form strong bonds in the presence of water. Catechols are oxidized to form highly reactive quinones which can covalently crosslink with other catechols within the adhesive film (cohesive crosslinking) [27] or with functional groups such as amine and thiol found on tissue surfaces (adhesive crosslinking) [28, 29]. While the oxidant (NaIO4) used in the activation process is an irritant, it is reduced to benign iodide through the red-ox reaction with catechol. Additionally, a NaIO4-cured sealant was demonstrated to be non-toxic [30] and elicited minimal inflammatory responses in vivo [31], indicating that the use of NaIO4 appears to be a potentially viable method for biomedical applications.
The adhesive catechol is chemically attached to biocompatible and biodegradable multiblock copolymers—poly(ethylene glycol) (PEG) and polycaprolactone (PCL). Both polymers are routinely used in various medical applications such as tissue sealants and biodegradable sutures. The presence of PEG allows the adhesive polymer to remain relatively hydrophilic in order to achieve good ‘wetting’ or adhesive contact with a biologic scaffold or tissue substrate, while the hydrophobic PCL segments increase cohesive strength and prevent rapid dissolution of the film in the presence of water. The adhesive film degrades through hydrolysis of ester linkages in the PCL (12% mass loss over 4 months in vitro) [24]. The adhesive properties, mechanical properties, and degradation rate can be easily tailored for a specific application [24].
The adhesive polymer was solvent cast onto two biologic scaffolds to demonstrate the feasibility of using the adhesive-coated construct in Achilles tendon repair. Bovine pericardium was chosen as one of the backing materials because it is an inexpensive and readily abundant extracellular matrix with suitable mechanical properties (tensile failure load of 41 ± 9.8 N cm−1). Additionally, several acellular bovine pericardium-based products (e.g., Veritas®, Synovis Surgical Innovations, St Paul, MN; Tutomesh®, RTI Biologics, Alachua, FL) have been approved by the FDA for soft tissue reconstruction [32-34]. On the other hand, Biotape is a commercially available decellularized porcine dermal matrix that has been evaluated for Achilles tendon repair [35].
Biologic scaffolds pre-coated with the proposed adhesives demonstrated strong adhesion to soft tissue. Both AC1 and AC2 failed predominately through cohesive failure of the adhesive film, indicating strong adhesion to both the wetted tissue substrates and the scaffolds. SEM images also revealed that the adhesive film was tightly bound to the scaffold. These constructs exhibited significantly higher adhesive strengths when compared to Tisseel (fibrin glue), while Dermabond (cyanoacrylate) demonstrated the highest adhesive strength among the adhesives tested. Both of these commercially available tissue adhesives have been evaluated for tendon repair. Although Achilles tendons repaired with fibrin glue demonstrated good healing histologically [36], significant lengthening of the tendon occurred during the early phase of healing, which has been associated with reduced strength of the repaired tendons [37, 38]. Due to its lack of adhesive strength, fibrin glue is not likely to contribute significantly to the mechanical properties of the repaired tendon immediately after surgery. On the other hand, cyanoacrylate-based adhesives have been found to augment the mechanical properties of suture-repaired tendons in vitro [39, 40]. However, Evans et al demonstrated that cyanoacrylate adhesives are cytotoxic to cells derived from human tendons [41], likely due to toxic degradation products (i.e., formaldehyde) [42]. Surgical meshes fixated with cyanoacrylate have also been shown to impair tissue integration, elicit inflammation, and unfavorably alter the biomechanical properties of the repaired tissue [43]. Additionally, these commercially available tissue adhesives require preparation prior to use, thereby complicating surgical work flow.
Adhesive constructs were used to augment transected porcine tendons, which were mechanically compared to tendons primarily repaired with two suture patterns. The parallel sutures were used to keep the two ends of the transected tendon in intimate contact in order to minimize gap formation, while the three-loop pulley was intended to be the main structural component that held the severed tendon together. However, despite the use of parallel sutures, gap formation was observed for suture-repaired tendons even at relatively low loads, with the gap typically reaching approximately 3 mm at 100 N. In some studies, gap formation of 1–3 mm has been used as a measure of failure [10, 44], with a gap greater than 3 mm correlated with increased risk of re-rupture during the first 6 weeks postoperatively [37]. For AC-augmented repairs, the repair site was obstructed by the adhesive constructs, so that gap formation was not used as the measure of failure. Instead, the onset of irreversible failure of the repair was identified from the load/displacement data. For suture-repaired tendons, failure occurred when the parallel sutures were pulled out of the tendon, whereas the AC-augmented repairs failed at the adhesive interface. While the three-loop suture was intended to be the primary structural component that holds the tendon together, its failure occurred well after the irreversible failure load, where the repaired tendon was significantly lengthened (>35% elongation) with an even larger gap formation at the repair site. Thus, the initial failure load, and not peak load, is likely the more important failure metric when considering repeated loading of a healing tendon.
AC-reinforced tendons exhibited significantly higher stiffness, failure load, and energy to failure when compared to primary suture repair alone, indicating that higher load and energy is necessary to lengthen the repair. Excessive elongation at the repair site has been associated with a poor functional outcome after tendon repair [37, 38]. Additionally, a stiffer repair also strongly correlated with increased failure properties of repaired tendons during the early phase of healing [38]. This study demonstrated that the AC-augmentation can be utilized to reinforce the primary suture repair immediately after surgery, which may be beneficial when postoperative management includes an early and aggressive rehabilitation program. Conventional postoperative treatment for surgically repaired Achilles tendons has meant immobilization in a below-the-knee plaster cast for 6–8 weeks with little to no weight bearing. However, complications of prolonged immobilization include muscle atrophy, joint stiffness, tendocutaneous adhesions, deep vein thrombosis, and ulceration of joint cartilage [45]. Recent clinical studies, including randomized controlled follow-ups, strongly suggest that early mobilization and weight bearing, when compared with immobilization, produce less tendon elongation, greater isokinetic calf muscle strength, improved quality of life, and more rapid resumption of normal activities without re-rupture [6, 45-49].
Various biologic scaffolds or meshes (e.g., bovine pericardium, small intestinal submucosa, acellular human and porcine dermal matrix) have been evaluated for tendon repair augmentation. In addition to mechanical support, these biologic graft materials serve as a matrix for tissue in-growth, and have become well incorporated into the tendon tissue in animal models [17] and clinics [18]. An augmented repair allowed patients to undergo an aggressive rehabilitation program with subsequent early return to activity without re-rupture or complications [19, 20]. However, these non-adhesive biologic grafts require multiple intratendinous, interlocking sutures placed throughout the construct to prevent motion along the tendon/graft interface, thereby potentially disrupting local blood flow [21, 22]. The adhesive-coated constructs reported here can potentially reduce the number of sutures or completely replace the use of sutures in graft fixation to the tendon.
In this study, a static tensile failure test was performed to characterize the biomechanical properties of transected tendons repaired by different methods. These results yielded important information on the efficacy of a novel adhesive biomaterial in tendon repair at an immediate post-surgery time point. Cyclic loading, which may more closely simulate physiologic conditions experienced by the tendons, was not performed. However, given that the AC-augmented repairs exhibited significantly higher failure energy and stiffness compared to the primary suture repair, it is possible that the reinforced tendon can better resist repetitive motions. Additionally, the biocompatibility and efficacy of these adhesive constructs on healing tendons will require further assessment in in vivo animal models. Future testing will involve evaluating AC-augmentation under cyclic loading, as well as the effect of the construct on long-term tendon healing.
Percent elongation based on the initial grip-to-grip length was used to evaluate lengthening of the tendon during mechanical testing, rather than strain measured at points along the length of the tendon surface. These strain measurements are typically based on optical techniques applied in the mid-region to the tendon, which was not feasible in our experiment since the adhesive constructs obscured the tendon surface. However, this does not affect the reported metrics, which are comparative and structural. The two ends of the tendons were held in grips rated to withstand up to 1000 N of tensile load, which is an order of magnitude higher than the failure loads in this study. Slippage of the tissue in the grips was not observed throughout testing. This was further confirmed by the statistical significance of the load measurements between test groups.
5. Conclusion
The data reported here indicate that a synthetic bioadhesive coated onto biologic scaffolds can impart strong adhesive properties to these biomaterials. These adhesive constructs demonstrated adhesive strengths that were significantly higher than that of Tisseel, and up to 60% of that of Dermabond. These coated adhesives were able to secure two biologic scaffolds to transected porcine tendons while improving their biomechanical properties when compared to the primary suture repair alone. A follow-on in vivo animal study will be performed to further elucidate the effect of adhesive construct-augmented repair on the healing of injured Achilles tendons, as well as integrating this biomaterial with a more aggressive rehabilitation regimen.
Acknowledgments
This work was supported by NIH grant 1R43AR056519-01A1. NMR was performed at the National Magnetic Resonance Facility at Madison (Madison, WI), which is supported by NIH (P41RR02301, P41GM66326, RR02781, RR08438), NSF (DMB-8415048, OIA-9977486, BIR-9214394), the University of Wisconsin, and the U.S. Department of Agriculture. The authors were employees of Nerites Corporation. They gratefully acknowledge Professor Ray Vanderby, Jr (Departments of Biomedical Engineering and Orthopedics and Rehabilitation, University of Wisconsin–Madison) for input related to mechanical testing and data analysis.
References
- [1].Leppilahti J, Orava S. Total Achilles tendon rupture: a review. Sports Med. 1998;25:79–100. doi: 10.2165/00007256-199825020-00002. [DOI] [PubMed] [Google Scholar]
- [2].Strauss EJ, Ishak C, Jazrawi L, Sherman O, Rosen J. Operative Treatment of acute Achilles tendon ruptures: an institutional review of clinical outcomes. Injury. 2007;38:832–8. doi: 10.1016/j.injury.2006.06.005. [DOI] [PubMed] [Google Scholar]
- [3].White DW, Wenke JC, Mosely DS, Mountcastle SB, Basamania CJ. Incidence of major tendon ruptures and anterior cruciate ligament tears in US army soldiers. Am. J. Sports Med. 2007;35:1308–14. doi: 10.1177/0363546507301256. [DOI] [PubMed] [Google Scholar]
- [4].Maffulli N. Rupture of the Achilles tendon. J. Bone Joint Surg. 1999;81A:1019–36. doi: 10.2106/00004623-199907000-00017. http://www.ejbjs.org/cgi/content/full/81/7/1019. [DOI] [PubMed] [Google Scholar]
- [5].Houshian S, Tscherning T, Riegels-Nielsen P. The epidemiology of Achilles tendon rupture in a Danish county. Injury. 1998;29:651–4. doi: 10.1016/s0020-1383(98)00147-8. [DOI] [PubMed] [Google Scholar]
- [6].Pajala A, Kangas J, Ohtonen P, Leppilahti J. Rerupture and deep infection following treatment of total Achilles tendon rupture. J. Bone Joint Surg. 2002;84A:2016–21. doi: 10.2106/00004623-200211000-00017. http://www.ejbjs.org/cgi/content/abstract/84/11/2016. [DOI] [PubMed] [Google Scholar]
- [7].Williams GRJ, et al. Rotator cuff tears: Why do we repair them? J. Bone Joint Surg. 2004;86A:2764–76. http://www.ejbjs.org/cgi/content/full/86/12/2764. [PubMed] [Google Scholar]
- [8].Lee SJ, Sileo MJ, Kremenic IJ, Orishimo K, Ben-Avi S, Nicholas SJ, McHugh M. Cyclic loading of 3 Achilles tendon repairs simulating early postoperative forces. Am. J. Sports Med. 2009;37:786–90. doi: 10.1177/0363546508328595. [DOI] [PubMed] [Google Scholar]
- [9].Lee SJ, Goldsmith S, Nicholas SJ, McHugh M, Kremenic I, Ben-Avi S. Optimizing Achilles tendon repair: effect of epitendinous suture augmentation on the strength of Achilles tendon repairs. Foot Ankle Int. 2008;29:427–32. doi: 10.3113/FAI.2008.0427. [DOI] [PubMed] [Google Scholar]
- [10].Shepard ME, Lindsey DP, Chou LB. Biomechanical testing of epitenon suture strength in Achilles tendon repairs. Foot Ankle Int. 2007;28:1074–7. doi: 10.3113/FAI.2007.1074. [DOI] [PubMed] [Google Scholar]
- [11].Korenkov M, Sauerland S, Arndt M, Bograd L, Neugebauer EAM, Troidl H. Randomized clinical trial of suture repair, polypropylene mesh or autodermal hernioplasty for incisional hernia. Br. J. Surg. 2002;89:50–6. doi: 10.1046/j.0007-1323.2001.01974.x. [DOI] [PubMed] [Google Scholar]
- [12].Herbort M, Haber A, Zantop T, Gosheger G, Rosslenbroich S, Raschke MJ, Petersen W. Biomechanical comparison of the primary stability of suturing Achilles tendon rupture: a cadaver study of Bunnell and Kessler techniques under cyclic loading conditions. Arch. Orthop. Trauma Surg. 2008;128:1273–7. doi: 10.1007/s00402-008-0602-1. [DOI] [PubMed] [Google Scholar]
- [13].Pasternak B, Missios A, Askendal A, Tengvall P, Aspenberg P. Doxycycline-coated sutures improve the suture-holding capacity of the rat Achilles tendon. Acta Orthop. Scand. 2007;78:680–6. doi: 10.1080/17453670710014392. [DOI] [PubMed] [Google Scholar]
- [14].Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J. Bone Joint Surg. 1981;63A:394–9. http://www.ejbjs.org/cgi/content/abstract/63/3/394. [PubMed] [Google Scholar]
- [15].Winter E, Weise K, Weller S, Ambacher T. Surgical repair of Achilles tendon rupture: comparison of surgical with conservative treatment. Arch. Orthop. Trauma Surg. 1998;117:364–7. doi: 10.1007/s004020050267. [DOI] [PubMed] [Google Scholar]
- [16].Kummer FJ, Iesaka K. The role of graft materials in suture augmentation for tendon repairs and reattachment. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2005;74 B:789–91. doi: 10.1002/jbm.b.30296. [DOI] [PubMed] [Google Scholar]
- [17].Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J. Bone Joint Surg. 2007;89:621–30. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- [18].Liden BA, Simmons M. Histologic evaluation of a 6-month GraftJacket matrix biopsy used for Achilles tendon augmentation. J. Am. Podiatr. Med. Assoc. 2009;99:104–7. doi: 10.7547/0980104. http://www.japmaonline.org/cgi/content/abstract/99/2/104. [DOI] [PubMed] [Google Scholar]
- [19].Lee DK. Achilles tendon repair with acellular tissue graft augmentation in neglected ruptures. J. Foot Ankle Surg. 2007;46:451–5. doi: 10.1053/j.jfas.2007.05.007. [DOI] [PubMed] [Google Scholar]
- [20].Lee DK. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J. Foot Ankle Surg. 2008;47:8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- [21].Hohendorff B, Siepen W, Spiering L, Staub L, Schmuck T, Boss A. Long-term results after operatively treated Achilles tendon rupture: fibrin glue versus suture. J. Foot Ankle Surg. 2008;47:392–9. doi: 10.1053/j.jfas.2008.05.006. [DOI] [PubMed] [Google Scholar]
- [22].Hohendorff B, Siepen W, Staub L. Treatment of acute Achilles tendon rupture: fibrin glue versus fibrin glue augmented with plantaris longus tendon. J. Foot Ankle Surg. 2009;48:439–46. doi: 10.1053/j.jfas.2009.04.005. [DOI] [PubMed] [Google Scholar]
- [23].Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res. C. 2005;75:226–36. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- [24].Murphy JL, Vollenweider L, Xu F, Lee BP. Adhesive performance of biomimetic adhesive-coated biologic scaffolds. Biomacromolecules. 2010;11:2976–84. doi: 10.1021/bm1007794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Waite JH. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987;7:9–14. [Google Scholar]
- [26].Yamamoto H. Marine adhesive proteins and some biotechnological applications. Biotechnol. Genet. Eng. Rev. 1996;13:133–65. doi: 10.1080/02648725.1996.10647926. http://www.ncbi.nlm.nih.gov/pubmed/8948111. [DOI] [PubMed] [Google Scholar]
- [27].Lee BP, Dalsin JL, Messersmith PB. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecule. 2002;3:1038–47. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- [28].Guvendiren M, Messersmith PB, Shull KR. Self-assembly and adhesion of DOPA-modified methacrylic triblock hydrogels. Biomacromolecule. 2008;9:122–8. doi: 10.1021/bm700886b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee H, Scherer NF, Messersmith PB. Single molecule mechanics of mussel adhesion. Proc. Natl Acad. Sci. 2006;103:12999–3003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bilic G, et al. Injectible candidate sealants for fetal membrane repair: bonding and toxicity in vitro. Am. J. Obstet. Gynaecol. 2010;202:1–9. doi: 10.1016/j.ajog.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brubaker CE, Kissler H, Wang L, Kaufman DB, Messersmith PB. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials. 2010;31:420–7. doi: 10.1016/j.biomaterials.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Santillan-Doherty P, et al. Thoracoabdominal wall repair with glutaraldehyde-preserved bovine pericardium. J. Invest. Surg. 1996;9:45–55. doi: 10.3109/08941939609012459. [DOI] [PubMed] [Google Scholar]
- [33].Burger JWA, Halm JA, Wijsmuller AR, ten Raa S, Jeekel J. Evaluation of new prosthetic meshes for ventral hernia repair. Surg. Endosc. 2006;20:1320–5. doi: 10.1007/s00464-005-0706-4. [DOI] [PubMed] [Google Scholar]
- [34].Lo Menzo E, Martinez JM, Spector SA, Iglesias A, Degennaro V, Cappellani A. Use of biologic mesh for a complicated paracolostomy hernia. Am. J. Surg. 2008;196:715–9. doi: 10.1016/j.amjsurg.2008.07.012. [DOI] [PubMed] [Google Scholar]
- [35].Turner TM, Urban RM, Hall DJ, Dahlmeier EL. Achilles tendon repair augmented with a decellularized porcine dermal graft compared to two other collagen xenografts; Am. College Foot Ankle Surg. Ann. Meeting; Washington, DC. 2009. [Google Scholar]
- [36].Thermann H, Frerichs O, Biewener A, Krettek C. Healing of the Achilles tendon: an experimental study. Foot Ankle Int. 2001;22:478–83. doi: 10.1177/107110070102200604. http://www.ncbi.nlm.nih.gov/pubmed/11475454. [DOI] [PubMed] [Google Scholar]
- [37].Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons: an experimental study on the early stages of tendon-healing in dogs. J. Bone Joint Surg. 1999;81A:975–82. doi: 10.2106/00004623-199907000-00010. http://www.ejbjs.org/cgi/content/abstract/81/7/975. [DOI] [PubMed] [Google Scholar]
- [38].Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair an experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J. Bone Joint Surg. 2001;83:891–9. http://www.ejbjs.org/cgi/content/abstract/83/6/891. [PubMed] [Google Scholar]
- [39].Bonutti PM, Weiker GG, Andrish JT. Isobutyl cyanoacrylate as a soft tissue adhesive: an in vitro study in the rabbit Achilles tendon. Clin. Orthop. Relat. Res. 1988;229:241–8. http://journals.lww.com/corr/Abstract/1988/04000/SECTION_III_BASIC_SCIENCE_AND_PATHOLOGY_Isobutyl.34.aspx. [PubMed] [Google Scholar]
- [40].Powell ES, Trail IA, Noble J. Non-suture repair of tendons. J. Biomed. Eng. 1989;11:215–8. doi: 10.1016/0141-5425(89)90144-1. [DOI] [PubMed] [Google Scholar]
- [41].Evans CE, Lees GC, Trail IA. Cytotoxicity of cyanoacrylate adhesives to cultured tendon cells. J. Hand Surg. 1999;24B:658–61. doi: 10.1054/jhsb.1999.0279. [DOI] [PubMed] [Google Scholar]
- [42].Ikada Y. Tissue adhesives. In: Chu CC, von Fraunhofer JA, Greisler HP, editors. Wound Closure Biomaterials and Devices. CRC Press; Boca Raton, FL: 1997. pp. 317–46. [Google Scholar]
- [43].Fortelny RH, Petter-Puchner AH, Walder N, Mittermayr R, Öhlinger W, Heinze A, Redl H. Cyanoacrylate tissue sealant impairs tissue integration of macroporous mesh in experimental hernia repair. Surg. Endosc. 2007;21:1781–5. doi: 10.1007/s00464-007-9243-7. [DOI] [PubMed] [Google Scholar]
- [44].Moores AP, Owen MR, Tarlton JF. The three-loop pulley suture versus two locking-loop sutures for the repair of canine Achilles tendons. Vet. Surg. 2004;33:131–7. doi: 10.1111/j.1532-950x.2004.04020.x. [DOI] [PubMed] [Google Scholar]
- [45].Strom AC, Casillas MM. Achilles tendon rehabilitation. Foot Ankle Clin. 2009;14:773–82. doi: 10.1016/j.fcl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- [46].Costa ML, MacMillan K, Halliday D, Chester R, Shepstone L, Robinson AH, Donell ST. Randomized controlled trials of immediate weight-bearing mobilization for rupture of the tendo Achillis. J. Bone Joint Surg. Br. 2006;88:69–77. doi: 10.1302/0301-620X.88B1.16549. http://www.web.jbjs.org.uk/cgi/content/abstract/88-B/1/69. [DOI] [PubMed] [Google Scholar]
- [47].Sorrenti SJ. Achilles tendon rupture: effect of early mobilization in rehabilitation after surgical repair. Foot Ankle Int. 2006;27:407–10. doi: 10.1177/107110070602700603. http://www.ncbi.nlm.nih.gov/pubmed/16764795. [DOI] [PubMed] [Google Scholar]
- [48].Suchak AA, Bostick GP, Beaupré LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J. Bone Joint Surg. Am. 2008;90:1876–83. doi: 10.2106/JBJS.G.01242. [DOI] [PubMed] [Google Scholar]
- [49].Suchak AA, Spooner C, Reid DC, Jomha NM. Postoperative rehabilitation protocols for Achilles tendon ruptures: a meta-analysis. Clin. Orthop. Relat. Res. 2006;445:216–21. doi: 10.1097/01.blo.0000203458.05135.74. [DOI] [PubMed] [Google Scholar]